Abstract
Nuclear Medicine is the science and practice of using radionuclides for diagnosis or therapy. There is a long tradition of the use of nuclear medicine in urological cancers stretching back to the origin of the specialty 60 years ago. Imaging devises have been refined and more recently added to CT so that functional and morphological information can be obtained at the same time. This has introduced the idea of the “one stop imaging shop” which improves the accuracy of imaging and reduces costs and the time taken for the patient to be imaged.
The types of nuclear medicine tests commonly performed in urological cancer setting include glomerular filtration rate (GFR) measurement and dynamic renography to assess renal function and bone scintigraphy to identify and quantify bone metastases. More recent work has concentrated on the use of positron emission tomography (PET) techniques which were generally less used which F-18 FDG (Fluorodeoxyglucose) was the only tracer available but now tracers which are much more avid for urological cancers such as F-18 and C-11 choline are becoming more widely available. In tandem radionuclide therapy has concentrated on palliation of bone pain but a recent trial has suggested that Ra-223 treatment can also have a survival benefit.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
In nuclear medicine administering to the patient orally or intravenously a radioactive tracer material visualizes the target organ. Imaging is performed with a gamma camera, which is able to form an image from the gamma photons emitted, by the radioactive tracer material. There are a wide range of applications for radiopharmaceuticals in the investigation and management of urological cancers. The advantage is that nuclear medicine techniques allow physiological processes to be followed in a non-invasive way, which can not only be done with imaging but by measuring the clearance of radioactivity from the blood or the appearance of radioactivity in urine.
The information provided is different from that obtained by other forms of radiology in that it is primarily physiological and not anatomical. Therefore the results of nuclear medicine results are complementary to other forms of radiology and can often be combined to improve diagnostic yield as can be seen in PET-CT. The type of study used will depend on the clinical situation and the question the clinician needs answering.
Atomic Structure and Radioactivity
All matter is made up of atoms. Each atom is made up of a nucleus, which is positively charged and surrounded by negatively charged electrons. The nucleus is made from positive charged protons and neutral neutrons. The number of protons and neutrons determine the atomic weight of the atom. The number of protons determines the atomic number and the chemical nature of the atom. A single element will have the same number of protons as any other atom of the same element. This is seen simply with hydrogen, in its simplest form Hydrogen has a single proton only so its atomic weight is 1 and its atomic number is 1. However there are two other forms of hydrogen each of these are called the isotopes of hydrogen the first is deuterium which has a proton and a neutron in the nucleus. The atomic weight is now 2 but atomic number remains 1. Likewise tritium has an atomic weight of 3 with 2 neutrons and one proton but atomic number remains 1. Though they have different atomic weight chemically hydrogen, deuterium and tritium all have the same chemical qualities.
The addition of neutrons (and in some cases removing neutrons) results in the nucleus being unstable and it can spontaneously break apart releasing energy. This is called radioactive decay and the isotopes that undergo these changes are called radioisotopes.
Finally when radioactive decay occurs the resulting isotope may itself not be stable and may have a transitory phase before decaying to a more stable form called the metastable form and marked with the letter “m”. Therefore a radioisotope may have both a metastable form such as Tc-99m with a half-life of 6 h and a more stable form Tc-99 with a half-life of thousands of years. These different forms of the same radioisotope are called radionuclides.
Positrons
A variation on radioactive decay is the fate of nuclei, which are proton rich. When these decay they produce a positively charged electron called a positron. This will travel a short distance typically 0.5–1 mm in tissue where it meets an electron. When the two combine to annihilate each other and all their mass is converted into energy. As the mass of a positron and electron cannot be changed the energy released is defined by Einstein’s equation E = mc2 in this case the energy is 1,022 keV and is released as 2 gamma rays each of energy 511 keV. These can then be picked up by the ring of detectors in a positron emission tomography (PET) machine.
The Tracer Principle
All nuclear medicine studies independent of their simplicity are based on de Heversey’s tracer principle. In a nuclear medicine study a small amount of a radioactive tracer is administered to the patient to track a specific physiological process without having a pharmacological action, which may change the effect you are observing. To do this tiny amounts of a radioactive tracer are used, often less than a billionth of a gram. Then the radioactivity in a particular fluid in the body is measured or imaged with an external imaging devise. These radioactive substances are called radiopharmaceuticals.
Tracers Used in Nuclear Medicine
There is a range of radiopharmaceuticals used in assessment of a patient’s renal tract. These can be split into three groups:
-
1.
Those with poor imaging characteristics used for in vitro testing.
-
2.
Those that can be used for imaging using gamma camera.
-
3.
Those that are positron emitter and can be imaged using PET.
Most rely on gamma ray emission as they decay to be detected either in a well counter or on an imaging device. When imaging the renal tract directly most of the agents used are highly hydrophilic allowing for rapid excretion through the kidneys. In the case of Diethylenetriaminepentaacetic acid (DTPA) excretion is purely glomerular, with hippuran it is purely tubular whereas with mercaptoacetylglycylglycylglycine (MAG3) it is both ways. In addition to the pharmaceutical there also needs to be an isotope. Together they make a specific radiopharmaceutical, which is designed to perform a specific task such as look at renal blood, flow, uptake and excretion as in the case of DTPA.
