Abstract
Aim
The aim of this study was to assess the utility of dual time point 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) imaging in differentiating benign from malignant pleural disease.
Methods
Fifty-five consecutive patients of suspected malignant pleural mesothelioma (MPM) and recurrence of MPM who were referred for the evaluation underwent two sequential 18F-FDG-PET scans (dual time point imaging). The average percent change in the maximum standardized uptake values (Δ%SUVmax) of the lesion/lesions between time point 1 (SUVmax1) and time point 2 (SUVmax2) was calculated. All PET results were correlated with the histopathological or cytopathology results. Patients were divided into three principal groups (A = newly diagnosed MPM, B = recurrent MPM, and C = benign pleural disease). The parameters of 18F-FDG uptake (SUVmax values and its changes over time) were compared among groups.
Results
Among the 55 patients who had undergone dual time point 18F-FDG-PET studies, 44 were diagnosed with MPM (28 newly diagnosed and 16 had recurrence). The PET studies demonstrated 229 malignant pleural lesions in these patients. The remaining 11 patients were proven to have benign pleural disease. The mean ± SD of the SUVmax1, SUVmax2, and the Δ%SUVmax of the all lesions of each patient in groups A, B, and C were 5.0 ± 2.2%, 5.8 ± 2.8%, and 12.8 ± 8.4%; 4.6 ± 1.7%, 5.3 ± 2.0%, 13.8 ± 9.2%; and 1.6 ± 0.4%, 1.4 ± 0.3%, and–9.6 ± 19.1%, respectively. The mean ± SD of the SUVmax1, SUVmax2, and Δ%SUVmax in patients with both newly diagnosed and recurrent MPM were significantly higher than those of benign pleural disease group (p < 0.0001). For each patient, the most intense (hottest) lesion’s SUVmax1, SUVmax2, and Δ%SUVmax were also compared among the aforementioned groups, and these results again confirmed that MPM lesions had significantly higher values than those of benign pleural lesions (p < 0.0001).
Conclusions
There is an increasing uptake of 18F-FDG over time in pleural malignancies, whereas the uptake in benign pleural disease generally stays stable or decreases over time. Therefore, dual time point imaging appears to be an effective approach in differentiating benign from malignant pleural disease, which increases the sensitivity and is also helpful in guiding the biopsy site for a successful diagnosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) has been proven to be valuable in diagnosis, staging, detecting early recurrences, and assessing response to therapy in a multitude of malignant disorders [1]. Despite its proven utility, the application of PET is limited by its variable sensitivity and specificity estimates. One of the most common reasons for this is that many inflammatory lesions also demonstrates increased 18F-FDG uptake and thereby results in false-positive 18F-FDG-PET study [2, 3]. On the other hand, some types of cancers, for example, well-differentiated and lobular carcinomas of the breast, thyroid, bronchoalveolar carcinomas, and epithelial mesotheliomas, have significantly low 18F-FDG uptake, which is well below the diagnostic threshold for 18F-FDG uptake in malignant lesions [4–6]. This causes false-negative 18F-FDG-PET study and results in a lower sensitivity of PET in detecting these malignancies.
Malignant pleural mesothelioma (MPM) is a relatively rare (incidence of 2,000–3,000 cases per year) neoplasm of the mesothelial cells of the pleura with a poor prognosis [7]. Exposure to asbestos can cause thickening and fibrosis of the pleura and can result in an increased risk of developing MPM. The latency period between asbestos exposure and mesothelioma development is 35–40 years, and as a result, the number of mesothelioma patients has continued to rise despite decreased asbestos production [8]. Early diagnosis and aggressive surgical extirpation are considered important for optimal long-term survival since distant metastases occur later in the course of the disease. Imaging plays an essential role in the evaluation of MPM. These pleural changes can be detected by noninvasive anatomical imaging techniques, such as chest radiography, ultrasound, computed tomography (CT) and magnetic resonance (MR). However, the differentiation of malignant from benign pleural lesions is often difficult and sometimes unreliable with currently available imaging techniques. There is a significant overlap between the radiological appearances of benign and malignant pleural disease [9, 10]. Diffuse pleural thickening, the hallmark of MPM, is not a specific finding on cross-sectional imaging and may be caused by asbestos exposure, as a consequence of hemorrhagic effusion, or by a number of infectious processes, such as tuberculosis [11]. Therefore, the anatomical imaging modalities cannot reliably differentiate benign from malignant pleural thickening. Since neither CT nor MR imaging provides a definitive diagnosis of mesothelioma, tissue biopsy is frequently required for a definitive diagnosis [12]. Thoracentesis, thoracoscopic biopsy, and open biopsy are the invasive methods of tissue sampling, which have many potential complications such as pneumothorax, persistent air leaks, hemorrhage, subcutaneous emphysema, wound infections, and the seeding of tumor along the chest wall [13, 14].
