Abstract
Hepatitis B virus (HBV) infection is a major risk factor for deaths from liver cirrhosis and hepatocellular carcinoma (HCC). With a long-term goal of improving early diagnosis, we aimed to identify specific biomarkers associated with the development of HCC and liver cirrhosis in patients with HBV infection. Serum samples from 46 HBV infected patients with HCC and liver cirrhosis and 24 age–gender matched healthy subjects were profiled by liquid chromatography–mass spectrometry and gas chromatography–mass spectrometry. It was found that fatty acids and long-chain acylcarnitines were significantly elevated in HBV-related HCC patients, whereas most of the carbohydrates, amino acids, short- and medium-chain acylcarnitines, and glycerophospholipids were decreased, when compared to healthy subjects. The up-regulation of fatty acids and long-chain acylcarnitines in HCC was positively correlated with liver cirrhosis state. Logistic regression analysis indicated that palmitoylcarnitine together with arginine was an effective “combined marker” for diagnosing HBV-related HCC with 97.3 % sensitivity and 100 % specificity. Linoleic acid plus glucose was useful for discrimination of liver cirrhosis in HCC patients with 79.2 % sensitivity and 75 % specificity. These findings demonstrate that mass-spectrometry-based metabolomics is a promising tool that could provide special insights into tumor metabolism and identify novel biomarkers for detection of HCC and liver cirrhosis from HBV infected patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer that causes more than 0.6 million deaths worldwide every year (Jemal et al. 2011). Among the various etiological factors being implicated in the cause of HCC, the major risk factors are viral hepatitis infection (hepatitis B or C) and liver cirrhosis (Simonetti et al. 1991). In Asia, hepatitis B virus (HBV) infection is considered the most important cause for HCC, accounting for 80–90 % of HCC cases (Yin et al. 2009). The majority (70–80 %) of HBV-related HCC cases occur in patients accompanied with liver cirrhosis; however, HBV can cause HCC even when individuals have not developed formal liver cirrhosis (Chi et al. 2013). According to the estimation of the World Gastroenterology Organisation (WGO), more than 350 million people are suffering from chronic HBV infection throughout the world (http://www.worldgastroenterology.org/hepatitis-b.html). The large population infected with HBV makes the prevention of HCC a formidable task. It is believed that early diagnosis of liver cirrhosis and HCC from high-risk populations, such as those with HBV infection, is vital to extend survival time and decrease the pain and cost for patients (Onodera et al. 1995). However, current approaches for diagnosis of liver cirrhosis and HCC using liver imaging and tumor marker alpha-fetoprotein (AFP) lack sufficient sensitivity and specificity (Ressom et al. 2012). Therefore, discovery of novel biomarkers for early detection of liver cirrhosis and HCC in HBV infected patients is of great clinical benefit.
Metabolomics surveys employ comprehensive metabolic profiling methods for analysis of small-molecule metabolites within biological fluids and tissues, providing systemic views of biological processes (Nicholson and Lindon 2008). Nuclear magnetic resonance (NMR) and mass spectrometry (MS) are two commonly used analytical platforms in metabolomics studies (Moco et al. 2007). Both NMR and MS produce comprehensive findings on small molecules in biological samples, but they also have their own specific advantages. NMR has the advantage of requiring little sample preparation, and of producing datasets that are more easily mined (Zhou et al. 2010). The main advantage of MS is sensitivity. The state-of-the-art mass spectrometers coupled with liquid chromatography (LC–MS) and gas chromatography (GC–MS) can detect analytes routinely in the femtomolar to attomolar range (Veenstra 2012). Being the newest of the post-genomics technologies, metabolomics provides a new insight into the progression of cancers, and it has shown promise in identifying metabolite-based biomarkers of various cancers (Denkert et al. 2006; Matsumura et al. 2010; Sreekumar et al. 2009; Tan et al. 2013; Zhang et al. 2013; Zhu et al. 2014). Although there have been decades of metabolomics studies carried out on liver cirrhosis