Abstract
Background
Periodontitis is resulted from a complex interaction between genetics and epigenetics, microbial factors, and the host response. Metabolomics analyses reflect both the steady-state physiological equilibrium of cells or organisms as well as their dynamic metabolic responses to environmental stimuli.
Aim of review
This systematic review of the literature aimed to assess which low molecular weight metabolites are more often found in biological fluids of individuals with periodontitis compared to individuals with gingivitis or periodontal health.
Key scientific concepts of review
All the included studies employed untargeted analysis. One or more biological fluids were analyzed, including saliva (n = 14), gingival crevicular fluid (n = 6), mouthwash (n = 1), serum (n = 3) and plasma (n = 1). Fifty-six main metabolites related to periodontitis have been identified in at least two independent studies by NMR spectroscopy or MS-based metabolomics. Saliva was the main biological fluid sampled. It is noteworthy that 14 metabolites of the 56 detected were identified as main metabolites in all studies that sampled the saliva. The majority of metabolites found consistently among studies were amino acids, organic acids and derivates: acetate, alanine, butyrate, formate, GABA, lactate, propionate, phenylalanine and valine. They were either up- or down-regulated in the studies or this information was not mentioned. The main metabolic pathway was related to phenylalanine, tyrosine and tryptophan biosynthesis. Metabolites more frequently found in individuals with periodontitis were related to both the host and to microorganism responses. Future studies are needed, and they should follow some methodological standards to facilitate their comparison.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Periodontal diseases are a chronic inflammatory condition and involve multiple causal components that play a role simultaneously, and interact with each other, in an unpredictable way (Loos & Van Dyke, 2020). This condition is the result of a complex interaction between genetics and epigenetics, microbial factors and the host response (Loos & Van Dyke, 2020) and can be divided into gingivitis and periodontitis. Gingivitis is considered an incipient dysbiosis because in non-susceptible individuals it does not progress beyond inflammatory signs in the gingival tissue. In contrast, periodontitis is a frank dysbiosis that perpetuates chronic non-resolving and destructive inflammation due to an aberrant host response (Meyle & Chapple, 2015). Periodontitis involves the destruction of the periodontal ligament, bone, and gingival tissues, and can lead to tooth loss. Periodontitis behaves in a nonlinear fashion. It has been established that the disease can involve bursts of activity followed by periods of quiescence or stability, which are not easily measured clinically (Loos & Van Dyke, 2020). For oral health-care clinicians, there can be a level of uncertainty in predicting successful periodontal site-specific treatment outcomes and stability (Korte & Kinney, 2016). Thus, the need exists for supplemental diagnostic tools (Korte & Kinney, 2016).
Personalized medicine is a medical model that uses genetic, genomic, environmental and clinical diagnostic testing to individualize patient care (Nguyen et al., 2020). This approach utilizes clinical assessment and subclinical profiles to develop highly individualized diagnosis, prognosis and treatment algorithms (Korte & Kinney, 2016). Personalized medicine for periodontal diseases may involve utilization of biological fluids such as gingival crevicular fluid (GCF) and saliva to develop subclinical profiles, identifying and measuring specific genotypes, phenotypes, putative pathogens, inflammatory markers and collagen-degradation biomarkers to make informed clinical decisions about disease susceptibility, site-specific risk and treatment interventions (Korte & Kinney, 2016). When considering the periodontal pathogenic processes, periodontitis can generally be divided into three phases: inflammation; connective tissue degradation; and bone turnover. During each phase of the disease, specific host-derived biomarkers have been identified and therefore might provide a general sense of what stage of pathologic breakdown the patient is currently experiencing (Korte & Kinney, 2016).
Metabolomics is emerging as an important tool to characterize patient phenotypes in parallel with other -omics platforms. Metabolomics analyses reflect both the steady-state physiological equilibrium of cells or organisms as well as their dynamic metabolic responses to environmental stimuli. In comparison to other human biological measurements, the metabolome is uniquely suited to depict the phenotype and measure the impact of environmental factors on the end products of metabolism (Tolstikov et al., 2020). Changes in metabolite composition are important in understanding the microbial–host response in periodontal disease (Nguyen et al., 2020). Identification of metabolites as potential biomarkers of periodontal disease status or pathogenesis is of interest to understand the metabolic mechanisms underlying periodontal disease and to target patient treatment. Changes in metabolite composition associated with states of disease may allow the identification of metabolic biomarkers that can be used in a variety of applications, including early disease detection, evaluation of current disease status, and examination of pathways triggered or altered in the diseased state (Almeida et al., 2017; de Oliveira et al., 2016; Fidalgo et al., 2013, 2015; Freitas-Fernandes et al., 2020; Nguyen et al., 2020).
The challenges associated with metabolomic studies in periodontal disease include the high variability that exists in the chemical structure and properties of the metabolites (Nguyen et al., 2020). Metabolic biomarkers of periodontal disease have been identified via the analysis of saliva, serum, plaque, and GCF (Nguyen et al., 2020). Such metabolic phenotyping studies are able to provide new insights into disease pathophysiology (Beger et al., 2016). The objective of the present systematic review was to summarize the existing information about human biofluids metabolomic findings in periodontal disease.
2 Material and methods
2.1 Protocol and registration
This review adhered to the PRISMA statement (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidelines (Moola et al., 2020). This systematic review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO), registration number: CRD42021253486.
