Abstract
Patients with high-grade gliomas usually have heterogeneous response to surgery and chemoirradiation. The objectives of this study were (1) to evaluate serial changes in tumor volume and perfusion imaging parameters and (2) to determine the value of these data in predicting overall survival (OS). Twenty-nine patients with World Health Organization grades III and IV gliomas underwent magnetic resonance (MR) and computed tomography (CT) perfusion examinations before surgery, and 1, 3, 6, 9, and 12 months after radiotherapy. Serial measurements of tumor volumes and perfusion parameters were evaluated by receiver operating characteristic analysis, Cox proportional hazards regression, and Kaplan–Meier survival analysis to determine their values in predicting OS. Higher trends in blood flow (BF), blood volume (BV), and permeability-surface area product in the contrast-enhancing lesions (CEL) and the non-enhancing lesions (NEL) were found in patients with OS < 18 months compared to those with OS ≥ 18 months, and these values were significant at selected time points (P < 0.05). Only CT perfusion parameters yielded sensitivities and specificities of ≥70 % in predicting 18 and 24 months OS. Pre-surgery BF in the NEL and BV in the CEL and NEL 3 months after radiotherapy had sensitivities and specificities >80 % in predicting 24 months OS in patients with grade IV gliomas. Our study indicated that CT perfusion parameters were predictive of survival and could be useful in assessing early response and in selecting adjuvant treatment to prolong survival if verified in a larger cohort of patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High-grade gliomas account for over 70 % of all malignant brain tumors, and survival remains dismal even after maximal safe resection, radiotherapy, and temozolomide chemotherapy. The median survival is typically 12–15 months and 2–5 years for patients with World Health Organization (WHO) grade IV and grade III gliomas, respectively [1]. However, there is considerable heterogeneity in patients’ treatment response. At present, widely accepted prognostic factors of survival in high-grade gliomas are WHO grade, extent of surgical resection, age, and performance status [2]. Molecular biomarkers, such as isocitrate dehydrogenase 1 (IDH1) mutation status, O6-methylguanine-DNA-methyltransferase (MGMT) methylation status and 1p19q loss of heterozygosity are also recognized as prognostic biomarkers in these patients [3].
Magnetic resonance (MR) is the standard imaging method for assessing high-grade gliomas [4]. For radiographic assessment, the typical MR protocol includes a T2-weighted or fluid-attenuated inversion recovery (FLAIR) MR and a gadolinium-enhanced T1-weighted MR [5]. The gadolinium-enhanced T1-weighted MR images depict the contrast-enhancing lesion (CEL) with disrupted blood–brain barrier. The T2-weighted or FLAIR MR images detect regions of T2 hyperintensity that are suspicious of tumor infiltration and vasogenic edema. This T2 hyperintense region is the non-enhancing lesion (NEL).
In addition to anatomical imaging, functional imaging, such as perfusion imaging, provides quantitative information regarding tumor biology that may be related to patient prognosis [6]. Measurements of tumor blood flow (BF), blood volume (BV), and permeability obtained by perfusion imaging have been shown to correlate with WHO grade [7, 8], histopathologic marker of tumor angiogenesis (e.g. microvessel density) [9,10], and outcome [11–13]. Most perfusion imaging studies that evaluated the relationships between tumor perfusion parameters and overall survival (OS) have focused on MR perfusion imaging at a single time point [14] or two time points [15]. CT perfusion has not been studied extensively despite the ubiquity of CT scanners [16]. However, CT perfusion can help distinguish between high and low grade gliomas [7, 17–19] and between tumor recurrence and radiation necrosis [20, 21]. Therefore, CT perfusion should be considered as a potentially valuable perfusion imaging modality in neuro-oncology. To our knowledge this study is the first to investigate serial changes in tumor BF, BV, and permeability-surface area (PS) in the CEL and NEL of patients with high-grade gliomas for up to a year after radiotherapy. In this analysis, we used these data to evaluate the prognostic value of CT perfusion imaging in predicting OS of these patients.