and HCC (Beyoglu et al. 2013; Gao et al. 2009; Ressom et al. 2012; Shariff et al. 2011; Shariff et al. 2010; Wang et al. 2013), to the best of our knowledge, so far there have only been a few metabolomics studies that specifically focus on HBV-related liver cirrhosis and HCC. In 2009, Yin et al. investigated the serum profiles of hepatitis B-induced liver cirrhosis and HCC using LC–MS. Glycocholic acid, glycochenodeoxycholic acid, taurocholic acid, and taurochenodesoxycholic acid were identified as potential biomarkers related to liver cirrhosis; dihydrosphingosine and phytosphingosine as potential diagnostic biomarkers for HCC (Yin et al. 2009). In 2012, Zhou et al. further investigated the serum metabolic alterations of HCC infected with HBV or hepatitis C virus (HCV), and they found that the decreased polyunsaturated lysophosphatidylcholines (lysoPCs) and elevated arachidonic acid may be mainly related to the initiation and progression of malignant hepatic tumor. Their data also revealed that endocannabinoids anandamide (AEA) and palmitylethanolamide (PEA) significantly elevated in HCC groups compared to healthy control, and their combination showed better sensitivity and specificity for distinguishing HCC from chronic liver diseases (Zhou et al. 2012a). In 2013, Liu et al. explored the tissue metabolic profiling of HBV-related HCC using LC–MS, and 14 metabolites were identified as biomarkers for HCC (Liu et al. 2013). There is currently no systematic metabolomics study based on GC–MS on HBV-related liver cirrhosis and HCC. LC–MS is suitable for analyzing high-polarity and secondary metabolites, while GC–MS is ideal for detecting volatile and derivatized compounds, and importantly they can produce complementary findings on the same sample sets (Halket et al. 2005; Lu et al. 2013).
In this study, we report an enhanced metabolic profiling study on the metabolic disturbances of HBV-related liver cirrhosis and HCC using a combined LC–MS and GC–MS analytical platform, and aim to identify specific biomarkers associated with liver cirrhosis and HCC in a HBV infected cohort to assist in early diagnosis. In brief, serum samples from 46 HBV infected patients with HCC and liver cirrhosis and 24 age–gender matched healthy volunteers were analyzed by liquid chromatography quadrupole time-of-flight mass spectrometry (LC-Q-ToF–MS) and gas chromatography quadrupole time-of-flight mass spectrometry (GC-Q-ToF–MS). Multivariate analyses such as principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were applied to identify differential metabolites that are related to HCC and liver cirrhosis. On the basis of identified metabolites, the related pathways and correlation networks were investigated and their potential clinical value was discussed.
2 Materials and methods
2.1 Chemicals
HPLC grade acetonitrile, methanol, and formic acid were purchased from Merck. Distilled water was purified “in-house” using a Milli-Q system Millipore. N-(9-Fluorenylmethoxycarbonyl)-glycine (FMOC-glycine), N-methyl-N-trimethyl-silyl-trifluoroacetamide (MSTFA), d-galactose, d-glucose, l-serine, l-proline, fumarate, malate, stearic acid, myo-inositol, and cholesterol were purchased from Sigma-Aldrich. Ammonium bicarbonate and oleic acid were purchased from Fluka. Isotopically labeled acetylcarnitine and palmitoylcarnitine were purchased from Cambridge Isotope Laboratories.
2.2 Participants
A total of 46 HBV infected patients with HCC (36 males and 10 females) were recruited at the Shanghai Huashan Hospital between February 2011 and August 2012. Among them, 28 patients (22 males and 6 females) were accompanied with liver cirrhosis. In parallel to these patients, 24 age- and gender-matched healthy subjects (19 males and 5 females) with no known history of chronic liver diseases were recruited as control at the same environment. The detailed clinical information of collected subjects is presented in Table 1. All participants voluntarily joined this study, gave written informed consent, and completed a questionnaire that provided demographical information including age, gender, lifestyle factors, and medical family history. The study protocol was approved by the Ethics Committees of Shanghai Huashan Hospital and National University of Singapore, and was conducted in accordance with the Helsinki Declaration of 1964, as revised in 1983.
