Abstract
Purpose
Chemical exchange saturation transfer MRI using an infusion of glucose (glucoCEST) is sensitive to the distribution of glucose in vivo; however, whether glucoCEST is more related to perfusion or glycolysis is still debatable. We compared glucoCEST to computed tomography perfusion (CTP), [18F] fluorodeoxyglucose positron emission tomography (FDG-PET), and hyperpolarized [1-13C] pyruvate magnetic resonance spectroscopy imaging (MRSI) in a C6 rat model of glioma to determine if glucoCEST is more strongly correlated with measurements of perfusion or glycolysis.
Methods
106 C6 glioma cells were implanted in Wistar rat brains (n = 11). CTP (including blood volume, BV; blood flow, BF; and permeability surface area product, PS) and FDG-PET standardized uptake value (SUV) were acquired at 11 to 13 days post-surgery. GlucoCEST measurements (∆CEST) were acquired the following day on a 9.4 T MRI before and after an infusion of glucose solution. This was followed by MRSI on a 3.0 T MRI after the injection of hyperpolarized [1-13C] pyruvate to generate regional maps of the lactate:pyruvate ratio (Lac:Pyr). Pearson’s correlations between glucoCEST, CTP, FDG-PET, and Lac:Pyr ratio were evaluated.
Results
Tumors had significantly higher SUV, BV, and PS than the contralateral brain. Tumor ∆CEST was most strongly correlated with CTP measurements of BV (ρ = 0.74, P = 0.01) and PS (ρ = 0.55, P = 0.04). No significant correlation was found between glycolysis measurements of SUV or Lac:Pyr with tumor ∆CEST. PS significantly correlated with SUV (ρ = 0.58, P = 0.005) and Lac:Pyr (ρ = 0.75, P = 0.005). BV significantly correlated with Lac:Pyr (ρ = 0.57, P = 0.02), and BF significantly correlated with SUV (ρ = 0.49, P = 0.02).
Conclusion
This study determined that glucoCEST is more strongly correlated to measurements of perfusion than glycolysis. GlucoCEST measurements have additional confounds, such as sensitivity to changing pH, that merit additional investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glioblastoma multiforme (GBM) is both the most common and the most aggressive type of malignant brain tumor. Regardless of technical advances, the median survival period for patients who are diagnosed with GBM remains approximately 12–15 months [1]. Angiogenesis, the formation of blood vessels, is regarded as one of the hallmarks of GBM. These newly formed vessels facilitate the delivery of nutrients such as glucose to cancer cells, contributing to the observed increase in tumor metabolism (glycolysis) and, ultimately, tumor proliferation [2]. Inevitably, measurements of tumor perfusion and glucose metabolism are interrelated and are important imaging biomarkers for predicting tumor cell proliferation and assessing treatment response. Chemical exchange saturation transfer (CEST), a magnetic resonance imaging (MRI) technique, may be used to monitor both tumor perfusion and glucose metabolism [3]. In particular, CEST is sensitive to endogenous glucose, potentially allowing us to monitor the change in metabolite concentration over time after an injection of glucose (glucoCEST) [4, 5]. Originally, researchers proposed that the change in concentration monitored using glucoCEST in the tumor region was associated with glucose metabolism [6, 7]. However, recent studies have shown that these changes are also sensitive to tumor perfusion [8, 9].
To evaluate glucoCEST as a surrogate biomarker of tumor perfusion and/or tumor glycolysis, we compared various well-defined in vivo perfusion and glycolysis measurements in the C6 rat glioma model. Tumor perfusion maps such as blood flow (BF), blood volume (BV), and permeability surface-area product (PS) were derived with computed tomography perfusion (CTP) measurements acquired during a bolus injection of iodinated contrast agent (Isovue). Glucose metabolism was evaluated with positron emission tomography (PET) and 18F-fluorodeoxyglucose (FDG), a glucose analogue which is phosphorylated in the cell but does not go through subsequent steps of glycolysis. Aerobic glycolysis was measured with MR spectroscopic imaging (MRSI) after an injection of hyperpolarized [1-13C]pyruvate.
Methods
The experimental timeline is shown in Fig. 1a. In short, C6 glioma tumor cells were implanted on day 0. A CT perfusion check was performed on day 7 to check if the diameter of the tumor has reached 4 mm [10,11,12]. FDG-PET followed by CT perfusion acquisition was performed between days 11 and 13 after C6 cell implantation. GlucoCEST MRI followed by hyperpolarized [1-13C] pyruvate MRI experiments was performed on the next day immediately prior to euthanization. All imaging experiments took place within a span of 24 h for each animal: FDG-PET and CT perfusion in the afternoon/evening, followed by 9.4 T MRI (glucoCEST) and 3 T MRI (hyperpolarized MRSI) in the morning/afternoon of the subsequent day. All procedures were performed in compliance with the Canadian Council for Animal Care and approved by the Western University Animal Care Committee.
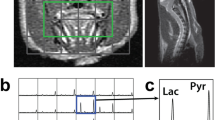
a Study overview. Day 0 corresponds to the tumor cell implantation. A CT perfusion check was performed on day 7 to monitor if the tumor had reached optimal size (diameter > 4 mm). An FDG-PET acquisition followed by CT perfusion experiment was carried out between days 11 and 13. The glucoCEST experiment and hyperpolarized 13C-pyruvate MRSI took place on the following day (days 12 to 14). b Dynamic CEST experiment. There were three CEST acquisitions: a baseline CEST acquisition (pre), a second CEST acquisition during the first 30 min of glucose infusion (during 1), and a final CEST acquisition during the last 30 min of glucose infusion (during 2).