Radionuclides
The first used isotopes tended to be those, which were produced as a by-product of the fission of Uranium-238. The most common of these is Iodine-131. On its own it is of little use in urology but when added to hippuran to produce Iodine-131 hippuran (also written by convention as I-131 hippuran or 131I-hippuran). It is possible using either blood samples to measure the clearance of the agent from the blood to give the effective renal plasma flow (EPRF). Using sodium iodide scintillation probes and later an Anger gamma camera, it is possible to measure uptake of the I-131 hippuran into the kidney and its excretion. The images are of poor quality and the radiation dose is high but this remains a valid test though often a different isotope of Iodine (I-123 or 123I) is now used as it gives slightly better images and at a lower radiation dose.
Technetium 99m
Technetium 99m (Tc-99m or 99Tcm) is the most commonly used isotope in nuclear medicine; however it is an artificial isotope with a very short half-life of only 6.02 h. This is a metastable form of technetium-99 with the conventionally written as 99Tcm. This isotope is the decay product (called a daughter) of another isotope molybdenum-99 (99Mo) which is a product of nuclear fission of 238U. However, to get sufficient quantities for medical use worldwide special high neutron flux reactors are used because on any given day about one million 99Tcm tests are performed for various different reasons worldwide.
Almost all the 99Mo is created in just four reactors (one in Canada, one in South Africa and two in Europe). All but the South African reactors are over 40 years old and due for de-commissioning. The age of the reactors meant that twice since 2009 there have been multiple breakdowns and there has been little or no 99Mo produced. This resulted in many millions of nuclear medicine studies being cancelled worldwide. At present there is no long-term solution to this problem and 99Mo supplies remain fragile.
As the half life of 99Tcm is so short it is best to have the equipment to separate 99Tcm from the parent 99Mo based as close to the patient as possible. Many hospitals will have their own 99Tcm/99Mo generator on site and it can be used once or twice a day for about 1 week.
The 99Tcm is drawn off as a column of insoluble molybdenum oxide and is oxidised to valency-7 (therefore is chemically soluble sodium pertechnetate). Then using transitional metal chemistry it can be added to vials containing the required pharmaceutical with buffers and a reducing agent to allow for binding. Most of these radiopharmaceuticals take 10–30 min to prepare depending on whether or not the product has to be boiled. Again worldwide over 80 % of all nuclear medicine tests use this isotope and these techniques are widely spread in developing countries.
Physical Characteristics
The 99Tcm produces a single burst of radiation when it decays, called its characteristic radiation. This radionuclide has energy of 140 KeV. This is ideal for the simple gamma cameras used based on a sodium-iodide scintillator. The range of radiopharmaceuticals that are available for use in urological cancers is wide and described in Table 6.1. It should be noted by the use of a variety of radiopharmaceuticals the same radionuclide can be used for a wide range of indications.
Measurement of Glomerular Filtration Rate
For in vitro testing of renal physiology, a long living isotope is preferable, attached to a product, which is excreted via a single part of the nephron. For this Chromium-51 (51Cr) is ideal when attached to ethylenediaminetetracetic acid (EDTA) which is filtered by the glomerulus and not re-absorbed. The gamma irradiation is not good for imaging but in small quantities such as 3–4 MBq will be measured efficiently in a well counter and therefore can be used via the radio-activity in sequential blood specimens to calculate the glomerular filtration rate (GFR). This is used in assessment of renal function during chemotherapy but in combination with 99Tcm DMSA can be used to predict residual renal function after nephrectomy.
Positron Emitters
The second main class of imaging radiopharmaceuticals are those that are positron emitters. Positrons are positively charged electrons emitted during decay of proton rich nuclei. To obtain these isotopes means adding protons or proton rich nuclei to the nucleus of the target atom. This cannot be done in a reactor instead a machine called a cyclotron accelerates the These positively charged particles, such as protons or alpha particles to close to the speed of light and then bombards them into a precise target. The resulting positron emitting isotopes tend to be of low atomic number such as oxygen nitrogen or fluorine. These have short half-lives so have to be used close to the site of production. For example, the most commonly used of these positron emitters is fluorine-18 (18F). This has a 2-h half-life and as after production some time will be taken up combining the positron emitter with a physiologically useful organic molecule such as glucose to make FDG. It is generally considered practical if the images are performed within a 2-h radius of production. This might mean 30 miles by road transport in a big city but 600 miles when the product can be moved by air.
