Abstract
Introduction
Early diagnosis of periodontitis by means of a rapid, accurate and non-invasive method is highly desirable to reduce the individual and epidemiological burden of this largely prevalent disease.
Objectives
The aims of the present systematic review were to examine potential salivary metabolic biomarkers and pathways associated to periodontitis, and to assess the accuracy of salivary untargeted metabolomics for the diagnosis of periodontal diseases.
Methods
Relevant studies identified from MEDLINE (PubMed), Embase and Scopus databases were systematically examined for analytical protocols, metabolic biomarkers and results from the multivariate analysis (MVA). Pathway analysis was performed using the MetaboAnalyst online software and quality assessment by means of a modified version of the QUADOMICS tool.
Results
Twelve studies met the inclusion criteria, with sample sizes ranging from 19 to 130 subjects. Compared to periodontally healthy individuals, valine, phenylalanine, isoleucine, tyrosine and butyrate were found upregulated in periodontitis patients in most studies; while lactate, pyruvate and N-acetyl groups were the most significantly expressed in healthy individuals. Metabolic pathways that resulted dysregulated are mainly implicated in inflammation, oxidative stress, immune activation and bacterial energetic metabolism. The findings from MVA revealed that periodontitis is characterized by a specific metabolic signature in saliva, with coefficients of determination ranging from 0.52 to 0.99.
Conclusions
This systematic review summarizes candidate metabolic biomarkers and pathways related to periodontitis, which may provide opportunities for the validation of diagnostic or predictive models and the discovery of novel targets for monitoring and treating such a disease (PROSPERO CRD42020188482).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Periodontitis is a chronic inflammatory disease characterized by the irreversible destruction of periodontal tissues and is one of the most common non-communicable diseases worldwide (Tonetti et al. 2018). In its more severe form it affects around 5–20% of the adult population, whereas in mild to moderate forms it reaches a prevalence of 50% (Bernabe et al. 2020). Since severe periodontitis impairs significantly the quality of life, by reducing functional capacity, deteriorating aesthetics, and decreasing self-esteem (Tonetti et al. 2017), early diagnosis is of utmost importance to prevent these negative consequences. Thus, non-invasive accurate diagnostic methods are sought to discriminate current periodontitis from gingivitis and/or past periodontitis (Korte and Kinney 2016).
To this regard, salivary diagnostics represents a promising strategy (Dawes and Wong 2019). As key component of the host defense against the microbial attacks from the dental biofilm, saliva provides both information on the whole mouth inflammatory status and on pathogen microbiota (Buduneli and Kinane 2011; Proctor 2016). Furthermore, it can be collected rapidly and non-invasively, and is convenient for repetitive sampling, even in patients with low compliance (Kinney et al. 2011; Jaedicke et al. 2016). Because of these reasons, salivary analysis has a great potential in large clinical trials, epidemiological studies and in self-management of disease, also where access to clinical facilities is scarce (Kim et al. 2013; Zhang et al. 2016).
Many analytical techniques have been used in the last years to discover early markers of periodontal disease in saliva samples (de Lima et al. 2016; Kc et al. 2020). Among these, metabolomics deals with the characterization of low molecular weight compounds and their metabolic pathways in biofluids or tissues using spectroscopic assay techniques (Wishart 2007). The metabolome encompasses a wide range of endogenous and exogenous compounds that can be affected by both genetic and environmental factors (Nicholson et al. 2012; Patti et al. 2012; Sun et al. 2020). Salivary metabolome can give valuable information on the complex pathogenic mechanisms of periodontitis, and salivary metabolites can be candidate biomarkers to identify periodontal status (Aimetti et al. 2012; Mikkonen et al. 2016; Gardner et al. 2020) and severity of periodontal breakdown (Liebsch et al. 2019).
Despite the increasing number of studies focusing on the relationship between salivary metabolome and periodontal conditions (Barnes et al. 2009; Romano et al. 2018), a systematic review on the diagnostic ability of salivary metabolomics for periodontitis is still lacking. Non-invasive and accurate salivary tests may be helpful for early diagnosis, thereby increasing the success of active periodontal treatment as well as the maintenance phase. Therefore, the aims of the present systematic review were to assess the potential of salivary untargeted metabolomics in the diagnosis of periodontitis, providing a methodological grade assessment of the included studies. Furthermore, we aimed to summarize methodological differences among studies in order to provide a comprehensive overview for future research implementation.
2 Materials and methods
2.1 Report and protocol
This research has been reported according to the Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) (Shamseer et al. 2015). A detailed protocol was registered on Prospero (CRD42020188482).
2.2 Focused question
Can levels of metabolites or metabolomic profiling of saliva be used to discriminate between periodontally healthy conditions and gingivitis or periodontitis?
2.2.1 Inclusion criteria
The inclusion criteria of this systematic review were organized according to the PECOS framework.
-
(P) Population Adult patients in good systemic health.
-
(E) Exposure Subjects diagnosed with gingivitis or periodontitis.
-
(C) Comparison Subjects with periodontal health.
-
(O) Type of outcome measures Differences in detectable metabolites in whole saliva assessed through nuclear magnetic resonance (NMR) spectrometry or mass spectrometry (MS) analytic platforms.
-
(S) Types of Studies Original studies in humans with observational design (cross-sectional, case–control and cohort) with the characterization of salivary analytes by untargeted metabolomics. Both prospective and retrospective studies were included.
2.2.2 Exclusion criteria
Studies that deal with targeted metabolomics, studies investigating salivary metabolic profiling in specific medical conditions (i.e. diabetes), as well as studies evaluating metabolomic changes after periodontal treatment were excluded. Clinical case reports, literature reviews, editorials, animal studies, and studies involving in vitro experiments were not included, as well as studies not published in English.
2.3 Search methods for the identification of studies
A literature search in Medline (via PubMed), Embase and Web of Science electronic databases was conducted up to and including April 14th, 2020. A search strategy based on a combination of Medical Subject Headings (MeSH) and free text words included the following keywords “periodontitis”, “gingivitis”, “metabolomics”, “metabonomics”, “metabolic fingerprinting”, “metabolites”, “nuclear magnetic resonance spectroscopy” and “mass spectrometry” combined with Boolean operators “AND" and “OR”. The search was conducted without any restriction on date of publication or publication status. In addition, two independent reviewers manually cross-checked the reference lists of all included studies and relevant systematic reviews. Duplicates were identified using reference manager software (EndNoteTM, version 7, Thomson Reuters, Philadelphia, PA, USA).
2.4 Study selection
The titles and abstracts of all studies identified by the search were screened independently by two reviewers, and those that fulfilled the inclusion criteria were selected for full review. Any disagreements was resolved by discussion and, when necessary, by consulting a third reviewer. The full texts were assessed independently by two reviewers for inclusion. The inter-reviewer agreement was verified by kappa coefficient.
2.5 Data extraction and management
The following information of included studies was extracted by two independent reviewers using predefined data extraction forms:
-
General information: first author, year of publication and country/region of origin.
-
Population: number of patients, inclusion/exclusion criteria, age at baseline, smoking habits.
-
Exposures and controls: case and control definition, case and control number, periodontal status assessment, pre-sampling procedures, saliva collection method, sample storage method, sample preparation, analytical platform employed [nuclear magnetic resonance (NMR) or mass spectrometry (MS) coupled with capillary electrophoresis, liquid or gas chromatography (CE, LC or GC, respectively)].