C6 Glioma Model
Male Wistar rats (Charles River, Canada, age 8 to 10 weeks at surgery) weighing on average 401 ± 42 g (N = 11) were used in this study. The animals were induced with 5 % isoflurane and were maintained at 2–3 % for all surgical and imaging procedures.
C6 glioma cells (CCL-107, American Type Culture Collection, Manassas, VA) were cultivated in F12k 15 % horse serum, 2.5 % bovine serum, and 1 % penicillin-streptomycin. Animals were placed into a stereotactic surgical frame during C6 glioma cell implantation. The animals were anesthetized with 2 % isoflurane and were injected with Metacam (Boehringer Ingelheim Vetmedica Inc., Duluth, USA, 10 mg/kg). The bregma was exposed after a scalp incision; a 1-mm-diameter burr hole was drilled at a point 1 mm anterior and 3 mm right of the bregma. A total of 106 C6 glioma cells suspended in 10 μL were slowly injected over a period of 5 min at a depth of 3–4 mm from the skull surface with a Hamilton syringe. The burr hole was sealed with bone wax, and the scalp was closed with sutures. The subjects were recovered and returned to animal care housing where they received an additional subcutaneous injection of Metacam (10 mg/kg) 12 h after surgical recovery.
CT Perfusion Imaging and Analysis
Detailed CT perfusion acquisition and analysis procedures can be found in previous research publications [10, 11]. In short, a non-contrast CT scan was performed prior to the perfusion scan to identify sixteen slices covering the entire brain with each section 1.25-mm thick (GE Discovery RCT). CT perfusion data were acquired over two back-to-back time periods. A fine temporal resolution (1.4-s intervals) was used during the first time period (32-s duration), and a coarse temporal resolution (15-s intervals) was used in the second period (165-s duration). Two to 3 s after the start of the first phase, a bolus of contrast (Isovue, Bracco Diagnostics Inc, Vaughan, Canada, 300 mg iodine/mL, 2.5 mL/kg body weight) was injected into a lateral tail vein at a rate of 0.13 mL/s using a syringe pump (New Era Pump Systems, Inc, Farmingdale, NY, USA). The scan parameters were 80 kVp, 120 mAs, and 10 cm field-of-view with a high-definition bone filter. A change of CT number as a function of time can be measured using the dynamic series of CTP images. The subjects were able to proceed to the subsequent experiments when the axial diameter of the tumor reached 4 mm [11, 12]. The CT perfusion measurements that were used in the perfusion analysis were acquired days 11–13 on a clinical CT scanner (Discovery 750 HD, GE Healthcare, Waukesha, WI) after a 75-min dynamic FDG-PET acquisition.
CT perfusion 5 (GE Healthcare) was used to generate maps of BF, BV, and PS. The time attenuation curve (TAC) from the carotid artery was selected as the arterial input. The arterial TAC was deconvolved with tissue TACs measured from 2 × 2 pixel blocks of CT images using the Johnson-Wilson model to calculate maps of BF, BV, and PS [12, 13]. The tissue enhancement curve can be expressed as the convolution between the blood flow-scaled impulse residue function (IRF), BF·R(t), and the arterial TAC, Ca(t). The shape of the BF-scaled IRF has two distinct phases, and it is solved by deconvolving the arterial TAC with the tissue enhancement curve. The plateau of the BF-scaled IRF defines the BF, while the area under the first phase of the BF-IRF is the BV. The second phase of the BF-scaled IRF starts at the height of the extraction fraction, which is the fraction of contrast agent that leaks into the interstitial space. The second phase of the BF-scaled IRF decays with time, and PS can be calculated as
FDG-PET Imaging
The FDG-PET acquisition took place 11–13 days after the surgery on a small animal PET system (Inveon DPET, Siemens Healthineers, Knoxville, TN, USA). The blood glucose level of each subject was tested using a glucometer prior to FDG-PET acquisition (CONTOUR meter, Ascensia Diabetes Care, Parsippany, NJ, USA). A 75-min emission scan was performed with a timing window of 3.432 ns and 350–640 keV discrimination energy range. Longitudinal PET data were binned into successive time intervals comprised of 10 frames of 30 s, 5 frames of 60 s, 10 frames of 300 s, and 1 frame of 900 s, totaling 75 min. An iterative, 3D ordered subset expectation maximization (OSEM3D) reconstruction was used to generate dynamic images for all frames with 2 iterations and 18 subsets. Approximately 30 s after the acquisition had started, a bolus of 18F-FDG (31.5 ± 3.1 MBq) was injected through a lateral tail vein. Anatomical CT images acquired after the PET acquisition were used to generated attenuation corrected PET images. The CT images were co-registered with the PET images using ASIPro (Siemens Healthineers, Knoxville, TN, USA). The co-registered CT images were used to generate a mu-map by converting Hounsfield units into linear attenuation coefficients for 511 keV gamma rays. The mu-map was then converted into an attenuation sinogram. The PET images were reconstructed using the attenuation sinogram to create the attenuation corrected PET images.
Standardized uptake values (SUV) were calculated using the last 15 min of the dynamic PET images as a surrogate for glucose metabolism. SUV was defined as SUV = Activity Concentration of ROI/(Injected Dose/Weight of the Subject) [14].