Imaging with Positrons
All positron emitters produce two 511 kEV gamma rays after their annihilation with an electron. The two gamma rays travel at 180° and can be identified by a paired detection system. Though traditional gamma cameras can be used the 511 jeV energy is a little high for them to work efficiently so a special crystal such as Beryllium-Germanium-Oxide (BGO) is used. These are set in a ring to collect the pairs of 511 keV photons, called co-incidence events. This enabled a 3 dimensional map of positron emission to be produced. This type of machine is a positron emission tomography (PET) camera. The most common form available at present is a combination of a PET machine and CT. The CT component allows accurate localisation of any site of abnormal uptake and is also used to correct the PET image for any attenuation of the gamma signal through the patient. In the last 12 months PET combined with MRI has also been launched. This technique must be considered experimental and there has been no evidence that it will be of use in urological cancers.
Therapy Isotopes
The third type of radiopharmaceutical has a particulate form of emission such as a beta or alpha particle. These have mass and therefore can deliver a higher radiation dose to any tissue they interact with but cannot be used for imaging unless there is an accompanying gamma emission. In urological cancers there use until recently has been limited to pain relief from bone metastases, however new agents which directly target renal cancer are entering phase II and phase III clinical trials.
Imaging Devices
To be able to image radiopharmaceuticals efficiently, a range of imaging devices have been produced. They all relay on the same principle. They use a scintillator, which is a type of crystal, which is temporarily energised by being hit by a gamma ray resulting in the production of visible light. This is picked up by a photo-multiplier tube (PMT), which converts this signal into the electrical energy, which is amplified and then passed on to a computer. The simplest form of such a system is found in a well gamma counter which can be used to measure small amounts of radioactivity in blood.
The Anger Camera
Invented in 1959 by Hal Anger, the gamma camera uses a large single scintillation crystal often 40 × 60 cm and about 2 cm thick. To pick up the light signal as array of 30–70 PMTs are needed and are normally hexagonal so they fit together in a honeycomb fashion to ensure the surface is covered. As the amount of light produced by the scintillation crystal is small both the scintillation crystal and PMTs are held in a light proof box. Gamma rays cannot be focused like light so any scattered photos would degrade the image and make it look more “fuzzy”. To remove these scattered photons the gamma rays pass through a series of parallel lead lined tubes called a collimator. The width of the septa in the collimator is determined by the energy of the gamma rays and divided into “low energy” (0–180 keV), medium energy (180–300 keV) and high energy (>300 keV). The computer that is attached to the gamma camera system then determines where on the crystal a gamma ray arrived and its energy. These camera cameras can be mounted on gantries that can move along the patient to perform whole body images and rotate around the patient producing a three-dimensional image called single photon emission computed tomography (SPECT). The associated computer system can detect a series of images (every 0.5 s upwards) of a particular part of the body. This is called a dynamic image then the computer can calculate the activity of a radiopharmaceutical at any area of interest to perform a time activity curve as seen in renography.
Positron Emission Tomography (PET) Imaging
PET imaging does not need collimation, as a scattered gamma ray will be deflected much like a billiard ball hitting one another. Therefore it will not arrive 180° from its partner gamma ray released at the same time and will not make a co-incidence event and will not be recorded. As no lead collimation is needed in PET less gamma rays are stopped and the system is more efficient than a standard gamma camera.
Tomography
Both SPECT and PET are excellent methods to look at functional images of the body and are finding increasing roles in the imaging of urological cancers. However spatial resolution is limited to about 7 mm for SPECT and 3 mm for PET, though these figures are optimistic and describe the best attainable. Also identifying the site of any abnormal uptake can be difficult due to the lack of anatomical markers. Therefore both SPECT and PET machines have been added to CT scanners to allow for simultaneous SPECT/CT and PET/CT a technique called image registration.
Radiation Dosimetry
The radiation burden for nuclear medicine tests tends to lie in the low to medium band compared to other radiological studies The unit of measurement for radiation is the Sievert (Sv) which equals a joule of received energy per kg of tissue Clearly as this is a lot of energy normally a mSv is used.
Residents of London receive on average 2–5 mSv of radiation per year. Residents of Cornwall or Aberdeen who live on granite may receive an annual radiation dose of 5–10 mSv due to Radon gas seeping into their home. The area of the world with the highest annual background radiation dose is the Southern Caucasus with an annual radiation rate of 25 mSv. The radiation dose from X-rays studies may vary depending on the number of views taken, energy of the X-ray beam and exposure time. In Nuclear Medicine the radiation dose is determined by the activity of radioisotope given. In the UK this is itself governed by a statuary instrument and administered through a committee of the Department of Health called the Administration of Radioactive Substances Advisory Committee (ARSAC) who publish typical maximum activities and radiation doses received (Table 6.2) [1]. A few radiological investigations are included for comparison. However, it can be seen that the excess radiation of a single nuclear medicine test is not great compared to background radiation.
Assessment of Renal Function
The most commonly used isotopic test for the assessment of renal function is the measurement of GFR with a radiopharmaceutical, which is exclusively filtered in the glomerulus. There are several agents which can do this including 99Tcm-DTPA. However the short half-life of 99Tcm at 6 h means that any counts must be decay corrected and correct timing of samples taken is vital. Also in patients with impaired GFR (that is below 30 ml/min), a 24-h sample is needed, which is not practical with 99Tcm-labeled pharmaceuticals. The method of choice then is to use 51Cr-EDTA, which has a half-life of 27 days. This helps make counting simple with no real need to decay correct and samples may be counted 2–3 days after the test has been performed. In addition taking a late sample at 24 h is simple.
