Abstract
Functional gastrointestinal disorders (FGID) such as functional dyspepsia (FD) and irritable bowel syndrome (IBS) are highly prevalent and debilitating attributed to altered gut function and gut-brain interactions. FGID can be reliably diagnosed based upon the symptom pattern; but in the clinical setting FD or IBS a frequent diagnoses of exclusion after relevant structural causes of symptoms have been ruled out by appropriate testing. Thus far, there is no established biomarker for FGIDs. To address this limitation, we utilised multi-omics and chemometrics integration to characterise the blood plasma biochemistry in patients with IBS, FD, an overlap of FD/IBS, and controls using liquid chromatography-mass spectrometry (LC-MS) techniques.
Cholesterol metabolism products Cholest-5,24-dien-3β-ol, 3-O-β-D-glucopyranoside, energy pathway metabolites, immunoglobulin-γ2 and immunoglobulin-κ, and carbonic anhydrase-1 proteins were particularly elevated in IBS. Furthermore, arginine and proline metabolisms, thyroid hormone synthesis, ferroptosis and, complementary and coagulation cascades were particularly upregulated in patients with IBS. Cer(d18:1/26:1(17Z)) and PI(14:0/22:1(11Z)) lipids were elevated in FD and FD-IBS but were depleted in IBS. Markers of central carbon metabolism and lipidome profiles allowed better discrimination and model predictability than metaproteome profile in healthy and FGID conditions.
Overall, the multi-omics integration allowed the discrimination of healthy controls and FGID patients. It also effectively differentiated the biochemistry of FGID subtypes including FD, IBS and FD-IBS co-occurrence. This study points towards the possibility of multi-omics integration for rapid and high throughput analysis of plasma samples to support clinicians screen and diagnose patients with suspected FGIDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Authors.
Utilising integrated multi-omics to differentiate biochemical expressions among FGIDs in blood plasma.
FormalPara Disclosuresand Conflict of Interest.
No disclosures and conflict of interest for A.V.K., J-W.L., A.S., N.K. and D.J.B. G.H received unrestricted educational support from the Falk Foundation. Research support was provided via the Princess Alexandra Hospital, Brisbane by GI Therapies Pty Ltd, Takeda Development Center Asia, Pty Ltd, Eli Lilly Australia Pty Ltd, F. Hoffmann-La Roche Ltd, MedImmune Ltd, Celgene Pty Ltd, Celgene International II Sarl, Gilead Sciences Pty Ltd, Quintiles Pty Ltd, Vital Food Processors Ltd, Datapharm Australia Pty Ltd Commonwealth Laboratories, Pty Ltd, Prometheus Laboratories, FALK GmbH & Co KG, Nestle Pty Ltd, Mylan, and Allergan (prior to acquisition by AbbVie Inc.). G.H is also a patent holder for a biopsy device to take aseptic biopsies (US 20,150,320,407 A1).
1 Introduction
It is now widely accepted that functional gastrointestinal disorders (FGIDs) are caused by a variety of pathophysiologic mechanisms that include altered gastrointestinal sensory or motor function, immune activation with minimal mucosal inflammation and microbial abnormalities (Drossman, 2016; Enck et al., 2017; Koloski et al., 2020; Talley et al., 2015).
In recent years The Rome IV committee (https://theromefoundation.org/) has developed and refined in the previous iterations of the Rome Criteria a rational approach to diagnose and categorise FGID. While there is now wide agreement that the Rome Criteria allow the diagnosis and categorisation of FGID, overlap of different FGIDs and the lack of a ‘test’ or biomarkers that provides reassurance to patients and clinicians that symptoms are indeed caused by a FGID presents a challenge and in the routine clinical setting FGIDs are frequently a diagnosis of exclusion. As a consequence patients presenting with symptoms consistent with a FGID undergo tests to rule out structural abnormalities and after various laboratory tests, many patients have endoscopies, colonoscopies or crossectional imaging and other tests (Fischbach et al., 2016; Madisch et al., 2018; Rodiño-Janeiro et al., 2018). Due to multiple tests required, a diagnostic confirmation can take weeks or months (Madisch et al., 2018). Furthermore, many IBS and FD patients report an overlap of symptoms (Haag et al., 2004) that seems to be in particular relevant in patients with severe symptom manifestation (Bierman et al., 1987; Sperber et al., 2021). This presents a challenge for the current Rome criteria (Holtmann & Talley, 2018).
Therefore, diagnostic tests utilsing less invasive samples such as blood (Linsalata et al., 2018; Mainoli et al., 2021; Talley et al., 2020) or non-invasive samples such as urine (Linsalata et al., 2018) might be of value for the work-up of suspected FGID patients (Jones et al., 2014; Talley et al., 2019). Blood proteins such as zonulin, FABP2 (Stevens et al., 2018), C-reactive protein, erythrocyte sedimentation rate, faecal calprotectin and lactoferrin (Menees et al., 2015) have been suggested to be the biomarkers for differentiating IBS from inflammatory bowel disease (IBD) and other functional gastrointestinal diseases. However, more recent studies have shown no association of some of these biomarkers (Ohlsson et al., 2017; Talley et al., 2020) with FD and IBS, indicating these biomarkers are at best controversial (Talley et al., 2020; Waluga, 2020). In this context, novel platforms involving multi-omic techniques have a potential towards understanding the underlying molecular mechanisms (Jeffery et al., 2020) and could provide robust identification and validation of biomarkers. This approach combines two or more techniques among microbial community genetics, proteomics and metabolomics.
