Abstract
Purpose
Arousals may contribute to the pathogenesis of sleep-disordered breathing (SDB) and central sleep apnea (CSA). We aimed to determine the effect of the nonbenzodiazepine hypnotic zolpidem on the frequency of respiratory-related arousals and central apnea in patients with moderate-to-severe SDB. We hypothesized that zolpidem decreases the severity of SDB by decreasing the frequency of respiratory-related arousals.
Methods
Patients with apnea–hypopnea index ≥ 15 events/hour and central apnea–hypopnea index ≥ 5 events/hour underwent a sleep study on zolpidem 5 mg and a sleep study with no medication in a randomized order. The respiratory arousal index was compared between the two studies using a randomized crossover design. Sleep, respiratory, and physiologic parameters, including the CO2 reserve and the respiratory arousal threshold, were also compared.
Results
Eleven participants completed the study. Compared to no treatment, zolpidem reduced the respiratory arousal index (39.7 ± 7.7 vs. 23.3 ± 4.4 events/h, P = 0.031). Zolpidem also lowered the total apnea–hypopnea index (55.6 ± 8.5 vs. 41.3 ± 7.5 events/hour, P = 0.033) but did not affect other clinical and physiologic parameters. Compared to control, zolpidem did not widen CO2 reserve (− 0.44 ± 1.47 vs. − 0.63 ± 0.86 mmHg, P = 0.81). The respiratory arousal threshold did not show a significant change on zolpidem compared to control (− 8.72 ± 2.1 vs. − 8.25 ± 2.81 cmH2O, P = 0.41).
Conclusion
Nocturnal arousals and overall SDB severity were reduced with a single dose of zolpidem in patients with moderate-to-severe sleep-disordered breathing with increased susceptibility for central apnea. Zolpidem did not widen the CO2 reserve or increase the arousal threshold.
Trial Registration
Clinicaltrials.gov. Sleep and Breathing in the General Population — Chemical Stimuli (NCT04720547).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep-disordered breathing (SDB) poses cardiovascular risks and worsens outcomes if left untreated [1, 2]. Central SDB, or central sleep apnea (CSA), is characterized by a reduction or cessation of the airflow in the upper airways. Current recommendations for the treatment of CSA include positive airway pressure (PAP) therapy and nocturnal oxygen; however, their efficacy remains limited [3,4,5].
Hypnotic agents such as benzodiazepines and nonbenzodiazepines can effectively reduce nocturnal arousals, which have been implicated in the pathogenesis of SDB and CSA, thus offering a potentially effective target for treatment [6,7,8]. Studies have shown mixed results concerning the effect of the hypnotic nonbenzodiazepine zolpidem on the respiratory arousal threshold [9,10,11], the frequency of arousals [6, 10], and SDB severity [6, 10,11,12]. For the most part, these studies are limited to patients with obstructive sleep apnea (OSA), and there have not been any randomized controlled trials assessing the effect of zolpidem in patients with CSA. Thus, we aim to investigate the efficacy of zolpidem in individuals with moderate-to-severe sleep-disordered breathing who have increased susceptibility for central apnea. We hypothesized that zolpidem lowers the severity of sleep-disordered breathing by decreasing the propensity to develop hypocapnic central apnea and decreasing the frequency of respiratory-related arousals.
Methods
The study protocol for the clinical trial Sleep and Breathing in the General Population — Chemical Stimuli (NCT04720547) was approved by the Wayne State University Institutional Review Board and the John D. Dingell VA Medical Center in Detroit, Michigan, USA. Participants were recruited via flyers, recruitment letters, and word of mouth. Additionally, hospital databases were searched to identify patients who completed polysomnography (PSG) studies at a sleep clinic and were diagnosed with CSA. Patients with no record of a previous PSG study or who underwent a PSG study earlier than 2 years at the screening date completed an in-laboratory PSG study to determine eligibility. Furthermore, patients who underwent medical procedures, surgeries, or had changes in health conditions or active medication that may affect sleep following the most recent PSG, underwent an in-lab PSG study irrespective of the date of the previous PSG study. Eligibility criteria included an apnea–hypopnea index (AHI) ≥ 15 and a central AHI (CAHI) ≥ 5. In addition, participants with OSA (AHI ≥ 15) and narrow CO2 reserve (> − 2.0 mmHg) were eligible due to their increased propensity to develop CSA [13]. Participants who met the inclusion criteria and signed an informed consent completed an in-laboratory night study on 5 mg zolpidem and a control night study with no medication. The dose of zolpidem of 5 mg was selected according to current FDA recommendations to avoid adverse effects [14]. Participants whose control night study did not show CSA or a narrow CO2 reserve as defined above were excluded. Additionally, patients with a history of severe respiratory, cardiac, renal, and neurologic disease and those with co-morbid sleep conditions were excluded.