Dynamic glucoCEST Imaging
MRI was acquired on a 9.4-T small animal MRI (Agilent, Palo Alto, CA, USA) the day after PET/CT (12–14 days after the surgery). Axial T2-weighted images using a 2-dimensional fast spin-echo (FSE) sequence (repetition time, TR = 3000 ms; echo time, TE = 10 ms; effective TE = 40 ms; echo train length = 4; number of slices = 29; slice thickness = 1 mm; field-of-view, FOV = 3.84 × 3.84 cm; matrix size = 128 × 128; acquisition time ≈ 3 min) were acquired first to locate the slice that contained the largest tumor cross section. CEST spectra were acquired using a continuous wave pre-saturation pulse (saturation time, TS = 5 s; B1 saturation pulse amplitude = 1. μT; offset frequency pulse alternated between negative and positive, from − 5/+5 to 0 ppm with 0.2 ppm steps) preceding a series of FSE images acquired on the slice that contained the largest tumor cross-section (TR = 3 s, effective TE = 40 ms, echo train length = 32, slice thickness = 2 mm, FOV = 3.84 × 3.84 cm FOV, matrix size = 128 × 128; acquisition time ≈ 30 min). Three spectra were obtained from three consecutive 30-min CEST acquisitions. The first CEST spectrum was acquired prior to a 20 % D-glucose infusion. A bolus of 20 % D-glucose solution (0.3 g/kg) was injected at the end of the first spectrum acquisition, following by a 60-min constant infusion of the same 20 % D-glucose solution (1.5 g/kg/h). The acquisition of the second and third CEST spectra corresponded to the first and second half of the constant infusion of glucose, respectively. The CEST imaging workflow is illustrated in Fig. 1b.
All CEST data were analyzed using MATLAB 2018b. A mask of the brain was delineated using the T2-weighted MR images and applied to the CEST images. A smoothing spline with a fitting parameter of 0.998 was used to generate Z-spectra at each pixel using all 53 CEST images. B0 correction was performed by shifting the frequency associated with the Z-spectra minima to 0 ppm. A magnetization transfer ratio asymmetry (MTRasym) spectrum [15] at each pixel is calculated as shown in Eq. (1).
The area under the curve (AUC) is calculated using MTRasym (AUCMTR) values from 1.2 to 2.8 ppm [6], and the relative change after glucose infusion is calculated as ∆CEST (see Eq. (2)). Note that AUC(during-infusion) corresponds to the last 30 min of the glucose infusion (the third CEST spectra), as a different imaging protocol was used in some of the experiments, while the blood glucose was still increasing in the first 30 min of the glucose infusion.
Hyperpolarized [1-13C]pyruvate
Detailed hyperpolarization and imaging procedures can be found in Lim et al. [16]. Rats were transported to a GE Discovery MR750 3.0 T MRI (General Electric Healthcare, Waukesha WI, USA) under anesthesia with 2 % isoflurane immediately after the glucose CEST experiment. An FSE sequence (80 × 80 mm FOV, 0.3-mm in-plane resolution, 3-mm slice thickness, TR = 4000 ms, TE = 85 ms, 16 echo trains, and 9 averages) was used to acquire axial T2-weighted 1H images which were later used to determine the axial slice(s) that contained the tumor and located at the approximate the same location using the CEST slice information. ProHance gadolinium contrast agent (Bracco Diagnostics, Monroe Township, NJ, USA) was added at a concentration of 1 mM to [1-13C] pyruvate acid (Sigma Aldrich, Miamisburg OH, USA), and the sample was hyperpolarized using a HyperSense dynamic nuclear polarizer (DNP, Oxford Instruments, Abingdon, UK). The resulting buffered hyperpolarized [1-13C] pyruvate solution (80 mM) had a final volume of ~ 4 mL with a pH of 7.4 at 37 °C. A 3-mL volume of hyperpolarized [1-13C] pyruvate solution was rapidly transferred to the MRI where it was injected through a lateral tail vein of the rat in a single 12-second bolus. After waiting a further 13 s for circulation and metabolism, 13C spectra in the axial plane (12 × 12 spectral imaging matrix, TR = 80 ms, bandwidth = 5 kHz, 2048 pts, flip angle = 10°) was acquired using a 2D chemical shift imaging with a free induction decay, chemical shift imaging (FID-CSI) pulse sequence (60 × 60 mm FOV, slice thickness approximately equal to the tumor extent). One FID-CSI with 144 spectra was acquired in each animal with a total scan time of 12 s. Regional maps of the ratio of lactate to pyruvate (Lac:Pyr) were calculated using a customized MATLAB script [16]. The animal was euthanized immediately after the experiment.
Benchtop Blood Glucose Measurement
The blood glucose change was measured by sampling blood from a rat tail artery (weight = 508 g) with a glucometer (Contour 7152B, Bayer HealthCare, Leverkusen, Germany) on the benchtop using the same glucose infusion protocol as the glucoCEST experiment. Baseline blood glucose was measured before the injection of 20 % glucose solution (0.3 g/kg) and the start of a 60-min constant infusion (1.5 g/kg). The blood glucose of the subject was measured every 5 min after the start of glucose infusion.
ROI Selection and Image Registration
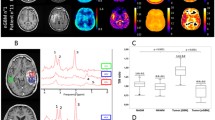
Tumor volume was measured on both imaging days using CT perfusion and T2-weighted MRI datasets, respectively. T2-weighted MRI was used as guidance for manual delineation of tumor and contralateral brain regions. All voxels that contained tumor were included in the tumor ROIs. The T2-weighted MRI was also used as a reference to co-register the CT perfusion, CEST, FDG-PET, and hyperpolarized [1-13C] pyruvate images using 3D Slicer (www.slicer.org). Manual rigid registration relied on anatomical landmarks identified in both T2-weighted images (e.g., major white matter features such as the corpus callosum, ventricles, and brain surface), features in the anatomical and perfusion CT (tumour, brain surface, and skull), and uptake in the FDG-PET volume (tumor uptake and normal uptake in the brain, Harderian glands, and surrounding tissue). The T2-weighted image from the 9.4 T MRI was subsequently used for ROI delineation and applied to all of the co-registered parameter maps (CEST, MRSI, CTP, and FDG-PET). The average BF, BV, and PS values from CT perfusion measurements; average ∆CEST measurement from glucoCEST; SUV from FDG-PET; and Lac:Pyr ratios from hyperpolarized [1-13C] pyruvate MRSI in tumor and contralateral brain regions were extracted. An example of registered images and ROIs is shown in Fig. 2.
