Abstract
Introduction
Ractopamine, a β-agonist used as growth promoter in livestock, is with great controversy, and it has been forbidden in most countries worldwide. However, due to economic benefits, the possibility of widespread abuse of ractopamine still exists. “Omics” strategies, based on the observation of physiological perturbations, are promising approaches to tackle drug misuse in breeding animals.
Objectives
A study was performed to determine if serum-metabolomics could be used to establish a predictive tool for identifying ractopamine misuse in pigs.
Methods
Our aim was to set up a high performance liquid chromatography—high resolution mass spectrometry based metabolomics workflow for screening pig serum for ractopamine administration. Therefore, an untargeted metabolomics approach was developed to characterize and compare serum metabolic profiles from control and treated pigs. Two different extraction strategies were investigated, and the results showed that the combination of methanol extraction and methanol–water extraction protocols significantly improve the metabolites coverage. A two-level data analysis using univariate and multivariate statistical analyses was carried out to establish descriptive and predictive models.
Results
The discrimination of treated animals from control animals could be achieved. A number of candidate biomarkers that contributed the most in the observed discrimination could be listed.
Conclusion
This research indicates that metabolomics approach can be considered as a powerful strategy to highlight biomarkers related to ractopamine treatment in pig which may subsequently be implemented as screening strategy to predict for such illicit practices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ractopamine is a phenylethanolamine β-adrenoceptor agonist (β-agonist) which contains two phenolic groups, an amino functional group, and a benzylic hydroxyl group. Its commercial product, ractopamine hydrochloride, exits under two diastereomeric forms, which are identified as RS, SR and RR, SS, resulting from the presence of two chiral carbons (WHO 1993). Isomer-specific treatment studies in rats have demonstrated that the RR isomer, butopamine, is the most biologically active and is associated with growth properties (Ricke et al. 1999). Such property has been widely studied in livestock and in particular in pigs where ractopamine has been shown to mediate the growth response through the β2ARβ2-adrenoceptor (Mills et al. 2003a, b). So, ractopamine is used for the improvement of weight gain, carcass leanness and feed efficiency in animal feed in more than 20 countries, including the United States, Canada, Japan, South Africa and Mexico (Anderson et al. 2009).
During its 2010 meeting, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) established maximum residue limits (MRLs) for ractopamine in muscle (10 ppb), fat (10 ppb), liver (40 ppb) and kidney (90 ppb) in pig and cattle (JECFA 2010). On 6 July 2012, the international reference standard Codex Alimentarius Commission narrowly approved the adoption of the MRLs although setting any limit was a controversial move. However, recent research confirmed that using ractopamine can affect heart, thyroid, urethra and prostate (Catalano et al. 2012), and also cause cardiac toxicity (Yaeger et al. 2012). As a consequence, the practice of ractopamine in food-producing animals is still with controversy. Most countries, such as the countries of the European Union and China, have not approved the usage of any β-agonist, including ractopamine, as growth promoters (Council Directive 96/22/EC 1996; MOA 2002; EFSA 2009). Yet due to economic benefits, the possibility of widespread abuse of ractopamine still exists. And recent international food trade restrictions (Bottemiller 2012, 2013) make the detection of ractopamine a high priority.
In order to guide method development and ensure compliance with EU prohibition in a general context of guarantying the consumers with safe food on a chemical point of view, the European Union laid down the requirements for residue analysis and performance of analytical methods in Council Directive 96/23/EC (1996) and Commission Decision 2002/657/EC (2002). The confirmation of ractopamine administration is generally not an issue now. However, the extent to which such practices occur is still difficult to evaluate since its screening is an enormous challenge. To meet the requirement for screening illicit practices, “Omics” strategies, based on the observation of physiological perturbations, are promising approaches to tackle drug misuse in breeding animals (Riedmaier et al. 2009; Pinel et al. 2010; Nebbia et al. 2011; Dervilly-Pinel et al. 2012; Gallart Ayala et al. 2015). Particularly, metabolomics depended on mass spectrometry analytical platforms have proven their efficiency in highlighting candidate biomarkers of anabolic treatments in livestock (Dervilly-Pinel et al. 2011; Jacob et al. 2015; Kouassi Nzoughet et al. 2015) and sport animals (Kieken et al. 2009, 2011; Boyard-Kieken et al. 2011).
During the past few years, several metabolomics studies have been reported for the control of β-agonists in livestock. Courant et al. (2009) developed an untargeted metabolomics approach based on high performance liquid chromatography coupled to high resolution mass spectrometry (HPLC–HRMS) measurement and made it possible to highlight metabolic modifications in calves’ urine consecutively to a clenbuterol administration. Later on, Dervilly-Pinel et al. (2015) proposed a validated HPLC–HRMS metabolomics strategy enabling implementing a robust statistical model to predict for β-agonists treated calves. Regarding the porcine specie, Li et al. (2016) reported a metabolomics investigation of porcine muscle and fatty tissue after clenbuterol treatment using gas chromatography–mass spectrometry (GC–MS). Differences in metabolomics profiles between control and treated groups were characterized by multivariate statistical analysis. Wu et al. (2015) outlined a metabolomics based strategy for detecting the use of “cocktails” composed of mixtures of low amounts of three β-agonists (clenbuterol, salbutamol, and ractopamine) via pig urine profiling, which was analyzed by ultra-high performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Until now however, most studies have been focusing on clenbuterol as a model of β-agonists. While ractopamine, the most controversial β-agonist, is even not included or focused on. Although Wu et al. (2015) mentioned the metabolomics profiling of “cocktails” including ractopamine, it is still not enough to underline significant difference between control and treated animals in terms of the global metabolic profiling after ractopamine administration. In the current international context, it has however become a matter of urgency to propose efficient high-throughput screening strategies to reveal biological effects in drug treated animals and tackle illegal practices.