Materials and methods
Patient population
This study was conducted in compliance with the institutional research ethics committee, and informed consent was obtained from patients. Patients with suspected WHO grade III and IV gliomas were prospectively recruited prior to surgery. Exclusion criteria were (1) prior diagnosis or therapy of a brain lesion, (2) clinically unstable, (3) contraindications to contrast materials, and (4) contraindications to MR imaging such as metallic implants, claustrophobia, and obesity. Our study included 29 patients with high-grade gliomas. The median age at diagnosis was 61 (range 31–81) years. Patients underwent serial MR and CT perfusion examinations prior to surgery and one, three, six, nine, and 12 months after radiotherapy. After surgery, genomic DNA was extracted from formalin-fixed paraffin-embedded tissue samples using a DNA extraction kit (Qiagen, Germany) after micro-dissection. MGMT promoter methylation status was determined for WHO grade IV glioma patients by methylation specific polymerase chain reaction after prior DNA bisulfite modification [22]. For WHO grade III glioma patients, IDH1 mutation was assessed by exon 4 direct DNA sequencing [23]. Primer extension sequencing was performed with the use of the Big Dye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). Sequences were determined using the ABI PRISM 310 Genetic Analyzer (Applied Biosystems) and the Sequencing analysis 5.2 software (Applied Biosystems).
All patients underwent surgery followed by radiotherapy (60 Gy in 30 fractions, N = 28; 45 Gy in 15 fractions with stereotactic boost of 24 Gy in 3 fractions, N = 1). Twenty-six patients received concurrent and adjuvant temozolomide chemotherapy. Six patients underwent second surgical resection after radiotherapy, and corticosteroids were administered to patients before the second surgery. WHO grade IV patients were also given Fotemustine at the time of progression. The median follow-up was 18.2 (range, 4.7–60.4) months.
MR and CT perfusion examinations
MR images were acquired with a 1.5 T Signa HDXT (GE Healthcare, Milwaukee, WI) or a 1.5 T Achieva scanner (Philips Medical Systems, Best, The Netherlands). The MR protocol included the following sequences: axial T1-weighted spin-echo, axial T2-weighted spin-echo or axial FLAIR, coronal FLAIR, and post-gadolinium axial T1-weighted spin echo.
All CT perfusion studies were performed using a multidetector-row CT scanner (Lightspeed VCT, GE) that covered eight 5-mm sections of tissue. Ten patients were imaged with a one-phase CT perfusion protocol while 19 patients were imaged with a two-phase CT perfusion protocol. For the one-phase protocol, a bolus of non-ionic contrast (Iomeron, Bracco Imaging, Konstanz, Germany; 350 mg I/ml, 40 ml) was injected at a rate of 4 mL/s. A cine scan of 50 s duration was initiated at 5 s after the injection (100 mA, 80 kV, 1 rotation/s), and the images were reconstructed at 0.5 s intervals. For the two-phase protocol, images were reconstructed at 0.5 s intervals in the initial 44 s cine scan, followed by eight additional axial images at 15 s intervals for another 105 s. The total scan duration was 150 s.
Image analysis
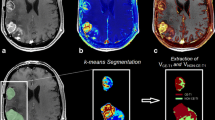
Maps of BF, BV, and PS were computed using CT Perfusion 4D (GE Healthcare) [24, 25]. Averaged CT images were produced by averaging the cine CT images of the same sections. MR images were rigidly registered to the averaged CT images using 3D Slicer [26]. A radiologist with 8 years of experience delineated the entire contrast-enhancing lesion (CEL) and the entire non-enhancing lesion (NEL). The NEL was the peritumoral T2 hyperintense region outside the CEL, central necrosis, and surgical cavity. Thus, the NEL included peritumoral edema and post-radiation T2 changes around the CEL. Mean volume, BF, BV, and PS in both the CEL and NEL were used for analysis.
Statistical analysis
Statistical analysis was performed using SPSS (IBM® SPSS®, version 21.0, Chicago, IL) and R (http://www.r-project.org/) to evaluate whether the imaging parameters were candidate biomarkers of OS. Patients were stratified by their OS using 12, 18, and 24 months OS as endpoints. Patients were designated as “long-term survivors” (OS ≥ 18 months) or “short-term survivors” (OS < 18 months) based on the 18 months OS endpoint. The imaging parameters in patients with short-term versus long-term OS were examined longitudinally. The Friedman test followed by the Wilcoxon signed-rank test were used for longitudinal comparisons within-group, and the Mann–Whitney U test examined the differences between groups. Only imaging data from pre-surgery, one, three, and 6 months post-radiotherapy were included for analysis due to patient dropouts resulting from deaths or complete regression of tumors at nine and 12 months post-radiotherapy.