18F-FDG-PET imaging is a unique, noninvasive modality that has been successfully used to evaluate several pleural diseases [5, 15–18]. The 18F-FDG-PET technique has been shown to be highly sensitive in detecting both malignant and inflammatory processes. However, there is still a need for decreasing the false positive rate in the technique. The concept of performing dual time point 18F-FDG-PET scans may be helpful for this purpose. Studies in literature have demonstrated that the uptake of FDG continues to rise in malignant tumors for several hours after the administration of FDG [4, 19–23]. This may be explained by the increased glucose uptake through the glucose transporter proteins and low concentration of glucose-6-phosphatase activity in malignant cells. Knowing that such prolonged period of 18F-FDG uptake is rare in inflammatory lesions and normal tissues, the dual time point approach may be helpful to differentiate them from malignant [2]. Thus, dual time point 18F-FDG-PET imaging (imaging at two time points following one single dose administration of 18F-FDG) has been shown to differentiate benign processes from malignant tumors [2–4, 20, 21, 23–25]. Therefore, despite being very sensitive, single time point SUV analysis may not be the best method in assessing pleural diseases. The aim of this study was to assess whether 18F-FDG uptake and its change over time can be helpful in differentiating benign from malignant pleural disease.
Materials and Methods
Patient Population
Fifty-five patients (mean age = 61.4 years, seven female, 48 male) who were referred to the Hospital of the University of Pennsylvania between 2000 and 2007 for the evaluation of suspected MPM and suspected recurrences of known MPM by 18F-FDG-PET imaging were analyzed. The disease was suspected on the basis of clinical symptoms and chest radiograph or CT scan results: effusion, pleural masses, or pleural thickening. All patients had a prior history of asbestos exposure in the past. Informed consent was obtained in all patients before the procedure. This study was Health Insurance Portability and Accountability Act compliant and approved by the Institutional Review Board.
FDG-PET Imaging and Assessment
Patients fasted for at least 4 h before the PET scan and had blood glucose levels less than 140 mg/dL at the time of injection. FDG (5.2 Mbq/kg of body weight) was administered intravenously through an indwelling catheter inserted into an antecubital vein. Two sets of PET imaging were performed in all patients using a dedicated whole-body PET scanner (Allegro Philips Medical System, Philadelphia, PA, USA). The first scan was performed as a whole-body image, which included the entire trunk (from neck to the groin). Immediately following the whole-body scan a second set of images of the chest were acquired. The mean time interval between the injection of 18F-FDG and the first and second scans were approximately 60 and 90 min, respectively. Using a Cesium-137 point source, transmission scans were performed to provide attenuation correction. The patients did not leave the scanning table between the two acquisitions, minimizing patient motion artifacts. The ordered subsets–expectation maximization method was used to reconstruct all of the PET images [26].
Image Analysis
Two nuclear medicine physicians analyzed the data together for this study. There was no inter-observer variability. Regions of interest (ROIs) were carefully drawn around the sites of active lesions on the consequent four to six PET scan slices (slice thickness and interval were both =4 mm). The maximum standardized uptake values (SUVmax) were measured for each ROI on both time points PET scans (SUVmax1 and SUVmax2). When there was linear increased FDG uptake pattern at the pleura, the most active site was found, and SUVmax was calculated from there. This pattern was counted as one lesion. Only pleural lesions were analyzed for the purpose of the study. All results were correlated with histopathology results and clinical follow-up.
From these ROIs, the SUV was calculated according to the formula described below:
where ‘MBq’ = mega-Becquerel and ‘g’ = grams.
The maximum standardized uptake value (SUVmax) of FDG was measured from ROI, which was placed at the site of the lesion clearly visualized or appeared suspicious on the PET scans from first time point (SUVmax1) and second time point (SUVmax2). The percent change in SUVmax (Δ%SUVmax) between SUVmax1 and SUVmax2 was calculated.
Statistical Analysis
Conventional methods were used to generate descriptive statistical results. Groups were compared by using Student’s t test. p values of less than 0.05 were considered to represent significant differences among populations sets examined.
