Abstract
Purpose
Assessment of renal masses with conventional imaging may be challenging. Anti-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid (anti-[18F]FACBC) is a synthetic l-leucine analog with relatively little renal excretion. The present study examines anti-[18F]FACBC positron emission tomography uptake in patients with renal masses.
Procedures
Six patients with seven renal lesions were imaged dynamically for 2 h after injection of 10–10.9 mCi (370–403 MBq) anti-[18F]FACBC. Lesions were evaluated qualitatively and quantitatively and correlated with histology.
Results
Four clear cell and one Rosai–Dorfman lesion were hypo/isointense to normal cortex; two papillary lesions in the same patient were hyperintense. Mean SUVmax ± SD at 30 min was 2.8 ± 0.24 for clear cell carcinomas and 4.5 ± 1.7 for papillary cell lesions. Mean SUVmax/SUVmean ratios ± SD of lesion to normal cortex at 30 min was 1.15 ± 0.19 for the clear cell carcinomas and 2.3 ± 0.84 for papillary cell.
Conclusions
In this small patient sample, relative amino acid transport compared with renal cortex is elevated in renal papillary cell carcinoma but not in clear cell carcinoma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There were approximately 51,190 new cases of renal cancer in the United Sates in 2007 [1]. An overlap in the appearance of some benign and malignant renal masses detected by computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound (US) may lead to patient management difficulties [2–6]. In addition, preoperative differentiation of more aggressive clear cell renal cell carcinoma from that of less aggressive papillary carcinoma subtypes may be helpful [7]. The role of 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography (FDG PET) for the detection of primary renal cell carcinoma is limited [6, 8]. Thus, a noninvasive exam utilizing molecular imaging techniques to add other metabolic data to anatomic imaging would be desirable in the investigation of renal masses
Anti 1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid (anti-[18F]FACBC) is a synthetic l-leucine analog which has been demonstrated to have excellent uptake within primary and metastatic prostate carcinoma with little renal excretion compared with 18F-FDG [9]. The uptake of anti-[18F]FACBC is likely mediated via the sodium-independent “L” large-neutral amino acid transport system (LAT) [10, 11]. The present study examines anti-[18F]FACBC uptake in patients with newly diagnosed renal masses.
Materials and Methods
Preparation of anti-[18F]FACBC
The preparation of anti-[18F]FACBC has been previously reported [12]. The decay corrected radiochemical yield of the desired product was 24%, and its radiochemical purity was 99% at 80 min after end of bombardment. The injected mass was approximately 5 mg or 38 µmol, and the specific activity was 3.7–5.2 Ci/mmol.
Patient Selection
All studies were performed under the auspices of the Emory University Institutional Review Board, Radioactive Drug Research Committee, and the Atlanta VA Medical Center Research and Development Committee. Inclusion criteria included patients with a solid renal mass and/or complex cystic Bosniak 3 or 4 renal mass on CT, MRI, and/or ultrasound, larger than 1 cm in size, and who were already scheduled for biopsy and/or surgical resection.
PET Imaging Protocol
All scanning was conducted on a Discovery DLS integrated PET-CT scanner (GE, Milwaukee, WI, USA), and the images were interpreted on an Xeleris workstation (GE, Milwaukee, WI, USA), as well as a program developed by the authors on the IDL platform (RSI, Boulder, CO, USA) running on a Pentium 4 computer (IBM, Armonk, NY, USA). Patients fasted for 4–6 h before the anti-[18F]FACBC scan. This is our standard practice for [18F]FDG PET scanning, though it has not been established if a fasting or non-fasting state optimizes anti-[18F]FACBC imaging. The patient first underwent a CT scan of the abdomen (80–120 mA) without oral or IV contrast for anatomic correlation and attenuation correction of emission data. The patient then received a bolus of anti-[18F]FACBC (370-403 MBq) injected IV over 1–2 min. PET imaging consisted of a 21-frame dynamic sequence lasting 125 min [number of frames, time (min): 6,0.5; 4,3; 11,10] centered upon the kidneys.