2.2 Search strategy
The search procedures were performed independently by two examiners (F.B. and H.F.). The electronic databases PubMed/Medline, SCOPUS, EMBASE, ISI Web of Knowledge and Google-Scholar were searched for studies published until November 2020, without language or year restrictions. The electronic search strategy was developed using the most cited descriptors in previous publications on this theme, combining Medical Subject Heading terms (MeSH) and free terms as title/abstract (tiab). Initially the search strategy was developed for PubMed and then adapted to the other databases. The following MeSH and tiab terms were used to search PubMed: (“Metabolome”, “Metabolomics”), “periodontitis”, periodontal disease, “humans”. The boolean operators “AND” and “OR” were applied to combine the terms and create the search strategy. The search strategies defined for each database are detailes in the Open Science Framework reppository (https://doi.org/10.17605/OSF.IO/ZK5HE). No filters or limits were applied in the searches. Disagreements related to the inclusion of studies were resolved via discussion and consultation with the third author (T.K.S.F.). A complementary screening of the references in the selected studies was performed and a hand search in the Journal of Periodontology, Metabolomics, Journal Clinical Periodontology and Periodontology 2000 was performed to find any additional studies that did not appear in the primary database search. Articles from different sources were imported to the EndNote Web reference manager (EndNote™), to catalog the references and automatically remove duplicate records. Duplicate studies in the database search were considered only once.
2.3 Eligibility criteria
Human studies that evaluated the low molecular weight metabolites of subjects with and without periodontal disease were included without age restriction. The criteria adopted for the eligibility of the studies to be included in this research were defined based on the elements of the PECOS strategy, as follows:
P (Population)—Biological fluids
E (Exposure)—Periodontitis
C (Comparison)—Gingivitis or periodontal health
O (Outcome)—Low molecular weight metabolites analyzed through metabolomic techniques
S (Study design)—Cross-sectional, prospective, parallel, and crossover designs
2.4 Exclusion criteria
Articles, or at least the abstract, must have been written in English. Conference abstracts, reviews, meta-analyses, case reports, ecological studies, and letters to the editor were excluded.
Patients with periodontal disease and other systemic diseases other than diabetes mellitus were excluded from the study. Diabetes was not excluded since is an important modifying factor of periodontitis, and according to the New Classification of Periodontal Diseases (Caton et al., 2018), diabetes should be included in a clinical diagnosis of periodontitis as a descriptor since there are no characteristic phenotypic features that are unique to periodontitis in patients with diabetes mellitus (Jepsen et al., 2018).
2.5 Data extraction and metabolic pathway
Two authors (F.B. and H.F.) independently collected the data from the included studies. Eventual disagreements were resolved consensually, and if the disagreement remained, a third experienced author made a decision (T.K.S.F.). Information regarding publication (author and publication year), study design, methods, statistical analysis, source of biological sample, number of subjects in the study, oral condition, number of metabolites detected, main metabolites that presented statistical difference, and perturbed pathways was identified. In the case of missing data, up to three attempts to contact the respective authors were made by email.
Based on the list of the most frequently found metabolites identified in the manuscripts, a pathway analysis was performed using Metaboanalyst 4.0. The following metabolites that presented statistical difference in saliva between groups were included in the analysis: butyrate, choline, ethanol, isopropanol and methanol, acetate, alanine, butyrate, formate, GABA, lactate, propionate, phenylalanine and valine. The pathway analysis was based on saliva since it is easily collected by untrained individuals, it is painless, and especially because the main metabolites found in saliva were not discrepant among the studies. The pathway analysis was obtained using the input type “compound name”, the visualization method was scatter plot, the enrichment method was hypergeometric test, the topology analysis was relative-betweenness centrality, the reference metabolome was all compounds in the selected pathway library, and the pathway library was Homo sapiens (KEGG).
2.6 Quality assessment
The methodological quality of the studies was assessed by two independent evaluators (T.K.S.F. and F.B.) using the Joanna Briggs Institute (JBI) Critical Appraisal Tool for cross-sectional studies (Moola et al., 2020). The JBI tool consists of eight questions that address the following parameters: inclusion criteria, description of the population included, risk of bias, outcomes and statistical analysis. The following variables were considered:
-
Were the criteria for inclusion in the sample clearly defined?
Whether the inclusion/exclusion criteria were addressed in the studies.
-
Were the study subjects and the setting described in detail?
Whether the sample of participants was described with information such as age and gender.
-
Was the exposure measured in a valid and reliable way?
Whether the study clearly described which reference was followed to determine periodontal status.
-
Were objective, standard criteria used for measurement of the condition?
Whether the study mentioned how the exam was done and if there was training and calibration.
-
Were confounding factors identified?
Whether there were confounders such as the inclusion of diseases, patients who used antibiotics, pregnant women, smokers and even saliva collection at a standardized time due to the variation in the production of metabolites related to the circadian cycle of saliva.
-
Were strategies to deal with confounding factors stated?
Whether strategies were used to deal with the confounders, such as multivariate analysis or separate analysis of data.
-
Were the outcomes measured in a valid and reliable way?
Whether the metabolomic evaluation methods were correct, that is, validated in the literature.
-
Was appropriate statistical analysis used?
Whether an adequate statistical analysis was performed.
The classification system was determined by the authors, judging each answer as a possible concern, with “No” being assigned for low risk and “Yes” for high risk. When at least one “high risk” response was attributed to studies, the study was classified as high risk of bias.
2.7 Strength of evidence
The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) tool was used to evaluate the strength of the evidence of the included studies (Guyatt et al., 2011). This tool assesses information about the overall included studies, such as the number of participants, the risk of bias, inconsistency, indirectness, imprecision, and publication bias. It allows the classification of the quality of the evidence as very low, low, moderate or high strength of evidence, based on the confounders present, in addition to the summary of the findings.
3 Results
Figure 1 shows the flow diagram of the search strategy. Initially, the search resulted in 353 studies published in the relevant databases and 6 studies were found in other sources. Of these, 114 were excluded due to duplication, 212 were excluded because they were not associated with the topic, and 13 were excluded because they did not satisfy the inclusion criteria. The analysis of titles and abstracts culminated in the selection of 21 suitable published studies (Tables 1, 2, 3).