For the entire cohort of 29 patients, receiver operating characteristic analyses were performed to consider all possible cut points to differentiate patients with long-term and short-term OS. The optimal cut point for each imaging parameter was selected for use in Cox proportional hazards regression and Kaplan–Meier survival analyses. Cox proportional hazards regression analyses were performed to determine the relationships between OS and the imaging parameters. A separate Cox proportional hazards regression model was computed for each imaging parameter at each time point while adjusting for age, Karnofsky performance status, extent of surgical resection, and WHO grade. The hazard ratios (HR) and their 95 % confidence intervals (CI) were computed. IDH1 mutation status was not adjusted in the Cox proportional hazards regression due to the small number of WHO grade III patients (N = 5). Kaplan–Meier survival analysis and log-rank test compared the OS of patients with high versus low values of imaging parameters.
For patients with grade IV gliomas (N = 24), Cox proportional hazards regression and Kaplan–Meier survival analysis were repeated. MGMT promoter status, age, Karnofsky performance status, and the extent of resection were also included in the Cox proportional hazards regression. A P ≤ 0.05 was considered statistically significant. Lastly, joint modeling of OS and the longitudinal covariates was performed to evaluate whether changes in these imaging parameters correlate with OS [27].
Results
Patient characteristics and outcomes
A total of 150 CT perfusion and 150 MR examinations were acquired, and 109 CT perfusion and 109 MR scans from the first 4 times points were analyzed. Twenty-two patients had imaging studies available from the first four time points while seven patients had imaging studies available from the first three time points. No radiation-induced side effects (e.g. skin erythema) from the serial CT perfusion scans was noted. The median OS was 18 months (range, 5–60 months) with three patients alive at the end of the study. Table 1 summarizes patient characteristics and the corresponding OS data. In univariate analyses, gender, extent of surgical resection, Karnofsky performance status, re-operation, and second-line Fotemustine were not significant predictors of OS. Older patients (≥50 years) had worse OS than younger patients, but this was marginally significant (P = 0.06). WHO grade IV was associated with worse OS when compared to grade III (P = 0.04), while patients with unmethylated MGMT promoter status was associated with worse OS compared to patients with methylated MGMT promoter (P < 0.001). IDH1 mutation could be associated with OS (P = 0.05), but the sample size was too small to be conclusive (wild-type N = 1).
Serial analysis
Figure 1a shows the serial changes in the CEL of patients stratified by 18 months OS. Patients appeared to show a reduction in CEL volumes after surgery and radiotherapy compared to baseline, and this was significant for long-term survivors (OS ≥ 18 months) (P < 0.04). There were also decreasing trends in BF, BV, and PS in the CEL after surgery and radiotherapy. These trends were significant for BF and BV in long-term survivors (P < 0.05 and P < 0.03, respectively). In general, CEL volumes, BF, BV, and PS appeared to be higher in short-term survivors (OS < 18 months) compared to long-term survivors. Serial changes in these patients stratified by 12 and 24 months OS are provided in Fig. S1.
Serial changes in a the contrast-enhancing lesion (CEL) and b the non-enhancing lesion (NEL) of patients stratified by 18 months (mo) overall survival (OS) for volume (top row), blood flow (BF, second row), blood volume (BV, third row), and permeability-surface area product (PS, last row). Horizontal line connects significant changes between two time points of the same group. Dotted box encloses a significant difference between the groups at a particular time point. Error bar represents 1 SD
Figure 1b illustrates the serial changes in the NEL of patients stratified by 18 months OS. NEL volumes decreased after surgery and radiotherapy. The change in NEL volume (compared to baseline) was significant for long-term survivors (P < 0.03). However, there was no between-group difference in NEL volumes in long-term versus short-term survivors. BF values in the NEL before surgery were significantly higher in short-term survivors (P = 0.003) compared with long-term survivors, and they were also significantly higher at 3 months post-radiotherapy (P = 0.05) in short-term survivors compared to long-term survivors. BV values in the NEL were significantly higher at 1 and 3 months post-radiotherapy in short-term survivors (P = 0.04 and P = 0.02, respectively) when compared to long-term survivors. PS values in the NEL significantly decreased after surgery and radiotherapy in long-term survivors (P < 0.01 and P ≤ 0.03, respectively), and also in short-term survivors (P < 0.04). PS values in the NEL at one and three months post-radiotherapy were significantly higher in short-term survivors when compared to long-term survivors (P < 0.01 and P = 0.02, respectively). Figure S2 shows these serial changes in patients stratified by 12 and 24 months OS.