2.3 Training set and test set
A portion (~80 %) of the 70 subjects (22 HBV-related HCC patients with liver cirrhosis, 14 HBV-related HCC patients without liver cirrhosis, and 19 healthy individuals) was used as a training set to explore the specific biomarkers associated with HCC and liver cirrhosis. The remaining ~20 % samples (six HBV-related HCC patients with liver cirrhosis, four HBV-related HCC patients without liver cirrhosis, and five healthy individuals) was used as a test set to validate the diagnostic capabilities of two combined markers discovered from the training set. The detailed information of subjects in training and test sets is presented in Supplementary Material Tables S1 and S2. No significant differences in the age and gender were found between HCC patients in the training and test sets (p > 0.05) (Supplementary Material Table S3).
2.4 Serum collection and preparation
The morning fasting antecubital venous blood samples were collected from the 70 participants using a sterile 21-gage needle syringe, allowed to clot at 4 °C overnight and centrifuged at 1699×g for 10 min. The sera (100 μL) were collected and diluted four times with cold methanol containing 10 μg/mL FMOC-glycine as internal standard (IS). The mixture was shaken vigorously for 30 s. After centrifugation at 20,817×g for 10 min at 4 °C, the supernatant fraction was collected and divided into two parts: one (100 μL) for LC–MS analysis and the other one (10 μL) for GC–MS analysis after derivatization. The 10 μL supernatant was dried under nitrogen and then derivatized with 100 µL of methoxyamine in pyridine (5 mg/mL) at 37 °C for 2 h, and followed by 100 µL MSTFA (37 °C × 16 h). After centrifugation (4 °C, 3824g × 1 min), the supernatant fraction was injected into GC–MS. Pooled quality control (QC) sample was prepared by mixing equal amounts (20 μL) of each sample. The QC sample was analyzed at the beginning, the end and randomly throughout the whole assay, for both LC–MS and GC–MS measurements to evaluate the stability of analytical performance.
2.5 Liquid chromatography–mass spectrometry (LC–MS)
LC–MS analysis was performed on an Agilent 1290 ultrahigh pressure liquid chromatography system (Waldbronn, Germany) coupled to a 6540 Q-ToF mass detector equipped with an electrospray ionization (ESI) source. The samples were analyzed in both ESI-positive and -negative ion modes. The separation was performed on an Agilent rapid resolution HT Zorbax SB-C18 column (2.1 × 50 mm2, 1.8 µm, Aglient) at a column temperature of 50 °C. The mobile phases A (water with 0.1 % formic acid) and B (acetonitrile with 0.1 % formic acid) were employed in the positive mode, whereas C (water with 5 mmol/L NH4HCO3) and D (95 % acetonitrile and 5 % water with 5 mmol/L NH4HCO3) were used in the negative mode. The gradient program was: 0–9 min, 5–45 % B (or D); 9–15 min, 45–100 % B (or D); 15–18 min, 100 % B (or D); 18–20 min, 100–5 % B (or D). The flow rate was set at 0.4 mL/min. A 10 μL aliquot of sample was injected for each. Mass data were collected between m/z 100 and 1000 at a rate of two scans per second. The ion spray voltage was set at 4000 V, and the heated capillary temperature was maintained at 350 °C. The drying gas and nebulizer nitrogen gas flow rates were 12.0 L/min and 50 psi, respectively. The targeted MS/MS analysis was carried out to study the structure of potential biomarkers. In this section, we used three different collision energies at 10, 20, and 40 V for each target ion.
2.6 Gas chromatography–mass spectrometry (GC–MS)
GC–MS analysis was performed on an Agilent 7683B Series Injector (Agilent, Santa Clara, CA) coupled to an Agilent 7890A Series gas chromatograph system and a 7200 Q-ToF mass detector (Agilent, Santa Clara, CA). A fused-silica capillary column HP-5MSI (30 m × 0.25 mm i.d., 0.25 μm film thickness) was used. The injector was kept at 250 °C. A 1 μL of sample was splitlessly injected for each individual analysis. Helium was used as the carrier gas with a constant flow rate 1 mL/min through the column. The GC oven temperature was maintained at 70 °C for 1 min, and then increased to 250 °C at a rate of 10 °C/min and further increased at 25 °C/min to 300 °C and held for 6 min. The transfer line temperature was kept at 280 °C. Detection was achieved using MS in electron impact mode (70 eV) and full scan monitoring (m/z 50–550). The temperature of the ion source was set at 230 °C, and the quadrupole was set at 150 °C.