Due to the non-invasive nature of the sample collection, urine of, e.g. bovine and pig, has primarily been the matrix of choice (Courant et al. 2009; Dervilly-Pinel et al. 2015; Jacob et al. 2015; Wu et al. 2015). Although easy to collect in some animal species (e.g. bovine), urine is not considered an easy matrix to be obtain in pigs which constitutes a shortcoming for further implementation at the farm level. As an alternative and complementary biological fluid, serum or plasma, which fully contains biological information and is less prone to rearing environmental contamination, was also used in metabolic profiling of some other drugs (Dunn et al. 2011; Kieken et al. 2011; Kouassi Nzoughet et al. 2015). In the scope of the present research, we aimed at investigating metabolic perturbations in pig serum after oral administration of ractopamine. And in order to characterize and compare the global metabolic profiles, two extraction strategies (methanol extraction and methanol–water extraction) were used for sample preparation. HPLC–HRMS analytical platforms were employed for metabolomics profiling in polarity switching mode H-ESI+/−; data generated were processed and further analysed using univariate and multivariate statistical analysis, and resulting models were evaluated for their descriptive and prediction capabilities. To the best of our knowledge, no specific metabolomics investigation of ractopamine has been performed for treated animal before, and this is the first time that metabolomics profiling of serum has been combined to tackle β-agonists abuse in pig.
2 Materials and methods
The recommendations of the metabolomic standards initiative (MSI) (Sumner et al. 2007) were considered during the present investigation, i.e., sample preparation, instrumental analysis, data processing and metabolite identification steps.
2.1 Animal experimentation and serum samples collection
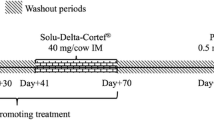
Ten 4 months’ female pigs (Axior Porc Charcutier) (Terrena, Ancenis, France) weighting 40 kg were randomly divided into control (n = 5 animals) and treated (n = 5 animals) groups. Animals were allowed a 3-day acclimatization period, following which, each animal received 1.8 kg feed/day. Pigs from the treatment group received then on a daily basis and during 29 days, a feed containing ractopamine hydrochloride (Sigma Aldrich) (10 mg/kg feed). The 10 ppm daily dose was selected as corresponding to classical doses used in finishing pigs. Blood samples were collected at day 3, 9, 16, 18, 23, 29, for both control and treated animals. The blood samples were allowed to clot at room temperature and the serum samples, obtained by centrifugation, were frozen and then stored at −80 °C.
2.2 Chemicals and reagents
Unless otherwise specified, all solvents and reagents used in this study were of LC–MS grade quality. Chloroform (CHCl3, analytical grade) was purchased from Carlo Erba Reactifs (SDS, Peypin, France). Acetic acid, acetonitrile (MeCN), methanol (MeOH) and water were sourced from Sigma–Aldrich (Saint Quentin Fallavier, France). Isotope labeled internal standards, namely, l-Leucine-5,5,5-d3, l-Tryptophan-2,3,3-d3, Indole-2,4,5,6,7-d5-3-acetic acid and 1,14-Tetradecanedioic-d24 acid were purchased from Sigma–Aldrich (Saint Quentin Fallavier, France) and CDN Isotopes (Québec, Canada). MSCAL6 ProteoMass LTQ/FT-Hybrid, standard mixtures used for calibration of the MS instrument (positive and negative ionization mode) were obtained from Sigma–Aldrich (Saint Quentin Fallavier, France).
2.3 Sample preparation
Prior to the extraction, serum samples were allowed to thaw on ice. In order to investigate and compare the global metabolomics profiles, two extraction strategies were used for sample preparation.
2.3.1 Methanol extraction
This strategy is similar to the procedure published by Dunn et al. (2011). 400 µL serum sample was extracted with 1400 µL MeOH containing 1 ng/µL of each labeled internal standards. Then the mixture was vortexed for 20 s before centrifugation (2000 r/min, 20 min, 4 °C). 370 µL supernatant was collected and then evaporated to dryness under gentle N2 at 30 °C, and subsequently reconstituted with 150 µL H2O before HPLC–HRMS analysis.
2.3.2 Methanol–water extraction
The extraction procedure firstly reported by Dyer and Bligh (1959) and further re-adapted by Bird et al. (2011) was employed. The procedure consisted of a triphasic liquid–liquid extraction with MeOH:CHCl3:H2O (1:2:0.63). 190 µL of MeOH was added to 30 µL serum sample and the mixture was vortexed for 20 s. Following this, 380 µL of CHCl3 was added and vortexed for 20 s. Finally, 120 µL of H2O was added to induce phase separation. The sample was further shaken for 10 s and allowed to equilibrate at room temperature for 10 min before centrifugation (2000 r/min, 10 min, 4 °C). 150 µL the upper phase (MeOH+H2O) was then collected and evaporated to dryness under gentle N2 at 30 °C, and subsequently reconstituted with 30 µL H2O containing 2 ng/µL of each labeled internal standards before HPLC–HRMS analysis.
2.3.3 QC sample preparation
A pooled quality control (QC) sample was prepared by pooling aliquots of all individual study samples. In our study, QC sample was extracted and processed according to the procedures described in Sects. 2.3.1 and 2.3.2, respectively. Then, these two reconstitution solutions were combined and mixed thoroughly before HPLC–HRMS analysis. The QC solution is therefore representative of both extraction strategies tested. The same QC solution was injected at the beginning, the end, but also intervals of every five samples in each batch run to assure the analytical repeatability and the quality of the fingerprinting process throughout the study.