Survival analysis
For the entire cohort of patients (N = 29), the Cox proportional hazards regression model of the “classical” prognostic factors (i.e. age, performance status, extent of resection, and WHO grade) showed that higher WHO grade (HR = 49.6, 95 % CI = 5.1–483.2, P < 0.001) and lower extent of resection (HR = 19.0, 95 % CI = 3.6–99.5, P < 0.001) were associated with significant hazards of death. Cox proportional hazards regression models of the imaging parameters were evaluated while adjusting for age, performance status, extent of resection and WHO grade. Figure 2 illustrates imaging parameters with cut points that were associated with both significant HRs and significant differences in OS for all 29 patients.
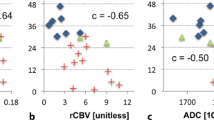
Kaplan–Meier survival plots of a blood flow in the non-enhancing lesion (BFNEL) measured at pre-surgery, b volume of the contrast-enhancing lesion (VOLCEL), volume in the NEL (VOLNEL), permeability-surface area product in the NEL (PSNEL) measured at 1 month post-radiotherapy, c blood volume in the CEL (BVCEL) measured at 3 months post-radiotherapy, and d VOLCEL and BV in the NEL (BVNEL) measured at 6 months post-radiotherapy for all patients (N = 29). Higher values in these parameters were associated with worse overall survival (log-rank P < 0.05 for all comparisons). These parameters were associated with significant hazard ratios (HR) after adjusting for tumor grade, age, Karnofsky performance status, and extent of surgical resection (P ≤ 0.05). Numbers in parentheses are the 95 % confidence interval (CI) of the HR. Asterisk represents significant HR and significantly different OS when considering only grade IV glioma patients with adjustments for MGMT promoter status, age, extent of resection, and Karnofsky performance status in the Cox proportional hazard regression
For WHO grade IV glioma patients, Cox proportional hazards regression of MGMT promoter status, age, extent of resection, and performance status showed methylated MGMT promoter status was associated with better OS (HR = 0.28, 95 % CI = 0.09–9.85, P = 0.02). With the exception of CEL volume at 6 months post-radiotherapy, the Kaplan–Meier analysis results presented in Fig. 2 were also significant for patients with grade IV gliomas and the Cox proportional hazards regression results were significant even with the adjustment for MGMT status, age, extent of resection, and performance status (P ≤ 0.05). In addition, grade IV glioma patients with high BV in the NEL 3 months post-radiotherapy had significantly shorter OS (BV ≥ 1.1 ml/100 g, median OS = 11.4, 95 % CI = 9.8–13.0 months) than those with low BV (BV < 1.1 ml/100 g, median OS = 23.6, 95 % CI = 20.3–26.9, log-rank P = 0.01), and this was associated with a significant HR 7.4 (95 % CI 1.6–34.6, P = 0.01). Joint modeling of longitudinal covariates and OS showed that changes in any of the imaging parameters did not affect survival.
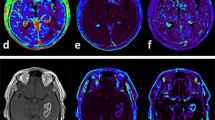
Table 2 shows the sensitivities and specificities of imaging parameters that are ≥70 % in stratifying OS of grade IV glioma patients. BF in the NEL at pre-surgery could stratify patients by 18 and 24 months OS with sensitivities and specificities ≥80 %. Similarly, BV in the CEL and NEL 3 months post-radiotherapy could stratify patients by 24 months OS with sensitivities and specificities ≥80 %. It is important to note that volumes of NEL and CEL could not stratify OS with sensitivities and specificities ≥70 %. The extent of resection could predict 12 months OS with 100 % sensitivity and 78 % specificity, but failed to achieve a specificity of >50 % when predicting 18 and 24 months OS. MGMT promoter status could predict 12 months OS with 87 % sensitivity and 89 % specificity, but specificities fell below 60 % when predicting 18 and 24 months OS. Figure 3 illustrates representative pre-surgery CT perfusion and MR images of two patients with different survival (16.7 vs. 41.6 months). Higher BF, BV, and PS in the NEL can be seen in the patient with shorter survival.
Illustrative pre-surgery CT perfusion and MR images of patients with WHO grade IV gliomas. Both patients presented with a contrast-enhancing lesion on post-gadolinium T1-weighted MR images, which also had elevated blood flow (BF), blood volume (BV), and permeability-surface area product (PS). Patient A presented with low BF, BV, and PS in the non-enhancing lesion (NEL, red asterisks). However, Patient B presented with regions of elevated BF, BV, and PS in the NEL (red arrows). The survival for patient A was 41.6 months, and patient B was 16.7 months. Identical window and level were used for the color maps
Discussion
In our analysis of patients treated for malignant gliomas, we found that CT perfusion parameters could potentially identify patients with favorable outcomes based on pre-treatment parameters as well as at early time points post-treatment. Based on our findings, we suggest CT perfusion could potentially serve as a valuable imaging biomarker that is complementary to conventional MR imaging.