The order of the night studies (zolpidem and control) was randomized according to a randomized crossover study design. The randomization sequence for treatment order was generated via Microsoft Excel v16. A placebo was not used, and thus, participants were not blinded to the treatment order. Lab staff performing the night studies had to administer the treatment and were not blinded to the treatment order either. Analysis of physiologic parameters and sleep studies scoring were performed by lab personnel and reviewed by the principal investigator or a co-investigator, all of whom were blinded to the study treatment arm at the time of the scoring, analysis, and review. Statistical analysis of outcomes was also performed without knowledge of the treatment arms. Night studies were scheduled based on participants’ availability but had to be within 6 months of each other. Zolpidem has a short elimination half-life, and a washout period for a single 5 mg dose is unnecessary. Therefore, consecutive night studies were scheduled when possible. Participants were contacted by phone, and their medical records were reviewed during each sleep study to ensure that no changes in medical conditions or medications occurred.
In-laboratory night studies
An enhanced PSG was performed during both nights. The PSG included electroencephalography, electrooculography, surface electromyography, electrocardiography, and pulse oximetry (Carefusion, SomnoStar z4 Sleep System, San Diego, CA, USA) consistent with the technical criteria of the American Academy of Sleep Medicine (AASM) [15]. Additionally, a nasal mask was connected to an in-line pneumotachometer (Hans Rudolph; model 3700A, Shawnee, KS, USA). An RSS 100HR Research Pneumotach System acquired data (Hans Rudolph) to achieve airflow, pressure, and volume measurements. The end-tidal partial pressure of carbon dioxide (PETCO2) was measured by connecting a tube placed in the nasal vestibule to a respiratory gas analyzer (CWE, Inc., GEMINI Respiratory Monitor, Ardmore, PA, USA). Airflow and PETCO2 were recorded by a PowerLab data acquisition system (AD Instruments, Inc., model 16SP, Colorado Springs, CO, USA). Tidal volume (VT) was derived by integrating the airflow channel, and minute ventilation (VE) was calculated by multiplying VT by breathing frequency (FB). Arterial oxygen saturation was monitored by an ear clip oximeter (Ohmeda Medical Inc., Biox 3740, Laurel, MD, USA). PSG variables, including apnea, arousal, and desaturation indices, were calculated during both nights.
Physiologic parameters
In addition to PSG, a noninvasive positive air pressure ventilation protocol was used to calculate the CO2 reserve and the controller gain as described in an earlier study [16]. Briefly, the CO2 reserve was defined as the difference between the eupneic end-tidal CO2 pressure and the end-tidal CO2 pressure (PETCO2) induced by the ventilation protocol that is sufficient to trigger a central apnea as defined by the AASM. In the case of participants in which we could not acquire a period of stable eupneic breathing due to the persistence of spontaneous central events, we administered a 40% CO2 gas mixture balanced with nitrogen to stabilize breathing. The combination was administered at 5-min intervals in 0.5 L/min increments. The volume per unit time of the mixture gas required to resolve central events was determined, and PETCO2 was allowed to return to baseline. We performed three 5-min trials at the therapeutic volume per unit of the mixture gas separated by sufficient time to return to baseline PETCO2. Here, the CO2 reserve was defined as the difference between baseline PETCO2 and the PETCO2 during the administration of the gas mixture at which central events were resolved.