2.4 HPLC–HRMS analysis
An UltiMate® 3000 Series high performance liquid chromatography (HPLC) system coupled to a hybrid quadrupole-orbitrap (Q-Exactive™) mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) equipped with a heated electrospray (H-ESI II) source was used for sample analysis. The chromatographic separation was achieved on a Hypersil Gold C18 column (2.1 × 100 mm2, 1.9 µm particle size, Thermo Fisher Scientific) together with the corresponding guard column maintained at 35 °C, without splitting to the electrospray interface operating in a polarity switching ion mode (positive/negative). The mobile phase consisted in water containing 0.1% acetic acid (A) and acetonitrile containing 0.1% acetic acid (B). The used elution gradient (A:B, v/v) was as follows: 95:5 from 0 to 2.40 min; 75:25 at 4.50 min; 30:70 at 11 min; 0:100 at 14–16.5 min; 95:5 at 19–25 min. The injected volume was 5 µL and the flow rate was 400 µL/min. High resolution MS fingerprints were acquired using the following parameters: spray voltage ±3 kV; capillary temperature 350 °C; sheath gas flow rate 55 a.u. (arbitrary units), auxiliary gas flow rate 10 a.u., spare gas flow rate 0; max spray current ±100 µA; probe heater temperature 300 °C; S-lens RF level 50. Full scan mass spectra were acquired from m/z 65 to 975 at a mass resolving power of 70,000 Full Width at Half Maximum (FWHM) at m/z 200. The scan speed was 2 Hz in profile mode. Daily instrument calibration was performed using a MSCAL6 ProteoMass LTQ/FT-Hybrid. Xcalibur 2.2 (Thermo Fisher Scientific, San Jose, CA, USA) was used for data acquisition and analysis.
In order not to bias statistical analyses or the metabolomics data structure, all the samples for analysis arising from control and treated animals, which were prepared separately by two extraction strategies, were randomized across the whole data acquisition sequence.
2.5 Data processing and inter-batch normalization
HPLC–HESI (±) HRMS raw data files were initially preprocessed with Xcalibur 2.2. The raw (*.raw) files were subsequently converted to a more exchangeable format (*.mzXML) and polarity split using MSConvert (Kessner et al. 2008). Further data processing was performed using XCMS (Smith et al. 2006) software implemented with the R statistical language; multiple steps including: peak alignment, peak picking, retention time alignment followed by a peak grouping and integration step. The resulting XCMS matrix containing MS features for each sample, was subsequently exported to Excel® for univariate analysis, inter-batch normalization and later to SIMCA-P+® (Version 13, Umetrics AB, Sweden) software for multivariate statistical analysis.
The inter-batch instrumental drift may be confused with biological differences if it is not minimized. Therefore, after data processing, the removal of between-batch offsets and drifts was carried out by following the procedure described by van der Kloet et al. (2009), assuming that the measurement errors in a single batch are randomly distributed, and that different batches can be compared and corrected using the average or median value of the QC samples in a batch.
2.6 Data analysis
In order to examine the MS features responsible for differences observed between control and ractopamine treated animals, a two-level data analysis was performed. Global data, methanol extraction data and methanol–water extraction data were first analyzed individually, and then the latter two were subjected to a second-order analysis.
2.6.1 Univariate analysis
The resulting XCMS matrix containing MS features for each sample was subsequently exported to Excel® for univariate data analysis evaluation. Univariate statistics were exploited to estimate instrumental variability. The coefficient of variation (CV) of each feature was calculated using the intensities detected in QC samples. Computation of the ratio of abundance observed between control and treated animals (fold change) and the statistical significance calculated from a student t test (p value) further served for metabolites selection. MS features selection was based on the following criteria: CV <30%, p value <0.05, fold change >1.5 and retention time between 30 and 1080 s.
2.6.2 Multivariate analysis
The dataset was subsequently exported to SIMCA-P+® software and subjected to analysis. Before multivariate statistical analysis, the data was log-transformed to improve its variance homogeneity and to make classification and clustering more effective. Pareto scaling was also applied to all variables (ions) in order to reduce the noise in the data and thereby enhance the information content and quality. Both unsupervised Principal Component Analysis (PCA) and supervised (Orthogonal) Partial Least Squares Discriminant Analysis [(O)PLS-DA] were employed. The PCA aims at providing an overview of the data structure, while the (O)PLS-DA models attempt to explain one Y variable (in our study the status of the animals involved, i.e., control or treated animals) from a X matrix of all the ions constituting the fingerprint. The validation and robustness of each model were evaluated by R2X(cum), R2Y(cum) and Q2(cum) parameters, cross validation-analysis of variance (CV-ANOVA) (Eriksson et al. 2008) and permutation test.
2.6.3 The second-order analysis
A second-order analysis was performed on the methanol extraction and methanol–water extraction matrices with metaXCMS R package (Tautenhahn et al. 2011; Patti et al. 2012) to study the common and unique features of the different datasets (between different extraction strategy). The second-order analysis was applied with m/z tolerance 0.008 and retention time tolerance 15 s, with or without filtering by parameters of fold change >1.5 and p value <0.05, in order to consider that a feature is common between compared datasets.
2.7 Identification of candidate biomarkers
Metabolites of interest were firstly identified on the basis of an internal databank and using Advanced Chemistry Development/Labs software (Advanced Chemistry Development, Inc. Toronto, ON, Canada). The details contained in the database for each metabolite are, mainly: retention time, monoisotopic accurate mass and fragmentation pattern (Courant et al. 2012). An in-house Excel® Macro file was used to improve the annotation of the metabolic fingerprints automatically. Then, metabolites of interest were searched against online databases, i.e. METLIN (Smith et al. 2005; Tautenhahn et al. 2012; Zhu et al. 2013) and HMDB (Wishart et al. 2007, 2009, 2013). Metabolite hits were searched for using mass tolerance of ±5 ppm, molecular species, charge state and adduct types. Additionally, results generated after database searches were assessed in relation to metabolites physiochemical properties, spectral similarities, matrix of occurrence and reports in related publications.