The standard method is to take a background sample and then three samples 2, 3 and 4 h after injection of 2–4 MBq of 51Cr-EDTA. The activity in a millilitre of plasma is then plotted on a graph and the rate of reduction of counts gives the GFR. However, a simpler 1 blood sample method and some mathematical modelling by Martenssen et al. [2] has shown that if the GFR is more than 50 ml/min then it is just as accurate as a three sample method. Likewise if the GFR is less than 30 ml/min, a 24 sample should be added. As it may not be possible to pre-predict the exact GFR before a test is done then more than one method may be needed. The creatinine clearance or estimated GFR can be used as a guide but may be inaccurate if the patient is in a catabolic state from malnutrition (the anorexia of cancer or post-chemotherapy), or has impaired renal function or has suffered a recent muscle injury including an operation. Also the GFR is more accurate for sequential measurements especially if potentially nephrotoxic treatment is being given [3, 4].
The main use of a GFR in urological cancer management is the correct dosing of nephrotoxic chemotherapy (especially platinum based drugs) before and during treatment. The combination of the GFR and divided renal function as determined by a 99Tcm DMSA study can be used to predict post nephrectomy renal function. The EPRF may be a better measure of early drug induced renal toxicity than GFR but it has not been accepted into standard clinical practice.
Cardiac Assessment
Though not directly connected to the urological system, a well functioning heart it is required for the efficient treatment of some patients. Many nephrologists think that the heart is there merely to perfuse the kidneys and there is some element of truth in this. However, there are two specific areas in which assessment of the heart using radionuclide techniques may be of particular value. The first is a general assessment of myocardial perfusion before any major surgery such as nephrectomy as not only is cancer more common in the elderly but so is heart disease. Therefore the high negative predictive value of stress and rest myocardial perfusion scintigraphy using 99Tcm-methyliso-butyliso nitrile (MIBI), 99Tcm-tetrofosmin or thallous-201 chloride (201TlCl) will determine, if normal with no evidence for myocardial ischaemia that a major operation can proceed without risk to the patient.
In addition gated blood pool imaging using 99Tcm-labeled red blood cells allow an accurate determination of left ventricular ejection fraction (LVEF) which is less dependent of left ventricular geometry than stress echocardiography and may be more accurate in serial studies [5]. This is used to assess the LVEF before and after chemotherapy with cardiotoxic drugs of doxorubicin type and may be of particular use if the patient has co-existent hypertension and or diabetes for which the commonly used nomograms for maximal tolerated drug may not apply.
Renography
Renography, acquired by dynamic gamma camera imaging normally with 300 MBq 99Tcm-DTPA or preferably with 100 MBq 99Tcm-MAG3 again are not primary methods used to assess urological cancers. However, they have two main roles. Firstly, in combination with a GFR it may be used to determine the expected result of a nephrectomy on residual renal function. For example, if a renogram demonstrates that each kidney contributes 50 % of renal function and the total GFR is 80 ml/min, removal of one kidney will result in a residual GFR of 40 ml/min. Interestingly as the most common agent used for bone imaging 99Tcm-MDP is up to 80 % excreted in the kidneys during the first 20 min post injection it may be possible to combine a 99Tcm-MDP renogram with a staging bone scan, which if combined with a GFR may provide all the staging required for the patient, in nuclear medicine terms, quickly and efficiently with reduced cost and radiation dose to the patient.
The second use of renography is to assess renal function if there is pelvic tumor, which is suspected of blocking one or both ureters, in which case a high urine flow rate needs to be achieved to prevent false positive studies due to ureteric dilatation form old obstruction. Frusemide can be given at any time during the renogram but if ureteric obstruction is suspected giving the Frusemide 15 min before the imaging agent (the Manchester protocol or F-15 protocol) means that the maximum diuresis occurs when the imaging agent in injected [6]. A flat or rising excretion phase curve suggests partial or complete obstruction of the ureter on that side (Fig. 6.1). If the ureter is stented or a nephrostomy placed the F-15 renogram should be repeated about 48 h later to determine if the kidney is now clearing. The divided function on the affected side will give some idea of the age of the obstruction, but if it is within a few days, the function of the affected kidney would not have been reduced by a huge margin and relief of the obstruction should result is a rapid return to full function of that kidney. If the function of the obstructed kidney is poor then a full recovery becomes less likely.
Ureteric Obstruction
In very acute obstruction, which is complete, the renogram with 99Tcm-MAG3 demonstrates not hold up in the renal pelvis but in the kidney parenchyma-a sort of “shock kidney” picture. The clues are divided function is normal, the other kidney drains and ultrasound suggests a dilated collecting system. If this pattern is seen early drainage procedures work well, often without any residual loss of function on the affected side.