We first investigated the serial changes in volume, BF, BV, and PS. The initial decline of all imaging parameters in the CEL after surgery and radiotherapy could be attributed to surgical debulking of the tumor and therapeutic effect of radiotherapy and chemotherapy. After stratifying patients based on OS, all three perfusion parameters in the CEL started to diverge as early as 3 months after radiotherapy with elevated BF, BV, and PS seen in patients with shorter OS. BF, BV, and PS in the NEL were also higher in patients with shorter OS. Although statistical differences were identified only at selected time points, our data provided corroborating evidence that the variation in tumor perfusion characteristics in the CEL and NEL were associated with differences in OS. This variation in perfusion characteristics reflects differences in angiogenesis and vascularity (and by extension differences in aggressiveness and treatment response) between patients. Angiogenesis leads to an increase in tumor microvessel area that facilitates tumor growth, and it is an active target in cancer therapies [28]. It has been shown to correlate with tumor BV and patient survival [10]. More recently, vasculogenesis, a process by which bone marrow-derived cells are recruited to form new tumor blood vessels, has been shown to govern tumor recurrence after radiotherapy [29]. Given that recurrence occurs within 2 cm of the irradiated volume [30], it is critical to assess perfusion parameters in both the CEL and NEL. In fact, we found perfusion parameters in the CEL and NEL to be prognostic of OS. The importance of perfusion parameters in the NEL reflect the limitation of conventional contrast-enhanced MR where enhancement may be more reflective of changes in vessel architecture and permeability (i.e. breakdown of the blood–brain barrier) rather than reflecting the overall vascular supply to the tumor.
None of the imaging measurements made in the CEL before surgery showed a significant association with OS. This could be because total resection of the tumor (mostly CEL) is a stronger predictor of OS. Over 60 % of patients underwent total resection, and it showed a significant association with OS. Prediction of survival using pre-surgery CT perfusion parameters in the CEL may be more appropriate for patients who are not candidates for total resection. For example, pre-surgery tumor BV is a predictor of survival in a study with a mixture of patients that received biopsy, subtotal resection, and total resection [11]. However, pre-surgery measurement of BF in the NEL proved to be useful in predicting OS in this study. This result points to the significance of tumor burden in the T2 hyperintense lesion that is not typically removed by surgery.
Although a larger sample size is required to show consistent associations between OS and CT perfusion parameters across all time points, our results are consistent with previous reports that higher BV and PS are associated with poor outcomes [11, 12, 20, 31, 32]. It is also noteworthy that while we showed significant hazards of death associated with both volumetric and CT perfusion parameters, only CT perfusion parameters resulted in sensitivities and specificities ≥70 % in stratifying OS while volumetric parameters did not. Volume of the CEL may not be a reliable predictor of outcome because the volume of contrast-enhancement may be affected by many nontumoral processes including inflammation, postsurgical changes, pseudoprogression, and treatment-induced necrosis [33–36]. Similarly, the volume of the NEL encompasses many causes of T2 hyperintensity such as vasogenic edema, gliosis, cystic changes, inflammation, and tumor infiltration [37–39]. It is evident from Fig. 3 that tumors can have regions of high BF, BV, and PS that are bigger than the CEL. These regions can potentially lead to future sites of recurrence. Although this is hypothesis-generating, there is preliminary evidence to suggest that there could be better spatial concordance between CT perfusion parameters with the site of future recurrence than the concordance between CEL and the site of future recurrence [24].
Some limitations must be noted. Firstly, a one-phase rather than two-phase CT perfusion protocol was used in one-third of the patients. Although a shorter scan duration of the CT perfusion protocol can affect measurements of PS, the effects on BF and BV are smaller [25]. The identified predictors of OS are unlikely to change, but the cut points of these predictors could change with a larger cohort of patients studied with a uniform two-phase protocol. It is important to use a two-phase CT perfusion protocol in future studies. Secondly, we could not adjust for the effect of IDH1 mutation status in our regression models due to a small number of WHO grade III glioma patients. Finally, a CT perfusion scan at the time point of pre-radiotherapy but post-surgery could be a better baseline scan than a pre-surgery perfusion imaging scan. The prognostic importance of CT perfusion parameters obtained pre-radiotherapy compared to post-radiotherapy remains to be demonstrated.