This multi-omics approach has previously been applied to characterise the important biochemical pathways and biomarkers of oral cavity and oropharyngeal cancer (Lim et al., 2021), mild cognition impairment-Alzheimer’s disease transition (François et al., 2021), and cryptosporidiosis (Karpe et al., 2021). This approach has also been applied towards a better characterisation of different IBS subtypes and their discrimination from healthy controls (Linsalata et al., 2018) and, gut and serum biochemistry during IBS (Vijayvargiya et al., 2018).
In the current study, we utilised liquid chromatography-mass spectroscopy (LC-MS) based platforms to analyse blood plasma metabolome, lipidome and metaproteome of FD, IBS and FD-IBS patients and compared them to healthy controls. The data from these omics platforms were integrated to obtain a more comprehensive metadata analysis and interpretation of prominent biochemical pathways, and establish the causation of these gut disorders. Additionally, the analyses also aided in the determination of a biochemical framework of IBS- FD and FD-IBS cooccurrence, indicating a combination of key lipids, metabolites and proteins that could act as biochemical panel for IBS and FD diagnosis.
2 Materials and methods
2.1 Ethics approval, sample collection and processing
All the sample collection and follow-up procedures were conducted in accordance to the National Statement on Ethical Conduct in Human Research (2007) guidelines set by National Health and Medical Research Council (NHMRC et al., 2018) and CONSORT reporting guidelines. Sample and data handing and all other aspects of the study were approved by the human research ethics committees of Metro South Hospital and Health Services (HREC/12/HNE/267, HREC/13/QPAH/690, HREC/2020/QMS/67504) and CSIRO (2020_096_RR).
Plasma samples (n = 48), in a randomised and double blind controlled trial from a mixed male and female cohort, were collected from biobanked samples (Burns et al., 2021; Hoedt et al., 2021). These samples were earlier collected from patients diagnosed with functional dyspepsia (FD) (n = 10), irritable bowel syndrome (IBS) (n = 10) and co-occurring FD-IBS (n = 28) by an independent clinician. These samples were compared with commercial human male plasma (n = 16) collected from healthy donors (Product H4522, Sigma Aldrich Pty Ltd) free from Hepatitis B & C and Human Immunodeficiency Virus (HIV) antibodies (Product Certificate of Analysis, Figure S4). The commercial plasma samples were not known to be FD or IBS positive.
2.2 Sample processing and metabolite and lipid extraction
Plasma samples (100 µL) was transferred to high extraction tubes (Thermo Fisher, Melbourne, Australia) and immediately quenched and protein crashed in 450 µL methanol: ethanol solvent mix (1:1 v/v; Sigma Aldrich, Castle Hill, NSW, Australia), containing 1 µg/mL L-phenylalanine (1-13 C) and succinic acid (1,4-13C2) as internal standards (Novachem Pty. Ltd., Heidelberg West, VIC, Australia). The samples were then processed in line with the previously reported protocol (Gyawali et al., 2021a). Briefly, the quenched samples were vortexed (4°C/ 1 h) (Thermomixer®, Eppendorf Australia, Sydney, NSW, Australia) and centrifuged (2000 g, 4°C, 15 min.) for protein crash. Supernatants were then transferred and filtered onto Captiva EMR-Lipid cartridges (Agilent, Mulgrave, Australia) on a Bravo metabolomics workbench (Agilent Technologies, Mulgrave, VIC, Australia). The filtered samples were vacuum dried (2000 rpm, 37 °C, 4 h), followed by reconstitution in 50 µL methanol/water (20:80, v/v). After brief vortexing (5 min.), reconstituted samples were then analysed for the determination of metabolites associated with central carbon metabolism on an Agilent 6470 LC-QqQ-MS coupled with an Agilent Infinity II Flex UHPLC system using the Agilent Metabolomics dMRM Database and Method (Staratain, 2016). To analyse the variability between the samples, quality control (QC) mixture of amino acids (Part number: 5061 − 3332, Agilent Technologies) and organic acids (Part number: 8500 − 6900, Agilent Technologies) were analysed (Table S1). Metabolite annotation was performed using the Agilent Metabolomics dMRM database and method, with default settings.
The lipids which remained adsorbed to the Captiva EMR-Lipid cartridges were eluted by adding 500 µL of dichloromethane: methanol (1:2, v/v) on Bravo metabolomics workbench. Eluted lipids were then air-dried overnight, followed by reconstitution in 50 µL BuOH:MeOH (1:1, v/v). The reconstituted samples were transferred for lipidomic profiling on an Agilent 6546 liquid chromatography time-of-flight mass spectrometer (LC-QToF-MS/MS) with an Agilent Jet Stream source coupled to an Agilent Infinity II UHPLC system (Agilent Technologies, USA). Chromatographic separation was performed according to the previously reported protocol (Beale et al., 2021). Pooled PBQC samples were subjected to the collisions of 20 eV and 35 eV to obtain an MS/MS-based lipid data. The acquired MS/MS lipid data were annotated using the Agilent Lipid Annotator tool (V1.0; Santa Clara, CA, USA) at Level 2 identification as per the chemical analysis working group of Metabolomics Standards Initiative. The annotated lipids were assigned MSMS fragmentation pattern-based isometric structures into a PCDL database that included accurate mass, retention and mass spectral patterns in combination with screening against the Agilentd METLIN lipid PCDL database. The PCDLs were then used annotate lipids from all the samples with retention time thresholds (± 0.15 min), MS/MS spectra, and a library threshold (> 80%) score.