Controller gain was calculated by dividing the difference in minute ventilation between the post-mechanical ventilation induced by the ventilation protocol and steady-state respiration by the difference in the end-tidal CO2 pressure between the mechanical ventilation and steady-state respiration. In participants administered the gas mixture as described above, the controller gain was not calculated due to the absence of mechanical ventilation in that protocol. Steady-state plant gain was calculated from the ratio of end-tidal CO2 to minute ventilation during stable respiration. All physiologic parameters were calculated during non-rapid eye movement (non-REM) sleep in both the control and the zolpidem night studies.
Arousal threshold
Measuring the respiratory arousal threshold has been previously described [17, 18]. During both nights, a supraglottic catheter was inserted in the patient’s nostril to measure epiglottic pressure throughout the PSG portion of the night study. The epiglottic nadir pressure occurring immediately before cortical arousal and during an obstructive respiratory event as defined by the AASM marked one instance of effort sufficient to trigger the arousal [18]. These instances were measured for all obstructive events followed by arousals during the PSG portion and averaged to produce the respiratory arousal threshold during REM and non-REM sleep.
Outcomes
The primary endpoint was the frequency of respiratory-related arousals, or the respiratory arousal index (R-ArI). R-ArI represents the number per hour of arousals resulting from respiratory events. Clinical outcomes were also compared between the two-night studies and included the total respiratory arousal index (ArI), AHI, and CAHI. The total ArI represents the number per hour of all arousals, whether spontaneous or due to a respiratory event. Physiologic outcomes, including the CO2 reserve, plant gain, controller gain, and arousal threshold, were calculated. Sleep and desaturation parameters are reported as well.
Statistical analysis
Demographic characteristics and clinical outcome measures were summarized as the mean and standard error of the mean (SEM), or frequency and percentage scores, as applicable. A crossover design was used to investigate the potential influence of sequence (the order of medication administration) and period (the time of medication administration) on the effects of the two treatments (control and zolpidem) on the clinical outcomes. The significance level was set at a p-value equal to or less than 0.05. For the primary endpoint and clinical outcomes, the difference between the control night and the intervention night was calculated for each participant, and Grubb’s test was used to identify outliers (alpha = 0.05). All statistical analyses were carried out using SPSS (Version 27), SAS (Version 9.4), and R (Version 4.1.1). In an equivalence test of means using a two-period crossover design, a total sample size of 10 achieved 80% power at a 5% significance level to detect a difference of 0.85 SD in means R-ArI, when the actual difference between the means was 0.00. The PASS computer software (version 11, Hintze, 2011) was used to estimate the required sample size.
Results
Participants
Eleven participants completed the study (nine men; seven self-identified as African American and four as White; average age 62.9 ± 11.8 years; average body mass index was 30.8 ± 5.3 kg/m2). Two participants had a previous spinal cord injury. Before randomization, participants completed the Epworth Sleepiness Scale (ESS), the Fatigue Severity Scale (FSS) questionnaire, the Pittsburgh Sleep Quality Index (PSQI) questionnaire, and the Berlin questionnaire. The demographics at baseline are reported in Table 1. Nine participants had AHI ≥ 15 and CAHI ≥ 5, and the remaining two were included after the baseline study revealed a narrow CO2 reserve. The sample included two participants receiving 10 mg of zolpidem for other reasons. Study participants were free of significant cardiopulmonary disease or morbid obesity. None of the participants had a history of heart failure or history and/or current use of opioid medications. CSA was likely idiopathic, resulting from an injury or unknown cause.
Effect of Zolpidem on the R-ArI
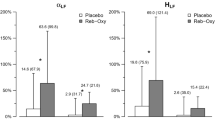
PSG data were collected for all participants in the study, and R-ArI was calculated for the treatment night and the control night. One outlier was identified (two-sided Grubb’s test p = 0.042) and removed from the final analysis. Thus, the final R-ArI study included a total of 10 participants. Zolpidem significantly decreased R-ArI compared to no treatment (R-ArI 23.3 ± 4.4 vs. 39.7 ± 7.7 events/h, F = 6.79, p = 0.031) Fig. 1 and Table 2. Individual data are summarized in Fig. 1. No period or sequence effects were detected.