3 Results and discussion
3.1 Quality assurance of HPLC–HRMS data
In metabolomics analysis, where the samples contain a huge number of components covering a wide range of concentrations and structural types, of variable and unknown MS responses, it is very important to ensure the quality of the data acquired. In the present study, internal standards and QC sample have been used for quality assurance. The mixture of four isotope labeled internal standards had been added to each sample before HPLC–HRMS analysis in order to evaluate the retention time stability, the consistency of the signal intensities and mass accuracy within and between measurement series. Peaks for the internal standards were clearly visible on the selected ion monitoring (SIM) chromatograms, no significant drift in retention time or decline in sensitivity was observed within and between injection batches, and the deviation from standard accurate mass remained below 5 ppm.
3.2 Investigation of pig serum metabolome under HPLC–HRMS in both polarity modes
In the scope of the present study, fingerprinting conditions based on reverse phase chromatography combined to electrospray ionization in polarity switch mode (+/−) using an acetonitrile/water gradient elution were implemented, as already reported efficient in terms of application range and particularly suited for the detection of markers of β-agonists practices (Dervilly-Pinel et al. 2015; Jacob et al. 2015). Representative total ion chromatograms (TICs) obtained from control and treated samples (Supplementary Fig. 1) illustrated that acceptable separation and intensity enhancement were achieved in these metabolic fingerprints using the HPLC–HRMS conditions.
3.2.1 Global data profiling
Data processing and analysis as described in Sects. 2.5 and 2.6 were applied to the whole dataset consisting of profiling data using both extraction strategies. Application of the protocol led to the selection of 1161 ions in positive mode and 983 ions in negative mode. A non-supervised PCA was first applied to the global dataset to explore the data structure (Fig. 1). As can be observed, QC samples were well-clustered, providing some evidence regarding the stability and repeatability of the methodology. Moreover, even these two extraction strategies could be clearly separated both in positive and negative modes in PCA model, which showed the great difference of each extraction strategy on both qualitative (metabolites) and quantitative (intensities) points of views. Although strong differences in the extracted metabolome profiles using different solvents mixture are expected and have already been documented previously (Winder et al. 2008; Courant et al. 2014), it is worthwhile to report such a pronounced difference using close extraction conditions, as observed in the present study. These differences have been further investigated in Sect. 3.3 of the present article.
Score plots for PCA model built with global datasets obtained from both methanol extraction (M) and methanol–water extraction (M+W) strategies under HPLC–HRMS positive (a) and negative (b) modes. QC samples are represented by blue filled circle; control samples are represented by green filled circle; ractopamine treated samples are represented by red filled circle
Since samples from control and treated groups could not be separated using such PCA data analysis strategy, a supervised multivariate OPLS-DA was then performed on the basis of all acquired metabolic fingerprints in order to investigate deeper potential differences between the conditions to compare. The resulting models (Fig. 2) obviously showed four separate clusters both in positive and negative modes. Samples collected from control pigs had Y-axis coordinates opposed to samples collected from treated pigs; however, samples processed by different extraction protocols had X-axis coordinates opposed to each other. Such performances in differentiating the samples were confirmed by the high values calculated for R2X(cum) = 0.766, R2Y(cum) = 0.959, Q2(cum) = 0.685 and CV-ANOVA p value = 1.53e−19 in positive mode and R2X(cum) = 0.753, R2Y(cum) = 0.993, Q2(cum) = 0.859 and CV-ANOVA p value = 7.01e−34 in negative mode, respectively, indicating that most of the variance between the status of the animals is explained by this model and it presents good descriptive ability.
Score plots for OPLS-DA model built with global datasets obtained from both methanol extraction (M) and methanol–water extraction (M+W) strategies under HPLC–HRMS positive (a) and negative (b) modes. Control samples are represented by green filled circle; ractopamine treated samples are represented by red filled circle
According to the large differences observed between both extraction protocols, it was decided not to study further the global dataset but to focus on each of them separately to highlight the most relevant one in addressing our research question.
3.2.2 Methanol extraction data profiling
Methanol extraction data were thus processed with XCMS software separately. Application of the same parameters and filters as in global data profiling led to the selection of 989 ions in positive mode and 696 ions in negative mode in the normalized data matrices, which were subjected to statistical analysis. No significant difference between samples was observed in PCA analysis when all features were used. In order to investigate any separation between the control and ractopamine treated groups, OPLS-DA was then performed. The OPLS score plots (Fig. 3) clearly showed that control samples had coordinates opposed to the samples from treated animals by the first principal component both in positive and negative modes. The OPLS fit criteria were calculated as follows: R2X(cum) = 0.495, R2Y(cum) = 0.968, Q2(cum) = 0.684 in positive mode and R2X(cum) = 0.326, R2Y(cum) = 0.993, Q2(cum) = 0.796 in negative mode. As expressed by these high values, the models demonstrated good descriptive and predictive performances.
In the scope of targeting candidate biomarkers of ractopamine administration, a selection of ions more particularly involved in the discrimination of control and treated populations was performed from the OPLS model presented in Fig. 4. The S-plots associated to this model were examined to visualize the contribution of each ion to the model and thus highlight which ions are the most responsible for the discrimination of the animals. A particular attention was paid to those ions and furthermore, only those exhibiting p value lower than 0.05 and fold change higher than 1.5 were selected. Finally, a visual examination of the XCMS overlaid extracted ion chromatograms (EICs) was completed in order to eliminate any potential artefacts MS signals and redundant information mainly due to adducts or fragments. The successive application of all these criteria finally led to the selection of 9 ions in positive mode and 13 ions in negative mode (Table 1), which were strongly correlated to the axis and presented a reasonable confidence interval.
S-plots associated to the OPLS-DA of datasets obtained from methanol extraction strategy under HPLC–HRMS positive (a) and negative (b) modes as illustrated in Fig. 3. The ions highlighted in red are responsible for the observed discrimination
Using these selected ions, and to confirm the ability of these ions to discriminate control from ractopamine treated samples, a new OPLS-DA (Supplementary Fig. 2) was performed. The two component model built showed the separation between control and treated animals by the first component with good descriptive and predictive capabilities (R2X(cum) = 0.587, R2Y(cum) = 0.988, Q2(cum) = 0.991 in positive mode and R2X(cum) = 0.459, R2Y(cum) = 0.979, Q2(cum) = 0.985 in negative mode).