Example images and ROIs in a single rat. A T2-weighted 9.4 T MR image (T2W) was used as a reference to co-register CT perfusion maps (BF, BV, PS), FDG-PET, CEST maps (AUCMTR), and hyperpolarized [1-13C] pyruvate MRSI (Lac:Pyr). The T2W image was subsequently used to delineate tumor (red dotted line) and contralateral brain tissue (blue dotted line), with the same ROI applied to all imaging measurements.
Statistical Analysis
Paired T tests were used to compare the similarity of CT perfusion, CEST, FDG-PET, and hyperpolarized [1-13C] pyruvate measurements between the tumor and contralateral brain regions. Paired T tests were also performed on the voxel-wise AUCMTR values in the tumor pre-infusion and during infusion in order to determine significant changes in AUCMTR during glucose infusion in each subject. The Pearson correlation between average tumoral CT perfusion parameters, ∆CEST measurement from glucoCEST, SUV from FDG-PET, and Lac:Pyr ratios from hyperpolarized [1-13C] pyruvate MRSI was evaluated using IBM SPSS version 22. A P value ≤ 0.05 was considered statistically significant.
Results
Tumor Size
As shown in Supplementary Fig.1, the average tumor volume on the day of PET and CT perfusion measurements is 98.4 ± 37.5 mm3 (measured using PS and contrast-enhanced images from CT perfusion), and the average tumor volume on the day of CEST and hyperpolarized [1-13C] pyruvate measurements was 99.4 ± 39.5 mm3 (measured in 9.4 T T2-weighted MRI), with no significant difference in volume between the two imaging days based on a paired t test (P = 0.54).
Blood Glucose Measurements
Benchtop measurements of blood glucose as a function of time during the glucose infusion protocol are plotted in Supplementary Fig. 2. The baseline blood glucose of the subject was 7.3 mmol/L and slowly increased to > 20 mmol/L during the 60-min constant infusion. Blood glucose was 7.6 ± 1.8 mmol/L prior to the FDG-PET and CT perfusion measurements and 6.7 ± 1.3 mmol/L prior to the CEST and hyperpolarized [1-13C] pyruvate measurements.
Dynamic CEST Trends
No significant change in applied frequency shifts (P = 0.52) was found between pre- (− 0.09 ± 0.05 ppm) and during glucose infusion (− 0.10 ± 0.04 ppm). Two different trends are observed in the dynamic CEST results (Fig. 3). A significantly increased AUCMTR in the tumor region at 30–60 min during infusion compared to the pre-infusion AUCMTR was denoted as an expected trend (n = 5). Significantly decreasing (n = 5) or no significant change (n = 1) in AUCMTR in the tumor region was denoted as an unexpected trend.
Illustrative examples of two different trends observed from the dynamic glucoCEST measurements: (a) expected and (b) unexpected change in AUCMTR. The expected trend was observed in 5 subjects and is shown in (c), where during infusion AUCMTR values are significantly higher than pre-infusion AUCMTR. The unexpected trend was observed in 6 subjects and is shown in (d), where during infusion AUCMTR is significantly lower (n = 5, grey-dotted line) than or is not significantly different (n = 1, red line, P = 0.46) from pre-infusion AUCMTR. Error bars = standard deviation.
Tumor vs. Contralateral Brain Tissue
After comparing CEST, perfusion, and glycolysis measurements, no statistically significant difference is found in BF and ∆CEST between tumor and contralateral brain tissue (P = 0.069 and 0.45, respectively) as shown in Fig. 4a and f (the pixel-wise ∆CEST values in contralateral region that were more than 2 standard deviations away from the mean were discarded as noise); statistically significant increases in BV (P < 0.05), PS (P < 0.001), SUV (P < 0.001), and Lac:Pyr (P < 0.001) are found in the tumor region vs. contralateral brain tissue as shown in Fig. 4b–e. The tumor AUCMTR in both pre- and during infusion is significantly higher than the contralateral side (P < 0.001) as shown in Supplementary Fig. 3.
Mean values from ROIs defined in both tumor and contralateral brain tissue in CT perfusion maps (a) BF, (b) BV and (c) PS, (d) FDG-PET (SUV), (e) hyperpolarized [1-13C] pyruvate (Lac:Pyr), and (f) glucoCEST (ΔCEST). A statistically significant (P < 0.05) difference was found in BV, PS, SUV, and Lac:Pyr between tumor and contralateral brain. No statistically significant difference was found in BF and ΔCEST between tumor and contralateral brain tissue. Error bars = standard deviation.
CEST vs. Perfusion and Glycolysis
∆CEST measurements had statistically significant and strong correlations with CT perfusion measurements of BV (0.74, P = 0.01) and PS (0.55, P = 0.04); however, no statistically significant correlations were found between BF and ∆CEST (P = 0.27). The Pearson correlation coefficients between ∆CEST and CT perfusion measurements are shown in Table 1. ∆CEST is directly proportional with BV and PS as shown in Fig. 5a and b, respectively.