3.1 Data extraction and metabolic pathway analysis
All the included studies employed untargeted analysis. Three studies did the assessment pre and post biofilm debridement (Kuboniwa et al., 2016; Romano et al., 2019; Sakanaka et al., 2017). According to the results reported by the authors, we divided the studies into three main categories. The first main category included studies in which metabolites were reported to be related to microorganisms (Table 1), the second main category included studies in which metabolites were reported to be related to the host response (Table 2) and the third main category included studies in which metabolites were reported to be related to both microorganisms and to the host response (Table 3).
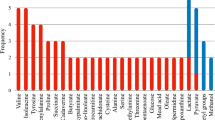
Identified metabolites that were considered as the main metabolite in at least two independent studies and their expression in periodontal disease (up or downregulated) are shown in Table 4. Figure 2 shows each metabolite’s super class (HMDB, 2022), the biological medium from which it was sampled and the type of response to which it was reported to be associated. Figure 3 shows the impacted pathway, the main metabolic pathway was related to phenylalanine, tyrosine and tryptophan biosynthesis. The following 6 metabolic pathways were related (Table 5): Aminoacyl-tRNA biosynthesis, Butanoate metabolism, Pyruvate metabolism, Glycolysis/Gluconeogenesis, Glyoxylate and dicarboxylate metabolism, and Phenylalanine, tyrosine and tryptophan biosynthesis.
Overview of salivary biofluid pathway analysis. The metabolic pathway analysis highlighted the main pathways. Aminoacyl-tRNA biosynthesis, Butanoate metabolism, Pyruvate metabolism, Glycolysis/Gluconeogenesis, Alanine, aspartate and glutamate metabolism, Glyoxylate and dicarboxylate metabolism, Phenylalanine, tyrosine and tryptophan biosynthesis
3.2 Assessment of the risk of bias
The 17 included studies presented a cross-sectional design (Tables 1, 2 and 3). Citterio et al., (2020) performed a treatment but only their baseline data was included in this systematic review. Similarly, Liebsch et al. (2019) data was part of a bigger cohort study; however, the authors collected the data without follow-up. The quality assessment and risk of bias demonstrated that six studies (Chen et al., 2018; Citterio et al., 2020; Kuboniwa et al., 2016; Pei et al., 2020; Sakanaka et al., 2017; Shi et al., 2020) presented a low risk of bias and 10 high risk (Aimetti et al., 2012; Barnes et al., 2009, 2011, 2014; Huang et al., 2014; Liebsch et al., 2019; Ozeki et al., 2016; Romano et al., 2018; Rzeznik et al., 2017; Singh et al., 2017) (Table 6).
The major concerns were the absence of a standard method, especially the absence of inter-individual assessment of the clinical measurements, and confounding, with the inclusion of patients with systemic diseases of smokers without controlling for these variables in the statistical analysis. Ozeki et al. (2016), Sakanaka et al. (2017) and Singh et al. (2017) did not mention their inclusion criteria, which was considered a concern. Ozeki et al. (2016) and Barnes et al. (2009) did not mention the reference used for classification of the periodontal condition. Romano et al. (2018) and Rzeznik et al. (2017) included smokers, but controlled for this in the statistical analysis. Liebsch et al. (2019) did not mention whether they excluded patients with comorbidities, smokers, those taking antibiotics etc. Barnes et al. (2014) included patients with diabetes, but considered this condition in the statistical analysis; they did not report the cigarette smoking data. Aimetti et al. (2012) included systemic diseases in the analysis such as hypertension, heart vessel alterations, Parkinson’s disease, chronic bronchitis, diabetes, and osteoporosis. Barnes et al. (2009) did not mention any exclusions related to smokers.
3.3 Strength of evidence
The strength of evidence was rated very low (Table 7). Regarding the "risk of bias" domain, there was a downgrade due to the presence of confounders, such as the inclusion of patients with systemic diseases and smokers. The “inconsistency” domain was not downgraded, since the results found were consistent among the different studies. The “indirect” domain was not downgraded since the studied population was compatible with the groups. The "imprecision" domain was downgraded because the studies had samples smaller than 300 individuals.
4 Discussion
The aim of the current review was to conduct an in-depth analysis of metabolomic approaches to determine the metabolomic profile of periodontitis. It revealed 56 main metabolites related to periodontitis that have been identified in at least two independent studies by HNM or MS-based metabolomics, using untargeted approaches. Due to the large number of metabolites detected, herein we discuss the most commonly detected metabolites, focusing on the biological medium in which they were sampled.
The studies varied considerably in terms of total number of participants and even more in the number of individuals regarding their periodontal diagnosis. The main focus of the studies was to compare periodontitis patients with healthy controls, and some assessed whether the severity of periodontitis could, in any way, influence metabolites (Kuboniwa et al., 2016; Sakanaka et al., 2017; Singh et al., 2017). Four studies compared the two previous extinctic forms of periodontitis, aggressive periodontitis and chronic periodontitis (Aimetti et al., 2012; Romano et al., 2018; Rzeznik et al., 2017; Shi et al., 2020), and the results showed that the metabolic profiles were similar. Three studies assessed only periodontitis patients without a control group (Barnes et al., 2009, 2010; Kuboniwa et al., 2016). In general, this heterogeneity prevented us comparing the results regarding periodontal disease diagnosis.