Static Imaging of the Kidneys
Static renal scintigraphy in Europe is performed using 99Tcm-DMSA. This radiopharmaceutical is filtered by the glomerulus and re-absorbed in the tubules and effectively maps working nephrons (Fig. 6.2). As such it can be used to characterise space-occupying lesions seen on other imaging such as ultrasound or CT. A study showing uptake of 99Tcm-DMSA is useful in showing that cancer in unlikely. However a defect may be due to cancer, scar, infarct or cyst. More commonly DMSA imaging is used in combination with a GFR to calculate residual renal function after nephrectomy or renal call carcinoma.
Imaging normally involves a series of static images including a posterior image and right and left posterior obliques performed 2.5–3 h after injection of about 150 MBq of 99Tcm-DMSA. SPECT imaging can also be performed producing 3- dimensional images of the kidney (Fig. 6.3). It could be of additional use if patient suffers from congenital recurrent potentially bilateral renal cell carcinomas in which case an assessment of residual working renal tissue can be made by the surgeon if they will attempt to maintain some working renal tissue.
Bone Scanning
Bone scintigraphy is probably the test that most often associated with urological cancers. It is primarily used in prostate cancer but has a role in bladder and renal cell cancers well. The mechanism by which bone scintigraphy works is that 550 MBq 99Tcm-MDP (or one of its close associates) is injected into the vein and 3 h later a static bone scan is performed. This can be done as a series of “spot” views or as a whole body image. With both methods it is essential that the parts of the skeleton that includes the red bone marrow, namely the skull, spine, ribs, sternum, scapulae, the pelvis, proximal humeri and proximal femora be covered (Fig. 6.3). The aetiology of bone metastases being primarily through haematogenous spread any metastases tends to be deposited in the red marrow containing bones. The 99Tcm-MDP does not attach directly to the metastases but is actually incorporated into new bone formation around the metastasis as the bone tries vainly to repair the injury caused to itself by the metastatic cancer deposit. This laying down of new bone may be seen as increased density on X-ray and is therefore described as sclerotic type metastases. Purely lytic lesions will vary rarely have increased uptake of 99Tcm-MDP. Fortunately in almost all cases urological cancer metastases are slow growing and tend to produce sclerotic lesions, which may be seen on radiology but the radiological changes may lag behind the scintigraphic changes by 6 months. The actual target cell for the 99Tcm-MDP is unclear and could be either the osteoblast or the fibroblast or both.
Sensitivity and Specificity
Although the sensitivity and specificity are rarely formally tested it has been assumed from clinical experience that bone scintigraphy is sensitive but not specific. This is generally the case, though methods which look at bone marrow disease such as magnetic resonance imaging MRI may be able to see metastases in the spine, pelvis or long bones before they become apparent on bone scintigraphy, again by as much as 6 months. The main difficulty with bone scintigraphy has been the non-specific uptake of the 99Tcm-MDP to any injury however trivial. Bone is slow to heal and defects can be active for over 12 months. Typically the problem is in the ribs where trivial injuries such as walking into a door handle 9 months before may not be remembered. Also in older men who smoke spontaneous cough fractures are often seen in winter and early spring. Other causes of significant trauma include falls, which if accompanied by a stroke or excessive alcohol may not be remembered by the patients. This type of trauma often produces a characteristic appearance on the bone scintigram with a linear uptake of equal intensity across several ribs. If multiple, such injuries are seen with different intensities in which case then frequent falls (from a cardiovascular or alcoholic cause) can be assumed. If these are ruled out then a more sinister non-accidental injury any need to be considered especially if the patient is incapacitated due to co-morbidity and needs help from caregivers.
The other area of non-specific uptake is in the spine. Metastases tend to favour the body of the vertebra and the pedicles producing areas of focal uptake within the vertebra. Degenerative disease tends to affect the body of the vertebra adjacent to the discs, the facet joints and anteriorly on the body if there is an active osteophyte. Correlative radiology confirming these areas show degenerative disease is sufficient. If however the X-ray or CT is normal then metastases cannot be excluded and MRI or even bone biopsy may be needed. If there is uptake across the vertebral body with a smooth outline a collapsed vertebrae may need to be considered. Often these, which are osteoporotic in nature, often affect more than one vertebra and do so at different times. This will lead to a number of vertebrae having increased uptake at various intensities often along with some cough and rib fractures. To the unwary observer they look like multiple bone metastases but are not. The accuracy of bone scintigraphy may be helped by SPECT-CT [7].
Paget’s disease also commonly occurs in the elderly male especially those from a North European background. Which is just the same population that suffers more from prostate cancer. The scintigraphic appearances are characteristic of active Paget’s disease in the whole of one bone (mono-ostotic) or more than one bone (polyostotic) is involved [8]. In active Paget’s disease uptake is intense throughout the bone, which is also expanded and in the case of the long bones bowed. If the long bones are involved then Paget’s is the most likely explanation, however in pelvic disease Paget’s can look like very sclerotic metastases on both scintigraphy and radiography. This confusion can be increased if the patient has a high PSA suggesting high cancer load. A trial of anti-androgens may help but it is possible for both diseases to co-exist.