ΔCEST vs. blood volume (a) and permeability-surface area product (b). Tumor SUV vs. blood flow (c) and permeability-surface area product (d). Tumor Lac:Pyr vs. blood volume (e) and permeability-surface area product (f). Solid line indicates the best fit and the dotted lines denote 95 % confidence interval. Error bar = standard deviation.
No statistically significant correlations between ∆CEST and glycolysis measurements using FDG-PET or hyperpolarized [1-13C] pyruvate are found (Table 1).
Glycolysis vs. Perfusion Measurements
As shown in Table 1, statistically significant moderate correlations between FDG-PET measurements of SUV and CT perfusion measurements of BF (0.49, P = 0.02) and PS (0.58, P = 0.005) are found. SUV is directly proportional with BF and inversely proportional with PS as shown in Fig. 5c and d, respectively.
Statistically significant moderate correlations between hyperpolarized [1-13C] pyruvate measurements of Lac:Pyr and CT perfusion measurements of BV (0.57, P = 0.02) and PS (0.75, P = 0.005) are also found (Fig. 5e and f).
As expected, there was a statistically significant moderate correlation between Lac:Pyr and SUV (0.56, P = 0.038).
It is important to note that the blood glucose conditions were different prior to the two glycolysis measurements: the average blood glucose level was 7.6 ± 1.8 mmol/L before FDG-PET acquisition, whereas the average blood glucose level was above 33 mmol/L prior to hyperpolarized [1-13C] pyruvate MRSI.
Expected vs Unexpected Group
As indicated in Supplementary Table 1, in the expected group, a significant correlation between ∆CEST and PS is found (0.60, P = 0.032), but no significant correlations were found between other measurements. No significant correlations are found in the unexpected group, as shown in Supplementary Table 2.
Discussion
In this study, the association between perfusion and glycolysis was investigated in vivo in the C6 rat model using CTP, FDG-PET, glucose CEST, and hyperpolarized [1-13C] pyruvate MRSI. All imaging experiments were performed within 24 h, and the tumor size did not significantly change within the 24-h span.
Tumor ∆CEST values were both significantly correlated and directly proportional to CT perfusion measurements of BV and PS. Increased BV in the tumor would correspond to a higher blood fraction in the measurement, where glucose concentration will be the highest, and a corresponding increase in glucose available to the tumor—both reflected by increased ∆CEST values. Tumor PS represents the leakiness of the tumor. Higher PS or leakiness would lead to increased glucose delivery throughout the constant infusion of glucose. No significant correlation was found between tumor ∆CEST measurements and tumor perfusion measurements of BF. This could be due to tumor heterogeneity as brain tumors will contain a mixture of viable, hypoxic, and necrotic regions [17]. The AUCMTR maps generated both pre- and during infusion of glucose most strongly resembled the PS maps, as illustrated in Fig. 6. According to previous work, lower intensity regions on BV and BF maps are associated with tumor necrosis [10]; however, only one subject had an AUCMTR that resembled tumor necrosis co-localized with reduced BV and BF indicative of possible necrosis within the tumor region (Fig. 6 a-c). Studies have shown CEST can distinguish tumor necrosis based on decreased MTR values from amide, attributed to decreased endogenous proteins and peptides in necrotic tissue [18, 19]. CEST measurements from this study could potentially identify tumor necrosis more accurately compared to our CTP measurements of BF and BV as MRI has superior soft tissue contrast [20]. No significant correlations were found between ∆CEST and tumor glycolysis measurements of SUV or Lac:Pyr ratio. Previous studies have suggested that D-glucose CEST enhancement comes from plasma and extracellular/extravascular spaces [6, 21], whereas SUV and Lac:Pyr ratios will correspond to glucose transport and metabolism in the cell. Our study provides important additional evidence that D-glucose enhanced ∆CEST is more sensitive to perfusion changes than glycolysis. However, CEST measurements of the immediate response during glucose infusion were missing because CEST experiments for the first 30 min of the glucose infusion were performed with two different protocols.
The tumor perfusion measurements of PS significantly correlated with glycolysis measurements of SUV and Lac:Pyr ratio. PS or leakiness are also related to the aggressiveness of the tumor [22]. Higher PS values (i.e., leakier tumors) are associated with more aggressive tumors, and more aggressive tumors would have a higher metabolism; hence, a directly proportional relationship was found between tumor PS and glycolysis measurements. A moderate but significant correlation was found between tumor BF and SUV and tumor BV and Lac:Pyr ratio. Higher tumor blood flow and blood volume could indicate increased vascularity, allowing more nutrients to be transferred to the tumor for glycolysis. Due to the sequential design of the study, glucose conditions before FDG-PET (normoglycemic) were distinct from the hyperpolarized [1-13C] pyruvate experiment (hyperglycemic) since it was conducted on the next day and immediately followed the glucose CEST experiment; as a result, Lac:Pyr and SUV provided different glycolysis measurements. In a recent study by Hundshammer et al. investigating a subcutaneous breast cancer model in rats, there was moderate correlation between SUVmean and the Lac:Pyr (0.41) that improved when looking at tumors with similar apparent diffusivity coefficients (0.59) [23]. This is quite similar to the correlation we found between SUV and Lac:Pyr (0.56) in our glioma model. Glucose and pyruvate take distinct metabolic pathways [23], which may help explain the moderate but not strong correlation between the two measurements. In our study, hyperglycemia, tumor glycolytic activity, and lactate production will be elevated during the hyperpolarized [1-13C] pyruvate experiment, while mitochondrial activity and lactate oxidation will be suppressed [24, 25]. Because of the hyperglycemic conditions, more pyruvate produced from glycolysis will be produced and later converted to lactate acid, which may help explain the slightly stronger correlation we found between Lac:Pyr and SUV. Although prolonged hyperglycemic conditions will lead to increased acidity and may eventually lead to saturation of both glucose transport and lactate export in tumor cells, our results, with elevated Lac:Pyr in the tumor and moderate correlation between Lac:Pyr and SUV, suggest that there is still elevated conversion of pyruvate to lactate, even in the hyperglycemic conditions present in the experiment.