-
Outcomes: number of metabolites identified, statistically significant metabolites in cases and controls, overall differences in metabolic profiling.
2.6 Risk of bias and quality assessment of included studies
The methodological quality of selected studies was assessed in duplicate by two reviewers according to the criteria of the modified version of the NIH Quality Assessment Tool for Cross-Sectional Studies and the QUADOMICS tool, specific to -omics research (Lumbreras et al. 2008). We composed a tool made of 15 items to evaluate research question, study population, exposure, salivary sampling procedures (including antibiotic prescription, time of sampling food assumption or toothpaste use), confounding factors (smoking and systemic diseases affecting salivary metabolic profile, i.e. diabetes mellitus), outcomes and statistics applied to metabolomic analysis. Every item was given either 0 or 1 point apart from item 3 and item 13 on sampling procedures and confounders in which each aspect accounted for 0.25 points. Studies were rated based on total score. Studies with a final score ≥ 13 were considered as high quality, from 10 to 12 as moderate quality and < 10 as low quality.
2.7 Pathway enrichment analysis
After identification of salivary metabolites from the selected articles, the Human Metabolome Database (HMDB) version 4.0 was searched manually to obtain metabolite identification prior to the analysis (Wishart et al. 2017). The pathway analysis was run through MetaboAnalyst version 4.0 (https://www.metaboanalyst.ca) (Chong et al. 2019). To characterize relevant pathways, the Kyoto Encyclopedia of Genes and Genomes (KEGG) and the small molecule pathway (SMP) were regarded as reference libraries. The analysis algorithm was a hypergeometric test for over-representation analysis, and the relative betweenness centrality was selected for pathway topology analysis. An adjusted P-value (false discovery rate, FDR) less than 0.05 was considered in order for a pathway to be identified as significantly enriched.
2.8 Data synthesis
Due to the limited number of studies reporting measures of diagnostic accuracy and the heterogeneity in methodologies, data were not pooled in a meta-analysis.
3 Results
3.1 Study selection
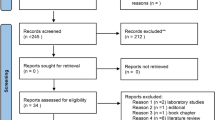
Supplementary Figure 1 depicts the flow chart of selection process: 2791 titles were retrieved from electronic and hand searches. After excluding duplicates, the total number of entries was 2108. Once title and abstract were evaluated, 2080 studies were excluded (agreement 88.89%; K = 0.77), resulting in 28 articles subjected to full-text analysis. Finally, 12 studies met the inclusion criteria and were eligible for the systematic review (Sugimoto et al. 2010; Barnes et al. 2011, 2014; Aimetti et al. 2012; Marchesan et al. 2015; Kuboniwa et al. 2016; Singh et al. 2017; Rzeznik et al. 2017; Romano et al. 2018, 2019; García-Villaescusa et al. 2018; Gawron et al. 2019). Reasons for exclusion are reported in Supplementary Table 1.
3.2 Characteristics of the included studies
Tables 1 and 2 summarize the characteristics of the included items. All studies presented a cross-sectional design, except one intervention study in which the metabolic profile of periodontitis patients was compared before and after non surgical treatment (Romano et al. 2019). In the latter, only baseline differences between healthy individuals and periodontitis patients were considered for this systematic review. The sample size ranged from 19 to 130 participants. All studies considered chronic periodontitis (CP) as cases, except 2 studies that additionally evaluated the metabolic profile of aggressive periodontitis (AgP) (Rzeznik et al. 2017; Romano et al. 2018). Only 6 studies excluded gingivitis from controls or performed a subgroup analysis. Seven of the included studies used 1H-NMR as analytic platform, whereas 5 studies used MS, coupled with CE, LC and/or GC. All studies collected unstimulated saliva, except two works in which saliva secretion was stimulated by using paraffin wax (Rzeznik et al. 2017), or employed mouth washouts with saline (Gawron et al. 2019). Seven studies collected saliva in the morning, one (Kuboniwa et al. 2016) in the afternoon, whereas the other four did not report the time of sample collection. Kuboniwa et al. (2016) were the only authors that performed debridement of supra gingival plaque and calculus 15 min before saliva sampling. In all studies, participants were asked to avoid eating, drinking, brushing or using mouthwash at least 1 h prior to saliva samples collection.
3.3 Quality assessment of the studies
The results of quality assessment according to the modified QUADOMICS tool for metabolomic research are shown in Table 3. While only one study (Romano et al. 2019) performed a sample size calculation, most studies (n = 8) properly controlled participants for antibiotics, food intake and toothpaste use before saliva collection. Only three studies reported the exact time of saliva sampling in relation to periodontal examination and only five studies excluded or properly controlled for smoking or systemic diseases in the statistical analysis. Four studies were not able to avoid overfitting due to lack of an independent validation set or cross-validation. No studies interpreted data without knowledge of the clinical diagnosis. According to these criteria, only one study was considered as high quality (Romano et al. 2019), five studies moderate quality, while the others were rated as low quality.
3.4 Altered metabolites identified in gingivitis and periodontitis
Table 4 includes results for upregulated and downregulated metabolites by periodontal conditions. Number of metabolites varied from 10 to 390. Only data for significantly distinctive metabolites were extracted.
3.4.1 Amino acid metabolites
Five studies reported elevated salivary levels of two amino acids synthesized from pyruvate in CP, valine (Aimetti et al. 2012; Kuboniwa et al. 2016; Singh et al. 2017; Romano et al. 2018, 2019), and isoleucin (Sugimoto et al. 2010; Barnes et al. 2014; Romano et al. 2018, 2019; García-Villaescusa et al. 2018). Tyrosine was found at increased concentrations in saliva of periodontitis patients in four studies (Sugimoto et al. 2010; Barnes et al. 2011; Romano et al. 2018, 2019), while cadaverine and spermidine were jointly upregulated in CP patients in 3 studies (Sugimoto et al. 2010; Barnes et al. 2014; Kuboniwa et al. 2016), and tyrosine and phenylalanine in both CP and AgP in four studies (Sugimoto et al. 2010; Barnes et al. 2011; Romano et al. 2018, 2019). Alanine, cysteine, leucine, serine, and threonine were the other amines consistently found at significantly higher concentrations in CP in at least two studies. On the other hand, data on salivary proline levels were controversial, with three studies reporting upregulated concentrations in periodontitis patients (Kuboniwa et al. 2016; Romano et al. 2018, 2019) and one study (García-Villaescusa et al. 2018) in healthy individuals and gingivitis cases. Cyclo (-leu-pro) and cyclo (-phe-pro) were the two cyclodipeptides positively correlated with increased severity of periodontitis and subgingival levels of Synergistetes (Marchesan et al. 2015).