2.7 Data processing and analysis
The LC–MS and GC–MS spectral data were exported as mzData files using Agilent MassHunter Qualitative analysis software (version B.05), and pretreated by the open-source software MZmine 2 for peak detection, peak alignment and peak area normalization in each dataset. The preprocessed metabolomics data were uploaded into SIMCA 13.0.3 software for multivariate statistical analyses, including PCA and OPLS-DA. Univariate statistical tests (student t test and one-way ANOVA), receiver operating characteristic (ROC) analysis, binary logistic regression, and Pearson correlation analysis were performed on SPSS 16.0 software. The p values less than 0.05 were considered significant. The false discovery rate (FDR) method of Benjamini and Yekutieli was used to correct for multiple hypothesis testing & reduce false positives.
2.8 Metabolite identification
The structure identification of metabolic candidates was based on our previously described strategy (Gao et al. 2014; Wen et al. 2013). In brief, NIST 11 mass spectral library was used to identify metabolites out of GC–MS spectra based on retention index and mass-spectral similarity (more than 80 %) match. For LC–MS analysis, the metabolites were temporarily identified based on accurate mass, MS/MS information (fragment pattern) pattern, and retention time by matching features in HMDB (http://www.hmdb.ca/), METLIN (http://metlin.scripps.edu/), and Lipid MAPS (http://www.lipidmaps.org/) databases (Supplementary Material Table S4). Finally, some of these identified metabolites were further confirmed using commercial standards.
3 Results and discussion
HCC is currently one of the most common malignancies worldwide that causes significant mortality, and its incidence is increasing dramatically in many parts of the world (Davis et al. 2008). The 5-year overall survival rate for patients with HCC is less than 5 % (El-Serag 2011). Liver cirrhosis and infection with hepatitis B or C are the more common causes of HCC. It should be noted that HBV is the major risk factor for HCC in the Asian region. Although tremendous efforts have been made over the past few decades to discover novel biomarkers for early diagnosis of HCC in clinical practice, our knowledge of HBV-related HCC is still limited. Therefore, for the current study we focused on the metabolic profiling of HBV-related HCC and liver cirrhosis, hoping to identify specific biomarkers associated with HCC and liver cirrhosis in a HBV infected cohort.
3.1 Subject characteristics
A total of 46 hepatitis B surface antigen (HBsAg) positive patients with HCC and liver cirrhosis and 24 age–gender matched (p > 0.05) healthy volunteers were enrolled in the present study. All of the 46 patients have HCC, and 28 patients were accompanied with liver cirrhosis. Edmonson stages of HCC ranged from stages I to IV. Serum tumor marker AFP was monitored to assist in diagnosis of HCC. It was found that the level of AFP in 16 patients was less than the clinical cut-off value (i.e., 20 mg/L) (Shen et al. 2012). In addition, serum levels of alanine transaminase (ALT) and aspartate transaminase (AST) were determined in patients to indicate the extent of liver damage. The detailed information is presented in Table 1. No significant differences in AFP and AST were found between HCC patients with and without liver cirrhosis, but the levels of ALT were significantly different. These parameters were not determined in healthy volunteers.
3.2 Reliability of analytical methods
A non-targeted serum metabolomics analysis using a combination of LC–MS and GC–MS analytical methods was applied in the training set. The 55 serum samples were analyzed in a random order, and pooled QC samples were inserted into the analysis sequence to evaluate the stability of analytical systems. Firstly, the spectral data of QC samples were analyzed by PCA together with all serum samples collected. The QC samples were tightly clustered in the PCA scores plots (Supplementary Material Fig. S1). In parallel to qualifying the overall performance of the methods, variations of retention time, mass accuracy and peak area of the peaks across all QC samples were calculated in both LC–MS and GC–MS: the retention time shift was less than 0.2 min; the mass accuracy deviation was less than 5 mDa; the relative standard deviations (RSDs) of peak areas were below 30 %. These results demonstrated that our analytical methods provided measurement stability and reliability for the present study.