Among the six studies that reported metabolites that were related to microorganisms, three studies used the NMR spectroscopy by 1H-NMR method (Gawron et al., 2019; Rzeznik et al., 2017; Singh et al., 2017) and three used the GC–MS method (Kuboniwa et al., 2016; Sakanaka et al., 2017; Shi et al., 2020). Among the 11 studies that reported metabolites that were related to the host response, four studies used the NMR spectroscopy by 1H-NMR method (Aimetti et al., 2012; Citterio et al., 2020; Romano et al., 2018, 2019) and seven used different MS methods (Barnes et al., 2009, 2010, 2011, 2014; Elabdeen et al., 2013; Huang et al., 2014; Ozeki et al., 2016). All four studies that reported metabolites that were related to both microorganisms and the host response used different methods of MS (Chen et al., 2018; Liebsch et al., 2019; Marchesan et al., 2015; Pei et al., 2020). Indeed, these two platforms are the most common analytical platforms used in metabolomics (Klupczyńska et al., 2015). It has been observed that the biomarkers indicated by NMR spectroscopy and MS techniques differ, which suggests the complementary nature of these two analytical platforms (Klupczyńska et al., 2015). It seems that the results of this review corroborate this statement. The studies that the heat map matched most were Aimetti et al. (2012), Citterio et al. (2020), Gawron et al. (2019), Romano et al. (), Rzenik et al. (2017) and Singh et al. (2017). All these studies used analytical NMR spectroscopy, mostly 1H-NMR.
Metabolite profiling was the metabolomic approach most employed in the studies included in this review. Metabolite profiling concentrates on the analysis of a wide range of metabolites (for example, amino acids, sugars, lipids, bile acids) and compounds of potential interest that are not known a priori. This approach aims to identify as many compounds as possible, as well as to quantify them (Klupczyńska et al., 2015). This strategy enables the detection of changes in unexpected parts of the metabolome and such changes can be attributed to specific metabolic pathways. Thus, metabolite profiling often leads to the formulation of new scientific hypotheses and the identification of novel metabolic biomarkers (Klupczyńska et al., 2015), which is the main aim in regard to metabolomics in periodontology. Half of the studies in this review identified perturbed pathways (Barnes et al., 2009, 2011, 2014; Chen et al., 2018; Elabdeen et al., 2013; Huang et al., 2014; Liebsch et al., 2019; Pei et al., 2020; Romano et al., 2018; Shi et al., 2020). The pathways identified were mostly related to oxidative stress, purine and the metabolism of pyrimidine and arachidonic acid.
Another common finding among the studies that the heat map matched most (Aimetti et al., 2012; Gawron et al., 2019; Romano et al., 2018, 2019; Rzeznik et al., 2017; Singh et al., 2019) was that saliva was the biological medium sampled (in six out of seven studies). In only one study the saliva was stimulated (Rzeznik et al., 2017) while in the other five studies unstimulated saliva was sampled (Aimetti et al., 2012; Citterio et al., 2020; Romano et al., 2018, 2019; Singh et al., 2019). Gawron et al. (2019) obtained the sample from mouth washout and tongue swab. Jo et al. (2019) demonstrated a very high correlation with the microbiome composition from mouth rinse water, stimulated and unstimulated saliva and suggested that mouth rinse water or mouth washout is a suitable collection method instead of saliva for oral microbiome analysis. To date, no comparison has been made for the oral metabolome but the findings of the above studies suggest the same tendency, as the metabolites from mouth washout were similar to those obtained from the saliva. Interestingly, four of these studies reported that their results were more related to the host response (Aimetti et al., 2012; Citterio et al., 2020; Romano et al., 2018, 2019) while the three other studies reported that their results were more related to the microorganisms’ response (Gawron et al., 2019; Rzeznik et al., 2017; Singh et al., 2019), although the main metabolites were coincident. Except for butyrate, ethanol, and methanol, all the following metabolites were coincident among the studies: acetate, alanine, butyrate, formate, lactate, propionate and phenylalanine (Aimetti et al., 2012; Gawron et al., 2019; Romano et al., 2018, 2019; Rzeznik et al., 2017; Singh et al., 2019). They were either up- or down-regulated in these studies, in some of them this information was not mentioned. In general, these metabolites are related to oxidative stress.
Phenylalanine was highlighted as the main metabolite in ten studies in different biological mediums: unstimulated saliva (Aimetti et al., 2012; Barnes et al., 2011; Citterio et al., 2020; Romano et al., 2018, 2019; Singh et al., 2019), stimulated saliva (Rzeznik et al., 2017; Sakanaka et al., 2017) blood and GCF (Chen et al., 2018) and GCF (Barnes et al., 2010; Ozeki et al., 2016). In five of these studies this metabolite was increased (Aimetti et al., 2012; Barnes et al., 2010, 2011; Ozeki et al., 2016; Romano et al., 2018). Phenylalanine is an essential amino acid that is highly concentrated in the human brain and plasma (Blau et al., 2010; Pilotto et al., 2021; Waisbren et al., 2007).
The second most reported main metabolite in seven and six studies respectively was valine and succinate. Valine is an essential branched-chain amino acid (BCAA) that is critical to human life and is particularly involved in stress, energy and muscle metabolism. Succinic acid is also a microbial metabolite that is produced by Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Enterobacter, Acinetobacter, Proteus mirabilis, Citrobacter frundii, and Enterococcus faecalis. Succinic acid is also found in Actinobacillus, Anaerobiospirillum, Mannheimia, Corynebacterium and Basfia. Succinate was increased in three studies (Aimetti et al., 2012; Gawron et al., 2019; Romano et al., 2018) whereas valine was increased in four studies (Aimetti et al., 2012; Romano et al., 2018; Shi et al., 2020; Singh et al., 2019) and decreased in one (Gawron et al., 2019).