3.4.2 Cellular respiration/carbohydrate metabolism
Many metabolic changes in the glycolysis, the tricarboxylic acid cycle (TCA) and the anaerobic respiration were associated with periodontal disease. Decrease in the salivary levels of N-acetyl groups (Aimetti et al. 2012; Rzeznik et al. 2017; Romano et al. 2018), but increase in succinate, a dicarboxylic acid generated in mitochondria via the TCA, were found in periodontitis patients compared to healthy controls (Aimetti et al. 2012; Barnes et al. 2014; Singh et al. 2017), While glucose increased more in periodontitis than healthy samples in 2 studies (Barnes et al. 2011, 2014), the trend of other mono- and di-saccharides from carbohydrate metabolism identified by Barnes (fructose, mannose and mannitol) failed to reach replication. Analysis of metabolites related to energy production pathways showed contradictory results. As intermediate in several metabolic pathways throughout both prokaryotic and eukaryotic cells, pyruvate was found to be depleted in saliva of periodontitis patients in four studies (Aimetti et al. 2012; Rzeznik et al. 2017; Romano et al. 2018, 2019), but upregulated in the study by Singh et al. (2017). Lactate, as product of anaerobic glycolysis, was found to be upregulated in periodontitis compared to healthy controls in 2 studies (Singh et al. 2017; Gawron et al. 2019), while in 4 studies followed the opposite trend (Rzeznik et al. 2017; Romano et al. 2018, 2019; García-Villaescusa et al. 2018), and in the study by García-Villaescusa et al. (2018) it increased in gingivitis patients. Finally, acetate, that is an end product of anaerobic fermentation, was upregulated in periodontitis patients in the subset of Aimetti et al. (2012), while it followed an inverse tendency in the study by Rzeznik et al. (2017).
3.4.3 Lipid metabolites
Free fatty acids and metabolites related to fatty acid oxidation are frequently altered in periodontitis patients. Butyrate is a short chain fatty acid (SCFA) metabolyzed by oral bacteria and was found upregulated in periodontitis across three studies (Aimetti et al. 2012; Rzeznik et al. 2017; García-Villaescusa et al. 2018). Barnes et al. (2011, 2014) reported increased salivary detection of linoleate, docosapentaenoate, dihomo-linoleate, arachidonate, 2-hydroxypalmitate, consistently with higher inflammatory degradation of lipid macromolecules, together with two byproducts of carnitine, 3-dehydrocarnitine and acetylcarnitine. Propionate, isovalerate, caproate and isocaproate are among the other fatty acids upregulated in periodontal diseases. Finally, glycerol-3-phosphate (G3P) was the only untargeted metabolite significantly discriminating periodontitis patients from healthy controls in the study by Marchesan et al. (2015).
3.4.4 Other significant metabolites
Methanol is a volatile alcohol catabolyzed by bacteria, that was downregulated in CP and AgP in two studies (Rzeznik et al. 2017; Gawron et al. 2019); while trimethylamine, synthetized during the reaction between ammonia and methanol, was upregulated in CP (Sugimoto et al. 2010; Aimetti et al. 2012). Hydrocinnamate, a carboxylic acid derived from phenylalanine, was significantly elevated in periodontitis patients in the study of Kuboniwa et al. (2016).
3.5 Pathway enrichment analysis of potential biomarkers
From the included studies, we extracted 114 potential biomarker candidates. Among them, only 27 compounds that had statistically significant trend in more than one paper were submitted to pathway enrichment analysis (Fig. 1). As shown in Fig. 2, phenylalanine, tyrosine and tryptophan biosynthesis pathway (impact value of 1, FDR of 0.023); glycine, serine and threonine metabolism pathway (impact value of 0.21, FDR of 0.023); phenylalanine metabolism pathway (impact value of 0.35, FDR of 0.091) and pyruvate metabolism pathway (impact value of 0.22, FDR of 0.29) were the most prominent ones derived from the selected biomarkers. Detailed information regarding the reported metabolites and enriched pathways can be found in Supplementary Table 2.
3.6 Discrimination of metabolic profiling by multivariate analysis
Among multivariate data analysis (MVDA) techniques aimed at grouping similar subsets of samples together, principal component analysis (PCA) was adopted in three studies (Sugimoto et al. 2010; Romano et al. 2018, 2019). This unsupervised modeling approach yielded from 81 to 82.5% of predictive accuracy in discriminating healthy from CP and AgP patients, and 60% in distinguishing CP against AgP patients. Conversely, supervised modeling approaches for regression discriminant analysis (DA), including partial least squares (PLS) and orthogonal PLS (OPLS), were adopted in 6 studies. In Aimetti et al. (2012), PLS-SVM yielded sensitivity of 91.1%, specificity of 79.4% and predictive accuracy of 84.1% in discriminating CP versus healthy status. PLS-DA yielded coefficient of determination (R2) and cross-validated coefficient of determination (Q2) values ranging from 0.71 to 0.999 and from 0.616 to 0.79, respectively (Kuboniwa et al. 2016; Singh et al. 2017; García-Villaescusa et al. 2018). Lower values in OPLS analysis were obtained in the studies by Rzeznik et al. (2017) and by Gawron et al. (2019) with R2 values of 0.57 and 0.52, respectively and Q2 values of 0.48 and 0.32, respectively. Finally, three studies did not perform or did not report results for MVDA (Barnes et al. 2011, 2014; Marchesan et al. 2015).
The area under the curve of the receiver operating characteristic curve (AUROC) values were reported only in 5 studies to assess the ability of candidate metabolites in discriminating healthy controls from periodontitis patients. They ranged between 0.69 and 0.982 (Sugimoto et al. 2010; Kuboniwa et al. 2016; Singh et al. 2017; Rzeznik et al. 2017; García-Villaescusa et al. 2018).
4 Discussion
4.1 Principal findings
This systematic review provided a qualitative synthesis of the literature dealing with untargeted metabolomic analysis of saliva for the diagnosis of periodontal diseases together with a methodological assessment of the 12 included studies. Compared to periodontally healthy subjects, patients with periodontitis presented statistically significant differences in 114 metabolites of different classes accounting for an elevated macromolecular degradation in periodontal disease. Pathway enrichment analysis revealed significant activity in the phenylalanine, tyrosine and tryptophan pathway, together within the phenylalanine and the pyruvate metabolism. The increased glycosidase, lipase, and protease activities associated with periodontal inflammation supply a more favorable energetic environment for oral bacteria, plausibly exacerbating the disease state (Barnes et al. 2011; Marsh and Zaura 2017). Due to the high complexity of systems targeted by omic research, multivariate analysis is often required in order to minimize random noise and to find latent trends within the data sets. The results of MVDA revealed that periodontitis is characterized by a specific metabolic signature in saliva, with model’s goodness-of-fit metrics ranging from 0.52 to 0.99. The distinctive clinical diagnosis of CP and AgP was not reflected in different metabolic profiling, as attested by the findings of Rzeznik et al. (2017) and Romano et al. (2018). This biological homogeneity has been operationalized by the 2017 World Workshop on the Classification of Periodontal Diseases that merged both forms in a single entity with different stagings and gradings (Tonetti et al. 2018). Due to the explorative nature and heterogeneity of the included studies, no meta-analysis of the results was possible. The relevant heterogeneity could be ascribed to differences in the sample size, case definitions, saliva collection methods, analytical platform and protocols adopted. The aforementioned issues are reflected in a general low/moderate quality scores of the studies, assessed by the modified QUADOMICS version.