The robustness of the models was further assessed by several steps, corresponding to the statistical validation of the models. First, cross-validation was performed. It consists to build a new OPLS-DA on the basis of 2/3 of the original dataset. The other 1/3 of the dataset is considered as validation test and is incremented in the model to be predicted. The discrimination ability is presented in Supplementary Fig. 3, while permutation tests consisting in 100 permutations of the status of the animals (i.e. treated or control) to demonstrate that the models are not the result of a chance factor but offers a valid and robust discrimination between controls and treated populations have been performed and are presented in Supplementary Fig. 4. Finally, CV-ANOVA was calculated. It allows attributing a degree of significance to the test-permutation. The results of CV-ANOVA variant suggested that the models are significant with p value of 6.79e−7 in positive mode and 2.94e−10 in negative modes.
3.2.3 Methanol–water extraction data profiling
Similarly, methanol–water extraction data were processed with XCMS software separately. Application of the same parameters and filters as in global data profiling led to the selection of 969 ions in positive mode and 886 ions in negative mode in the normalized data matrices, which were subjected to statistical analysis. No significant difference between samples was observed in PCA analysis when all features were used. Then, the OPLS-DA was performed. And the OPLS score plots (Supplementary Fig. 5) clearly showed that control samples had coordinates opposed to the samples from treated animals by the first principal component both in positive and negative modes. Totally, 7 ions in positive mode and 8 ions in negative mode in S-plots (Table 1, Supplementary Fig. 6) with p value lower than 0.05 and fold change higher than 1.5, and without any potential artefacts MS signals and redundant information mainly due to adducts or fragments, were selected to discriminate of control and treated populations.
Then the new OPLS-DA (Supplementary Fig. 7) performed on selected ions showed that the separation between control and treated animals with good descriptive and predictive capabilities (R2X(cum) = 0.373, R2Y(cum) = 0.873, Q2(cum) = 0.858 in positive mode and R2X(cum) = 0.683, R2Y(cum) = 0.992, Q2(cum) = 0.991 in negative mode).
The three-step validation of the models was also performed and results are reported in Supplementary Figs. 8 and 9. In particular robustness of the model was confirmed through cross-validation using 1/3 of the original samples. Finally, the results of CV-ANOVA variant suggested that the models are significant with a corresponding p value of 2.66e−3 in positive and 2.11e−12 in negative mode.
3.3 The second-order analysis
For the purpose of investigating and comparing the global metabolic profiles, two extraction strategies (methanol extraction and methanol–water extraction) were used for sample preparation which conducted to pronounced differences in the fingerprints. Methanol used as extraction solvent has the abilities of removing high molecular weight species including proteins, preserving the LC–MS system integrity and reducing matrix effects. While, the methanol–water extraction strategy using a triphasic liquid–liquid extraction with MeOH:CHCl3:H2O presents the advantage of obtaining both polar (upper polar methanol–water phase) and non-polar (lower non-polar CHCl3 phase) fractions of the serum which can respectively serve for metabolomics and lipidomics (data not shown here) investigations.
In order to investigate and compare deeper both extraction protocols, the results of the generated data matrices from two extraction strategies were subjected to a second-order analysis. 987 ions in positive mode (2 ions with invalid p value or fold change were deleted) and 696 ions in negative mode of methanol extraction data matrix, and 949 ions in positive mode (20 ions with invalid p value or fold change were deleted) and 881 ions in negative mode (5 ions with invalid p value or fold change were deleted) of methanol–water extraction data matrix were analyzed. A repartition of the selected ions into two groups was obtained as follows: common ions or specific ions for each ionization mode.
As shown in the Venn diagrams (Fig. 5a, b), the overlap among the two pairwise comparisons between control and treated animals revealed that more than 68% ions were in common in both extraction strategies for each ionization mode without filtering indicating that methanol extraction and methanol–water extraction exhibit to some extent globally similar extraction ability and efficiency. In addition, more than 55% of specific ions in both extraction strategies for each ionization mode with filtering indicated that methanol extraction and methanol–water extraction strategies are on the other hand quite different and complementary. And it can be deduced this is one of the main reasons that fingerprints obtained by two extraction strategies can be clearly separated in non-supervised PCA score plots in global data profiling as illustrated in Fig. 1.
Venn diagrams showing the repartition of the detected ions obtained from methanol extraction (M) and methanol–water extraction (M+W) strategies under HPLC–HRMS positive (a) and negative (b) modes in metaXCMS analysis with m/z tolerance 0.008 and retention time tolerance 15 s, with or without filtering by parameters of fold change >1.5 and p value <0.05
Signals of interest highlighted by metaXCMS after filtering the global dataset corresponding to both extraction strategies are presented in Supplementary Table 1. There were 20 ions detected in positive mode and 29 ions in negative mode, respectively. The candidate biomarkers proposed in methanol extraction data profiling (Table 1) and methanol–water extraction data profiling (Table 1) were all included in the list of detected ions of interest (Supplementary Table 1). So we can deduce that this verification is consistent with the individual result related to both extraction strategies. The reason why some ions highlighted in metaXCMS analysis with good reliability were not selected as candidate biomarkers in the previous data profilings is due to their poor correlation to the axis and reduced confidence interval in S-plots acquired by OPLS-DA.
Moreover, the detected ions of interest in methanol extraction strategy corresponded to small polar compounds as evidenced by their retention time upon reverse phase chromatography and their masses below 500 g/mol. While ions of interest detected in methanol–water extraction strategy showed longer retention time upon reverse phase chromatography and broader mass range from 100 to 900 g/mol (in negative mode) or 1000 g/mol (in positive mode). It highlights that methanol–water extraction strategy has a stronger extraction ability of relatively nonpolar compounds than methanol extraction strategy. This outcome is maybe due to CHCl3 used in triphasic liquid–liquid extraction, which is obviously miscible in the methanol–water layer and then increases the dissolution ability for lipid compounds.