Propionate was reported in six studies as one of the main metabolites (Table 4) whereas acetate and butyrate were reported in five studies as one of the main metabolites (Table 4). Propionic acid (PA) is an end-product of the microbial digestion of carbohydrates. It is a metabolite of Bacteroides, Clostridium, Dialister, Megasphaera, Phascolarctobacterium, Propionibacterium, Propionigenum, Salmonella, Selenomonas and Veillonella. Butyrate is produced as an end-product of a fermentation process solely performed by obligate anaerobic bacteria (Rivière et al., 2016). Acetate is an ionic form from acetic acid, one of the simplest carboxylic acids. The acetyl group, derived from acetic acid, is fundamental to the biochemistry of virtually all forms of life. When bound to coenzyme A it is central to the metabolism of carbohydrates and fats. Acetic acid is produced and excreted by certain bacteria, notably the Acetobacter genus and Clostridium acetobutylicum. Urinary acetic acid is produced by Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Enterobacter, Acinetobacter, Proteus mirabilis, Citrobacter frundii, Enterococcus faecalis, Streptococcus group B, and Staphylococcus saprophyticus. Acetic acid is also found in Akkermansia, Bacteroidetes, Bifidobacterium, Prevotella and Ruminococcus. Butyrate has diverse and apparently paradoxical effects on cellular proliferation, apoptosis and differentiation. It is produced as an end-product of a fermentation process solely performed by obligate anaerobic bacteria. It is a metabolite of Anaerostipes, Coprococcus, Eubacterium, Faecalibacterium and Roseburia (Duncan et al., 2002).
When analyzing studies in which the biological medium sampled was the GCF (Barnes et al., 2009, 2010; Ozeki et al., 2016; Pei et al., 2020; Shi et al., 2020), the most consistently reported metabolites were lysine and putrescine. Lysine is an essential amino acid. Lysine reduction can also affect immune system since there is a cellular proliferation role and ability to induce humoral and cell mediated immune responses (Datta et al., 2001). Putrescine is a polyamine. Putrescine is related to cadaverine,another polyamine. Both are produced by the breakdown of amino acids in living and dead organisms, and both are toxic in large doses. Putrescine can be found in Citrobacter, Corynebacterium, Cronobacter and Enterobacter (Wendisch, 2017). The GCF results reflect inflammatory processes at individual sites with the disease (Barros et al., 2016; Jaedicke et al., 2016; Nazar Majeed et al., 2016; Öngöz Dede et al., 2017; Wassall & Preshaw, 2016). Saliva results, on the other hand, reflect the inflammatory state of the total gingival fluid in the periodontal pockets of the entire mouth (Taylor & Preshaw, 2016). The results of this review demonstrate that the metabolites from the GCF and the metabolites from the saliva differ. More studies assessing both saliva and GCF simultaneously are necessary for metabolite profiling in periodontal disease, especially if the aim is to analyze the severity of the disease.
Studies in which the biological medium sampled was plasma or serum associated with other biological mediums compared serum and GCF (Chen et al., 2018), serum and unstimulated saliva (Huang et al., 2014), serum and unstimulated saliva and GCF (Elabdeen et al., 2013) and plasma and unstimulated saliva (Barnes et al., 2014). The only metabolites that were common to all studies were 12-HETE and 5-HETE. 12-hydroxyeicosatetraenoic acid, also known as 12-HETE, is an eicosanoid, a 5-lipoxygenase metabolite of arachidonic acid. 12-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid. 12-HETE is a neuromodulator that is synthesized during ischemia. 5-hydroxyeicosatetraenoic acid (5-HETE) is an endogenous eicosanoid. 5-HETE is an intermediate in arachidonic acid metabolism. It is also involved in the pathway of leukotriene synthesis (Fruteau de Laclos et al., 1984; Rodriguez-Lagunas et al., 2013). It is important to highlight that the main metabolites in GCF, serum and plasma did not correspond to metabolites in the saliva. This is an issue that should be better investigated in future studies.
As periodontitis is a complex chronic inflammatory disease, a question that arises is whether some metabolite biomarkers of other chronic inflammatory diseases can be detected in individuals with periodontitis. It was demonstrated that in diabetes, for example, phenylalanine, tyrosine, valine and isoleucine were upregulated and are considered metabolite biomarkers of diabetes (Barnes et al., 2014). Interestingly, these metabolites were reported as main metabolites in some studies included in this review. Ulven et al. (2019) identified metabolomic signatures in obese individuals and obesity-related metabolic alterations such as inflammation or oxidative stress that demonstrated the value of an integrative approach to the microbiome and metabolomics. In periodontology, based on the findings of the selected studies, this integrative approach might also help researchers to clarify and understand the interactions of the microbial metabolites with the host organism and to avoid misinterpretation.
It was noticed that the PLS-DA was used in studies with a small number of case–control individuals. Some caveats associated with PLS-DA that need to be considered when using this model are difficulties in the identification of small numbers of variables that are responsible for the separation between two or more groups (classes) and therefore a larger number of variables are required to achieve a good prediction accuracy, scores plot may present an overoptimistic view of the separation between the classes and there is a tendency to overfitting (Gromski et al., 2015). Mendez et al., (2019) suggested that for robust predictive models the most important consideration is statistical power (Mendez et al., 2019). The authors point out there is no magic formula for calculating the number of samples needed for robust metabolomics multivariate machine learning, where estimates are dependent on many factors, including: the dimensionality of the data, the strength of effect, the degree of covariance (strength of latent structure), the heterogeneity of the sample population, the repeatability of the measurement instrument, and the complexity of the model. As the number of individuals are not large in studies about metabolites in periodontal disease and many of it used PLS-DA analysis, we need to be careful to avoid over optimistic reporting of results. Thus, through this critical analysis of the literature, we have carefully to consider if the complete data set is a real representative sample of the biological question about periodontal disease. Studies with larger numbers of participating individuals (tests and controls) should be performed so that we have more consistent data on periodontal disease metabolites.