Results
Among 55 patients, 44 were diagnosed with MPM according to histopathological and cytopathology results (28 newly diagnosed and 16 had recurrent disease). The final diagnosis was established by thoracoscopic biopsy specimen, pleural biopsy specimen, and pleural fluid cytology. The remaining 11 patients were proven to have benign pleural disease. These patients were followed up clinically for 2 years. Patients were divided into three groups according to histopathology results: group A = MPM (newly diagnosed), group B = recurrent MPM, group C = benign pleural disease.
MPM patients had a total of 44 dual time point PET studies, which resulted in detecting of 229 malignant pleural lesions. The lesions showed different patterns such as linear or focal, sometimes multiple. Some patients had more than one lesion. Therefore, for each patient, average SUVmax of all numbers of malignant lesions were calculated. The benign pleural disease patients (group C) had a total of 11 dual time point PET studies. The mean ± SD of the SUVmax1, SUVmax2, and the Δ%SUVmax of groups A, B, and C were 5.0 ± 2.2%, 5.8 ± 2.8%, and 12.8 ± 8.4% (Table 1); 4.6 ± 1.7%, 5.3 ± 2.0%, and 13.8 ± 9.2% (Table 2); and 1.6 ± 0.4%, 1.4 ± 0.3% and −9.6 ± 19.1% (Table 3), respectively. The mean ± SD of the SUVmax1, SUVmax2, and Δ%SUVmax in both newly diagnosed and recurrent MPM were significantly higher than those of benign pleural disease group (p < 0.0001). There was no significant difference between the comparisons of mean SUVmax of groups A and B (p > 0.05) patients.
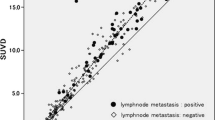
For each patient, we also calculated SUVmax1, SUVmax2, and Δ%SUVmax of the most intense (hottest lesion) and compared the three groups with regard to this parameter (Fig. 1 ). The mean ± SD of SUVmax1, SUVmax2, and Δ%SUVmax of the most intense (hottest) lesions of groups A, B, and C were 6.5 ± 3.3%, 7.5 ± 4.2%, and 13.3 ± 9.1% (Table 1); 6.1 ± 2.7%, 6.7 ± 3.0%, and 11.9 ± 10.0% (Table 2); and 1.6 ± 0.4%, 1.4 ± 0.3%, and −9.6 ± 19.1% (Table 3), respectively. In group C (benign pleural disease group), since every patient had only one lesion, their most intense lesion was the same lesion. The SUVmax calculation and its change over time of MPM patients in groups A and B were significantly higher than those of benign pleural disease group (p < 0.0001). In our study, dual time point 18F-FDG-PET imaging has proven to be useful in localizing the areas involved with MPM.
We visually observed that, especially the patients who had more than one malignant lesion, some of their lesions were not very intense (SUVmax1 were low) at the first time point image, but at the second time point, these lesions became more intense. Those lesions were small sized. However, since we calculated the average of all malignant lesions of a patient, the result of mean SUVmax1 of these patients were higher than the known malignancy threshold. Therefore, by employing this method despite the low initial SUV of small MPM lesions, the intensity of the uptake was higher on delayed images, and this resulted in higher detectability. We made another observation that mediastinal lesions that had focally increased FDG uptake at the first time point image (early) also became more intense at the second time point image (delayed).
Discussion
MPM carries a poor prognosis and the median survival for patients after diagnosis is between 12 and 18 months [27]. The disease is frequently staged with the tumor, node, metastases based system introduced by the International Mesothelioma Interest Group [28]. Patients with local extension of the tumor into mediastinum, chest wall, or diaphragm and those with hematogeneous dissemination are considered inoperable [29] and are treated with aggressive combined-modality therapeutic intervention. Thus, accurate detection of local spread and systematic dissemination of the tumor is important in selecting the appropriate treatment modalities. Chemotherapy, radiotherapy, surgery, and combined modality approaches are utilized in the treatment of MPM; however, these techniques are generally unsuccessful [30].