The results of metaXCMS analysis above illustrated that methanol extraction strategy as reported by Dunn et al. (2011) is more fitted for metabolomics analysis focusing on small polar compounds, while methanol–water extraction strategy (Dyer and Bligh 1959; Bird et al. 2011) appears as a good complementary protocol in metabolomics investigation for its ability to extract ions with broader range of polarity.
3.4 Identification of candidate biomarkers
The identification of potential metabolites is the most difficult and time-consuming step of a given metabolomics investigation. In our study, all the candidate biomarkers were identified based on the MSI (Sumner et al. 2007), and the current identified metabolites were considered as “level 1” identification.
Firstly, ions M478T267 (in positive mode) and M476T268 (in negative mode) (Fig. 5) located in the upper-right area and therefore more abundant in the serum of treated animals were easily assigned to the signals of mono-glucuronide metabolite originating directly from ractopamine metabolism, as described by Jiang et al. (2014). Next, the identification of the other metabolites of interest began with an in-house database query either directly on its experimental accurate measurement or on its deduced elemental composition. Then, results generated after database searches were assessed in relation to metabolites physiochemical properties, spectral similarities and matrix of occurrence. Table 1 provided the selected ions, which were pointed out in this study as candidate biomarkers with regard to the data obtained from methanol extraction strategy and methanol–water extraction strategy respectively, and their possible identities. Among these ions, in-house database research made it possible to assign ions M160T284 (in positive mode) and M158T285 (in negative mode) to 2-aminooctanoic acid, M193T171 to p-aminohippuric acid, M127T89 to thymine or 4-imizoleacetic acid, M100T104 to betaine or l-valine or 5-aminovaleric acid; the remaining ions could not be identified yet at this moment, even after METLIN and HMDB online databases interrogations. Further identification, such as comparison with corresponding commercially available standard compound on the same analytical system under the same conditions, will be ongoing.
It was observed interestingly that the concentration of 2-aminooctanoic acid increased among the treatment period, i.e. higher concentrations were found in serum samples collected on day 18 as observed in Supplementary Fig. 10. As well known, 2-aminooctanoic acid is as an endogenous water-soluble compound locates in cytoplasm, and belongs to the class of organic compounds known as alpha amino acids occasionally found in human blood and urine (Parry 1957); and it is widely considered that 2-aminooctanoic acid is involved in biofunctions of protein synthesis and amino acid biosynthesis, which may be associated to the expected anabolic effect upon ractopamine administration.
In a context where both screening and confirmatory strategies targeting ractopamine residues are already efficiently implemented for control purposes, the importance of discovering additional markers may be questioned. The rationale behind such metabolomics approach is to investigate biomarkers that would eventually allow the possibility to screen at the same time a wider range of practices, i.e. different β-agonists and (low dose-) cocktails… which are known to be an issue with current analytical approaches. In this objective, the ability of the present models to predict different β-agonists treatments than the ractopamine-based original one, its efficiency in screening also lower treatment doses, as well as mixtures will be studied. If successful such results would therefore allow an extended scope of application to the screening of any β-agonists related practice in porcine as already successfully reported for bovine animals (Dervilly-Pinel et al. 2015).
4 Concluding remarks
The need for new and alternative screening analytical strategies to highlight illegal anabolic practices have gathered scientifics’ attention over the last past decade. Metabolomics in particular was investigated in the hope of identifying biomarkers evidencing such practices in breeding animals. Although several studies have demonstrated the efficiency of mass spectrometric based fingerprinting to discriminate control animals from animals treated with a range of anabolic treatments, the issue of ractopamine use in pigs has not been addressed yet, although innovative screening strategies are expected to ensure chemical food safety. The present study has demonstrated the feasibility and relevance of the approach since modified serum profiles could be observed after ractopamine administration. The metabolites with modified profile may be considered as potential biomarkers of treatment and could be combined using bioinformatics to formulate a discriminant function which may be further used to predict ractopamine treatment in pigs in an inexpensive, accurate, feasible, high-throughput test as already implemented for β-agonists in calves (Dervilly-Pinel et al. 2015). It is expected that such screening strategy compared to the current implemented one in control laboratories focusing on the residue itself or its direct metabolites will enable extended detection time windows through more sensitive detection and will also allow for an increased number of animals to be tested. Future work will focus on the validation of the model: a challenge test using a new cohort of animals will be applied and in particular, the specificity of the markers toward other β-agonist drugs, anabolic agents (steroids, protein hormones…) will be investigated.
References
Anderson, D. B., Moody, D. E., & Hancock, D. L. (2009). Beta adrenergic agonists. In W. G. Pond & A. W. Bell (Eds.), Encyclopedia of animal science (pp. 104–107). New York: Marcel Dekker, Inc.
Bird, S. S., Marur, V. R., Sniatynski, M. J., Greenberg, H. K., & Kristal, B. S. (2011). Serum lipidomics profiling using LC-MS and high energy collisional dissociation fragmentation: Focus on triglyceride detection and characterization. Analytical Chemistry, 83, 6648–6657.
Bottemiller H. (2012). Dispute over drug in feed limiting US meat exports. Retrieved from Sept 15, 2016 from https://thefern.org/2012/01/dispute-over-drug-in-feed-limiting-u-s-meat-exports/.
Bottemiller H. (2013). Escalating trade dispute, Russia bans Turkey over ractopamine residues. Retrieved Sept 15, 2016 from http://www.foodsafetynews.com/2013/02/escalating-trade-dispute-russia-bans-turkey-over-ractopamine-residues/#.V7cJ_fmep8o.