Conclusions
BF, BV, and PS are potential biomarkers of OS in patients with high-grade gliomas treated with multi-modality therapies (surgery, radiotherapy, chemotherapy) even after adjustments for age, WHO grade, Karnofsky performance status, the extent of resection, and MGMT promoter status. The results of this study, if verified in a larger cohort of patients, could establish CT perfusion imaging as a reliable early predictor of survival.
References
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Curran WJ, Scott CB, Horton J et al (1993) Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst 85:704–710
Weller M, Stupp R, Hegi ME et al (2012) Personalized care in neuro-oncology coming of age: why we need MGMT and 1p/19q testing for malignant glioma patients in clinical practice. Neuro Oncol 14(suppl iv):100–108
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Essig M, Anzalone N, Combs SE et al (2012) MR imaging of neoplastic central nervous system lesions: Review and recommendations for current practice. Am J Neuroradiol 33:803–817
Cha S (2006) Update on brain tumor imaging: from anatomy to physiology. Am J Neuroradiol 27:475–487
Jain R, Ellika SK, Scarpace L et al (2008) Quantitative estimation of permeability surface-area product in astroglial brain tumors using perfusion CT and correlation with histopathologic grade. Am J Neuroradiol 29:694–700
Weber MA, Henze M, Tüttenberg J et al (2010) Biopsy targeting gliomas: do functional imaging techniques identify similar target areas? Invest Radiol 45:755–768
Jain R, Gutierrez J, Narang J et al (2011) In vivo correlation of tumor blood volume and permeability with histologic and molecular angiogenic markers in gliomas. Am J Neuroradiol 32:388–394
Hu LS, Eschbacher JM, Dueck AC et al (2012) Correlations between perfusion MR imaging cerebral blood volume, microvessel quantification, and clinical outcome using stereotactic analysis in recurrent high-grade glioma. Am J Neuroradiol 33:69–76
Jain R, Narang J, Griffith B et al (2013) Prognostic vascular imaging biomarkers in high-grade gliomas: tumor permeability as an adjunct to blood volume estimates. Acad Radiol 20:478–485
Shankar JJ, Woulfe J, Silva VD, Nguyen TB (2013) Evaluation of perfusion CT in grading and prognostication of high-grade gliomas at diagnosis: a pilot study. AJR Am J Roentgenol 200:W504–W509
Law M, Young RJ, Babb JS et al (2008) Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 247:490–498
Bisdas S, Kirkpatrick M, Giglio P, Welsh C, Spampinato MV, Rumboldt Z (2009) Cerebral blood volume measurements by perfusion-weighted MR imaging in gliomas: ready for prime time in predicting short-term outcome and recurrent disease? Am J Neuroradiol 30:681–688
Galbán CJ, Chenevert TL, Meyer CR et al (2011) Prospective analysis of parametric response map-derived MRI biomarkers: Identification of early and distinct glioma response patterns not predicted by standard radiographic assessment. Clin Cancer Res 17:4751–4760
Jain R (2011) Perfusion CT imaging of brain tumors: an overview. AJNR Am J Neuroradiol 32:1570–1577
Ding B, Ling HW, Chen KM, Jiang H, Zhu YB (2006) Comparison of cerebral blood volume and permeability in preoperative grading of intracranial glioma using CT perfusion imaging. Neuroradiology 48:773–781
Fainardi E, Di Biase F, Borrelli M et al (2010) Potential role of CT perfusion parameters in the identification of solitary intra-axial brain tumor grading. Acta Neurochir Suppl 106:283–287
Ellika SK, Jain R, Patel SC et al (2007) Role of perfusion CT in glioma grading and comparison with conventional MR imaging features. Am J Neuroradiol 28:1981–1987
Jain R, Narang J, Schultz L et al (2011) Permeability estimates in histopathology-proved treatment-induced necrosis using perfusion CT: Can these add to other perfusion parameters in differentiating from recurrent/progressive tumors? Am J Neuroradiol 32:658–663
Vidiri A, Guerrisi A, Pinzi V et al (2012) Perfusion Computed Tomography (PCT) adopting different perfusion metrics: recurrence of brain metastasis or radiation necrosis? Eur J Radiol 81:1246–1252
Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB (1996) Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 93:9821–9826
Hartmann C, Meyer J, Balss J et al (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 118:469–474
Yeung TPC, Yartsev Y, Lee TY et al (2014) Relationship of computed tomography perfusion and positron emission tomography to tumour progression in malignant glioma. J Med Radiat Sci 61:4–13
Yeung TPC, Yartsev S, Bauman G, He W, Fainardi E, Lee TY (2013) The effect of scan duration on the measurement of perfusion parameters in CT perfusion studies of brain tumors. Acad Radiol 20:59–65