2.3 Plasma protein extraction and metaproteomic analysis
Plasma samples (100 µL) were added to the spin columns consisting of albumin and immunoglobulin depletion resin (Product ID: PROTBA, Sigma Aldrich). The samples were then processed as per the manufacturer’s protocol, without any modification. The protein concentration was determined by Bradford assay and, a protein volume equivalent to 5 µg. Volume was adjusted to 10 µL by adding urea (8 M). Dithiothreitol (1µL, 15% v/v) was added, followed by a 30-minute room temperature incubation. Bis-acrylamide (1 µL, 40% v/v) was then added and followed with a further 30-minute incubation. Trypsin (0.1 µg in 20mM ammonium bicarbonate) was added, and the mixture was incubated overnight at 37 °C. Formic acid (1µL, 10% v/v) was added to terminate trypsin activity.
Tryptic peptides (100 ng) were desalted and concentrated with a trap column (PepMap100 C18 5 mm × 300 μm, 5 μm) and separated on a nano column (PepMap100 C18 150 mm × 75 μm, 2 μm) using an Ultimate™ 3000 RSLC nano LC system, and tandem mass spectra (MS/MS) analysis was performed using an Orbitrap Fusion MS (both, Thermo Scientific Australia Pty Ltd, Scoresby, VIC, Australia), as per the previously reported protocol (Karpe et al., 2021).
2.4 Statistical analysis, quality control and multi-omics integration
The raw data for metabolomics and lipidomic workflow was obtained from the Agilent MassHunter workstation (Quantitative Analysis, Version B.10.00, Agilent Technologies). The metabolic datasets were normalised to succinic acid (1,4-13C2) (1 µg/mL per sample, relative standard deviation (RSD) = 5.02%). Similarly, variability between the samples was indicated by the RSD of L-phenylalanine (1-13 C) (RSD = 8.66%) and QC mix (RSD = 3.35–9.82%; Table S1). The Overall, the samples were handled and data were analysed according to the Metabolomics Standards Initiative (MSI) (Fiehn et al., 2007; Griffin et al., 2007) and (Fiehn, 2016).
Protein Discoverer 2.2 (Thermo Scientific) and Sequest HT search engine were used to identify peptides/proteins and quantify relative abundance of proteins. The spectrum data was searched against the Uniprot Homo sapiens protein database (UP000005640). Precursor mass tolerance was set to 10 ppm and product ions were searched at 0.6 Da. Three missed tryptic cleavages were allowed. Modification included Oxidation (+ 15.995 Da), Deamidation (+ 0.984 Da), Amidation (− 0.984Da), and Propionamidation (+ 71.037 Da). Peptide spectral match was validated using the Percolar algorithm, based on q-values and 1% False Discovery Rate (FDR). Relative abundance was calculated from precursor abundance intensity, normalised to total peptide amount (Ratio calculation based on summed abundance; ANOVA test based on individual proteins).
The metabolomics, lipidomic and proteomic data, after normalisation, were then analysed through SIMCA (version 16, Sartorius Stedim Biotech, Umeå, Sweden) and MetaboAnalyst 5.0 (Chong et al., 2018) statistical software packages. The chromatographic data were subjected to Log10 transformation and Pareto scaling before univariate and multivariate statistical analysis according to previously reported workflows (Karpe et al., 2015; Shah et al., 2021). Briefly, the data were subjected to an unsupervised Principal component analysis (PCA), followed by partial least square discriminate analysis (PLS-DA) (Azizan et al., 2012). Also, univariate analysis through volcano plots, enrichment analysis and biomarker analysis was done in MetaboAnalyst 5.0 (Chong et al., 2018). All models were cross validated using CV-ANOVA in SIMCA, which is a diagnostic approach for assessing the reliability of PLS models.
Multi-omics integration was performed with the Joint-pathway analysis tool of MetaboAnalyst 5.0 and Paintomics 3 v0.4.5 (Hernández-de-Diego et al., 2018). A minimum threshold of p-value (P ≤ 0.05) and combined Log2 fold change (combinedLogFC ≥ 1.0) were defined for both categories. The metabolic pathway mapping and curation was performed by using Omix visualisation tool (Version 1.9.34; Omix Visualisation GmbH and Co. KG, Lennestadt, Germany).
3 Results
The metabolomics workflow indicated a presence of ca. 107, 123, 125 and 128 central carbon metabolites across control, IBS, FD and IBS-FD samples, respectively. Lipidomic analysis indicated ca. 663 lipids across the samples, of which 378 were statistically significant.
We observed that 36 metabolites and 96 lipids were statistically significant (FDR adjusted p value ≤ 0.05) across all the samples analysed across different FGID groups. Similarly, of 351 expressed proteins, only 62 proteins showed statistically significant discrimination (FDR adj. p value ≤ 0.05) in their expression across across different FGID groups.
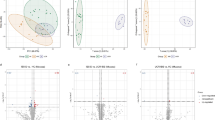
There was a considerable overlapping of the proteomic profiles in the plasma of FD and IBS groups (Figures S3), yielding a low model (\({Q}_{Cum.}^{2}<0.5\)) predictability (\({Q}_{proteome}^{2}=0.321\)). The overall model predictability was slightly better \({Q}_{Cum.}^{2}>0.5\)for the metabolomic \({Q}_{metabolome}^{2}=0.571\)and lipidomic profiles (\({Q}_{lipidome}^{2}=0.594\)), and a comparison indicated some discrimination between the FD and IBS groups (Figures S1 and S2).