Boyard-Kieken, F., Dervilly-Pinel, G., Garcia, P., Paris, A. C., Popot, M. A., Le Bizec, B., et al. (2011). Comparison of different liquid chromatography stationary phases in LC-HRMS metabolomics for the detection of recombinant growth hormone doping control. Journal of Separation Science, 34, 3493–3501.
Catalano, D., Odore, R., Amedeo, S., Bellino, C., Biasibetti, E., & Miniscalco, B. (2012). Physiopathological changes related to the use of ractopamine in swine: Clinical and pathological investigations. Livestock Science, 144, 74–81.
Commission Decision 2002/657/EC. (2002). Implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Official Journal of the European Communities, L221.
Council Directive 96/22/EC. (1996). Concerning the prohibition on the use in stockfarming of certain substances having a hormonal or thyrostatic action and of beta-agonists, and repealing Directives 81/602/EEC, 88/146/EEC and 88/299/EEC. Official Journal of European Communities, L125.
Council Directive 96/23/EC. (1996). On measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC. Official Journal of European Communities, L125.
Courant, F., Antignac, J. P., Dervilly-Pinel, G., Le Bizec, B. (2014). Basics of mass spectrometry based metabolomics. Proteomics, 14, 2369–2388.
Courant, F., Dervilly-Pinel, G., Bichon, E., Monteau, F., Antignac, J. P., Le Bizec, B. (2009). Development of a metabolomic approach based on liquid chromatography high resolution mass spectrometry to screen for clenbuterol abuse in calves. The Analyst, 134, 1637–1646.
Courant, F., Royer, A. L., Chereau, S., Morvan, M., Monteau, F., Antignac, J. P., et al. (2012). Implementation of a semi-automated strategy for the annotation of metabolomic fingerprints generated by liquid chromatography-high resolution mass spectrometry from biological samples. The Analyst, 137, 4958–4967.
Dervilly-Pinel, G., Chereau, S., Cesbron, N., Monteau, F., Le Bizec, B. (2015). LC–HRMS based metabolomics screening model to detect various β-agonists treatments in bovines. Metabolomics, 11, 403–411.
Dervilly-Pinel, G., Courant, F., Chereau, S., Royer, A. L., Boyard-Kieken, F., Antignac, J., et al. (2012). Metabolomics in food analysis: Application to the control of forbidden substances. Drug Testing and Analysis, 4, 59–69.
Dervilly-Pinel, G., Weigel, S., Lommen, A., Chereau, S., Rambaud, L., Essers, M., et al. (2011). Assessment of two complementary liquid chromatography coupled to high resolution mass spectrometry metabolomics strategies for the screening of anabolic steroid treatment in calves. Analytica Chimica Acta, 700, 144–154.
Dunn, W. B., Broadhurst, D., Begley, P., Zelena, E., Francis-McIntyre, S., Anderson, N., et al. (2011). Procedures for large scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nature Protocols, 6, 1060–1083.
Dyer, E. G., & Bligh, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917.
EFSA. (2009). Scientific opinion of the panel on additives and products or substances used in Animal Feed (FEEDAP) on a request from the European Commission on the safety evaluation of ractopamine. The EFSA Journal, 1041, 1–52.
Eriksson, L., Trygg, J., & Svante, W. (2008). CV-ANOVA for significance testing of PLS and OPLS® models. Journal of Chemometrics, 22, 594–600.
Gallart Ayala, H., Chéreau, S., Dervilly-Pinel, G., Le Bizec, B. (2015). Potential of mass spectrometry metabolomics for chemical food safety. Bioanalysis Review, 7, 133–146.
Jacob, C. C., Dervilly-Pinel, G., Biancotto, G., Monteau, F., & Le Bizec, B. (2015). Global urine fingerprinting by LC-ESI(+)-HRMS for better characterization of metabolic pathway disruption upon anabolic practices in bovine. Metabolomics, 11, 184–197.
JECFA. (2010). Evaluation of data on ractopamine residues in pig tissues. FAO JECFA Monographs 9, meeting 2010.
Jiang, X. F., Zhu, Y. H., & Liu, X. Y. (2014). Identification of ractopamine glucuronides and determination of bioactive ractopamine residues and its metabolites in food animal urine by ELISA, LC-MS/MS and GC-MS. Food Additives and Contaminants–Part A Chemistry, Analysis, Control, Exposure and Risk Assessment, 31, 29–38.
Kessner, D., Chambers, M., Burke, R., Agusand, D., & Mallick, P. (2008). ProteoWizard: Open source software for rapid proteomics tools development. Bioinformatics (Oxford, England), 24, 2534–2536.
Kieken, F., Pinel, G., Antignac, J. P., Monteau, F., Christelle Paris, A., Popot, M. A., et al. (2009). Development of a metabonomic approach based on LC-ESI-HRMS measurements for profiling of metabolic changes induced by recombinant equine growth hormone in horse urine. Analytical and Bioanalytical Chemistry, 394, 2119–2128.
Kieken, F., Pinel, G., Antignac, J. P., Paris, A. C., Garcia, P., Popot, M. A., et al. (2011). Generation and processing of urinary and plasmatic metabolomic fingerprints to reveal an illegal administration of recombinant equine growth hormone from LC-HRMS measurements. Metabolomics, 7, 84–93.
Kouassi Nzoughet, J. J., Dervilly-Pinel, G., Chereau, S., Biancotto, G., Monteau, F., Elliott, C. T., et al. (2015). First insights into serum metabolomics of trenbolone/estradiol implanted bovines; screening model to predict hormone-treated and control animals’ status. Metabolomics, 11, 1184–1196.
Li, G. L., Fu, Y. H., Han, X. S., Li, X. Y., & Li, C. C. (2016). Metabolomic investigation of porcine muscle and fatty tissue after Clenbuterol treatment using gas chromatography/mass spectrometry. Journal of Chromatography A, 1456, 242–248.