The dynamic changes of AUCMTR for all subjects are shown in Fig. 3c. Two different trends are found from the dynamic CEST experiment as shown in Fig. 3a and b. The expected trend illustrated an increasing intensity over the time of glucose infusion in the tumor region, assuming the glucose solution would act as either a glucose metabolism or perfusion contrast agent. An increase of glucose CEST signal in the tumor region was expected due to the increased glucose concentration caused by a constant infusion of glucose. Dynamic CEST maps at later infusion times appear to enhance the boundary between necrotic and viable tumor areas as shown in Fig. 3a. The glucose infusion captured the leaky vessels of the viable tumor region, helping to facilitate glucose perfusion and enhancing the contrast between viable tumor and necrotic tumor areas. In some cases, however, the CEST map would decrease after the start of the glucose infusion (Fig. 3b). The mechanisms for the unexpected decrease in CEST signal need to be elucidated in future studies but may be related to the complex vascularity of the tumor, the insulin response, and/or changes in tumor pH induced by the glucose infusion.
There are several limitations for this experiment. Large tumors will often encompass the ventricles; as a result, the ROI defined using the T2-weighted MRI will contain CSF. The inclusion of CSF in tumor ROIs could lead to underestimation of all measurements in the tumor regions as CSF is distinct from the contrast agent in the blood vessels and tissue. Although histology was not performed, in a previous study using the same C6 tumor model [10], we demonstrated the relationship between tumor perfusion measurements and tumor hypoxia and necrosis using CT perfusion and corresponding histology data. We found that CT perfusion measurements of BF and BV are associated with tumor necrosis and hypoxia: regions with higher values of BF and BV representing viable tumor would surround regions with lower values of BF and BV, which were associated with lack of vascularization due to tumor hypoxia, necrosis, or fibrosis [26]. T2-weighted MRI offers better soft tissue contrast and resolution for tumor delineation; however, the delineated region could potentially include non-tumor features such as edema [27]. Fasting was not done prior to FDG-PET or subsequent glucoCEST and hyperpolarized [1-13C] pyruvate MRSI experiments; instead, blood glucose was monitored and confirmed to be within the normoglycemic range prior to both imaging experiments. Although this could potentially decrease FDG uptake [28], there is evidence that glucose loading conditions may actually increase the conspicuity of brain tumors as normal brain uptake is decreased, but gliomas continue to utilize FDG, even in hyperglycemic conditions [29]. Fasting for an extended period of time in small animals may also have more significant deleterious consequences than in humans, particularly if repeated for 2 days in a row for both imaging experiments [30]. Finally, in humans, FDG uptake has been shown to be approximately the same across brain regions in hypo- and normoglycemic conditions [31]. Ultimately, we chose to monitor blood glucose instead of applying a fasting protocol prior to both imaging days in this fragile animal model. Blood glucose levels were comparable prior to the start of both imaging experiments, and FDG uptake was conspicuous in the tumor relative to the surrounding brain tissue.
Although the imaging measurements were conducted within 24 h, they were in the span of 2 days, and on multiple scanners, as a result, the tumor perfusion and glycemic environment were not identical during the measurements. During the hyperpolarized [1-13C ]pyruvate MRSI experiment, the subject will have received a relatively large injected volume that includes both the previous constant infusion of glucose and a bolus of [1-13C] pyruvate (approximately 6 mL total). Hypervolemia could lead to an elevated BV and PS [32, 33]; however, BF should not be significantly affected [32]. Renal function such as the glomerular filtration rate will be elevated during glucose infusion [34] in order to compensate for the volume increase. The study plan was designed to follow the radiation safety protocol at different sites; as a result, the subjects had to be housed at our PET facility overnight to allow the radioactivity to decay before the subjects could be transferred to the MR facility. A hybrid PET/MR system could be used in the future to provide simultaneous measurement under the same tumor microenvironment. Meanwhile, there are a number of factors that could affect the CEST signal, including pH [35, 36]. A lower pH environment would be induced by hyperglycemia [37] and in turn will lead to decreased CEST signal [6]. The glucose CEST measurements (AUCMTR) during the constant infusion of glucose would have been underestimated due to pH reduction under hyperglycemic conditions. The effect of hyperglycemia on the tumor pH environment induced by a constant infusion of glucose needs to be further investigated. One approach may be to use pH-sensitive CEST acquisitions such as acidoCEST [38, 39] or amine and amide concentration-independent detection (AACID) [40] in conjunction with an infusion of glucose. The role that mammalian target of rapamycin (mTOR) plays in glucoCEST was not identified in this study. mTOR is upregulated in aggressive tumors and leads to elevated glucose uptake [41]. During the last half of the infusion protocol (during infusion), tumor metabolism is likely to have plateaued as excess amounts of glucose are introduced into the system. In this scenario, glucoCEST would predominantly reflect perfusion effects. Xu et al. also concluded that glucoCEST signal originates mainly from the extravascular/extracellular space and blood vessels [41]. There are a couple of additional limitations using D-glucose as a contrast agent for CEST signal enhancement. The metabolism of glucose in cells will decrease glucoCEST signal as the metabolites from glycolysis cannot be detected with CEST [42]. In addition, insulin response to high blood glucose concentration will limit the amount of detectable free glucose in blood [42, 43].