The cross validation analysis using CV-ANOVA of PLS-DA indicated metabolomic model to be statistically sifnificant (regression sum of squares (SS) = 62.82, mean-squared error (MS) = 2.61 and p value = 1.69e− 06, standard deviation (SD) = 1.62). Lipidomics PLS-DA model was also shown as statistically significant (SS = 61.24, MS = 2.55 and p value = 3.41e− 06, SD = 1.59). In contrast, the proteomic PLS-DA model lacked statistical significance (SS = 20.47, MS = 1.14 and p value = 0.305, SD = 1.07). Overall, the lipidomic profile provided better discrimination between FD and IBS groups versus subjects with FD-IBS, indicating a more severly altered metabolic pattern in subjects with overlap of FD and IBS. Particularly phosphocholines (PC), phosphoethanolamines (PE) and, more interestingly, phosphoinositols (PI) (Fig. 1 A) appeared to be major lipid subclasses contributing to this discrimination.
The plots represent (A) Enrichment plot showing top enriched lipid subclasses contributing to metabolism in the blood plasma during IBS, FD and FD-IBS co-occurrence. The integrated proteomic-metabolomic heatmap, displaying omic component fold change (Log2FC) within the biochemical pathway components of (B) Cholesterol metabolism, (C) Thyroid hormone synthesis, (D) Ferroptosis and (E) Arginine and proline metabolism. Note: Values in the individual boxes of heatmaps represent Log2FC values of the individual omic components, represented in the respective legends. The protein identifiers (e.g., P02749) on Y-axis refer to the Uniprot IDs. The heatmaps were designed using Clustvis web tool (Metsalu and Vilo, 2015)
To determine how the individual disorder or a co-occurrence were changing the metabolic behaviour, the proteomic-metabolomic integration was performed. The joint pathway analysis indicated the presence of 238 biochemical pathways, of which 49 were observed to be significant during FD, IBS and FD-IBS conditions (FDR ≤ 0.05) (Table S4). Of these pathways, 4 pathways were particularly upregulated during the IBS condition. These pathways included thyroid hormone metabolism, cholesterol metabolism, ferroptosis and, arginine and proline metabolism (Figs. 1 and 2).
3.1 Biochemical discriminators between IBS and FD
As lipids showed higher discrimination with respect to the metabolites and proteins, biomarker analysis was done by examining the lipidomic behaviour and contribution from individual lipid/lipid classes. For this, lipidomic data were subjected to the lipid enrichment toolbox of MetaboAnalyst 5.0.
We observed that the most enriched lipids consisted of glycerophospholipids, fatty acyls and sterol lipids (Fig. 1 A). However, to filter out the most prominent lipids which could differentiate between the two IBS and FD conditions, a combined approach of univariate analysis (using one-way ANOVA) and multivariate (using Significance Analysis of Microarray (SAM)) analysis was performed. The analyses indicated that out of 96 most significant lipids (Table S2A), Cer(d18:1/26:1(17Z)) and PI(14:0/22:1(11Z)), were differentially expressed during IBS (Fig. 3).
Key statistically significant lipids and metabolites indicated by SAM and biomarker analysis, differentially expressed in plasma during the IBS condition with respect to others. Note: The boxes in the box-whisker plots indicate the normalised expression (or concentration) of the lipids and metabolites, with the average value represented by the horizontal black line, and yellow diamond representing median (or 50th percentile value) within each box. The whiskers represent standard deviation between the replicates. The upper whisker is located at the smaller of the maximum ‘x-value’ and Quartile 3 (Q)3 + 1.5 × Interquartile Range (IQR). Lower whisker is located at the larger of the smallest ‘x-value’ and Quartile 1 (Q1) − 1.5 × IQR. The Y-axis indicates Log10 normalised and pareto scaled values for lipids and metabolites, while the horizontal red line indicates the threshold cut-off for the true positive rate (sensitivity) of the metabolite within the 95% confidence interval
The biomarker analysis toolbox indicated that there were a few lipids and metabolites which were differentially elevated during either FD or IBS. Key biomarkers were selected and ranked using a combination of area under the ROC curve (AUROC) and T-statistics with a 95% confidence interval as reported previously (Gyawali et al., 2021a 2021b). The biomarker analysis was in line with the joint pathway analysis that cholesterol metabolism and, arginine and proline metabolism played an important role during both FD and IBS. The lipid intermediates or end products of cholesterol metabolism were prominent during the IBS condition with respect to FD and FD-IBS co-occurrence. Additionally, the key metabolites found to be important were related to arginine and proline metabolism were significantly elevated during FD, IBS and FD-IBS co-occurrence. Particularly, N-carbamoyl aspartate was found to be the only metabolite to be significantly elevated during FD, while it was depleted during other conditions (Fig. 3). Also, lipids such as cholest-5,24-dien-3beta-ol 3-O-beta-D-glucopyranoside and 1,6-Pentadecadiene were particularly elevated during IBS (Fig. 3; Table 1).