Pieper S, Lorensen B, Schroeder W, Kikinis R (2006) The NA-MIC Kit: ITK, VTK, pipelines, grids and 3D slicer as an open platform for the medical image computing community. In: Proceedings of the 3rd IEEE international symposium biomedical imaging: From Nano Macro, vol 1, pp 698–701
Rizopoulos D (2010) JM: an R package for the joint modelling of longitudinal and time-to-event data. J Stat Softw 35:1–33
Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT (2007) Angiogenesis in brain tumours. Nat Rev Neurosci 8:610–622
Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM (2010) Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 120:694–705
Milano MT, Okunieff P, Donatello RS et al (2010) Patterns and timing of recurrence after temozolomide-based chemoradiation for glioblastoma. Int J Radiat Oncol Biol Phys 78:1147–1155
Vöglein J, Tüttenberg J, Weimer M et al (2011) Treatment monitoring in gliomas: comparison of dynamic susceptibility-weighted contrast-enhanced and spectroscopic MRI techniques for identifying treatment failure. Invest Radiol 46:390–400
Mangla R, Singh G, Ziegelitz D et al (2010) Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology 256:575–584
van den Bent MJ, Vogelbaum MA, Wen PY, Macdonald DR, Chang SM (2009) End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's Criteria. J Clin Oncol 27:2905–2908
Finn MA, Blumenthal DT, Salzman KL et al (2007) Transient postictal MRI changes in patients with brain tumors may mimic disease progression. Surg Neurol 67:246–250
Ulmer S, Braga TA, Barker FG 2nd et al (2006) Clinical and radiographic features of peritumoral infarction following resection of glioblastoma. Neurology 67:1668–1670
Kumar AJ, Leeds NE, Fuller GN et al (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384
Oh J, Cha S, Aiken AH et al (2005) Quantitative apparent diffusion coefficients and T2 relaxation times in characterizing contrast enhancing brain tumors and regions of peritumoral edema. J Magn Reson Imaging 21:701–708
Hattingen E, Jurcoane A, Daneshvar K et al (2013) Quantitative T2 mapping of recurrent glioblastoma under Bevacizumab improves monitoring for non-enhancing tumor progression and predicts overall survival. Neuro Oncol 15:1395–1404
Li Y, Lupo JM, Polley MY et al (2011) Serial analysis of imaging parameters in patients with newly diagnosed glioblastoma multiforme. Neuro Oncol 13:546–557
Acknowledgments
This project was funded by The Project of Emilia-Romagna region on Neuro-Oncology (PERNO) study group, the Canadian Institutes of Health Research (CIHR), and the CIHR Strategic Training Program in Cancer Research and Technology Transfer.
Funding
Ting-Yim Lee licenses CT Perfusion software to and receives funding from GE Healthcare.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
A complete list of the members of the PERNO study group appears in the “Appendix”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2015_1766_MOESM1_ESM.tif
Supplementary Figure S1. Serial changes in mean volumes (top row), blood flow (BF, second row), blood volume (BV, third row), and permeability-surface area product (PS, bottom row) in the contrast-enhancing lesions (CEL) of patients stratified by a 12 and b 24 months (mo) overall survival (OS). Horizontal line connects significant changes between two time points of the same group. Dotted box encloses a significant difference between the groups at a particular time point. Error bar represents one standard deviation (TIFF 159 kb)
11060_2015_1766_MOESM2_ESM.tif
Supplemeantary Figure S2. Serial changes in mean volumes (top row), blood flow (BF, second row), blood volume (BV, third row), and permeability-surface area product (PS, bottom row) in the non-enhancing lesion (NEL) of patients stratified by a 12 and b 24 months (mo) overall survival (OS). Horizontal line connects significant changes between two time points of the same group. Dotted box encloses a significant difference between the groups at a particular time point. Error bar represents one standard deviation (TIFF 155 kb)
Appendix
Appendix
Steering committee
Baruzzi A. (Chair), Albani F., Calbucci F., D'Alessandro R., Michelucci R. (IRCCS Institute of Neurological Sciences, Bologna, Italy), Brandes A. (Department of Medical Oncology, Bellaria-Maggiore Hospitals, Bologna, Italy), Eusebi V. (Department of Hematology and Oncological Sciences “L. & A. Seràgnoli”, Section of Anatomic Pathology at Bellaria Hospital, Bologna, Italy), Ceruti S., Fainardi E., Tamarozzi R. (Neuroradiology Unit, Department of Neurosciences and Rehabilitation, S. Anna Hospital, Ferrara, Italy), Emiliani E. (Istituto Oncologico Romagnolo, Department of Medical Oncology, Santa Maria delle Croci Hospital, Ravenna, Italy), Cavallo M. (Division of Neurosurgery, Department of Neurosciences and Rehabilitation, S. Anna Hospital, Ferrara, Italy).