Diffusely increased uptake in the bones with little or no urinary activity but affecting the distal long bones equally to the axial skeleton may be due to a metabolic bone disease. Commonly this is due to hyperparathyroidism but also, surprisingly, a high proportion due to osteomalacia.
Patterns of Abnormality
With prostate cancer the primary site for metastases beyond the loco-regional area is bone. Therefore sequential bone scinigraphies can help map out the progress of disease and the effect of any interventions. The disease load at diagnosis can also predict the outcome. Of the methods used to grade bone scans the easiest and most robust is the one by Soloway et al. (Table 6.3) [9]. This has been shown with the Gleason score to be the one of the best predictors of survival (Table 6.4) [10].
The pattern described as a “superscan” is an unusual variant in bone imaging of prostate cancer. In the case of the superscan there is contiguous or almost contiguous metastases in the red marrow containing bone (Fig. 6.4). All the injected 99Tcm-MDP is deposited in these bones specifically the ribs, sternum, scapulae, spine at least part of the skull, the pelvis, proximal humeri and femora but not the distal long bones which are often invisible. It is likely that such a patient would also have a PSA greater than 1,000 and may have impaired bone marrow function.
In the 6 weeks following manipulation of androgens by drugs or surgery there may be an increase/start of bone pain accompanied by an apparent worsening of the bone scan this is a “flare” reaction. Therefore bone scanning should be avoided in this period but if done and a flare reaction suspected repeated 3 months later when it should return to its normal activity.
Other Urological Cancers
Bladder and renal cell cancers (RCC) seldom produce such a florid reaction in the bones; often only 2–3 lesions being seen before death from extensive soft tissue disease. However RCC metastases occur in unlikely places. An example being a patient presenting with a single metastases in the right patella with no further sites of disease for many years. These metastases are painful so any areas causing pain in these patients should always be imaged even if it is outside the normal area for metastases. A further rare appearance has been increased activity in the cortex of the tibiae, fibulae and distal tibiae, sometimes called tram-lining. Though variously named it is known as hypertrophic pulmonary osteo-arthropathy (HPAO) and may be painful or not. Though normally associated with small cell lung cancer it has been seen with pulmonary metastases from testicular and renal tumours.
Which Patient Should Undergo Bone Scintigraphy?
Does every man with prostate cancer need a bone scintigraphy at diagnosis? The answer is plainly no. If the PSA is less than 10 and the Gleason score less than 3 + 3 the yield in a patient without bone pain does not justify a bone scan [11]. However if the patient has bone pain a bone scintigram should be considered because if he has degenerative disease this will act as a baseline scan for comparison as new lesions tend to be metastatic. For a PSA greater than 20 and a Gleason score of 3 + 4 a bone scintigraphy, at least at diagnosis, should be performed. However this is a guideline and bone scintigraphy is cheap and normally readily available, therefore the threshold for use should be low [12].
In renal, bladder and testicular cancer the case is less clear. For renal and bladder a bone scan may be useful before surgery with curative intent. If not it should be directed by the patient’s symptoms. However, pre-surgical screening of testicular and renal cancers may be better done by using PET than a bone scintigraphy.
Alternative Methods
The role of the bone scintigraphy in routine assessment of bone metastases has been questioned. In particular there is good evidence that whole body MRI especially with diffusion weighted imaging (DWI) is highly accurate for bone metastases in prostate cancer especially those with sclerotic lesions [13]. There is a clear advantage in not using ionising radiation though that may be less of an issue in the normal population with metastatic prostate cancer where the rate of cancer induction 20 years hence is less worrisome. The scanning may take 60–70 min roughly twice the time of a bone scintigraphy so there could be an issue of cost and accessibility.
Therapy for Pain Relief
The same mechanism by which 99Tcm-MDP is taken into the bone around metastases can be exploited by therapeutic radiopharmaceuticals. There are three main groups of radiopharmaceuticals used.
-
1.
Phosphorus-32 (P-32) which is built into hydroxyapatite,
-
2.
Analogues of calcium.
-
3.
Di-phosphonates
Radiophosphorus is cheap but the long penetrating beta particle of P-32 (32P) means that it often results in significant bone marrow toxicity and repeat treatments cannot be given. Strontium-89 (89Sr) is a calcium analogue and Samarium-153 lexidronate (153Sm-lexidronate),- a diphosphonate. Both of these latter agents produce good pain relief in 80 % of patients though the onset of pain relief is faster at 7–10 days after injection of 153Sm-lexidronate though re-treatment is often needed after 3 months.
89Sr has a longer onset of action related to its longer physical half-life, but re-treatment is not normally needed for 6 months. In both agents a “flare” reaction may occur 24–48 h before pain relief. Though these treatments are primarily directed towards pain relief there is some evidence that repeat treatments can result in delay in advance or even retreat in bone metastases.