While proteomic analysis indicated a low model predictability, the biomarker analysis indicated that a few immunoglobulin proteins were particularly upregulated during the IBS condition only. These proteins included P01859 (AUC = 0.78, FDR = 0.01315), P01599 (AUC = 0.72, FDR = 0.0307), P01743 (AUC = 0.79, FDR = 0.0065) (all immunoglobulins) and P00915 (carbonic anhydrase, AUC = 0.72, FDR = 0.0392) (Fig. 4).
Key statistically significant proteins indicated by SAM and biomarker analysis, differentially expressed in plasma. Note: The Y-axis indicates Log10 normalised and pareto scaled abundance values for individual lipids, while the horizontal red line indicates the threshold cut-off for the true positive rate (sensitivity) of the metabolite within the 95% confidence interval. The IDs in parenthesis indicate Uniprot IDs of individual proteins
Metsalu, T. and Vilo, J. (2015) ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Research43, W566-W570
4 Discussion
The is the first study that used a metabolomic approach to differentiate patients with FD, IBS and FD/IBS overlap. While parameters such as myeloperoxidase activity can be used as biomarkers for inflammatory bowel disease (IBD) (Shinde et al., 2020), the method requires colonic biopsies limiting the benefits compared to routine histology. Non-biopsic matrices such as blood plasma have been used with some success, but complexities were reported during FGID co-occurrences (Stevens et al., 2018; Uhde et al., 2016). However, recent reanalyses (Talley et al., 2020) has suggested that while these biomarkers may be discriminatory between healthy people and IBS patients, they fail to be efficient biomarkers between IBS and FD and, during a FD-IBS co-occurrence.
Bile acid malabsorption in the gut has been reported in subgroups of IBS patients (Jeffery et al., 2020). This malabsorption has been indicated to cause a depletion of metabolites such as 7α-hydroxy-4-cholesten-3-one in serum (Vijayvargiya et al., 2018) during IBS compared to that in the healthy subjects. Similarly, the rat model of FD has suggested bile acid synthesis perturbations in faeces (Luo et al., 2018). Additionally, the recent study by James et al., (2021) has also indicated the differential increase in various faecal bile acids during different types of FGIDs such as IBS (IBS-constipation (C), IBS-diarrhea (D), IBS-mixed (M)), functional gut disorders (functional constipation (FC), functional diarrhea (FD)). Their study also indicated the gut microbial role in a conversion of primary bile acids to secondary bile acids. Furthermore, the depletion of taurocholate, combined with the increased levels of threonine and its downstream cholestenoid lipids such as cholest-5,24-dien-3beta-ol 3-O-beta-D-glucopyranoside, have indicated towards a likelihood of liver dyslipidemia (Huang et al., 2020; Xiao et al., 2021). Our results were in line with the previous studies (Bernelot Moens et al., 2017; Roy et al., 2019) that showed increased levels of cholesterol and lipid accumulation in the monocytes of the FD patients. Simialrly, in line with the outputs of the study by James et al., (2021), and Huang et al., (2020); (Xiao et al., 2021), it is likely that the bile acids and their products, resulting for a host-microbiome interaction, circulate much further than just the gut region into gut-liver axis, and into circulatory system. However, further studies on hepatic metabolomics would be able to shed further light on this phenomenon.
Amino acid metabolism leading to the biosynthesis of neurotransmitter metabolites such as gamma-aminobutyric acid (GABA) was observed in our study. In the rat models of functional dyspepsia, pathways such as arginine and proline metabolism and, aspartate and glutamate metabolism are greatly enriched in urine samples (Luo et al., 2018), although glutamate was observed to be depleted, resulting in the phenomenon of disturbed TCA cycle, causing central fatigue. Similarly, this depletion of glutamate and glutamine has also been indicated in the plasma of patients diagnosed with FD, likely causing the elevation in the downstream metabolites such as proline (Wu et al., 2016). Outputs of the current study showed a similar trend of these pathways being upregulated in FD patients.
The proteins such as carbonic anhydrases have been indicated to introduce tissue hypoxia in colonic biopsies during IBD conditions, especially during ulcerative colitis (Rowan et al., 2018). However, the expression of carbonic anhydrases is depleted in the colonic and ileal biopsies of rats during induced colitis(Khan & Khan, 2019) and in ulcerative colitis patients (Nakada et al., 2020). Our observations, on the contrary, showed an upregulated carbonic anhydrase activity in blood plasma, particularly during IBS rather than the FD. On the other hand, the proteins such as Ig kappa and apolipoproteins have been shown to be considerably upregulated in blood serum during IBS, the observations being similar to that observed recently (Mainoli et al., 2021; Tsigaridas et al., 2018) and, indicating an increased hypersensitivity response during IBS. Tsigaridas et al. (Tsigaridas et al., 2018) and Camilleri (Camilleri, 2009) have also shown an upregulation of apolipoproteins in plasma of IBS patients and their negative correlation with the blood serotonin levels and a positive correlation with increased constipation.
Our analyses suggests that a single platform was unable to discriminate between healthy subjects and IBS, FD and FD-IBS patients. However, an integration of different omics platforms has the potential to not only differentiate between healthy people and patients with gut disorders but also enable the discrimination between different disorders (Waluga, 2020). Moreover, the study also indicated that more accessible samples such as blood plasma can be used to diagnose gut disorders rather than biopsy samples. The benefit of this approach is not simply to observe a link between the results of the various platforms to assess metabolomic markers and symptoms that affect patients, but utilsing metabolomic data generated with various platforms to better categorise and ultimetaley target therapies towards the underlying disease mechanism.