Image Analysis
The PET/CT studies were reviewed by one experienced nuclear radiologist (DMS). Each mass was visually assessed for activity and recorded as less than, equal to, or greater than normal cortex. Circular regions of interest, 1.5 cm, were defined on transaxial planes conforming to the lesions on PET-CT and SUVmax values of each lesion were obtained, as well as SUV mean from aorta and representative normal uninvolved ipsilateral renal cortex at 5, 15, 30, 60, 90, and 120 min. The ratio of SUVmax of each mass to SUVmean of normal cortex was calculated for each time point and was chosen because it best replicates a typical visual identification of a “hot spot” upon a homogenous background.
Correlation of Imaging to Clinical and Pathologic Data
Anti-[18F]FACBC imaging findings were correlated with pathologic follow-up, either after nephrectomy or biopsy before cryoablation of the renal tumor. Correlation of quantitative and qualitative uptake was made to tumor histology.
In one patient, a cervical spine lesion was proven to be metastatic renal carcinoma on biopsy, and it was presumed that the renal mass had the same histology. In another patient, a lesion less than 1 cm was reported intraoperatively, but its location could not be localized on imaging; thus, it was not included in the analysis. Due to the small patient sample, only descriptive statistics were applied.
Results
Patient mean age (years ± SD) is 67.3 ± 15.1 with a range of 47–89. Five males and one female were studied. Tumor size ranged from 2.2 to 9 cm. Histology demonstrated four clear cell renal cell carcinomas, two papillary renal cell carcinomas, and one Rosai–Dorfman lesion. The two papillary cell carcinomas were in the same patient. Table 1 is a summary of lesion characteristics, SUV, and ratio to normal cortex at representative time points.
Upon visual analysis, the clear cell and Rosai–Dorfman lesions were hypo- or isointense to normal renal cortex except at one time point at 15 min when one of the clear cell lesions was slightly hyperintense. The two papillary lesions were hyperintense to normal renal cortex except at the 5-min frame in which one of the papillary lesions was hypointense to normal cortex.
Based upon analysis of time activity curves, imaging at 30 min resulted in the best lesion to background contrast. Mean SUV ± SD at 30 min for the clear cell carcinomas (n = 4) was 2.8 ± 0.24 and for the papillary cell lesions (n = 2) 4.5 ± 1.7. Mean ratios ± SD of lesion to normal cortex at 30 min for the clear cell carcinomas (n = 4) was 1.15 ± 0.19 and for the papillary cell (n = 2) 2.3 ± 0.84. At 30 min, the Rosai–Dorfman lesion had an SUV of 3.1 and a lesion to cortex ratio of 1.1.
Analysis of time activity curves of SUV (Fig. 1) demonstrates generalized decrease of SUV with time for all lesions. Analysis of time activity curves of lesion to cortex ratios (Fig. 2) reveals that only the papillary lesions had a sustained lesion to cortex ratio greater than 1.5 for the 15- to 60-min time frames.
Time activity curve of SUVmax of each renal lesion. One of the papillary cell lesions had greater and sustained SUV over time compared with the other renal lesions. The right-hand legend table notes histology (grade): RD Rosai–Dorfman, clear clear cell renal cell carcinoma, Pap papillary cell renal cell carcinoma.
Time activity curve of the ratio of lesion SUVmax to normal renal cortex. Only the two papillary cell lesions had a sustained uptake ratio greater than 1.5 for the 15- to 30-min time interval. The right-hand legend table notes histology (grade): RD Rosai–Dorfman, clear clear cell renal cell carcinoma, pap papillary cell renal cell carcinoma.
We also separately plotted (Fig. 3) the average of the four clear cell carcinoma SUVmax values and the average of the two papillary cell carcinoma SUVmax values versus the average of the aorta and normal renal cortex SUVmean values for all points of the dynamic data set. While clear cell carcinoma uptake and washout closely parallels that of normal cortex and aorta blood pool, papillary cell carcinoma uptake peaks higher and retains more activity for the 2 h of imaging. Figures 4 and 5 are examples of FACBC PET-CT images at the 30-min frame for papillary cell carcinoma and clear cell lesions, respectively.