Primary endpoint and main clinical outcomes following zolpidem treatment for n = 10 participants for R-ArI, AHI, and total ArI, and n = 8 for CAHI. CAHI for the two participants with CAHI < 5 events/hour at baseline who were recruited based on the presence of narrow CO2 were not included in the analysis. Gray and white boxplots represent the interquartile ranges and medians (solid lines) of the number of events for the no treatment condition and the zolpidem condition, respectively. The dashed lines represent the mean number of events, and individual data points are overlayed on the boxplots. *P-value ≤ 0.05. R-ArI; respiratory arousal index, ArI: arousal index, AHI: apnea–hypopnea index; CAHI: central apnea–hypopnea index
Clinical outcomes
The clinical outcomes are summarized in Fig. 1and Table 2. The single low dose of zolpidem did not result in changes in sleep efficiency and time spent in any sleep stage and was, therefore, well-tolerated. Additionally, there were no significant changes in oxygen desaturation. Zolpidem resulted in a statistically significant decrease in the total AHI and ArI. Main PSG and physiologic outcomes are summarized in Table 2, and individual data are summarized in Fig. 1.
Physiologic outcomes
The CO2 reserve and plant gain data were available for eight participants. For the remaining three participants, we could not complete the non-invasive ventilation protocol.
Controller gain data were available for 5 participants because the alternative protocol requiring a gas mixture (described in the Methods section) was performed in three participants, which prevented us from calculating the controller gain for those participants. The physiologic parameters did not change on zolpidem compared to no treatment (CO2 reserve − 0.44 ± 1.47 vs. − 0.63 ± 0.86 mmHg, F = 0.07, P = 0.81; plant gain 4.78 ± 0.28 vs. 5.27 ± 0.36 mmHg·L·min−1, F = 3.2, P = 0.12; controller gain 2.39 ± 0.49 vs. 3.95 ± 1.05 L·min−1·mmHg, F = 5.4, P = 0.06). Results are summarized in Table 3. The change in the CO2 reserve for each participant is shown in Fig. 3. The respiratory arousal threshold was measured for 4 participants and did not show a significant change on zolpidem (− 8.72 ± 2.1 vs. − 8.25 ± 2.81 cmH2O, F = 0.78, P = 0.41) Fig. 2. The respiratory arousal threshold was not measured for the remaining participants due to poor catheter signal or discomfort with the catheter that led to its removal. We also assessed the number of participants with a low arousal threshold according to PSG parameters as outlined by Edwards et al.. We found 5 out of 11 patients with a low arousal threshold during the no-treatment night and 4 out of 11 during the Zolpidem night study [17]. Out of the 5 patients with a low arousal threshold, one did not show a low arousal threshold during the zolpidem night study, whereas no changes were seen in others.
Discussion
Our study found that administering a single dose of zolpidem decreased the frequency of respiratory-related arousals (R-ArI) and total arousals. While this was associated with decreased AHI, there was no effect on CAHI or the propensity to hypocapnic central apnea.
Ventilatory overshoot in the aftermath of apnea or hypopnea is considered a potential mechanism of breathing instability and recurrent central apnea. The physiological premise is based on the notion that hyperventilation during the arousal would bring the arterial PCO2 below the apneic threshold when sleep is resumed. Empirical evidence supporting this potential mechanism has been modest. Bonnet et al. demonstrated that administration of triazolam in five men with central sleep apnea was associated with decreased apnea severity and arousal frequency [8]. More recently, Quadri et al. demonstrated a significant decrease in the arousal frequency in patients with idiopathic CSA after one night of zolpidem treatment in a nonrandomized open-label study [6]. The investigators also found a significant correlation between the change in R-ArI and CAHI. It is of note that the study excluded patients with heart failure, Cheyne-Stokes respiration, obstructive apnea–hypopnea index ≥ 5 events/hour, and a history of restless leg syndrome. In addition, patients undergoing PAP or oxygen therapy were also excluded. Therefore, the sample in that study may not be representative of the CSA patient population [7, 19].