3.3 Global metabolic shifts in HBV-related HCC
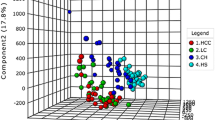
We first compared the serum metabolic profiles of 36 HBV-related HCC patients and 19 healthy individuals in the training set to explore the metabolic shifts in HBV-related HCC patients. The data from both LC–MS and GC–MS mass spectra were merged together for multivariate and univariate statistical analyses. The PCA scores plot (Fig. 1) showed that the healthy individuals (n = 19) cluster clearly apart from HCC patients (n = 36), indicating the metabolic profiles in HCC patients have changed. The model parameters of R2X (cum) and Q2 (cum) are 0.526 and 0.287, respectively. Finally, 26 metabolites through LC–MS analysis and 19 by GC–MS analysis were identified as significantly different in HBV-related HCC patients compared to healthy controls (p < 0.05), including carbohydrates, amino acids, fatty acids, acylcarnitines, glycerophospholipids, and other kinds of metabolite (Table 2). To have an overview on the differential expression of these 45 metabolites, we applied heat map based on the analysis of Pearson correlation coefficients to visualize their relative levels in each individual (Fig. 2). Compared to healthy individuals, HBV-related HCC patients had higher levels of fatty acids (stearic acid, oleic acid, linoleic acid, and linolenic aicd) and long-chain acylcarnitines (hexadecadienoylcarnitine, hexadecenoylcarnitine, linoleylcarnitine, palmitoylcarnitine, and tetradecadiencarnitine), together with lower levels of carbohydrates (glucose, and galactose), amino acids (serine, glycine, and threonine), glycerophospholipids [LysoPC(14:0), LysoPC(15:0), LysoPE(16:0), LysoPEe(16:0), LysoPEp(16:0), LysoPE(20:5)], short- and medium-chain acylcarnitines (acetylcarnitine, decanoylcarnitine, pimelylcarnitine, undecanoylcarnitine, and valerylcarnitine), and the metabolites (malate, fumarate, arginine, and urea) in tricarboxylic acid (TCA) and urea cycles.
Heat map of 45 identified differential metabolites in 19 healthy individuals and 36 HBV-related HCC patients (including 14 patients without liver cirrhosis and 22 patients with liver cirrhosis). Each row shows peak area for a specific metabolite after mean centering and unit variance scaling of the data. Each column shows the serum metabolic profile of each subject. ±, The metabolites were detected and identified by LC-MS. #, The metabolites were detected and identified by GC–MS (Color figure online)
3.4 Altered pathways in HBV-related HCC
Further, the altered pathways in HBV-related HCC were investigated based on these differential metabolites. On the basis of the online database of metabolic pathways (KEGG PATHWAY Database, http://www.genome.jp/kegg/), it was found that most of the metabolites identified in our study were mapped onto general biochemical pathways, such as glycolysis, TCA cycle, urea cycle, carbohydrate metabolism, amino acid metabolism, and lipid metabolism (Fig. 3). These pathways can be broadly classified into three types: (i) energy metabolism, (ii) amino acid metabolism, and (iii) lipid metabolism.