5 Conclusions
With an integrated omics platform utilsing blood plasma and capturing metaproteomics, central carbon metabolism and lipidomics it appears feasible to better characterise the biochemical mechanisms of IBS, FD and overlap of FD-IBS. LC-MS techniques combined with the multivariate chemometric analyses can be used to identify the plasma proteins, lipids and central carbon metabolism and characterise prominent biochemical pathways associated with the individual or a cooccurrence of these FGIDs. Utilising metaproteome expression in samples from controls, FD, IBS and FD-IBS, metabolome and lipidome profiles yielded appropriate discrimination and model predictability. Metabolomic-lipidomic-proteomic integration point towards upregulated cholesterol and arginine, proline metabolism, increased thyroid hormone synthesis, ferroptosis and, upregulated complementary and coagulation cascades in IBS. The results indicated that instead of the individual identifiers, a panel of multiple identifiers are likely required as biomarker for the various FGID. Further studies with larger sample sizes are required to delineate potential specific disease pathways and the ability to predict response to therapy.
References
Azizan, K. A., Baharum, S. N., Ressom, H. W., & Noor, N. M. (2012). GC-MS analysis and PLS-DA validation of the trimethyl silyl-derivatization techniques. American Journal of Applied Sciences, 9, 1124–1136
Beale, D. J., Shah, R., Karpe, A. V., Hillyer, K. E., McAuley, A. J., Au, G. G. … Vasan, S. S. (2021). Metabolic Profiling from an Asymptomatic Ferret Model of SARS-CoV-2 Infection
Bernelot Moens, S. J., Verweij, S. L., Schnitzler, J. G., Stiekema, L. C. A., Bos, M., Langsted, A. … Kroon, J. (2017). Remnant Cholesterol Elicits Arterial Wall Inflammation and a Multilevel Cellular Immune Response in Humans. Arteriosclerosis, Thrombosis, And Vascular Biology, 37, 969–975
Bierman, A. J., Tertoolen, L. G., de Laat, S. W., & Moolenaar, W. H. (1987). The Na+/H + exchanger is constitutively activated in P19 embryonal carcinoma cells, but not in a differentiated derivative. Responsiveness to growth factors and other stimuli. Journal Of Biological Chemistry, 262, 9621–9628
Burns, G. L., Bruce, J., Minahan, K., Mathe, A., Fairlie, T., Cameron, R. … Keely, S. (2021). Distinct Adaptive Immunophenotypes in duodenal mucosa but not in peripheral blood of patients with functional dyspepsia. 2021.11.22.21266508
Camilleri, M. (2009). Serotonin in the gastrointestinal tract. Current opinion in endocrinology diabetes and obesity, 16, 53–59
Chong, J., Soufan, O., Li, C., Caraus, I., Li, S., Bourque, G. … Xia, J. (2018). MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Research, 46, W486–W494
Drossman, D. A. (2016). Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology, 150, 1262–1279e2
Enck, P., Azpiroz, F., Boeckxstaens, G., Elsenbruch, S., Feinle-Bisset, C., Holtmann, G. … Talley, N. J. (2017). Functional dyspepsia. Nature Reviews Disease Primers, 3, 17081
Fiehn, O. (2016). Metabolomics by gas chromatography–mass spectrometry: Combined targeted and untargeted profiling. Current protocols in molecular biology114, 30.4. 1-30.4. 32
Fiehn, O., Robertson, D., Griffin, J., van der Werf, M., Nikolau, B., Morrison, N. … Taylor, C. (2007). The metabolomics standards initiative (MSI). Metabolomics, 3, 175–178
Fischbach, W., Malfertheiner, P., Lynen Jansen, P., Bolten, W., Bornschein, J., Buderus, S. … Vieth, M. (2016). [S2k-guideline Helicobacter pylori and gastroduodenal ulcer disease]. Zeitschrift Fur Gastroenterologie, 54, 327–363
François, M., Karpe, A., Liu, J. W., Beale, D., Hor, M., Hecker, J. … Leifert, W. R. (2021). Salivaomics as a Potential Tool for Predicting Alzheimer’s Disease During the Early Stages of Neurodegeneration. Journal of Alzheimer’s Disease, 82, 1301–1313
Griffin, J. L., Nicholls, A. W., Daykin, C. A., Heald, S., Keun, H. C., Schuppe-Koistinen, I. … Robertson, D. (2007). Standard reporting requirements for biological samples in metabolomics experiments: mammalian/in vivo experiments. Metabolomics, 3, 179–188
Gyawali, P., Karpe, A. V., Hillyer, K. E., Nguyen, T. V., Hewitt, J., & Beale, D. J. (2021a). A multi-platform metabolomics approach to identify possible biomarkers for human faecal contamination in GreenshellTM mussels (Perna canaliculus). Science of The Total Environment, 145363
Haag, S., Talley, N. J., & Holtmann, G. (2004). Symptom patterns in functional dyspepsia and irritable bowel syndrome: relationship to disturbances in gastric emptying and response to a nutrient challenge in consulters and non-consulters. 53,1445–1451