We did not find an improvement in CAHI or a correlation between the R-ArI and CAHI. However, extant literature regarding the effect of zolpidem on arousal threshold/frequency is inconclusive. For example, Messineo et al. conducted a randomized controlled trial in patients with OSA and a low or moderate arousal threshold. The study demonstrated an increased arousal threshold in the zolpidem arm compared to the placebo arm; however, there was no change in the arousal index following a one-night treatment with zolpidem [9]. Likewise, in a retrospective study comprising 444 patients, Smith et al. found no change in the arousal threshold type calculated via PSG variables described by Edwards et al. in patients with a low arousal threshold and OSA [11]. Overall, the effect of zolpidem on the severity of SDB remains uncertain. Some studies, such as Quadri et al., demonstrated decreased SDB severity, whereas no change was detected in several other studies in patients with OSA.
The biological plausibility of suppressing arousals on CSA is based on the premise that hypocapnia due to transient post-apneic hyperventilation will suppress the ventilatory motor output and precipitate central apnea. However, empirical proof supporting this postulate remains modest. Specifically, brief hyperventilation (1 min) is rarely associated with central apnea, except in the presence of concomitant hyperoxia. Similarly, apnea and hypopnea did not occur after induced brief auditory arousals in healthy individuals [20]. Overall, short periods of hyperventilation are unlikely to produce medullary hypocapnia of sufficient magnitude to induce central apnea. However, the development of central apnea following brief hyperventilation under hyperoxic conditions may reflect the interdependence of peripheral and central chemoreceptors. In addition, actively induced hyperventilation is associated with the activation of short-term potentiation, which mitigates the subsequent ventilatory decline [21].
The lack of reduction in CAHI, despite decreased AHI, is intriguing. One possible explanation is the small sample size. Thus, central hypopneas may manifest as obstructive events because they cause pharyngeal narrowing. Alternatively, hypocapnic hypopnea may be associated with upper airway narrowing and may be scored as obstructive despite a central etiology driving upper airway narrowing. Using fiberoptic pharyngeal imaging, Sankri-Tarbichi et al. demonstrated expiratory pharyngeal narrowing and increased expiratory resistance during central hypocapnic hypopnea [22].
Clinical implications
While a decrease in nocturnal arousals was achieved with a single dose of zolpidem, the reduction in AHI was modest and of questionable clinical significance. Furthermore, there was no decrease in CAHI. Therefore, zolpidem does not seem to offer an alternative to current CSA treatments, including PAP, oxygen, and acetazolamide. The long-term tolerability profile of zolpidem in patients with CSA and the effect of long-term treatment with zolpidem on the severity of CSA remain to be determined. Our data do not support the routine use of zolpidem for CSA treatment.
Methodological considerations
Our study has some methodological considerations to highlight. First, patients had moderate-to-severe SDB (AHI ≥ 15) with the presence of central events (CAHI ≥ 5 events /hour) and/or increased susceptibility for CSA but may not have fulfilled the diagnostic criteria for CSA based on ICSD-3 [23]. Second, although we used a low dose of zolpidem (5 mg) to minimize potential side effects should a favorable outcome be found, there were two exceptions in two participants receiving higher doses for other reasons, and we did not wish to use a less effective amount. Third, our study does not address the long-term effect on daytime function. However, the lack of effect on the arousal threshold or the CO2 reserve provides a mechanistic underpinning for the lack of clinical effect. While these physiological measurements were obtained in a subset of participants only, findings corroborate the study’s overall results. Fourth, we did not restrict recruitment to a low-baseline arousal threshold, identified as OSA phenotype but not CSA [24]. However, there was no change in the arousal threshold score derived from the PSG parameters by Edwards et al. [17]. This method has not been validated in patients with significant CSA, and it does not capture changes in the arousal threshold unless patients cross the threshold of the low arousal threshold in either direction to change the outcome of the equation. Fifth, while no worsening of obstructive events was noted in our study, we cannot make such inference in patients with significant co-morbid cardiopulmonary disease or morbid obesity. Lastly, the CO2 reserve protocol was carried out in the initial portion of the night study before the PSG portion in both the zolpidem and control studies. Since sleep architecture is known to change throughout the night, the reported parameters may be influenced by the portion of the night during which they were captured. However, we remained consistent with the order of the protocol and PSG to limit any potential effect on the results for all participants. In addition, a placebo was not used, and thus, participants were not blinded to the treatment order. The night studies’ research team had to administer the treatment and were not blinded to the treatment order either. However, all analysis and sleep studies scoring were blinded to investigators.