Although structural imaging techniques are essential for evaluating pleural diseases, they have certain limitations in reliably diagnosing the disease. For example, many infectious disorders such as tuberculosis or emphysema cannot be differentiated from pleural malignancies with this approach alone. Benign and malignant pleural diseases have similar appearances on conventional imaging techniques, such as CT, ultrasound, and chest radiography. CT is useful in localizing the areas of thickening but tends to underestimate the extent of the disease process [11]. Especially, it has been shown that CT is inefficient to differentiate pleural fibrosis following therapy from active benign or malignant diseases [31]. MR imaging has limited value in evaluating pleural diseases because of cardiac and respiratory motion artifacts [32].
Several studies have documented the superiority of PET over CT in differentiating benign from MPM and in detecting extrathoracic and medistinal nodal metastasis [5, 33]. In one study, 18F-FDG-PET correctly identified the presence or absence of metastatic sites in 89% of patients and, therefore, prevented inappropriate thoracotomy [33]. Other studies have directly compared 18F-FDG-PET with CT, mediastinoscopy, thoracoscopy, and pathological examination and have found that PET is useful in determining the true nature of doubtful CT findings, especially when lymph node involvement and distant metastases are of concern on actual scans [34].
Benign pleural disorders such as inflammatory asbestos reaction, pleuritis of various causes (e.g., bacterial infection, tuberculosis, parapneumonic effusion, sarcoidosis, and fungal infection), recent surgery, and radiotherapy can cause detectable FDG uptake. Sometimes, this increased metabolic activity in the pleura can have an SUV, which may exceed 2.5 that results in false-positive 18F-FDG-PET findings [5, 16, 29, 35]. Conversely, the researchers described the cause of false-negative findings on 18F-FDG-PET as a slow-growing epithelioid subtype of mesothelioma with a low mitosis rate, which also has a relatively better survival than other types [36]. Supporting this finding, it was shown that the degree of FDG uptake of tumor is predictive of patient survival [37].
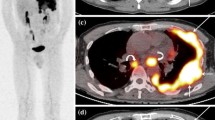
In a study of 28 patients by Benard et al. [5], 18F-FDG-PET achieved a sensitivity of 91% and specificity of 100% in differentiating benign from malignant disease, using an SUV of 2.0 as a cutoff threshold, and they reported that PET was more sensitive than CT scan in determining the extent of the disease process. Another group later evaluated 106 patients with cancer in whom the degree of 18F-FDG uptake was examined in pleura, and similarly, when an SUV threshold of 2.0 was chosen to separate malignant and benign disease, they reported that the sensitivity and specificity of 18F-FDG-PET for malignant pleural disease were 90% and 72%, respectively [38]. Several reports indicate that the degree of 18F-FDG uptake in the pleura and qualitative assessment of pleural thickening can accurately differentiate benign pleural plaques and inflammatory conditions from malignant pleural involvement [15–18], and therefore, 18F-FDG-PET is accurate noninvasive imaging technique in differentiating benign from malignant diseases and more accurately stage MPM. In addition, 18F-FDG-PET images provide excellent information about the active tumor sites especially in patients who are surgical candidates [39]. PET imaging has been used to detect MPM, which appears as a linear area of intense 18F-FDG uptake surrounding the lungs [5]. However, in our study, we observed that this is not always such typical and easy to decide. In our study, some of our patients had multiple focally increased 18F-FDG uptake on their pleura despite their having continuous thickening on their corresponding CT slices. These malignant lesions showed focally increased 18F-FDG uptake on pleura, which had become more intense on second time point image (delayed) (Fig. 2a–c) and showed appropriate biopsy site.