4.2 Interpretation in the context of the available literature
Valine, isoleucine, phenylalanine and tyrosine were the metabolites consistently upregulated in periodontitis in a large number of included studies. Also, cadaverine was significantly upregulated in 3 studies, in agreement with a recent research in which elevated concentrations of this product of protein decomposition were associated with higher periodontal pocket inflamed surface area (Sakanaka et al. 2017). The impaired catabolism of peptides in periodontitis is imputable to an increased activity of proteases, such as matrix metalloproteinases, which are usually upregulated in inflammatory conditions (Tervahartiala et al. 2000; Arias-Bujanda et al. 2020). In addition, the shift of the subgingival flora towards anaerobic proteolytic species also contributes to peptide degradation (Potempa et al. 2000). Valine and isoleucine are branched chain essential amino acids located at the crossroad of many metabolic pathways in eukaryotic and prokaryotic cells. Interestingly, valine is also synthetized by bacteria from pyruvic acid (Blombach et al. 2007), that was found significantly depleted in periodontitis patients in 4 studies (Aimetti et al. 2012; Rzeznik et al. 2017; Romano et al. 2018, 2019). Other two mechanisms may account for this finding. First, pyruvic acid represents the starting point of Krebs cycle, upregulated in periodontal ligament cells when facing infections (Su et al. 2020). Second, this compound is the principal substrate of L-lactate dehydrogenase, which manifests increased activity during periodontal inflammation (Wolff et al. 1988). Another metabolite associated with the depletion of the saccharolytic flora in favor of a more pathogenic one is lactate, upregulated in healthy controls in 4 studies (Rzeznik et al. 2017; Romano et al. 2018, 2019; García-Villaescusa et al. 2018). However, two studies reported an opposite trend for this molecule; but while Singh et al. (2017) did not provide a motivation, Gawron et al. (2019) could have favored the displacement in saliva of metabolites from the supragingival biofilm by making patients rinse their mouth with sterile saline before sampling. Intriguingly, lactate concentrations were upregulated in healthy controls in all three studies that distinguished between periodontitis and gingivitis (Romano et al. 2018, 2019; García-Villaescusa et al. 2018). Therefore, decrease of lactate seems to be a promising indicator of periodontitis rather than inflammation.
The complex interplay between the host immune system and the dysbiotic microflora is reflected by other changes in the salivary metabolites. Succinate and butyrate were found upregulated in periodontitis patients compared to healthy controls in 3 studies. This finding is consistent with the evidence demonstrating that butyrate, metabolyzed by bacteria, increases with the worsening of disease state, although its role in periodontal pathology remains controversial (Shirasugi et al. 2018; Liu et al. 2019). In one recent in vitro study, Porphyromonas gingivalis-infected periodontal ligament cells showed a marked pileup of succinate (Su et al. 2020), considered as an indicator of energetic stress. Barnes et al. (2011, 2014) reported increased concentrations of lysolipids and complex fatty acids in periodontitis. This can be justified by the enhanced secretion of pro-inflammatory lipids by the inflammatory cells and by the upregulation of fatty acid metabolism in response to oxidative stress (Huang et al. 2014). However, other studies which used different analytic platforms failed to detect an alteration in lipid profile. While Barnes et al. (2011, 2014) utilized MS for its higher sensitivity and wider range of measurement, NMR spectroscopy is the preferred method for untargeted analysis owing to its excellent reproducibility and simplicity of sample preparation (Emwas et al. 2013; Wishart 2019). Although in the majority of cases NMR and MS signals belong to the same metabolite, the bias arising from the analytical platform should not be neglected because the two techniques are not perfectly commutable (Hao et al. 2016).
Pathway enrichment analysis represents a first choice for gaining insight into the underlying biology of differentially expressed metabolites, as it reduces complexity and increases explanatory power (Hung 2013). This analysis showed how the pathway of phenylalanine, tyrosine and tryptophan biosynthesis had the highest impact on the overall metabolic profile. This pathway is highly expressed in conditions involving immune activation (Murr et al. 2014) and has a key role in bacterial anabolism (Parthasarathy et al. 2018). This finding is consistent with those by Liebsch et al. (2019), reporting positive correlation between probing depth and salivary levels of phenylalanine and tyrosine catabolites, as well as of ω-6 fatty acid dihomo-linolenate. Another significantly upregulated pathway was aminoacyl-tRNA biosynthesis, that plays a crucial role in regulating the maturation, transcription, activation and recruitment of immune cells (Nie et al. 2019).
Environmental factors should be considered when interpreting the oral metabolome. Smoking can affect the salivary expression of lactate, pyruvate, and sucrose (Takeda et al. 2009), hexanoic acid, cadaverine and G3P (Mueller et al. 2014). This may explain the conflicting increase of pyruvate in periodontitis patients of Singh et al. (2017), as smoking status of participants was not provided. Also, the alteration in the G3P levels detected by Marchesan et al. (2015) may be biased by the inclusion of a 43% of smokers within participants. Saliva collection method and amount of supragingival plaque may influence the metabolomic analysis of saliva. Passive drooling is regarded as the gold standard, because all other protocols may generate exogenous metabolites in samples (Pereira et al. 2019). Incidentally, increase in threonine and GABA reported by Rzeznik et al. (2017) could be partially explained by saliva stimulation with paraffin wax, as specified by Okuma et al. (2017). The concentration of SCFAs, amines, phenylalanine, glycine, and succinate is strongly correlated with the bacterial load in whole mouth saliva (Gardner et al. 2019). To this regard, Kuboniwa et al. (2016) collected saliva samples before and 15 min after supragingival debridement: while the metabolic phenotyping of periodontal patients remained similar and clearly distinct from healthy controls, the use of post-debridement saliva improved the predictive ability of their model, enhancing the detection of spermidine, ornithine and 5-oxoprolin secreted from sub-gingival locations.
In addition, concomitant systemic and/or oral diseases may also affect the salivary metabolites. Valine was found upregulated in oral, breast and prostate cancers (Sugimoto et al. 2010; Wei et al. 2011), and propionate in Alzheimer’s disease (Yilmaz et al. 2017) and dementia (Figueira et al. 2016), while butyrate levels were affected by the presence of dental caries (Fidalgo et al. 2013; Pereira et al. 2019). Furthermore, isoleucine, leucine, valine, and tyrosine were significantly upregulated in patients with type 2 diabetes (Sun et al. 2020). Due to the multifactorial character of periodontitis, diagnostic power can be enhanced by evaluating a panel of metabolites (Ramseier et al. 2009; Lee et al. 2012). Kuboniwa et al. (2016) reported that a combination of cadaverine, 5-oxoproline, and histidine increased the AUC for moderate and severe periodontitis to 0.881, while AUC values for single metabolites were not higher than 0.6.
Biostatistics methods are consistently used together with high-throughput techniques for reducing dimensionality and finding latent trend or clusters within the data. Despite the variance in the estimates, the majority of MVDA provided evidence for a distinctive salivary metabolomic signature of periodontitis. Therefore, metabolomics candidates itself as a support to achieve a biologically oriented classification of periodontal diseases (Kornman et al. 2017). Importantly, the generalized periodontal inflammation brought by a condition of gingivitis can be considered as a major confounding factor when interpreting the data projected into the score plots of PCA or PLS. Metabolomic profiles of gingivitis can partially overlap with both healthy state and periodontitis, although it seems that they present a uniquely specific fingerprint (García-Villaescusa et al. 2018).