Mills, S. E., Kissel, J., Bidwell, C. A., & Smith, D. J. (2003a). Stereoselectivity of porcine badrenergic receptors for ractopamine stereoisomers. Journal of Animal Science, 81, 122–129.
Mills, S. E., Spurlock, M. E., & Smith, D. J. (2003b). Beta-adrenergic subtypes that mediate ractopamine stimulation of lipolysis. Journal of Animal Science, 81, 662–668.
MOA. (2002). List of banned veterinary drugs and other compounds in food-producing animals. Ministry of Agriculture of China 235th Bulletin.
Nebbia, C., Urbani, A., Carletti, M., Gardini, G., Balbo, A., Bertarelli, D., et al. (2011). Novel strategies for tracing the exposure of meat cattle to illegal growth-promoters. Veterinary Journal, 189, 34–42.
Parry, T. E. (1957). Paper chromatography of 56 amino compounds using phenol and butanol-acetic acid as solvents with illustrative chromatograms of normal and abnormal urines. Clinica Chimica Acta, 2, 115–125.
Patti, G. J., Yanes, O., & Siuzdak, G. (2012). Metabolomics: The apogee of the omics trilogy. Nature Reviews Molecular Cell Biology, 13, 263–269.
Pinel, G., Weigel, S., Antignac, J. P., Mooney, M. H., Elliott, C., Nielen, M. W. F., et al. (2010). Targeted and untargeted profiling of biological fluids to screen for anabolic practices in cattle. TrAC Trends in Analytical Chemistry, 29, 1269–1280.
Ricke, E. A., Smith, D. J., Feil, V. J., Larsen, G. L., & Caton, J. S. (1999). Effects of ractopamine HCl stereoisomers on growth, nitrogen retention, and carcass composition in rats. Journal of Animal Science, 77, 701–707.
Riedmaier, I., Becker, C., Pfaffl, M. W., & Meyer, H. H. (2009). The use of omic technologies for biomarker development to trace functions of anabolic agents. Journal of Chromatograohy A, 1216, 8192–8199.
Smith, C. A., O’Maille, G., Want, E. J., Qin, C., Trauger, S. A., Brandon, T. et al. (2005). METLIN: A metabolite mass spectral database. Therapeutic Drug Monitoring, 27, 747–751.
Smith, C. A., Want, E. J., O’Maille, G., Abagyan, R., & Siuzdak, G. (2006). XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching and identification. Analytical Chemistry, 78, 779–787.
Sumner, L. W., Amberg, A., Barrett, D., Beale, M. H., Beger, R., Daykin, C. A., et al. (2007). Proposed minimum reporting standards for chemical analysis. Metabolomics, 3, 211–221.
Tautenhahn, R., Patti, G. J., Kalisiak, E., Miyamoto, T., Schmidt, M., Lo, F. Y., et al. (2011). metaXCMS: Second-order analysis of untargeted metabolomics data. Analytical Chemistry, 83, 696–700.
Tautenhahn, R., Patti, G. J., Rinehart, D., & Siuzdak, G. (2012). XCMS online: A web-based platform to process untargeted metabolomic data. Analytical Chemistry, 84, 5035–5039.
van der Kloet, F. M., Bobeldijk, I., Verheij, E. R., & Jellema, R. H. (2009). Analytical error reduction using single point calibration for accurate and precise metabolomic phenotyping. Journal of Proteome Research, 8, 5132–5141.
WHO (1993). Ractopamine. Toxicological evaluation of certain veterinary drug residues in food. Food Additives Series No. 31, nos 777 on INCHEM.
Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., & Goodacre, R. (2008). Global metabolic profiling of Escherichia coli cultures: An evaluation of methods for quenching and extraction of intracellular metabolites. Analytical Chemistry, 80, 2939–2948.
Wishart, D. S., Jewison, T., Guo, A. C., Wilson, M., Knox, C., Liu, Y., et al. (2013). HMDB 3.0—The human metabolome database in 2013. Nucleic Acids Research, 41, 801–807.
Wishart, D. S., Knox, C., Guo, A. C., Eisner, R., Young, N., Gautam, B., et al. (2009). HMDB: A knowledgebase for the human metabolome. Nucleic Acids Research, 37, 603–610.
Wishart, D. S., Tzur, D., Knox, C., Eisner, R., Guo, A. C., Young, N., et al. (2007). HMDB: The human metabolome database. Nucleic Acids Research, 3, 521–526.
Wu, Y. P., Bi, Y. F., Bingga, G. L., Li, X. W., Zhanga, S. X., Li, J. C., et al. (2015). Metabolomic analysis of swine urine treated with β2-agonists by ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Journal of Chromatography A, 1400, 74–81.
Yaeger, M. J., Mullin, K., Ensley, S. M., Ware, W. A., & Slavin, R. E. (2012). Myocardial toxicity in a group of greyhounds administered ractopamine. Veterinary Pathology, 49, 569–573.
Zhu, Z. J., Schultz, A. W., Wang, J., Johnson, C. H., Yannone, S. M., Patti, G. J., et al. (2013). Liquid chromatography quadropole-time-of-flight mass spectrometry characterization of metabolites guided by the METLIN database. Nature Protocols, 8, 451–460.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with animal studies and ethical standards
The animal study was approved by the national Ethical Committee (n°6) under agreement 2,015,092,516,084,715 / APAFIS 1914 (CRIP-2015-054).
Conflict of interest
The authors declare no conflicts of interest.
Compliance with ethical requirements
We confirm that this manuscript has not been published elsewhere and is not under consideration in another journal. All authors have approved the version of this manuscript and agree with its submission to Metabolomics.
Additional information
Study identifier in Metabolights: MTBLS384.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Peng, T., Royer, AL., Guitton, Y. et al. Serum-based metabolomics characterization of pigs treated with ractopamine. Metabolomics 13, 77 (2017). https://doi.org/10.1007/s11306-017-1212-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-017-1212-0