Despite that these results suggest that 18F-FDG-PET should have a growing role in the evaluation of mesotheliomas, dual time point imaging may be of value in reliably distinguishing intense uptake of 18F-FDG in benign inflammatory disease from that noted in the malignant disorders. The concept of performing dual time point 18F-FDG-PET scans in differentiating malignant from inflammatory processes was first described by Alavi, Zhuang. and their colleagues from the University of Pennsylvania [2, 3]. However, still in literature, there is a very few knowledge about the role of dual time point imaging in differentiating infection and/or inflammation from malignant disease [2, 3, 25]. The advantages of dual time point imaging in head and neck, lung, pancreatic, and breast malignancies demonstrated that imaging at two different time points reveal substantially higher SUV’s on delayed scans compared to those measured on the initial scans [2–4, 21–24, 40]. In these studies, this approach also improved the sensitivity of the technique for both the primary and metastatic sites. In the present study, we performed dual time point 18F-FDG-PET studies to the 55 consecutive patients who were referred for the evaluation of pleural disease. We analyzed a total number of 55 dual time point studies. In 44 dual time point studies, we detected 229 malignant mesothelioma lesions, and in the remaining 11 dual time point studies, we detected 11 benign pleural lesions. The average SUVmax values and changes over time of all malignant lesions of each patient were calculated. Our results were the mean ± SD of the SUVmax1, SUVmax2, and the Δ%SUVmax of MPM patient groups A, B, and C were 5.0 ± 2.2%, 5.8 ± 2.8%, and 12.8 ± 8.4%; 4.6 ± 1.7%, 5.3 ± 2.0%, and 13.8 ± 9.2%; and 1.6 ± 0.4%, 1.4 ± 0.3%, and −9.6 ± 19.1%, respectively. The mean ± SD of the SUVmax1, SUVmax2, and Δ%SUVmax in both newly diagnosed and recurrent MPM were significantly higher than those of benign pleural disease group (p < 0.0001). There was no significant difference between the comparisons of mean SUVmax of patients in group A and B (p > 0.05). Our other analyses were calculating SUVmax of the most intense lesion of each patient in three groups and comparing the results among them. The mean ± SD of SUVmax1, SUVmax2, and Δ%SUVmax of the most intense (hottest) lesions of groups A, B, and C were 6.5 ± 3.3%, 7.5 ± 4.2%, and 13.3 ± 9.1%; 6.1 ± 2.7%, 6.7 ± 3.0%, 11.9 ± 10.0% and 1.6 ± 0.4%, 1.4 ± 0.3%, and −9.6 ± 19.1%, respectively. The SUVmax calculations and its change over time of MPM patients in groups A and B were even more significantly higher than those of benign pleural disease group (p < 0.0001). We had only one patient who had benign pleural lesion, which showed an increase in FDG uptake over time, and we had one patient who had no change in FDG uptake over time. The histopathology of the patient who showed increase in uptake was reactive mesothelial cells. However, we had another patients in this group who had a decrease in uptake with the same pathological result. This exceptional patient showed that further investigations are needed in this area based on cellular level. The patient who had no change in uptake over time had no pathological cells in the specimen.
There are limitations that probably influenced the sensitivity in this study. First, there was a considerable degree of variation seen among patients with benign pleural lesions. In our study, while the malignant tissues had positive dual time changes in SUV, the benign pleural disease generally showed either no or negative dual time point changes (in nine patients among 11; Fig. 3). This would suggest that dual time point imaging will improve the sensitivity of the test, since it is expected that normal tissue would not accumulate 18F-FDG over an extended period of time. However, the two benign lesions in the present study and other findings in literature showed that some exceptional benign lesions’ FDG uptake increases over time [2, 3, 25]. This can be explained by Zhuang et al. [2], as mentioned in their study the uptake of 18F-FDG in benign lesions can be influenced by underlying etiology (infection vs. inflammation) and state of inflammation (acute vs. chronic). Secondly, this can be explained by selecting the time interval between the first and the second scans as approximately 30 min. As stated above, most cancers require several hours to reach maximum level in 18F-FDG uptake. Since 18F-FDG uptake by inflammatory cells reaches its peak at about 60 min [2], the time interval between the first scan and the second scan is also likely to be a factor that may affect the performance of this technique. Therefore, we believe that, if the time interval between the two scans could have been longer, our results would have been more striking. In our study, dual time point 18F-FDG-PET imaging has been proven to be useful in localizing the areas involved with MPM and can be helpful in guiding biopsy site. Dual time point imaging technique of 18F-FDG-PET in the diagnostic algorithm may reduce the number of open pleural biopsies and thoracotomies performed for benign pleural disease. We believe that the change in the dual time point SUVs would be a more valuable diagnostic tool than an early or a delayed single time point alone. Further studies are warranted about the utility of dual time point imaging technique and quantifying SUV, since our study showed that dual time point imaging technique can help in evaluating the stage and response to therapy (differentiation of benign and malignant activity), detecting disease recurrence, and pinpointing the most appropriate biopsy site (Figs. 4 and 5).
Top rows FDG-PET shows two foci (black arrows) of intense tracer uptake corresponding soft tissue masses on the CT images. Note that there is no significant tracer uptake corresponding to the pleural effusion. Bottom rows Another patient’s CT image shows nodular thickening of mediastinal pleura (white arrows). FDG-PET image of this patient shows mild FDG uptake indicating benign nature of the lesion.