Time–activity curves of the average of the four clear cell carcinoma SUVmax values in (a) and the average of the two papillary cell carcinoma SUVmax values in (b) plotted against the average of the aorta and normal renal cortex SUVmean values. Note that while clear cell carcinoma uptake and washout (a) closely parallels that of normal cortex and aorta blood pool, papillary cell carcinoma uptake (b) peaks higher and retains more activity for the 2 h of imaging.
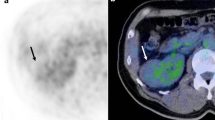
Axial CT (a), PET (b), and fused (c) anti-[18F]FACBC images in a patient with iso-cortical uptake within a large clear cell carcinoma of the left kidney (white arrow). A relatively hypointense center suggests central necrosis. White arrowhead and curved arrow in (b) are normal intense hepatic and pancreatic uptake, respectively.
Anecdotally, intense anti-[18F]FACBC uptake in enlarged retroperitoneal lymph nodes in the patient with the Rosai–Dorfman lesion proved to be metastatic prostate carcinoma and was further investigated in a different study [9].
Discussion
FDG PET has not demonstrated utility in the evaluation of primary renal masses [6, 8]. Other PET radiotracers such as 18F-fluoromisonidazole are being investigated for the evaluation of primary renal cell carcinoma [6]. One of the reasons 18F-FDG has limited value for renal tumors is its high level of renal excretion, limiting conspicuity of uptake adjacent to normal intense activity in the renal collecting system.
Unlike 18-FDG, anti-[18F]FACBC demonstrates relatively little renal excretion [9, 13, 14]. Thus, we hypothesized that anti-[18F]FACBC could serve as a valuable adjunct in the evaluation of renal masses much in the same way low native brain uptake helps makes this radiotracer potentially useful for cerebral tumors [15, 16]. Unfortunately, with the most predominant of renal cell carcinomas, the clear cell subtype, conspicuity was poor, being equal to or less than normal renal cortex. Only in the case of the two papillary lesions was visualization greater than renal cortex. Both of these carcinomas were in the same patient and thus may have been clonally similar.
The time activity curves of Figs. 1 and 3 show rapid uptake of anti-[18F]FACBC followed by a relatively rapid average washout out of 19%/min to 30 min and a sustained tracer concentration out to 120 min. Yet, the time–activity curve of Fig. 3 demonstrates that papillary cell carcinoma uptake peaks higher and retains more activity for the 2 h of imaging, allowing conspicuity against typical normal renal cortex background.
It is interesting to note that in literature examining differentiation of subtypes of renal cell carcinoma based on CT imaging with iodinated contrast [7, 17], papillary lesions were noted to be relatively hypovascular in terms of enhancement compared with clear cell carcinomas. Analysis of the time activity curves suggests that the uptake of anti-[18F]FACBC leading to conspicuity in the two papillary tumors in this series is not due to blood flow alone, but rather some type of active transport mechanism and an element of trapping leading to less washout for papillary cell carcinomas than for clear cell carcinomas. The preoperative differentiation of papillary from clear cell carcinoma may have some limited utility in terms of approach to that patient’s medical or surgical management [17].
A shortcoming of our study is that due to its pilot nature, a few patients were studied, and no definitive conclusions can be reached from the small sample. If anti-[18F]FACBC is to prove useful in differentiating papillary from clear cell subtypes, a much larger series will have to be undertaken since papillary cell subtypes constitute approximately 10–15% of all resected renal cell carcinomas.
Of note, uptake within the lymph nodes involved with metastatic prostate carcinoma spurred further research in the utility of anti-[18F]FACBC PET for patients with prostate carcinoma [9].
Conclusions
In conclusion, in this small patient sample, anti-[18F]FACBC did not prove useful in visualizing clear cell renal cell carcinoma but demonstrated better conspicuity for papillary cell tumors. In order to determine if anti-[18F]FACBC may have some utility in determining preoperatively if a lesion is a papillary cell subtype, a larger series would be necessary. Anti-[18F]FACBC PET has shown promise in evaluating patients with cerebral lesions and with prostate carcinoma [9, 15, 16].