4.3 Strengths and limitations of this study
To the best of our knowledge, this is the first systematic review focusing on the application of untargeted salivary metabolomics to diagnosis of periodontitis. Consistent with the previous narrative reviews (Mikkonen et al. 2016; Gardner et al. 2020), this review indicated that some metabolites can consistently discriminate between healthy and diseased periodontal conditions, and may be candidates for future steps in the diagnostic test validation process. Untargeted metabolite analysis is preferred for providing global profiling and for discovering new pathways (Gertsman and Barshop 2018). Although some articles dealing with targeted metabolites in saliva were found, they were mostly oriented at investigating their specific biological functions, and not on diagnostic purposes (Pradeep et al. 2007; Elabdeen et al. 2013). However, limiting the number of metabolites can lead to overlooking of some important byproducts, which might be a bias for targeted method (Roberts et al. 2012).
Some limitations of this review should be also acknowledged, mainly ascribed to the nature of the included studies. Firstly, most of them have small sample sizes preventing the generalizability of the results. Secondly, despite the strict inclusion criteria, there was heterogeneity in geographic area, case definitions, and eligibility criteria. For instance, smoking is a known risk factor for periodontitis, although a number of smokers was included in many of the studies (Nociti et al. 2015). Finally, the lack of analytical protocol homogeneity was a major problem and resulted in a broad range of metabolites with lack of replications. Also, multiple independent validations were not adopted for many studies.
4.4 Implications for future research
Prevention and early diagnosis of periodontitis are open challenges in periodontology. Developing diagnostic tests with high sensitivity and specificity requires mandatory steps (Gluud and Gluud 2005; Colli et al. 2014). Current knowledge about metabolomics in the periodontal field is limited to diagnostic biomarker discovery or preliminary comparative studies, i.e. phase 0 or phase IIa. In parallel, there is a need to better characterize the metabolic events occurring in the mouth in health and disease. Temporal stability and reproducibility of salivary fingerprinting are key features, both at circadian level and after oral activities or dental treatment (Wallner-Liebmann et al. 2016). Preliminary data (Romano et al. 2019) indicated that periodontally treated patients display a distinct metabolomic profiling compared to both healthy and periodontitis patients. Also, most included studies considered only generalized periodontitis for their analysis, but it would be more relevant for treatment to promptly detect the localized/initial forms (Papapanou et al. 2018). However further studies are needed to better characterize these groups by consistently applying the new classification of periodontal diseases and properly controlling for the presence of gingivitis. At present, it is not clear whether the state of disease activity has a major influence on the salivary metabotype or, alternatively, the latter is more dependent upon the genetic or epigenetic susceptibility factors.
Finally, integrating metabolomics with other omics platforms (e.g., genomics, transcriptomics and proteomics) will empower the knowledge on the biological stratification of periodontitis and help constructing the best discriminative diagnostic model in the view of personalized and precision medicine (Lyman and Moses 2016; Divaris et al. 2020). Periodontitis has been linked to numerous systemic conditions (Papageorgiou et al. 2017; Romandini et al. 2018; Beck et al. 2019). To this regard, salivary diagnostics has been proposed for biomarker discovery in other human pathologies, mostly in oral cancer, pancreatic cancer and Alzheimer’s disease (Dawes and Wong 2019; Assad et al. 2020). Expanding salivary metabolomics into other fields may help understanding the connection between molecular scale pathology and phenotypic expressions; and concomitantly refining the knowledge about metabolomic changes in periodontal health and disease. In order to achieve these highly desirable goals, general consensus and harmonization of protocols between different center is mandatory (Beger 2018).
5 Concluding remarks
This systematic review supports the use of salivary untargeted metabolomics for the diagnosis and monitoring of periodontal diseases. Several molecules and metabolic pathways detected in saliva were found to be associated with periodontitis, although no single biomarker could be considered specific for the disease. The combination of cadaverine, 5-oxoproline, and histidine seems particularly promising to improve diagnostic accuracy, but this panel was tested by only one study. Despite a meta-analysis was not possible, a high consistency of the findings was found across the studies, irrespective of the methods employed. These results may support the design of targeted analysis for those metabolites that were consistently associated to periodontitis or periodontal health either.
In addition, profiling by multiple logistic regression models confirmed that a distinct fingerprint of periodontitis is detectable in saliva through metabolomics. However, the discriminating ability has not reached the consistency of replication required for a diagnostic tests yet, especially for early state of disease. Overall, due to the heterogeneity and quality of the available evidence, more methodologically robust research is demanded in order to make advancements in salivary metabolomics for periodontal diseases. This requires harmonization of protocols to implement candidate metabolites, and sufficiently powered multi-centered cohort studies with blinded evaluation to validate them for clinical translation.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Aimetti, M., Cacciatore, S., Graziano, A., & Tenori, L. (2012). Metabonomic analysis of saliva reveals generalized chronic periodontitis signature. Metabolomics, 8(3), 465–474. https://doi.org/10.1007/s11306-011-0331-2.
Arias-Bujanda, N., Regueira-Iglesias, A., Balsa-Castro, C., Nibali, L., Donos, N., & Tomás, I. (2020). Accuracy of single molecular biomarkers in saliva for the diagnosis of periodontitis: A systematic review and meta-analysis. Journal of Clinical Periodontology, 47(1), 2–18. https://doi.org/10.1111/jcpe.13202.
Assad, D. X., Mascarenhas, E. C. P., de Lima, C. L., de Toledo, I. P., Chardin, H., Combes, A., et al. (2020). Salivary metabolites to detect patients with cancer: A systematic review. International Journal of Clinical Oncology, 25(6), 1016–1036. https://doi.org/10.1007/s10147-020-01660-7.
Barnes, V. M., Teles, R., Trivedi, H. M., Devizio, W., Xu, T., Mitchell, M. W., et al. (2009). Acceleration of purine degradation by periodontal diseases. Journal of Dental Research, 88(9), 851–855. https://doi.org/10.1177/0022034509341967.
Barnes, V. M., Ciancio, S. G., Shibly, O., Xu, T., Devizio, W., Trivedi, H. M., et al. (2011). Metabolomics reveals elevated macromolecular degradation in periodontal disease. Journal of Dental Research, 90(11), 1293–1297. https://doi.org/10.1177/0022034511416240.
Barnes, V. M., Kennedy, A. D., Panagakos, F., Devizio, W., Trivedi, H. M., Jonsson, T., et al. (2014). Global metabolomic analysis of human saliva and plasma from healthy and diabetic subjects, with and without periodontal disease. PLoS ONE, 9(8), e105181. https://doi.org/10.1371/journal.pone.0105181.
Beck, J. D., Papapanou, P. N., Philips, K. H., & Offenbacher, S. (2019). Periodontal medicine: 100 years of progress. Journal of Dental Research, 98(10), 1053–1062. https://doi.org/10.1177/0022034519846113.
Beger, R. D. (2018). Interest is high in improving quality control for clinical metabolomics: setting the path forward for community harmonization of quality control standards. Metabolomics, 15(1), 1. https://doi.org/10.1007/s11306-018-1453-6.
Bernabe, E., Marcenes, W., Hernandez, C. R., Bailey, J., Abreu, L. G., Alipour, V., et al. (2020). Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: A systematic analysis for the global burden of disease 2017 study. Journal of Dental Research, 99(4), 362–373. https://doi.org/10.1177/0022034520908533.
Blombach, B., Schreiner, M. E., Holátko, J., Bartek, T., Oldiges, M., & Eikmanns, B. J. (2007). L-valine production with pyruvate dehydrogenase complex-deficient Corynebacterium glutamicum. Applied and Environmental Microbiology, 73(7), 2079–2084. https://doi.org/10.1128/AEM.02826-06.