Hernández-de-Diego, R., Tarazona, S., Martínez-Mira, C., Balzano-Nogueira, L., Furió-Tarí, P., Pappas, G. J. Jr., & Conesa, A. (2018). PaintOmics 3: a web resource for the pathway analysis and visualization of multi-omics data. Nucleic Acids Research, 46, W503–w509
Hoedt, E. C., Shanahan, E. R., Keely, S., Shah, A., Burns, G. L., Holtmann, G. J. … Morrison, M. (2021). Draft Genome Sequence of Streptococcus salivarius AGIRA0003, Isolated from Functional Gastrointestinal Disorder Duodenal Tissue. Microbiology resource announcements, 10, e0075821–e0075821
Holtmann, G. J., & Talley, N. J. (2018). Inconsistent symptom clusters for functional gastrointestinal disorders in Asia: is Rome burning? Gut, 67, 1911–1915
Huang, H. T., Zhang, X. Y., Zhang, C., Ling, Q., & Zheng, S. S. (2020). Predicting dyslipidemia after liver transplantation: A significant role of recipient metabolic inflammation profile. World journal of gastroenterology, 26, 2374–2387
James, S. C., Fraser, K., Young, W., Heenan, P. E., Gearry, R. B., Keenan, J. I. … Roy, N. C. (2021). Concentrations of Fecal Bile Acids in Participants with Functional Gut Disorders and Healthy Controls. Metabolites, 11, 612
Jeffery, I. B., Das, A., O’Herlihy, E., Coughlan, S., Cisek, K., Moore, M. … O’Toole, P. W. (2020). Differences in Fecal Microbiomes and Metabolomes of People With vs Without Irritable Bowel Syndrome and Bile Acid Malabsorption. Gastroenterology, 158, 1016–1028e8
Jones, M. P., Chey, W. D., Singh, S., Gong, H., Shringarpure, R., Hoe, N. … Talley, N. J. (2014). A biomarker panel and psychological morbidity differentiates the irritable bowel syndrome from health and provides novel pathophysiological leads. Alimentary Pharmacology & Therapeutics, 39, 426–437
Karpe, A. V., Beale, D. J., Morrison, P. D., Harding, I. H., & Palombo, E. A. (2015). Untargeted metabolic profiling of Vitis vinifera during fungal degradation. Fems Microbiology Letters, 362, fnv060
Karpe, A. V., Hutton, M. L., Mileto, S. J., James, M. L., Evans, C., Shah, R. M. … Beale, D. J. (2021). Cryptosporidiosis Modulates the Gut Microbiome and Metabolism in a Murine Infection Model. Metabolites11, 380
Khan, I., & Khan, K. (2019). Uncoupling of Carbonic Anhydrase from Na-H exchanger-1 in Experimental Colitis: A Possible Mechanistic Link with Na-H Exchanger. 9,700
Koloski, N., Holtmann, G., & Talley, N. J. (2020). Is there a causal link between psychological disorders and functional gastrointestinal disorders? Expert Review of Gastroenterology & Hepatology, 14, 1047–1059
Lim, Y., Tang, K. D., Karpe, A. V., Beale, D. J., Totsika, M., Kenny, L. … Punyadeera, C. (2021). Chemoradiation therapy changes oral microbiome and metabolomic profiles in patients with oral cavity cancer and oropharyngeal cancer. 43,1521–1534
Linsalata, M., Riezzo, G., D’Attoma, B., Clemente, C., Orlando, A., & Russo, F. (2018). Noninvasive biomarkers of gut barrier function identify two subtypes of patients suffering from diarrhoea predominant-IBS: a case-control study. Bmc Gastroenterology, 18, 167
Luo, L., Hu, M., Li, Y., Chen, Y., Zhang, S., Chen, J. … Liao (2018). Association between metabolic profile and microbiomic changes in rats with functional dyspepsia. Q J R a, 8, 20166–20181
Madisch, A., Andresen, V., Enck, P., Labenz, J., Frieling, T., & Schemann, M. (2018). The Diagnosis and Treatment of Functional Dyspepsia. Deutsches Arzteblatt international, 115, 222–232
Mainoli, B., Filyk, A. K., Lu, C., & Dufour, A. (2021). A154 proteomic identification of inflammatory and fibrostenotic blood serum biomarkers in Crohn’s disease. Journal of the Canadian Association of Gastroenterology, 4, 159–161
Menees, S. B., Powell, C., Kurlander, J., Goel, A., & Chey, W. D. (2015). A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. American Journal Of Gastroenterology, 110, 444–454
Metsalu, Tauno and Vilo, Jaak. Clustvis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Research, 43(W1):W566?W570, 2015. doi: https://doi.org/10.1093/nar/gkv468
Nakada, N., Mikami, T., Horie, K., Nagashio, R., Sakurai, Y., Sanoyama, I. … Murakumo, Y. (2020). Expression of CA2 and CA9 carbonic anhydrases in ulcerative colitis and ulcerative colitis-associated colorectal cancer. 70,523–532
NHMRC, ARC and Universities-Australia. (2018). In N. H. .a.M.R. (Ed.), National Statement on Ethical Conduct in Human Research 2007 (Updated 2018) in Council. National Health and Medical Research Council
Ohlsson, B., Orho-Melander, M., & Nilsson, P. M. (2017). Higher Levels of Serum Zonulin May Rather Be Associated with Increased Risk of Obesity and Hyperlipidemia, Than with Gastrointestinal Symptoms or Disease Manifestations.Int J Mol Sci18
Rodiño-Janeiro, B. K., Vicario, M., Alonso-Cotoner, C., Pascua-García, R., & Santos, J. (2018). A review of microbiota and irritable bowel syndrome: Future in therapies. Advances in therapy, 35, 289–310