Conclusion
Our study results indicate that 18F-FDG uptake increases with time in MPM. On the other hand, the uptake of 18F-FDG in benign pleural disease decreases with time. Therefore, it can differentiate benign from malignant pleural disease and also be helpful in guiding the biopsy site for an accurate diagnosis. Dual time point imaging is a simple and noninvasive method that may improve the sensitivity and specificity of 18F-FDG-PET in detecting malignant pleural disease. Although more research is needed in this area, dual time point imaging appears to improve the accuracy of the technique when existing techniques cannot distinguish between inflammation and malignancy. Therefore, this technique may allow appropriate therapeutic interventions to be initiated early in the course of the disease.
References
Alavi A, Lakhani P, Mavi A, Kung JW, Zhuang H (2004) PET: a revolution in medical imaging. Radiol Clin North Am 42(6):983–1001
Zhuang H, Pourdehnad M, Lambright ES et al (2001) Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med 42:1412–1417
Hustinx R, Smith RJ, Benard F et al (1999) Dual time point fluorine-18 fluorodeoxyglucose positron emission tomography: a potential method to differentiate malignancy from inflammation and normal tissue in the head and neck. Eur J Nucl Med 26:1345–1348
Matthies A, Hickeson M, Cuchiara A, Alavi A (2002) Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med 43:871–875
Benard F, Sterman D, Smith RJ, Kaiser LR, Albelda SM, Alavi A (1998) Metabolic imaging of malignant pleural mesothelioma with fluorodeoxyglucose positron emission tomography. Chest 114:713–722
Buck A, Schirrmeister H, Kuhn T et al (2002) FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging 29:1317–1323
Price B (1997) Analysis of current trends in United States mesothelioma incidence. Am J Epidemiol 145:211–218
Bertram P, Adam W (2004) Mesothelioma trends in the United States: an update based on surveillance, epidemiology, and end results program data for 1973 through 2003. Am J Epidemiol 159:107–112
Muller N (1993) Imaging of the pleura. Radiology 186:297–309
Falaschi F, Boraschi P, Musante F, Volpini F, D'Alessandro F, Torri T, Barbieri L (1992) The computed tomographic diagnosis of malignant pleural mesothelioma. A multicenter study. Radiol Med (Torino) 84(1-2):43–7
Rusch V, Godwin J, Shuman W (1988) The role of computed tomography scanning in the initial assessment and follow-up of malignant pleural mesothelioma. J Thorac Cardiovasc Surg 96:171–177
Metintas M, Ozdemir N, Isiksoy S et al (1995) CT-guided pleural biopsy in the diagnosis of malignant mesothelioma. J Comput Assist Tomogr 19:370–374
Collins T, Sahn S (1987) Thoracocentesis. Clinical value, complications, technical problems, and patient experience. Chest 91:817–822
Menzies R, Charbonneau M (1991) Thoracoscopy for the diagnosis of pleural disease. Ann Intern Med 114:271–276
Carretta A, Landoni C, Melloni G, Ceresoli GL, Compierchio A, Fazio F et al (2000) 18-FDG positron emission tomography in the evaluation of malignant pleural diseases—a pilot study. Eur J Cardiothorac Surg 17:377–383
Kramer H, Pieterman RM, Slebos DJ, Timens W, Vaalburg W, Koeter GH et al (2004) PET for the evaluation of pleural thickening observed on CT. J Nucl Med 45:995–998
Gupta NC, Rogers JS, Graeber GM, Gregory JL, Waheed U, Mullet D et al (2002) Clinical role of F-18 fluorodeoxyglucose positron emission tomography imaging in patients with lung cancer and suspected malignant pleural effusion. Chest 122:1918–1924
Bury T, Paulus P, Dowlati A, Corhay JL, Rigo P, Radermecker MF (1997) Evaluation of pleural diseases with FDG-PET imaging: preliminary report. Thorax 52:187–189
Hamberg LM, Hunter GJ, Alpert NM, Choi NC, Babich JW, Fischman AJ (1994) The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med 35:1308–1312
Lodge MA, Lucas JD, Marsden PK, Cronin BF, O’Doherty MJ, Smith MA (1999) A PET study of 18FDG uptake in soft tissue masses. Eur J Nucl Med 26:22–30