Buduneli, N., & Kinane, D. F. (2011). Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. Journal of Clinical Periodontology, 38(Suppl 11), 85–105. https://doi.org/10.1111/j.1600-051X.2010.01670.x.
Chong, J., Wishart, D. S., & Xia, J. (2019). Using metaboanalyst 4.0 for comprehensive and integrative metabolomics data analysis. Current Protocols in Bioinformatics, 68, e86. https://doi.org/10.1002/cpbi.86.
Colli, A., Fraquelli, M., Casazza, G., Conte, D., Nikolova, D., Duca, P., et al. (2014). The architecture of diagnostic research: From bench to bedside—research guidelines using liver stiffness as an example. Hepatology, 60(1), 408–418. https://doi.org/10.1002/hep.26948.
Dawes, C., & Wong, D. T. W. (2019). Role of saliva and salivary diagnostics in the advancement of oral health. Journal of Dental Research, 98(2), 133–141. https://doi.org/10.1177/0022034518816961.
de Lima, C. L., Acevedo, A. C., Grisi, D. C., Taba, M., Jr., Guerra, E., & De Luca Canto, G. (2016). Host-derived salivary biomarkers in diagnosing periodontal disease: Systematic review and meta-analysis. Journal of Clinical Periodontology, 43(6), 492–502. https://doi.org/10.1111/jcpe.12538.
Divaris, K., Moss, K., & Beck, J. D. (2020). Biologically informed stratification of periodontal disease holds the key to achieving precision oral health. Journal of Periodontology. https://doi.org/10.1002/JPER.20-0096.
Elabdeen, H. R., Mustafa, M., Szklenar, M., Ruhl, R., Ali, R., & Bolstad, A. I. (2013). Ratio of pro-resolving and pro-inflammatory lipid mediator precursors as potential markers for aggressive periodontitis. PLoS ONE, 8(8), e70838. https://doi.org/10.1371/journal.pone.0070838.
Eke, P. I., Page, R. C., Wei, L., Thornton-Evans, G., & Genco, R. J. (2012). Update of the case definitions for population-based surveillance of periodontitis. Journal of Periodontology, 83(12), 1449–1454. https://doi.org/10.1902/jop.2012.110664.
Emwas, A.-H.M., Salek, R. M., Griffin, J. L., & Merzaban, J. (2013). NMR-based metabolomics in human disease diagnosis: Applications, limitations, and recommendations. Metabolomics, 9(5), 1048–1072. https://doi.org/10.1007/s11306-013-0524-y.
Fidalgo, T. K. S., Freitas-Fernandes, L. B., Angeli, R., Muniz, A. M. S., Gonsalves, E., Santos, R., et al. (2013). Salivary metabolite signatures of children with and without dental caries lesions. Metabolomics, 9(3), 657–666. https://doi.org/10.1007/s11306-012-0484-7.
Figueira, J., Jonsson, P., Nordin Adolfsson, A., Adolfsson, R., Nyberg, L., & Öhman, A. (2016). NMR analysis of the human saliva metabolome distinguishes dementia patients from matched controls. Molecular Biosystems, 12(8), 2562–2571. https://doi.org/10.1039/c6mb00233a.
García-Villaescusa, A., Morales-Tatay, J. M., Monleón-Salvadó, D., González-Darder, J. M., Bellot-Arcis, C., Montiel-Company, J. M., et al. (2018). Using NMR in saliva to identify possible biomarkers of glioblastoma and chronic periodontitis. PLoS ONE. https://doi.org/10.1371/journal.pone.0188710.
Gardner, A., Parkes, H. G., So, P.-W., & Carpenter, G. H. (2019). Determining bacterial and host contributions to the human salivary metabolome. Journal of Oral Microbiology, 11(1), 1617014. https://doi.org/10.1080/20002297.2019.1617014.
Gardner, A., Carpenter, G., & So, P. W. (2020). Salivary metabolomics: From diagnostic biomarker discovery to investigating biological function. Metabolites. https://doi.org/10.3390/metabo10020047.
Gawron, K., Wojtowicz, W., Lazarz-Bartyzel, K., Lamasz, A., Qasem, B., Mydel, P., et al. (2019). metabolomic status of the oral cavity in chronic periodontitis. In Vivo, 33(4), 1165–1174. https://doi.org/10.21873/invivo.11587.
Gertsman, I., & Barshop, B. A. (2018). Promises and pitfalls of untargeted metabolomics. Journal of Inherited Metabolic Disease, 41(3), 355–366. https://doi.org/10.1007/s10545-017-0130-7.
Gluud, C., & Gluud, L. L. (2005). Evidence based diagnostics. British Medical Journal (Clinical research ed.), 330(7493), 724–726. https://doi.org/10.1136/bmj.330.7493.724.
Hao, J., Liebeke, M., Sommer, U., Viant, M. R., Bundy, J. G., & Ebbels, T. M. (2016). Statistical correlations between NMR spectroscopy and direct infusion FT-ICR mass spectrometry aid annotation of unknowns in metabolomics. Analytical Chemistry, 88(5), 2583–2589. https://doi.org/10.1021/acs.analchem.5b02889.
Huang, Y., Zhu, M., Li, Z., Sa, R., Chu, Q., Zhang, Q., et al. (2014). Mass spectrometry-based metabolomic profiling identifies alterations in salivary redox status and fatty acid metabolism in response to inflammation and oxidative stress in periodontal disease. Free Radical Biology and Medicine, 70, 223–232. https://doi.org/10.1016/j.freeradbiomed.2014.02.024.
Hung, J. H. (2013). Gene Set/Pathway enrichment analysis. Methods in Molecular Biology, 939, 201–213. https://doi.org/10.1007/978-1-62703-107-3_13.
Jaedicke, K. M., Preshaw, P. M., & Taylor, J. J. (2016). Salivary cytokines as biomarkers of periodontal diseases. Periodontology 2000, 70(1), 164–183. https://doi.org/10.1111/prd.12117.
Kc, S., Wang, X. Z., & Gallagher, J. E. (2020). Diagnostic sensitivity and specificity of host-derived salivary biomarkers in periodontal disease amongst adults: Systematic review. Journal of Clinical Periodontology, 47(3), 289–308. https://doi.org/10.1111/jcpe.13218.
Kim, J. J., Kim, C. J., & Camargo, P. M. (2013). Salivary biomarkers in the diagnosis of periodontal diseases. Journal of the California Dental Association, 41(2), 119–124.
Kinney, J. S., Morelli, T., Braun, T., Ramseier, C. A., Herr, A. E., Sugai, J. V., et al. (2011). Saliva/pathogen biomarker signatures and periodontal disease progression. Journal of Dental Research, 90(6), 752–758. https://doi.org/10.1177/0022034511399908.
Kornman, K. S., Giannobile, W. V., & Duff, G. W. (2017). Quo vadis: What is the future of periodontics? How will we get there? Periodontology, 2000(75), 353–371. https://doi.org/10.1111/prd.12217.
Korte, D. L., & Kinney, J. (2016). Personalized medicine: an update of salivary biomarkers for periodontal diseases. Periodontology 2000, 70(1), 26–37. https://doi.org/10.1111/prd.12103.
Kuboniwa, M., Sakanaka, A., Hashino, E., Bamba, T., Fukusaki, E., & Amano, A. (2016). Prediction of periodontal inflammation via metabolic profiling of saliva. Journal of Dental Research, 95(12), 1381–1386. https://doi.org/10.1177/0022034516661142.