Conclusion
Administering a single, low dose of the hypnotic nonbenzodiazepine zolpidem reduced the frequency of respiratory arousals and the total AHI. Zolpidem, however, did not lower the CAHI or the propensity to develop CSA.
Data availability
The authors may provide access to data using the specified submission pursuant to the following criteria: Datasets meeting VA standards for disclosure to the public will be made available within 1 year of publication. Prior to distribution, a privacy officer will certify that all datasets contain no PHI. Guidance on request and distribution processes will be provided by the office of research and development (ORD). Those requesting data will be asked to sign a Letter of Agreement.
Abbreviations
- AHI:
-
Apnea-hypopnea index
- AI:
-
Apnea index
- ArI:
-
Arousal index
- FB :
-
Breathing frequency
- PETCO2 :
-
Carbon dioxide end-tidal partial pressure
- CAHI:
-
Central apnea–hypopnea index
- CAI:
-
Central apnea index
- CHI:
-
Central hypopnea index
- CSA:
-
Central sleep apnea
- ESS:
-
Epworth Sleepiness Scale
- FSS:
-
Fatigue Severity Scale
- HI:
-
Hypopnea index
- VE :
-
Minute ventilation
- MAI:
-
Mixed apnea index
- OAI:
-
Obstructive apnea index
- OAHI:
-
Obstructive apnea–hypopnea index
- OHI:
-
Obstructive hypopnea index
- OSA:
-
Obstructive sleep apnea
- ODI:
-
Oxygen desaturation index
- PSQI:
-
Pittsburgh Sleep Quality Index
- PSG:
-
Polysomnography
- PAP:
-
Positive airway pressure
- R-ArI:
-
Respiratory arousal index
- SDB:
-
Sleep-disordered breathing
- S-ArI:
-
Spontaneous arousal index
- VT :
-
Tidal volume
References
Tietjens JR, Claman D, Kezirian EJ et al (2019) Obstructive sleep apnea in cardiovascular disease: a review of the literature and proposed multidisciplinary clinical management strategy. J Am Heart Assoc 8(1):e010440. https://doi.org/10.1161/JAHA.118.010440
Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S (2017) INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists) Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation 136(19):1840–1850. https://doi.org/10.1161/CIRCULATIONAHA.117.029400
Aurora RN, Chowdhuri S, Ramar K, et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35(1):17–40. Published 2012 Jan 1. https://doi.org/10.5665/sleep.1580
Bradley TD, Logan AG, Kimoff RJ et al (2005) Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 353(19):2025–2033. https://doi.org/10.1056/NEJMoa051001
Bordier P, Lataste A, Hofmann P, Robert F, Bourenane G (2016) Nocturnal oxygen therapy in patients with chronic heart failure and sleep apnea: a systematic review. Sleep Med 17:149–157. https://doi.org/10.1016/j.sleep.2015.10.017
Quadri S, Drake C, Hudgel DW (2009) Improvement of idiopathic central sleep apnea with Zolpidem. J Clin Sleep Med 5(2):122–129
Eckert DJ, Jordan AS, Merchia P, Malhotra A (2007) Central sleep apnea: pathophysiology and treatment. Chest 131(2):595–607. https://doi.org/10.1378/chest.06.2287
Bonnet MH, Dexter JR, Arand DL (1990) The effect of triazolam on arousal and respiration in central sleep apnea patients. Sleep 13(1):31–41. https://doi.org/10.1093/sleep/13.1.31
Messineo L, Eckert DJ, Lim R et al (2020) Zolpidem increases sleep efficiency and the respiratory arousal threshold without changing sleep apnoea severity and pharyngeal muscle activity. J Physiol 598(20):4681–4692. https://doi.org/10.1113/JP280173
Carberry JC, Fisher LP, Grunstein RR, et al. Role of common hypnotics on the phenotypic causes of obstructive sleep apnoea: paradoxical effects of Zolpidem. Eur Respir J. 2017;50(6):1701344. Published 2017 Dec 28. https://doi.org/10.1183/13993003.01344-2017
Smith PR, Sheikh KL, Costan-Toth C, et al. Eszopiclone and Zolpidem do not affect the prevalence of the low arousal threshold phenotype. J Clin Sleep Med. 2017;13(1):115–119. Published 2017 Jan 15. https://doi.org/10.5664/jcsm.6402.