Conclusions
Dynamic glucose CEST measurements of tumor ∆CEST was significantly correlated with tumor perfusion measurements of BV and PS but not tumor glycolysis measurements of SUV and Lac:Pyr, suggesting that glucose CEST imaging using ∆CEST functions primarily as a perfusion tracer. Conversely, tumor glycolysis measurements of SUV and Lac:Pyr were significantly correlated with perfusion measurements of PS, reflecting increased tumor leakiness in tumors with higher glycolytic metabolism. Confounding these results is the fact that hyperglycemia induced by a constant infusion of glucose during dynamic CEST experiments may lead to a more acidic pH environment and give rise to underestimation of CEST measurements. Subsequently, in approximately half of the dynamic glucose CEST experiments, there was a drop in CEST signal post-glucose infusion. Although glucose CEST measurements may yield additional novel information about tumor status, the complicated dependency of glucose CEST signal on a variety of factors would benefit from additional in vivo mechanistic studies.
References
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT (2007) Angiogenesis in brain tumors. Nat Rev Neurosci 8(8):610–622
Sagiyama K, Mashimo T, Togao O, Vemireddy V, Hatanpaa KJ, Maher EA, Mickey BE, Pan E, Sherry AD, Bachoo RM, Takahashi M (2014) In vivo chemical exchange saturation imaging allows early detection of a therapeutic response in glioblastoma. Proc Natl Acad Sci U S A 111(12):4542–4547
Chan KW, McMahon MT, Kato Y et al (2012) Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn Reson Med 68(6):1764–1773
Torrealdea F (2016) Investigation of brain tumor metabolism using naturally occurring chemical exchange saturation transfer agents with magnetic resonance imaging. PhD Thesis, University College London
Walker-Samuel S, Ramasawmy R, Torrealdea F, Rega M, Rajkumar V, Johnson SP, Richardson S, Gonçalves M, Parkes HG, Årstad E, Thomas DL, Pedley RB, Lythgoe MF, Golay X (2013) In vivo imaging of glucose uptake and metabolism in tumors. Nat Med 19(8):1067–1072
Jin T, Mehrens H, Hendrick KS, Kim SG (2014) Mapping glucose uptake with chemical exchange-sensitive spin-lock magnetic resonance imaging. J Cerebral Metab 34:1402–1410
Xu X, Yadav N, Knutsson L et al (2015) Dynamic glucose-enhance (DGE) MRI: translation to human scanning and first results in glioma patients. Tomography 1(2):105–114
Jin T, Lordanova B, Hitchens TK et al (2018) Chemical exchange-sensitive spin-lock (CESL) MRI of glucose and analogs in brain tumors. MR Med 80:488–495
Qi Q, Yeung TPC, Lee TY, Bauman G, Crukley C, Morrison L, Hoffman L, Yartsev S (2016) Evaluation of CT perfusion biomarkers of tumor hypoxia. PLoS One 11(4):e0153569
Yeung TPC, Wang Y, He W et al (2015) Survival prediction in high-grade gliomas using CT perfusion imaging. J Neuro-Oncol 123:93–123
Yeung TPC, Bauman G, Yartsev S, Fainardi E, Macdonald D, Lee TY (2015) Dynamic perfusion CT in brain tumors. Eur J Radiol 84(12):2386–2392
Lee TY, Purdie TG, Stewart E (2003) CT imaging of angiogenesis. QJ Nucl Med 41:171–187
Tahari AK, Chien D, Azadi J et al (2014) Optimum lean body formulation for correction of standardized uptake value in PET imaging. JNM 55:1481–1484
Liu G, Song X, Chan KW et al (2013) Nuts and bolts of chemical exchange saturation transfer MRI. NMR Biomed 26(7):810–828
Lim, H (2017) A Longitudinal study of tumor metabolism using hyperpolarized carbon-13 magnetic resonance spectroscopic imaging in a preclinical model of glioma. PhD Thesis, Western University, London
Bonavia R, Inda M, Cavanee WK et al (2011) Heterogeneity maintenance in glioblastoma: a social network. Cancer Res 71:4055–4060
Mehrabian H, Desmon KL, Soliman H et al (2017) Differentiation between radiation necrosis and tumor progression using chemical exchange saturation transfer. Clin Cancer Res 23:3667–3675
Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, Wang S, Yan K, Fu DX, Ford E, Tyler B, Blakeley J, Laterra J, van Zijl P (2010) Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med 17(1):130–134
Hogeboom WR, Hoekstra HJ, Mooyaart EI et al (1991) MRI and CT in the preoperative evaluation of soft-tisue tumors. Arch Orthop Trauma Surg 110:162–164
Xu X, Chan K, Knutsson L et al (2015) Dynamic glucose enhanced (DGE) MRI for combined imaging of blood-brain barrier break down and increased blood volume in brain cancer. Magn Reason Med 74:1556–1563
Cao Y, Nagesh V, Hasmstra D et al (2006) The extent and severity of vascular leakage as evidence of tumor aggressiveness in high-grade gliomas. Cancer Res 66(17):8912–8917
Hundershammer C, Braeuer M, Müller CA et al (2018) Simultaneous characterization of tumor cellularity and the Warburg effect with PET, MRI and hyperpolarized 13C-MRSI. Theranostics 8(17):4765–4780