In continental Europe rhenium-186 diphosphonates (186Re-HEDP) are used instead of 153Sm-EDTMP [14]. A different approach has been the use of radium-223. This is an pure alpha emitter and radium is a calcium analogue. It is given in small quantities over 4–6 cycles at 4 weekly intervals and not only produced pain relief but there is evidence it prolongs overall survival [15]
Sentinel Node
Since Cabanas described the principle of sentinel node drainage 40 years ago in carcinoma of the penis it has been widely used in many cancers such as melanoma and breast. The sentinel node principle states that every tumour has a logical lymph drainage to a particular first lymph node-the sentinel node [16]. Other nodes and therefore other metastases are only involved after the sentinel node. Therefore if this node is identified and removed the possibility of cancer spreading beyond that node is very low. This node can be identified best by injecting a radio-colloid of about 100 nm such as 99Tcm-nanocoll peri-tumourly in the sub-dermas. Using a combination of imaging and a hand held gamma probe the node can be identified and removed for histological assessment. This process may be aided by addition of blue dye and accurate localisations of >98 % have been obtained in other organs. Interest had therefore been re-kindled in its use in penile cancer.
There has also been interest in using sentinel node imaging in high risk but possibly curable prostate cancer with one group in Germany claiming an accuracy of 96 % in their experience of over 2,000 patients [17]. The technique is technically difficult and has not been widely adopted.
Positron Emission Tomography (PET)
Positron emission tomography (PET) is normally performed with 18F-fluor-deoxyglucose (FDG). This acts as a false substrate for glucose metabolism which is increased in cancers compared to normal tissues. However this will depend on the metabolic rate of the cancer being imaged. Unfortunately the most common Urological cancer from the prostate appears to have a low metabolic rate and as a consequence 18F-FDG is rarely positive in this disease and therefore is of little use.
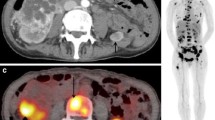
Likewise 18F-FDG is excreted via the kidneys so that it may be difficult to differentiate a renal primary from background renal activity. Though is an FDG PET scan is being performed for another indication and focal uptake of FDG is seen in the prostate, this can demonstrate not only the presence of possibly an unexpected prostatic primary but as it is FDG avid the more aggressive tumour type and further investigation is obligatory (Fig. 6.5). In renal and bladder cancers uptake of FDG can be variable but there is good evidence that 18F-FDG PET is superior to CT in identifying lymph node disease in such a way that management was changed in 35 % of patients. Overall accuracy in staging has been found to be 89 % [18].
F-18 FDG PET CT the top right hand image shows a CT at the level of the prostate, the top right image shows a “hot spot” on the F-18 FDG scan. The lower left image is a fused image showing focal uptake in the right side of the prostate. A small primary cancer is likely. The whole body image confirms this in the only site of cancer
Testicular Cancer
Again as there is high levels of 18F-FDG in the bladder little work has been done in bladder cancer, though theoretically using catheters and bladder washout, bladder tumours could be better delineated. In testicular cancer, 18F-FDG PET has been shown to have a very high accuracy in testicular cancers. In seminomas multi-centre trials have shown a sensitivity of 89 % and specificity of 100 % [19]. One area in which PET has been found to be useful it to look for cancer in those patients with rising tumour markers but CT/MRI negative. Again the most likely area found to be abnormal are lymph nodes which appear on CT or MRI to be less than 1 cm and morphologically normal.
Other Positron Emission Tomography Tracers
To overcome the problem of carcinoma of the prostate having a low metabolic rate work has looked at the increased uptake of amino-acids in cancers with promising results for 18F-choline or 11C-methionine, though the very short half life of 11C means that imaging can only occur in hospitals with their own cyclotron [20]. More recently there has been renewed interest with 11C-choline and 18F-choline. With the short half life of only 20 min limited imaging with 11C-Choline has been proposed looking at extent of primary disease and locoregional nodes. However, the high cost of 11C which must be produced near the PET scanner. It is proposed to use 18F-choline for staging of distant metastases but neither agent is in routine clinical use.
Antibody Imaging
After 30 years of false starts antibodies have finally entered medicine for both imaging and therapy. In North America there has been an antibody used which is directed against prostate membrane specific antigen (PSMA) in prostate cancer. This is labelled with indium-111 (111In) and designated at CYT-356 and is commonly called “Prostoscint” [21]. It has been shown to have a sensitivity of 86 % in nodal disease but only 55 % for bone disease. It has developed a role in characterising the nature of pelvic lymph nodes, which are equivocal on MRI. At present it is not available in the European Union on a routine basis. It is most likely this technique will be replaced by a PET technique.