Carberry JC, Grunstein RR, Eckert DJ (2019) The effects of Zolpidem in obstructive sleep apnea — an open-label pilot study. J Sleep Res 28(6):e12853. https://doi.org/10.1111/jsr.12853
Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS (2010) Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med 181:189–193. https://doi.org/10.1164/rccm.200810-1658OC
FDA (2018). Questions and answers: risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing Zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). https://www.springer.com/journal/11325/submission-guidelines. Accessed 15 November 2021
Berry RB, Brooks R, Gamaldo C, et al. AASM scoring manual updates for 2017 (Version 2.4). J Clin Sleep Med. 2017;13(5):665–666. Published 2017 May 15. https://doi.org/10.5664/jcsm.6576
Ginter G, Sankari A, Eshraghi M et al (2020) Effect of acetazolamide on susceptibility to central sleep apnea in chronic spinal cord injury. J Appl Physiol (1985) 128(4):960–966. https://doi.org/10.1152/JApplPhysiol.00532.2019
Edwards BA, Eckert DJ, McSharry DG et al (2014) Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med 190(11):1293–1300. https://doi.org/10.1164/rccm.201404-0718OC
Rizwan A, Sankari A, Bascom AT, Vaughan S, Badr MS (2018) Nocturnal swallowing and arousal threshold in individuals with chronic spinal cord injury. J Appl Physiol (1985) 125(2):445–452. https://doi.org/10.1152/japplphysiol.00641.2017
Bradley TD, Floras JS (2003) Sleep apnea and heart failure: Part II: central sleep apnea. Circulation 107(13):1822–1826. https://doi.org/10.1161/01.CIR.0000061758.05044.64
Badr MS, Morgan BJ, Finn L et al (1997) Ventilatory response to induced auditory arousals during NREM sleep. Sleep 20(9):707–714. https://doi.org/10.1093/sleep/20.9.707
Badr MS, Skatrud JB, Dempsey JA (1992) Determinants of poststimulus potentiation in humans during NREM sleep. J Appl Physiol (1985) 73(5):1958–1971. https://doi.org/10.1152/jappl.1992.73.5.1958
Sankri-Tarbichi AG, Rowley JA, Badr MS (2009) Expiratory pharyngeal narrowing during central hypocapnic hypopnea. Am J Respir Crit Care Med 179(4):313–319. https://doi.org/10.1164/rccm.200805-741OC
Sateia MJ (2014) International classification of sleep disorders-third edition: highlights and modifications. Chest 146:1387–1394. https://doi.org/10.1378/chest.14-0970
Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A (2013) Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 188(8):996–1004. https://doi.org/10.1164/rccm.201303-0448OC
Funding
The US Department of Veterans Affairs supported this study: Merit Review Award # CX001944-01 and NIH R01 HL 130552. The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Wayne State University Institutional Review Board and the John D. Dingell VA Medical Center in Detroit, Michigan, USA) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, B., Sankari, A., Eshraghi, M. et al. Effect of Zolpidem on nocturnal arousals and susceptibility to central sleep apnea. Sleep Breath 27, 173–180 (2023). https://doi.org/10.1007/s11325-022-02593-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-022-02593-3