This systematic review identified 56 metabolites that were detected in at least two independent studies. It is noteworthy that 14 metabolites of the 56 detected were identified as main metabolites in all six studies that sampled the saliva and in the study that sampled the mouthwash and the tongue swab. However, these 14 metabolites were related to both the host and microorganism responses. This finding supports the use of saliva as a potential biofluid for monitoring this disease state. Finally, further long term longitudinal studies considering the analysis before and after periodontal treatment would benefit this knowledge area in order to confirm this findings.
References
Aimetti, M., Cacciatore, S., Graziano, A., & Tenori, L. (2012). Metabonomic analysis of saliva reveals generalized chronic periodontitis signature. Metabolomics, 8(3), 465–474.
Almeida, P. A., Fidalgo, T. K. S., Freitas-Fernandes, L. B., Almeida, F. C. L., Valente, A. P., & Souza, I. P. R. (2017). Salivary metabolic profile after hemodialysis among children and adolescents with chronic kidney disease. Metabolomics, 13, 141–150.
Barnes, V. M., Ciancio, S. G., Shibly, O., Xu, T., Devizio, W., Trivedi, H. M., Guo, L., & Jönsson, T. J. (2011). Metabolomics reveals elevated macromolecular degradation in periodontal disease. Journal of Dental Research, 90(11), 1293–1297.
Barnes, V. M., Kennedy, A. D., Panagakos, F., Devizio, W., Trivedi, H. M., Jönsson, T., Guo, L., Cervi, S., & Scannapieco, F. A. (2014). Global metabolomic analysis of human saliva and plasma from healthy and diabetic subjects, with and without periodontal disease. PLoS ONE, 9(8), e105181.
Barnes, V. M., Teles, R., Trivedi, H. M., Devizio, W., Xu, T., Lee, D. P., Mitchell, M. W., Wulff, J. E., Milburn, M. V., & Guo, L. (2010). Assessment of the effects of dentifrice on periodontal disease biomarkers in gingival crevicular fluid. Journal of Periodontology, 81(9), 1273–1279.
Barnes, V. M., Teles, R., Trivedi, H. M., Devizio, W., Xu, T., Mitchell, M. W., Milburn, M. V., & Guo, L. (2009). Acceleration of purine degradation by periodontal diseases. Journal of Dental Research, 88(9), 851–855.
Barros, S. P., Williams, R., Offenbacher, S., & Morelli, T. (2016). Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontology, 70(1), 53–64.
Beger, R. D., Dunn, W., Schmidt, M. A., Gross, S. S., Kirwan, J. A., Cascante, M., Brennan, L., Wishart, D. S., Oresic, M., Hankemeier, T., et al. (2016). Metabolomics enables precision medicine: “A white paper, community perspective.” Metabolomics, 12(9), 149.
Blau, N., van Spronsen, F. J., & Levy, H. L. (2010). Phenylketonuria. Lancet, 376(9750), 1417–1427.
Caton, J. G., Armitage, G., Berglundh, T., Chapple, I. L. C., Jepsen, S., Kornman, K. S., Mealey, B. L., Papapanou, P. N., Sanz, M., & Tonetti, M. S. (2018). A new classification scheme for periodontal and peri-implant diseases and conditions - introduction and key changes from the 1999 classification. Journal of Clinical Periodontology, 45(Suppl 20), S1–S8.
Chen, H. W., Zhou, W., Liao, Y., Hu, S. C., Chen, T. L., & Song, Z. C. (2018). Analysis of metabolic profiles of generalized aggressive periodontitis. Journal of Periodontal Research, 53(5), 894–901.
Citterio, F., Romano, F., Meoni, G., Iaderosa, G., Grossi, S., Sobrero, A., Dego, F., Corana, M., Berta, G. N., Tenori, L., et al. (2020). Changes in the salivary metabolic profile of generalized periodontitis patients after non-surgical periodontal therapy: A metabolomic analysis using nuclear magnetic resonance spectroscopy. Journal of Clinical Medicine, 9(12), 3977.
Datta, D., Bhinge, A., & Chandran, V. (2001). Lysine: Is it worth more? Cytotechnology, 36(1–3), 3–32.
de Oliveira, L. R., Martins, C., Fidalgo, T. K., Freitas-Fernandes, L. B., de Oliveira, T. R., Soares, A. L., Almeida, F. C., Valente, A. P., & de Souza, I. P. (2016). Salivary metabolite fingerprint of type 1 diabetes in young children. Journal of Proteome Research, 15(8), 2491–2499.
Duncan, S. H., Barcenilla, A., Stewart, C. S., Pryde, S. E., & Flint, H. J. (2002). Acetate utilization and butyryl coenzyme a (coa): Acetate-coa transferase in butyrate-producing bacteria from the human large intestine. Applied and Environment Microbiology, 68(10), 5186–5190.
Elabdeen, H. R. Z., Mustafa, M., Szklenar, M., Rühl, R., Ali, R., & Bolstad, A. I. (2013). Ratio of pro-resolving and pro-inflammatory lipid mediator precursors as potential markers for aggressive periodontitis. PLoS ONE, 8(8), e70838–e70838.
Fidalgo, T. K. S., Freitas-Fernandes, L. B., Almeida, F. L., Valente, A. P., & Souza, I. P. R. (2015). Longitudinal evaluation of salivary profile from children with dental caries before and after treatment. Metabolomics, 11(3), 780–785.