Executive committee
Franceschi E., Tosoni A. (Department of Medical Oncology, Bellaria-Maggiore Hospitals, Bologna, Italy), Cavallo M. (Division of Neurosurgery, Department of Neurosciences and Rehabilitation, S. Anna Hospital, Ferrara, Italy), Fiorica F. (Department of Radiation Oncology, S. Anna Hospital, Ferrara, Italy), Valentini A. (Division of Neurosurgery, Nuovo Ospedale Civile S. Agostino-Estense, Baggiovara, Modena, Italy), Depenni R. (Department of Oncology, Policlinico di Modena, Italy), Mucciarini C. (Department of Oncology, Ramazzini Hospital, Carpi, Modena, Italy), Crisi G. (Department of Neuroradiology, Maggiore Hospital, Parma, Italy), Sasso E. (Department of Neurological Sciences, Maggiore Hospital, Parma, Italy), Biasini C., Cavanna L. (Department of Oncology and Hematology, Guglielmo da Saliceto Hospital, Piacenza, Italy), Guidetti D. (Department of Neurology, Guglielmo da Saliceto Hospital, Piacenza, Italy), Marcello N., Pisanello A. (Department of Neurology, Istituto in tecnologie avanzate e modelli assistenziali in oncologia, IRCCS, S. Maria Nuova Hospital, Reggio Emilia, Italy), Cremonini A.M., Guiducci G. (Division of Neurosurgery, M. Bufalini Hospital, Cesena, Italy).
Registry Coordination Office: de Pasqua S., Testoni S. (IRCCS Institute of Neurological Sciences, Bologna, Italy).
Participants
Agati R., Ambrosetto G., Bacci A., Baldin E., Baldrati A., Barbieri E., Bartolini S., Bellavista E., Bisulli F., Bonora E., Bunkheila F., Carelli V., Crisci M., Dall'Occa P., Ferro S., Franceschi C., Frezza G., Grasso V., Leonardi M., Morandi L., Mostacci B., Palandri G., Pasini E., Pastore Trossello M., Poggi R., Riguzzi P., Rinaldi R., Rizzi S., Romeo G., Spagnolli F., Tinuper P., Trocino C. (Bologna), Dall'Agata M., Frattarelli M., Gentili G., Giovannini A., Iorio P., Pasquini U., Galletti G., Guidi C., Neri W., Patuelli A., Strumia S. (Forlì-Cesena), Faedi M. (IRCCS Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori), Casmiro M., Gamboni A., Rasi F. (Faenza R.A.), Cruciani G. (Lugo, RA), Cenni P., Dazzi C., Guidi A.R., Zumaglini F. (Ravenna), Amadori A., Pasini G., Pasquinelli M., Pasquini E., Polselli A., Ravasio A., Viti B. (Rimini), Sintini M. (Cattolica, RN), Ariatti A., Bertolini F., Bigliardi G., Carpeggiani P., Cavalleri F., Meletti S., Nichelli P., Pettorelli E., Pinna G., Zunarelli E. (Modena), Artioli F., Bernardini I., Costa M., Greco G., Guerzoni R., Stucchi C. (Carpi M.O.), Iaccarino C., Ragazzi M., Rizzi R., Zuccoli G. (Istituto di Ricovero e Cura a Carattere Scientifico, Reggio Emilia), Api P., Cartei F., Colella M., Fallica E., Farneti M., Frassoldati A., Granieri E., Latini F., Monetti C., Saletti A., Schivalocchi R., Sarubbo S., Seraceni S., Tola M.R., Urbini B., Zini G. (Ferrara), Giorgi C., Montanari E. (Fidenza P.R.), Cerasti D., Crafa P., Dascola I., Florindo I., Giombelli E., Mazza S., Ramponi V., Servadei F., Silini E.M., Torelli P. (Parma), Immovilli P., Morelli N., Vanzo C. (Piacenza), Nobile C. (Padova).
Rights and permissions
About this article
Cite this article
Yeung, T.P.C., Wang, Y., He, W. et al. Survival prediction in high-grade gliomas using CT perfusion imaging. J Neurooncol 123, 93–102 (2015). https://doi.org/10.1007/s11060-015-1766-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1766-5