Antibody Therapy
A final development has been the use of antibodies labelled with therapeutic isotopes which emit beta particles such as yttrium-90 (90Y) and lutetium-177 (177Lu) [22]. These radiometals offer stable labelling of biomolecules via a linker molecule. New antibodies have been developed for example J591 which a genetically humanised antibody directed against prostate specific prostate antigen, thought to be more specific of liver and growing prostate cancer than prostate specific antigen which can remain expressed on dead tissue. After imaging with an 111indium labelled version of the antibody to ensure localisation on the tumour a therapeutic dose can be given. In 29 patients treated with 300–1,200 MBq 90Y-J591. At the higher activities there were 40–90 % reduction in measurable tumour and 70–85 % reduction in PSA. After a single treatment this was maintained for 6 months. Funding is being sought for further development of this agent.
References
Notes for Guidance on the Clinical Administration of Radiopharmaceuticals and use of Sealed Radioactive Sources ARSAC, NRBP, Chilton, 1998.
Martensson J, Groth S, Rehling M, Gref M. Chromium-51-EDTA clearance in adults with a single-plasma sample. J Nucl Med. 1998;39:2131–7.
Daugaard G. Cisplatin nephrotoxicity: experimental and clinical studies. Dan Med Bull. 1990;37:1–12.
Hjorth L, Wiebe T, Karpman D. Correct evaluation of renal glomerular filtration rate requires clearance assays. Pediatr Nephrol. 2002;17:847–51.
Vorobiof DA, Iturralde M, Falkson G. Assessment of ventricular function by radionuclide angiography in patients receiving 4′-epidoxorubicin and mitoxantrone. Cancer Chemother Pharmacol. 1985;15:253–7.
Upsdell SM, Testa HJ, Lawson RS. The F-15 diuresis renogram in suspected obstruction of the upper urinary tract. Br J Urol. 1992;69:126–31.
Helyar V, Mohan HK, Barwick T, Livieratos L, et al. The added value of multislice SPECT/CT in patients with equivocal bony metastasis from carcinoma of the prostate. Eur J Nucl Med Mol Imaging. 2010;37:706–13.
Lentle BC, Russell AS, Heslip PG, Percy JS. The scintigraphic findings in Paget’s disease of bone. Clin Radiol. 1976;27:129–35.
Soloway MS, Hardeman SW, Hickey D, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;61:195–202.
Richmond PJM, Dorman AM, Buscombe JR, et al. Extent of disease on initial isotope bone scan: an indicator of survival in prostate cancer patients. In Radionuclides and Nephrology Es Taylor A, Nally JV, Thomsen H, VA USA: Society of Nuclear Medicine, Reson. 1997:211–5.
Cook GJ, Fogelman I. The role of nuclear medicine in monitoring treatment in skeletal malignancy. Semin Nucl Med. 2001;32:206–11.
Oyen WJ, Witjes JA, Corstens FH. Nuclear medicine techniques for the diagnosis and therapy of prostate carcinoma. Eur Urol. 2001;40:294–9.
Eiber M, Holzapfel K, Ganter C, et al. Whole-body MRI including diffusion-weighted imaging (DWI) for patients with recurring prostate cancer: technical feasibility and assessment of lesion conspicuity in DWI. J Magn Reson Imaging. 2011;33:1160–70.
Lewington VJ. Bone-seeking radionuclides for therapy. J Nucl Med. 2005;46 Suppl 1:38S–47.
Nilsson S, Franzén L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–94.
Cabanas RM. An approach for the treatment of penile carcinoma. Cancer. 1977;39:456–66.
Holl G, Dorn R, Wengenmair H, Weckermann D, Sciuk J. Validation of sentinel lymph node dissection in prostate cancer: experience in more than 2,000 patients. Eur J Nucl Med Mol Imaging. 2009;36:1377–82.
Schoder H, Larson SM. Positron emission tomography for prostate, bladder, and renal cancer. Semin Nucl Med. 2004;34:274–92.
Hain SF, Maisey MN. Positron emission tomography for urological tumours. BJUI. 2003;92:159–71.
Macapinlac HA, Humm JL, Akhurst T, et al. Differential Metabolism and Pharmacokinetics of L-[1-(11)C]-Methionine and 2-[(18)F] Fluoro-2-deoxy-D-glucose (FDG) in Androgen Independent Prostate Cancer. Clin Positron Imaging. 1999;2:173–81.
Feneley MR, Jan H, Granowska M, Mather SJ, Ellison D, et al. Imaging with prostate-specific membrane antigen (PSMA) in prostate cancer. Prostate Cancer Prostatic Dis. 2000;3:47–52.
Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Bander NH. Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J Clin Oncol. 2004;22:2522–31.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag London
About this chapter
Cite this chapter
Buscombe, J. (2015). Nuclear Medicine in Urological Cancer. In: Nargund, V., Raghavan, D., Sandler, H. (eds) Urological Oncology. Springer, London. https://doi.org/10.1007/978-0-85729-482-1_6
Download citation
DOI: https://doi.org/10.1007/978-0-85729-482-1_6
Published:
Publisher Name: Springer, London
Print ISBN: 978-0-85729-481-4
Online ISBN: 978-0-85729-482-1
eBook Packages: MedicineMedicine (R0)