Fidalgo, T. K. S., Freitas-Fernandes, L. B., Angeli, R., Muniz, A. M. S., Gonsalves, E., Santos, R., Almeida, F. L., Valente, A. P., & Souza, I. P. R. (2013). Salivary metabolite signatures of children with and without dental caries lesions. Metabolomics, 9, 657–666.
Freitas-Fernandes, L. B., Fidalgo, T. K. S., de Almeida, P. A., Souza, I. P. R., & Valente, A. P. (2020). Salivary metabolome of children and adolescents under peritoneal dialysis. Clinical Oral Investigations, 25, 2345.
Fruteau de Laclos, B., Braquet, P., & Borgeat, P. (1984). Characteristics of leukotriene (lt) and hydroxy eicosatetraenoic acid (hete) synthesis in human leukocytes in vitro: Effect of arachidonic acid concentration. Prostaglandins, Leukotrienes, and Medicine, 13(1), 47–52.
Gawron, K., Wojtowicz, W., Łazarz-Bartyzel, K., Łamasz, A., Qasem, B., Mydel, P., Chomyszyn-Gajewska, M., Potempa, J., & Mlynarz, P. (2019). Metabolomic status of the oral cavity in chronic periodontitis. In Vivo, 33(4), 1165–1174.
Gromski, P. S., Muhamadali, H., Ellis, D. I., Xu, Y., Correa, E., Turner, M. L., & Goodacre, R. (2015). A tutorial review: Metabolomics and partial least squares-discriminant analysis: A marriage of convenience or a shotgun wedding. Analytica Chimica Acta, 879, 10–23.
Guyatt, G., Oxman, A. D., Akl, E. A., Kunz, R., Vist, G., Brozek, J., Norris, S., Falck-Ytter, Y., Glasziou, P., DeBeer, H., et al. (2011). Grade guidelines: 1. Introduction-grade evidence profiles and summary of findings tables. Journal of Clinical Epidemiology, 64(4), 383–394.
HMDB. (2022). Online document (2022, june 02). Human metabolome database. Retrieved June 02, 2022, from https://hmdb.Ca/classyfication
Huang, Y., Zhu, M., Li, Z., Sa, R., Chu, Q., Zhang, Q., Zhang, H., Tang, W., Zhang, M., & Yin, H. (2014). Mass spectrometry-based metabolomic profiling identifies alterations in salivary redox status and fatty acid metabolism in response to inflammation and oxidative stress in periodontal disease. Free Radical Biology and Medicine, 70, 223–232.
Jaedicke, K. M., Preshaw, P. M., & Taylor, J. J. (2016). Salivary cytokines as biomarkers of periodontal diseases. Periodontology 2000, 70(1), 164–183.
Jepsen, S., Caton, J. G., Albandar, J. M., Bissada, N. F., Bouchard, P., Cortellini, P., Demirel, K., de Sanctis, M., Ercoli, C., Fan, J., et al. (2018). Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. Journal of Clinical Periodontology, 45(Suppl 20), S219–S229.
Jo, R., Nishimoto, Y., Umezawa, K., Yama, K., Aita, Y., Ichiba, Y., Murakami, S., Kakizawa, Y., Kumagai, T., Yamada, T., et al. (2019). Comparison of oral microbiome profiles in stimulated and unstimulated saliva, tongue, and mouth-rinsed water. Scientific Reports, 9(1), 16124.
Klupczyńska, A., Dereziński, P., & Kokot, Z. J. (2015). Metabolomics in medical sciences–trends, challenges and perspectives. Acta Poloniae Pharmaceutica, 72(4), 629–641.
Korte, D. L., & Kinney, J. (2016). Personalized medicine: An update of salivary biomarkers for periodontal diseases. Periodontology 2000, 70(1), 26–37.
Kuboniwa, M., Sakanaka, A., Hashino, E., Bamba, T., Fukusaki, E., & Amano, A. (2016). Prediction of periodontal inflammation via metabolic profiling of saliva. Journal of Dental Research, 95(12), 1381–1386.
Liebsch, C., Pitchika, V., Pink, C., Samietz, S., Kastenmüller, G., Artati, A., Suhre, K., Adamski, J., Nauck, M., Völzke, H., et al. (2019). The saliva metabolome in association to oral health status. Journal of Dental Research, 98(6), 642–651.
Loos, B. G., & Van Dyke, T. E. (2020). The role of inflammation and genetics in periodontal disease. Periodontology 2000, 83(1), 26–39.
Marchesan, J. T., Morelli, T., Moss, K., Barros, S. P., Ward, M., Jenkins, W., Aspiras, M. B., & Offenbacher, S. (2015). Association of synergistetes and cyclodipeptides with periodontitis. Journal of Dental Research, 94(10), 1425–1431.
Mendez, K. M., Reinke, S. N., & Broadhurst, D. I. (2019). A comparative evaluation of the generalised predictive ability of eight machine learning algorithms across ten clinical metabolomics data sets for binary classification. Metabolomics, 15(12), 150.
Meyle, J., & Chapple, I. (2015). Molecular aspects of the pathogenesis of periodontitis. Periodontology 2000, 69(1), 7–17.
Moola, S., Munn, Z., Tufanaru, C., Aromataris, E., Sears, K., Sfetcu, R., Currie, M., Lisy, K., Qureshi, R., Mattis, P., et al. (2020). Jbi manual for evidence synthesis. In E. Aromataris & Z. Munn (Eds.), Chapter 7: Systematic reviews of etiology and risk (pp. 217–269). JBI.
Nazar Majeed, Z., Philip, K., Alabsi, A. M., Pushparajan, S., & Swaminathan, D. (2016). Identification of gingival crevicular fluid sampling, analytical methods, and oral biomarkers for the diagnosis and monitoring of periodontal diseases: A systematic review. Disease Markers, 2016, 1804727.