References
Jemal A, Siegel R, Ward E et al (2006) Cancer statistics, 2006. CA Cancer J Clin 56:106–130
Rodriguez R, Fishman EK, Marshall FF (1995) Differential diagnosis and evaluation of the incidentally discovered renal mass. Semin Urol Oncol 13:246–253
Montie JE (1991) The incidental renal mass. Management alternatives. Urol Clin North Am 18:427–436
Davidson AJ, Hartman DS, Choyke PL, Wagner BJ (1997) Radiologic assessment of renal masses: implications for patient care. Radiology 202:297–305
Kutikov A, Fossett LK, Ramchandani P et al (2006) Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology 68:737–740
Bolton DM, Wong P, Lawrentschuk N (2007) Renal cell carcinoma: imaging and therapy. Curr Opin Urol 17:337–340
Zhang J, Lefkowitz RA, Ishill NM et al (2007) Solid renal cortical tumors: differentiation with CT. Radiology 244:494–504
Kang DE, White RL Jr, Zuger JH, Sasser HC, Teigland CM (2004) Clinical use of fluorodeoxyglucose F 18 positron emission tomography for detection of renal cell carcinoma. J Urol 171:1806–1809
Schuster DM, Votaw JR, Nieh PT et al (2007) Initial Experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med 48:56–63
Martarello L, McConathy J, Camp VM et al (2002) Synthesis of syn- and anti-1-amino-3-[18F]fluoromethyl-cyclobutane-1-carboxylic acid (FMACBC), potential PET ligands for tumor detection. J Med Chem 45:2250–2259
McConathy J, Martarello L, Simpson NE et al (2002) Uptake profiles of six 18F-labeled amino acids for tumor imaging: comparison of in vitro and in vivo uptake of branched chain and cyclobutyl amino acids by 9LGliosarcoma tumor cells. J Nucl Med 43:41P
McConathy J, Voll RJ, Yu W, Crowe RJ, Goodman MM (2003) Improved synthesis of anti-[18F]FACBC: improved preparation of labeling precursor and automated radiosynthesis. Appl Radiat Isot 58:657–666
Nye JA, Schuster DM, Yu W, Camp VM, Goodman MM, Votaw JR (2007) Biodistribution and radiation dosimetry of the synthetic nonmetabolized amino acid analogue anti-18F-FACBC in humans. J Nucl Med 48:1017–1020
Oka S, Hattori R, Kurosaki F et al (2007) A preliminary study of anti-1-amino-3-18F-fluorocyclobutyl-1-carboxylic acid for the detection of prostate cancer. J Nucl Med 48:46–55
Schuster DM, Votaw JR, Halkar RK et al (2003) Uptake of the synthetic PET amino acid radiotracer 1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid (18F-FACBC) within primary and metastic brain cancer compared with 18F-fluorodeoxyglucose (18F-FDG). J Nucl Med 5:167
Shoup TM, Olson J, Hoffman JM et al (1999) Synthesis and evaluation of [18F]1-amino-3-fluorocyclobutane-1-carboxylic acid to image brain tumors. J Nucl Med 40:331–338
Ruppert-Kohlmayr AJ, Uggowitzer M, Meissnitzer T, Ruppert G (2004) Differentiation of renal clear cell carcinoma and renal papillary carcinoma using quantitative CT enhancement parameters. AJR Am J Roentgenol 183:1387–1391
Acknowledgements
This research was sponsored by a Society of Nuclear Medicine Research and Education Foundation Pilot Research Grant. We would like to acknowledge the hard work of Fenton G. Ingram, CNMT, PET, RT(R) on this protocol as well as all our technologists at Emory Healthcare. We also gratefully acknowledge the contributions of Ron Crowe, R.Ph. for radiotracer synthesis, Dr. Mahul B. Amin for histologic interpretation, Dr. John Carew for statistical consultation, and Drs. Fray F. Marshall, Kenneth Ogan, and Chad Ritenour for clinical urologic guidance and patient referrals. Dr. Mark Goodman and Emory University are eligible to receive royalties from this radiotracer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schuster, D.M., Nye, J.A., Nieh, P.T. et al. Initial Experience with the Radiotracer Anti-1-amino-3-[18F]Fluorocyclobutane-1-Carboxylic Acid (Anti-[18F]FACBC) with PET in Renal Carcinoma. Mol Imaging Biol 11, 434–438 (2009). https://doi.org/10.1007/s11307-009-0220-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-009-0220-5