Lee, A., Ghaname, C. B., Braun, T. M., Sugai, J. V., Teles, R. P., Loesche, W. J., et al. (2012). Bacterial and salivary biomarkers predict the gingival inflammatory profile. Journal of Periodontology, 83(1), 79–89. https://doi.org/10.1902/jop.2011.110060.
Liebsch, C., Pitchika, V., Pink, C., Samietz, S., Kastenmüller, G., Artati, A., et al. (2019). The saliva metabolome in association to oral health status. Journal of Dental Research, 98(6), 642–651. https://doi.org/10.1177/0022034519842853.
Liu, J., Wang, Y., Meng, H., Yu, J., Lu, H., Li, W., et al. (2019). Butyrate rather than LPS subverts gingival epithelial homeostasis by downregulation of intercellular junctions and triggering pyroptosis. Journal of Clinical Periodontology, 46(9), 894–907. https://doi.org/10.1111/jcpe.13162.
Lumbreras, B., Porta, M., Márquez, S., Pollán, M., Parker, L. A., & Hernández-Aguado, I. (2008). QUADOMICS: An adaptation of the quality assessment of diagnostic accuracy assessment (QUADAS) for the evaluation of the methodological quality of studies on the diagnostic accuracy of ’-omics’-based technologies. Clinical Biochemistry, 41(16–17), 1316–1325. https://doi.org/10.1016/j.clinbiochem.2008.06.018.
Lyman, G. H., & Moses, H. L. (2016). Biomarker tests for molecularly targeted therapies—the key to unlocking precision medicine. New England Journal of Medicine, 375(1), 4–6. https://doi.org/10.1056/NEJMp1604033.
Marchesan, J. T., Morelli, T., Moss, K., Barros, S. P., Ward, M., Jenkins, W., et al. (2015). Association of synergistetes and cyclodipeptides with periodontitis. Journal of Dental Research, 94(10), 1425–1431. https://doi.org/10.1177/0022034515594779.
Marsh, P. D., & Zaura, E. (2017). Dental biofilm: ecological interactions in health and disease. Journal of Clinical Periodontology, 44(Suppl 18), S12–S22. https://doi.org/10.1111/jcpe.12679.
Mikkonen, J. J., Singh, S. P., Herrala, M., Lappalainen, R., Myllymaa, S., & Kullaa, A. M. (2016). Salivary metabolomics in the diagnosis of oral cancer and periodontal diseases. Journal of Periodontal Research, 51(4), 431–437. https://doi.org/10.1111/jre.12327.
Mueller, D. C., Piller, M., Niessner, R., Scherer, M., & Scherer, G. (2014). Untargeted metabolomic profiling in saliva of smokers and nonsmokers by a validated GC-TOF-MS method. Journal of Proteome Research, 13(3), 1602–1613. https://doi.org/10.1021/pr401099r.
Murr, C., Grammer, T. B., Meinitzer, A., Kleber, M. E., März, W., & Fuchs, D. (2014). Immune activation and inflammation in patients with cardiovascular disease are associated with higher phenylalanine to tyrosine ratios: The Ludwigshafen risk and cardiovascular health study. Journal of Amino Acids, 2014, 783730. https://doi.org/10.1155/2014/783730.
Nicholson, J. K., Holmes, E., Kinross, J. M., Darzi, A. W., Takats, Z., & Lindon, J. C. (2012). Metabolic phenotyping in clinical and surgical environments. Nature, 491(7424), 384–392. https://doi.org/10.1038/nature11708.
Nie, A., Sun, B., Fu, Z., & Yu, D. (2019). Roles of aminoacyl-tRNA synthetases in immune regulation and immune diseases. Cell Death & Disease, 10(12), 901. https://doi.org/10.1038/s41419-019-2145-5.
Nociti, F. H., Jr., Casati, M. Z., & Duarte, P. M. (2015). Current perspective of the impact of smoking on the progression and treatment of periodontitis. Periodontology 2000, 67(1), 187–210. https://doi.org/10.1111/prd.12063.
Okuma, N., Saita, M., Hoshi, N., Soga, T., Tomita, M., Sugimoto, M., et al. (2017). Effect of masticatory stimulation on the quantity and quality of saliva and the salivary metabolomic profile. PLoS ONE, 12(8), e0183109. https://doi.org/10.1371/journal.pone.0183109.
Papageorgiou, S. N., Hagner, M., Nogueira, A. V., Franke, A., Jäger, A., & Deschner, J. (2017). Inflammatory bowel disease and oral health: Systematic review and a meta-analysis. Journal of Clinical Periodontology, 44(4), 382–393. https://doi.org/10.1111/jcpe.12698.
Papapanou, P. N., Sanz, M., Buduneli, N., Dietrich, T., Feres, M., Fine, D. H., et al. (2018). Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. Journal of Clinical Periodontology, 45(Suppl 20), S162–S170. https://doi.org/10.1111/jcpe.12946.
Parthasarathy, A., Cross, P. J., Dobson, R. C. J., Adams, L. E., Savka, M. A., & Hudson, A. O. (2018). A three-ring circus: Metabolism of the three proteogenic aromatic amino acids and their role in the health of plants and animals. Frontiers in Molecular Biosciences, 5, 29–29. https://doi.org/10.3389/fmolb.2018.00029.
Patti, G. J., Tautenhahn, R., & Siuzdak, G. (2012). Meta-analysis of untargeted metabolomic data from multiple profiling experiments. Nature Protocols, 7(3), 508–516. https://doi.org/10.1038/nprot.2011.454.
Pereira, J. L., Duarte, D., Carneiro, T. J., Ferreira, S., Cunha, B., Soares, D., et al. (2019). Saliva NMR metabolomics: Analytical issues in pediatric oral health research. Oral Diseases, 25(6), 1545–1554. https://doi.org/10.1111/odi.13117.
Potempa, J., Banbula, A., & Travis, J. (2000). Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontology 2000, 24, 153–192. https://doi.org/10.1034/j.1600-0757.2000.2240108.x.
Pradeep, A. R., Kumar, M. S., Ramachandraprasad, M. V., & Shikha, C. (2007). Gingival crevicular fluid levels of neopterin in healthy subjects and in patients with different periodontal diseases. Journal of Periodontology, 78(10), 1962–1967. https://doi.org/10.1902/jop.2007.070096.
Proctor, G. B. (2016). The physiology of salivary secretion. Periodontology 2000, 70(1), 11–25. https://doi.org/10.1111/prd.12116.
Ramseier, C. A., Kinney, J. S., Herr, A. E., Braun, T., Sugai, J. V., Shelburne, C. A., et al. (2009). Identification of pathogen and host-response markers correlated with periodontal disease. Journal of Periodontology, 80(3), 436–446. https://doi.org/10.1902/jop.2009.080480.
Roberts, L. D., Souza, A. L., Gerszten, R. E., & Clish, C. B. (2012). Targeted metabolomics. Current Protocols in Molecular Biology, 98, 30.2.1–30.2.24. https://doi.org/10.1002/0471142727.mb3002s98.
Romandini, M., Laforí, A., Romandini, P., Baima, G., & Cordaro, M. (2018). Periodontitis and platelet count: A new potential link with cardiovascular and other systemic inflammatory diseases. Journal of Clinical Periodontology, 45(11), 1299–1310. https://doi.org/10.1111/jcpe.13004.