Nguyen, T., Sedghi, L., Ganther, S., Malone, E., Kamarajan, P., & Kapila, Y. L. (2020). Host-microbe interactions: Profiles in the transcriptome, the proteome, and the metabolome. Periodontology 2000, 82(1), 115–128.
Öngöz Dede, F., Balli, U., Bozkurt Doğan, Ş, & Güven, B. (2017). Interleukin-32 levels in gingival crevicular fluid and saliva of patients with chronic periodontitis after periodontal treatment. Journal of Periodontal Research, 52(3), 397–407.
Ozeki, M., Nozaki, T., Aoki, J., Bamba, T., Jensen, K. R., Murakami, S., & Toyoda, M. (2016). Metabolomic analysis of gingival crevicular fluid using gas chromatography/mass spectrometry. Mass Spectrometry (tokyo), 5(1), A0047.
Pei, J., Li, F., Xie, Y., Liu, J., Yu, T., & Feng, X. (2020). Microbial and metabolomic analysis of gingival crevicular fluid in general chronic periodontitis patients: Lessons for a predictive, preventive, and personalized medical approach. EPMA Journal, 11, 197.
Pilotto, A., Zipser, C. M., Leks, E., Haas, D., Gramer, G., Freisinger, P., Schaeffer, E., Liepelt-Scarfone, I., Brockmann, K., Maetzler, W., et al. (2021). Phenylalanine effects on brain function in adult phenylketonuria. Neurology, 96(3), e399–e411.
Rivière, A., Selak, M., Lantin, D., Leroy, F., & De Vuyst, L. (2016). Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Frontiers in Microbiology, 7, 979.
Rodriguez-Lagunas, M. J., Storniolo, C. E., Ferrer, R., & Moreno, J. J. (2013). 5-hydroxyeicosatetraenoic acid and leukotriene d4 increase intestinal epithelial paracellular permeability. International Journal of Biochemistry & Cell Biology, 45(7), 1318–1326.
Romano, F., Meoni, G., Manavella, V., Baima, G., Mariani, G. M., Cacciatore, S., Tenori, L., & Aimetti, M. (2019). Effect of non-surgical periodontal therapy on salivary metabolic fingerprint of generalized chronic periodontitis using nuclear magnetic resonance spectroscopy. Archives of Oral Biology, 97, 208–214.
Romano, F., Meoni, G., Manavella, V., Baima, G., Tenori, L., Cacciatore, S., & Aimetti, M. (2018). Analysis of salivary phenotypes of generalized aggressive and chronic periodontitis through nuclear magnetic resonance-based metabolomics. Journal of Periodontology, 89(12), 1452–1460.
Rzeznik, M., Triba, M. N., Levy, P., Jungo, S., Botosoa, E., Duchemann, B., Le Moyec, L., Bernaudin, J. F., Savarin, P., & Guez, D. (2017). Identification of a discriminative metabolomic fingerprint of potential clinical relevance in saliva of patients with periodontitis using 1h nuclear magnetic resonance (NMR) spectroscopy. PLoS ONE, 12(8), e0182767.
Sakanaka, A., Kuboniwa, M., Hashino, E., Bamba, T., Fukusaki, E., & Amano, A. (2017). Distinct signatures of dental plaque metabolic byproducts dictated by periodontal inflammatory status. Scientific Reports, 7, 42818.
Shi, M., Wei, Y., Nie, Y., Wang, C., Sun, F., Jiang, W., Hu, W., & Wu, X. (2020). Alterations and correlations in microbial community and metabolome characteristics in generalized aggressive periodontitis. Frontiers in Microbiology, 11, 573196.
Singh, M. P., Saxena, M., Saimbi, C. S., Arif, J. M., & Roy, R. (2017). Metabolic profiling by 1h nmr spectroscopy of saliva shows clear distinction between control and diseased case of periodontitis. Metabolomics, 13(11), 137.
Singh, M. P., Saxena, M., Saimbi, C. S., Siddiqui, M. H., & Roy, R. (2019). Post-periodontal surgery propounds early repair salivary biomarkers by 1h nmr based metabolomics. Metabolomics, 15(11), 1–11.
Taylor, J. J., & Preshaw, P. M. (2016). Gingival crevicular fluid and saliva. Periodontology 2000, 70(1), 7–10.
Tolstikov, V., Moser, A. J., Sarangarajan, R., Narain, N. R., & Kiebish, M. A. (2020). Current status of metabolomic biomarker discovery: Impact of study design and demographic characteristics. Metabolites, 10(6), 224.
Ulven, S. M., Holven, K. B., Gil, A., & Rangel-Huerta, O. D. (2019). Milk and dairy product consumption and inflammatory biomarkers: An updated systematic review of randomized clinical trials. Advances in Nutrition, 10(suppl_2), S239-s250.
Waisbren, S. E., Noel, K., Fahrbach, K., Cella, C., Frame, D., Dorenbaum, A., & Levy, H. (2007). Phenylalanine blood levels and clinical outcomes in phenylketonuria: A systematic literature review and meta-analysis. Molecular Genetics and Metabolism, 92(1–2), 63–70.
Wassall, R. R., & Preshaw, P. M. (2016). Clinical and technical considerations in the analysis of gingival crevicular fluid. Periodontology 2000, 70(1), 65–79.
Wendisch, V. F. (2017). Microbial production of amino acid-related compounds. Advances in Biochemical Engineering/biotechnology, 159, 255–269.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brito, F., Curcio, H.F.Q. & da Silva Fidalgo, T.K. Periodontal disease metabolomics signatures from different biofluids: a systematic review. Metabolomics 18, 83 (2022). https://doi.org/10.1007/s11306-022-01940-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-022-01940-5