Mavi A, Urhan M, Yu JQ et al (2006) Dual time point 18F-FDG PET imaging detects breast cancer with high sensitivity and correlates well with histologic subtypes. J Nucl Med 47(9):1440–1446
Mavi A, Lakhani P, Zhuang H et al (2005) Fluorodeoxyglucose-PET in characterizing solitary pulmonary nodules, assessing pleural diseases, and the initial staging, restaging, therapy planning, and monitoring response of lung cancer. Radiol Clin North Am 43(1):1–21
Basu S, Mavi A, Cermik T, Houseni M, Alavi A (2008) Implications of standardized uptake value measurements of the primary lesions in proven cases of breast carcinoma with different degree of disease burden at diagnosis: does 2-deoxy-2-[F-18]fluoro-D-glucose-positron emission tomography predict tumor biology? Mol Imaging Biol 10(1):62–66
Demura Y, Tsuchida T, Ishizaki T et al (2003) 18F-FDG accumulation with PET for differentiation between benign and malignant lesions in the thorax. J Nucl Med 44:540–548
Mavi A, Cermik TF, Urhan M, Yu JQ, Zhuang H, Alavi A (2006) Dual time point FDG-PET imaging can help distinguishing residual invasive tumor from post biopsy inflammation in newly diagnosed breast cancer. 9th Congress of World Federation of Nuclear Medicine & Biology. World J Nucl Med (WJNM) 5(supp 1):S77
Bedigian MP, Benard F, Smith RJ, Karp JS, Alavi A (1998) Whole-body positron emission tomography for oncology using singles transmission scanning with segmentation and ordered subsets expectation maximization (OS-EM) reconstruction. Eur J Nucl Med 25:659–661
Aisner J (1995) Current approach to malignant mesothelioma of the pleura. Chest 107:332S–344S
Rusch VW (1995) A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest 108(4):1122–1128
Pisani R, Colby T, Williams D (1988) Malignant mesothelioma of the pleura. Mayo Clin Proc 63:1234–1244
Sugarbaker DJ, Jaklitsch MT, Liptay MJ (1995) Mesothelioma and radical multimodality therapy: who benefits? Chest 107:345S–350S
Erasmus JJ, McAdams HP, Rossi SE, Goodman PC, Coleman RE, Patz EF (2000) FDG PET of pleural effusions in patients with non-small cell lung cancer. Am J Roentgenol 175:245–249
Falaschi F, Battolla L, Mascalchi M, Cioni R, Zampa V, Lencioni R et al (1996) Usefulness of MR signal intensity in distinguishing benign from malignant pleural disease. AJR Am J Roentgenol 166:963–968
Schneider DB, Clary-Macy C, Challa S, Sasse KC, Merrick SH, Hawkins R et al (2000) Positron emission tomography with F18-fluorodeoxyglucose in the staging and preoperative evaluation of malignant pleural mesothelioma. J Thorac Cardiovasc Surg 120:128–133
Nanni C, Castellucci P, Farsad M, Pinto C, Moretti A, Pettinato C et al (2004) Role of F-18-FDG PET for evaluating malignant pleural mesothelioma. Cancer Biother Radiopharm 19:149–154
Patz EF Jr., Lowe VJ, Hoffman JM, Paine SS, Burrowes P, Coleman RE et al (1993) Focal pulmonary abnormalities: evaluation with F-18 fluorodeoxyglucose PET scanning. Radiology 188:487–490
Rusch VW, Venkatraman ES (1999) Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann Thorac Surg 68:1799–1804
Benard F, Sterman D, Smith RJ, Kaiser LR, Albelda SM, Alavi A (1999) Prognostic value of FDG PET imaging in malignant pleural mesothelioma. J Nucl Med 40:1241–1245
Alavi A, Gupta N, Alberini JL, Hickeson M, Adam LE, Bhargava P et al (2002) Positron emission tomography imaging in nonmalignant thoracic disorders. Semin Nucl Med 32:293–321
Haberkorn U (2004) Positron emission tomography in the diagnosis of mesothelioma. Lung Cancer 45 Suppl 1:S73–S76
Nakamoto Y, Higashi T, Sakahara H et al (2000) Delayed (18)F-fluoro-2-deoxy-D-glucose positron emission tomography scan for differentiation between malignant and benign lesions in the pancreas. Cancer 89:2547–2554
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mavi, A., Basu, S., Cermik, T.F. et al. Potential of Dual Time Point FDG-PET Imaging in Differentiating Malignant from Benign Pleural Disease. Mol Imaging Biol 11, 369–378 (2009). https://doi.org/10.1007/s11307-009-0212-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-009-0212-5