Romano, F., Meoni, G., Manavella, V., Baima, G., Tenori, L., Cacciatore, S., et al. (2018). Analysis of salivary phenotypes of generalized aggressive and chronic periodontitis through nuclear magnetic resonance-based metabolomics. Journal of Periodontology, 89(12), 1452–1460. https://doi.org/10.1002/jper.18-0097.
Romano, F., Meoni, G., Manavella, V., Baima, G., Mariani, G. M., Cacciatore, S., et al. (2019). Effect of non-surgical periodontal therapy on salivary metabolic fingerprint of generalized chronic periodontitis using nuclear magnetic resonance spectroscopy. Archives of Oral Biology, 97, 208–214. https://doi.org/10.1016/j.archoralbio.2018.10.023.
Rzeznik, M., Triba, M. N., Levy, P., Jungo, S., Botosoa, E., Duchemann, B., et al. (2017). Identification of a discriminative metabolomic fingerprint of potential clinical relevance in saliva of patients with periodontitis using 1H nuclear magnetic resonance (NMR) spectroscopy. PLoS ONE, 12(8), e0182767. https://doi.org/10.1371/journal.pone.0182767.
Sakanaka, A., Kuboniwa, M., Hashino, E., Bamba, T., Fukusaki, E., & Amano, A. (2017). Distinct signatures of dental plaque metabolic byproducts dictated by periodontal inflammatory status. Scientific Reports, 7, 42818. https://doi.org/10.1038/srep42818.
Shamseer, L., Moher, D., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. British Medical Journal, 349, g7647. https://doi.org/10.1136/bmj.g7647.
Shirasugi, M., Nakagawa, M., Nishioka, K., Yamamoto, T., Nakaya, T., & Kanamura, N. (2018). Relationship between periodontal disease and butyric acid produced by periodontopathic bacteria. Inflammation and Regeneration, 38, 23. https://doi.org/10.1186/s41232-018-0081-x.
Singh, M. P., Saxena, M., Saimbi, C. S., Arif, J. M., & Roy, R. (2017). Metabolic profiling by 1H NMR spectroscopy of saliva shows clear distinction between control and diseased case of periodontitis. Metabolomics. https://doi.org/10.1007/s11306-017-1245-4.
Su, W., Shi, J., Zhao, Y., Yan, F., Lei, L., & Li, H. (2020). Porphyromonas gingivalis triggers inflammatory responses in periodontal ligament cells by succinate-succinate dehydrogenase–HIF–1α axis. Biochemical and Biophysical Research Communications, 522(1), 184–190. https://doi.org/10.1016/j.bbrc.2019.11.074.
Sugimoto, M., Wong, D. T., Hirayama, A., Soga, T., & Tomita, M. (2010). Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics, 6(1), 78–95. https://doi.org/10.1007/s11306-009-0178-y.
Sun, Y., Gao, H. Y., Fan, Z. Y., He, Y., & Yan, Y. X. (2020). Metabolomics signatures in type 2 diabetes: A systematic review and integrative analysis. The Journal of Clinical Endocrinology & Metabolism. https://doi.org/10.1210/clinem/dgz240.
Takeda, I., Stretch, C., Barnaby, P., Bhatnager, K., Rankin, K., Fu, H., et al. (2009). Understanding the human salivary metabolome. NMR in Biomedicine, 22(6), 577–584. https://doi.org/10.1002/nbm.1369.
Tervahartiala, T., Pirilä, E., Ceponis, A., Maisi, P., Salo, T., Tuter, G., et al. (2000). The in vivo expression of the collagenolytic matrix metalloproteinases (MMP-2, -8, -13, and -14) and matrilysin (MMP-7) in adult and localized juvenile periodontitis. Journal of Dental Research, 79(12), 1969–1977. https://doi.org/10.1177/00220345000790120801.
Tonetti, M. S., Greenwell, H., & Kornman, K. S. (2018). Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. Journal of Clinical Periodontology, 45(Suppl 20), S149-s161. https://doi.org/10.1111/jcpe.12945.
Tonetti, M. S., Jepsen, S., Jin, L., & Otomo-Corgel, J. (2017). Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. Journal of Clinical Periodontology, 44(5), 456–462. https://doi.org/10.1111/jcpe.12732.
Wallner-Liebmann, S., Tenori, L., Mazzoleni, A., Dieber-Rotheneder, M., Konrad, M., Hofmann, P., et al. (2016). Individual human metabolic phenotype analyzed by (1)H NMR of saliva samples. Journal of Proteome Research, 15(6), 1787–1793. https://doi.org/10.1021/acs.jproteome.5b01060.
Wei, J., Xie, G., Zhou, Z., Shi, P., Qiu, Y., Zheng, X., et al. (2011). Salivary metabolite signatures of oral cancer and leukoplakia. International Journal of Cancer, 129(9), 2207–2217. https://doi.org/10.1002/ijc.25881.
Wishart, D. S. (2007). Current progress in computational metabolomics. Briefings in Informatics, 8(5), 279–293. https://doi.org/10.1093/bib/bbm030.
Wishart, D. S. (2019). NMR metabolomics: A look ahead. Journal of Magnetic Resonance, 306, 155–161. https://doi.org/10.1016/j.jmr.2019.07.013.
Wishart, D. S., Feunang, Y. D., Marcu, A., Guo, A. C., Liang, K., Vázquez-Fresno, R., et al. (2017). HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Research, 46(D1), D608–D617. https://doi.org/10.1093/nar/gkx1089.
Wolff, L. F., Smith, Q. T., Snyder, W. K., Bedrick, J. A., Liljemark, W. F., Aeppli, D. A., et al. (1988). Relationship between lactate dehydrogenase and myeloperoxidase levels in human gingival crevicular fluid and clinical and microbial measurements. Journal of Clinical Periodontology, 15(2), 110–115. https://doi.org/10.1111/j.1600-051x.1988.tb01003.x.
Yilmaz, A., Geddes, T., Han, B., Bahado-Singh, R. O., Wilson, G. D., Imam, K., et al. (2017). Diagnostic biomarkers of Alzheimer’s disease as identified in saliva using 1H NMR-based metabolomics. Journal of Alzheimers Disease, 58(2), 355–359. https://doi.org/10.3233/jad-161226.
Zhang, Y., Sun, J., Lin, C. C., Abemayor, E., Wang, M. B., & Wong, D. T. (2016). The emerging landscape of salivary diagnostics. Periodontology 2000, 70(1), 38–52. https://doi.org/10.1111/prd.12099.
Acknowledgements
The authors would like to acknowledge special gratitude to Gaia Meoni, Leonardo Tenori and the CERM institute of Florence for providing missing data and technical support for writing the manuscript.
Funding
This research was not supported by any fundings.
Author information
Authors and Affiliations
Contributions
GB, GI and MA made substantial contributions to conception of the study. GB, NB, FC and FR contributed to the study design. GI, SG, and GB searched and collected the data. GB and GNB performed data processing and interpretation. GB and NB prepared the first draft of the manuscript. All authors have read, revised critically, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baima, G., Iaderosa, G., Citterio, F. et al. Salivary metabolomics for the diagnosis of periodontal diseases: a systematic review with methodological quality assessment. Metabolomics 17, 1 (2021). https://doi.org/10.1007/s11306-020-01754-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-020-01754-3