Twarock S, Reichert C, Peters U, Gorski DJ, Röck K, Fischer JW (2017) Hyperglycemia and aberrated insulin signaling stimulate tumor progression via induction of extracellular matrix component hyaluronan. Int J Cancer 141:791–804
Lund J, Ouwens DM, Wettergreen M et al (2019) Increased glycolysis and higher lactate production in hyperglycemic myotubes. Cells 8(9):1101. https://doi.org/10.3390/cells8091101
Hsu T, Nguyen-Tran HH, Trojanowska M (2019) Active roles of dysfunctional vascular endothelium in fibrosis and cancer. J Biomed Sci 26:86
Blystad I, Warntjes JBM, Smedby Ö, Lundberg P, Larsson EM, Tisell A (2017) Quantitative MRI for analysis of peritumoural edema in malignant gliomas. PLoS ONE 12(5):e0177135. https://doi.org/10.1371/journal.pone.0177135
Furger BJ, Czernin J, Hildebrandt, et al. (2006) Impact of animal handling on the results of 18F-FDG PET study in mice. J Nucl Med 47(6):999–1006
Kim D, Ko HY, Lee S, Lee YH, Ryu S, Kim SY, Chung JI, Lee M, Moon JH, Chang JH, Yun M (2020) Glucose loading enhances the value of 18F-FDG PET/CT for the characterization and delineation of cerebral gliomas. Cancers 12(7). https://doi.org/10.3390/cancers12071977
Jensen TL, Kiersgaard MK, Sørensen DB, Mikkelsen LF (2013) Fasting of mice: a review. Lab Anim 47(4):225–240
Sarikaya I, Albatineh AN, Sarikayaa A (2020) Effect of various blood glucose levels on regional FDG uptake in the brain. Asia Ocean J Nucl Med Biol 8(1):46–53
Todd MM, Weeks JB, Warner DS (1993) The influence of intravascular volume expansion on cerebral blood flow and blood volume in normal rats. Anesthesiology 78:945–953
Cole DJ, Drummond JC, Mastumura JS et al (1990) Hypervolemic-hemodilution and hypertensive during temporary middle cerebral artery occlusion in rats: the effect on blood-brain barrier permeability. Can J Neurol Sci 17:372–377
Christiansen JS, Frandsen M, Parving HH (1981) Effect of intravenous glucose infusion on renal function in normal man and insulin-dependent diabetics. Diabetologia 21:368–373
van Zijl PCM, Yadav NN (2011) Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn Reson Med 65(4):927–948
Sun PZ, Sorensen AG (2008) Imaging pH using the chemical exchange saturation transfer (CEST) MRI: Correction of concomitant RF irradiation effects to quantify CEST MRI for chemical exchange rate and pH. Magn Reson Med 60(2):390–397
Roszinski S, Wiedemann G, Jiang SZ, Baretton G, Wagner T, Weiss C (1991) Effects of hyperthermia and/or hyperglycemia on pH and pO2 in well oxygenated xenotransplanted human sarcoma. Inl J Radiat Oncol Biol Phys 20(6):1273–1280
Chen LQ, Howison CM, Jeffery JJ, Robey IF, Kuo PH, Pagel MD (2014) Evaluation of extracellular pH within in vivo tumors using acidoCEST MRI. Magn Reson Med 72(5):1408–1417
Longo DL, Bartoli A, Consolino L, Bardini P, Arena F, Schwaiger M, Aime S (2016) In vivo imaging of tumor metabolism and acidosis by combining PET and MRI-CEST pH imaging. Cancer Res 76(22):6463–6470
McVicar N, Li A, Gonҫalves DF et al (2014) Quantitative tissue pH measurement during cerebral ischemia using amine and amide concentration-independent detection (AACID) with MRI. J Cereb Blood Flow Metab 34(4):690–698
Xu X, Xu J, Knutsson L, el al. (2019) The effect of the mTOR inhibitor rapamycin on glucoCEST singal in a preclinical model of glioblastoma. Magn Reson Med 81(6):3798–3807
Nasrallah FA, Pages G, Kuchel PW et al (2013) Imaging brain deoxyglucose uptake and metabolism by glucoCEST MRI. J Cereb Blood Flow Metab 33:1270–1278
Sehgal AA, Li Y, Lal B et al (2018) CEST MRI of 3-O-methyl-D-glucose and accumulation in brain tumors. Magn Reson Med 81:1993–2000
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts of interest for this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Figure 1
Average tumor size on first imaging day for PET and CT perfusion experiment and the next day for glucoCEST and hyperpolarized [1-13C]pyruvate. No statistical significance in tumor size were found between these two subsequent imaging days (P = 0.54). Error bars = standard deviation. (PNG 75 kb)
Supplementary Figure 2
An illustrative example of blood glucose change during the 60-min constant infusion. Time 0 was defined as the time at the start of constant infusion of 20 % glucose solution (1.5 g/kg) and after a bolus of 20 % glucose solution (0.3 g/kg) was injected. (PNG 26 kb)
Supplementary Figure 3
Mean values from ROIs defined in both tumor and contralateral brain tissue in AUCMTR pre- and During infusion (30-60 min). A statistically significant (P < 0.05) difference was found between tumor and contralateral side in AUCMTR pre- and during glucose infusion. (DOCX 20 kb)
ESM 1
(PNG 28 kb)
Rights and permissions
About this article
Cite this article
Qi, Q., Fox, M.S., Lim, H. et al. Multimodality In Vivo Imaging of Perfusion and Glycolysis in a Rat Model of C6 Glioma. Mol Imaging Biol 23, 516–526 (2021). https://doi.org/10.1007/s11307-021-01585-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-021-01585-1