Rowan, C. R., Brown, E., Ryan, E., Taylor, C., & Doherty, G. A. (2018). P022 Severe disease activity in UC is associated with mucosal hypoxia measured endoscopically. Journal of Crohn’s and Colitis, 12, S104–S104
Roy, N., Fraser, K., Young, W., Cooney, J., McNabb, W., & Gearry, R. (2019). The COMFORT Cohort: Identifying Biomarkers Relevant to Functional Gastrointestinal Disorders (P20-039-19).Current Developments in Nutrition3
Shah, R. M., Hillyer, K. E., Stephenson, S., Crosswell, J., Karpe, A. V., Palombo, E. A. … Beale, D. J. (2021). Functional analysis of pristine estuarine marine sediments. Science of The Total Environment, 781, 146526
Shinde, T., Perera, A. P., Vemuri, R., Gondalia, S. V., Beale, D. J., Karpe, A. V. … Stanley, R. (2020). Synbiotic supplementation with prebiotic green banana resistant starch and probiotic Bacillus coagulans spores ameliorates gut inflammation in mouse model of inflammatory bowel diseases. European Journal Of Nutrition, 59, 3669–3689
Sperber, A. D., Freud, T., Aziz, I., Palsson, O. S., Drossman, D. A., Dumitrascu, D. L. … Bangdiwala, S. I. (2021). Greater Overlap of Rome IV Disorders of Gut-Brain Interactions Leads to Increased Disease Severity and Poorer Quality of Life. Clin Gastroenterol Hepatol
Staratain, M. (2016). The Agilent Metabolomics DynamicMRM Database and Method (5991-6482EN), Agilent Technologies Technical Overview
Stevens, B. R., Goel, R., Seungbum, K., Richards, E. M., Holbert, R. C., Pepine, C. J., & Raizada, M. K. (2018). Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut, 67, 1555–1557
Talley, N. J., Holtmann, G., & Walker, M. M. (2015). Therapeutic strategies for functional dyspepsia and irritable bowel syndrome based on pathophysiology. Journal of Gastroenterology, 50, 601–613
Talley, N. J., Holtmann, G., Walker, M. M., Burns, G., Potter, M., Shah, A. … Keely, S. (2019). Circulating Anti-cytolethal Distending Toxin B and Anti-vinculin Antibodies as Biomarkers in Community and Healthcare Populations With Functional Dyspepsia and Irritable Bowel Syndrome. Clin Transl Gastroenterol, 10, e00064
Talley, N. J., Holtmann, G. J., Jones, M., Koloski, N. A., Walker, M. M., Burns, G. … Keely, S. (2020). Zonulin in serum as a biomarker fails to identify the IBS, functional dyspepsia and non-coeliac wheat sensitivity. 69,1719–1722
Tsigaridas, A., Anagnostopoulos, A. K., Papadopoulou, A., Ioakeim, S., Vaiopoulou, A., Papanikolaou, I. S. … Gazouli, M. (2018). Identification of serum proteome signature of irritable bowel syndrome: Potential utility of the tool for early diagnosis and patient’s stratification. Journal of Proteomics, 188, 167–172
Uhde, M., Ajamian, M., Caio, G., De Giorgio, R., Indart, A., Green, P. H. … Alaedini, A. (2016). Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. 65,1930–1937
Vijayvargiya, P., Busciglio, I., Burton, D., Donato, L., Lueke, A., & Camilleri, M. (2018). Bile Acid Deficiency in a Subgroup of Patients With Irritable Bowel Syndrome With Constipation Based on Biomarkers in Serum and Fecal Samples. Clinical Gastroenterology and Hepatology, 16, 522–527
Waluga, M. (2020). 9 - Biomarkers of irritable bowel syndrome in Fichna, J. (Ed), A Comprehensive Overview of Irritable Bowel Syndrome, Academic Press. pp. 107–127
Wu, Q., Zou, M., Yang, M., Zhou, S., Yan, X., Sun, B. … Yu, S. (2016). Revealing Potential Biomarkers of Functional Dyspepsia by Combining 1H NMR Metabonomics Techniques and an Integrative Multi-objective Optimization Method. Scientific Reports, 6, 18852
Xiao, Y., Wang, Y., Liu, Y., Wang, W., Tian, X., Chen, S. … Cai, W. (2021). A nonbile acid farnesoid X receptor agonist tropifexor potently inhibits cholestatic liver injury and fibrosis by modulating the gut–liver axis. Liver International, 41, 2117–2131
Funding
The operational funding for this study was provided through the CSIRO Probing Biosystems Future Science Platform (FSP).
Author information
Authors and Affiliations
Contributions
A.V.K., D.J.B., N.K., A.S. and G.H.: Concept, experiment design, planning, analysis, manuscript drafting, and critical review. A.V.K. and D.J.B.: Data analysis, interpretation, manuscript drafting, and critical review. J.-W.L.: Proteomic sample process and analysis. A.V.K and N.K.: sample handling and ethics approval process. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karpe, A.V., Liu, JW., Shah, A. et al. Utilising lipid and, arginine and proline metabolism in blood plasma to differentiate the biochemical expression in functional dyspepsia (FD) and irritable bowel syndrome (IBS). Metabolomics 18, 38 (2022). https://doi.org/10.1007/s11306-022-01900-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-022-01900-z