Major differential metabolites and pathways in HBV-related HCC patients: these metabolites in the Green box were decreased in patients’ serum when compared to healthy individuals, whereas the metabolites in Red box increased. Glycerate-3P, Glycerate 3-phosphate; Glycerol-3P, Glycerol 3-phosphate; LysoPC, including LysoPC(14:0), (15:0); LysoPE, including LysoPE (16:0), (20:5), LysoPEe(16:0), and LysoPEp(16:0) (Color figure online)
3.4.1 Energy metabolism
Significantly altered energy metabolism in serum metabolite concentrations were observed in HBV-related HCC patients. Compared with healthy subjects, glucose as a major metabolic precursor of glycolysis and the subsequent TCA cycle was presented with a significant down-regulation in HCC patients. Not surprisingly, the metabolic intermediates of glycolysis and TCA cycle such as pyruvate, malate, and fumarate were also observed with a significant reduction in the serum of HCC patients (Fig. 3). This unique feature of glycolysis and TCA cycle is consistent with the abnormal propensity of cancer cells to produce most energy for their survival and growth by aerobic glycolysis, in which significant amount of pyruvate was consumed and converted to lactate, rather than through oxidative phosphorylation in mitochondria (i.e., Warburg Effect) (Gatenby and Gillies 2004). These findings indicate that cancer cell changes energy metabolism by promoting glycolysis and suppressing the TCA cycle.
3.4.2 Amino acid metabolism
Several HCC metabolomics studies have linked amino acid metabolism aberrations to cancer development (Chen et al. 2011; Lai et al. 2005). Consistent with these reports, significant down-regulation of serine, glycine, and threonine in HCC patients was found in our present study (Fig. 3). Also, we observed down-regulation of dipeptides in HCC, e.g., phenylalanylphenylalanine and phenylalanylisoleucine (Fig. 2). The increased demand of amino acid in the presence of malignant tumors for tumor protein synthesis and energy supply is thought to be one of the reasons for this decrease in serum (Baniasadi et al. 2013). For instance, as an allosteric activator of pyruvate kinase M2 (PKM2), serine involves in the rate-limiting conversion of phosphoenolpyruvate to pyruvate during glycolysis (Chaneton et al. 2012). In addition, we found gamma-aminobutyric acid (GABA) was significantly increased in HCC patients. GABA primarily functions as an inhibitory neurotransmitter in the mature central nervous system (CNS) (Li et al. 2012). Recently, the abnormal levels of gene and protein expression of some GABA receptor subunits have been detected in malignant tumors, such as π subunit in pancreatic cancer (Takehara et al. 2007). Also it has been reported that GABA promotes human HCC tumor growth through overexpressed GABAA receptor α3 and theta subunits (Li et al. 2012; Liu et al. 2008). This indicates that GABAergic system may play an important role in the development of malignant tumors.
3.4.3 Lipid metabolism
The largest number of molecules of interest that were altered in the serum of HCC patients was in the lipid group, mainly on fatty acids, phospholipids, and acylcarnitines. Fatty acids are used for both structural purpose (membrane lipids synthesis) and energy production (beta-oxidation) in malignant tumors (Santos and Schulze 2012). We observed that the levels of saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) were increased in HCC patients, such as stearic acid (18:0), oleic acid (18:1), linoleic acid (18:2), linolenic acid (18:3) (Fig. 3). Such significant increase of fatty acids in HCC may have been the results of energy requirement and cell membrane synthesis due to aggressive cell proliferation. Phospholipids have been reported previously as candidate markers for HCC (Patterson et al. 2011). We found significantly abnormal expression of glycerophospholipids in HCC patients, such as LysoPC(14:0), LysoPC(15:0), LysoPE(16:0), LysoPEe(16:0), LysoPEp(16:0), and LysoPE(20:5) (Fig. 2). It is known that LysoPCs and LysoPEs regulate a variety of biological processes including cell proliferation, tumor cell invasiveness, and inflammation (Ressom et al. 2012). The down-regulation of them may mainly result from rapid membrane PC and PE turnover during the liver injury or malignant regeneration (Liu et al. 2014). Acylcarnitines (carnitine-fatty acid esters) are essential intermediates in lipid metabolism, which facilitate transportation of long-chain fatty acids into mitochondria for beta-oxidation to generate adenosine triphosphate (ATP), assist in the removal of short- and medium-chain fatty acids out of mitochondria, and maintain membrane phospholipid turnover (Sharma and Black 2009). Recent publication by Zhou et al. discussed the differential metabolism of acylcarnitines in serum of HCC patients, and concluded that acylcarnitines with short- and long-chain showed reverse accumulation in HCC (Zhou et al. 2012b). This matches our finding of up-regulation of long-chain acylcarnitines and down-regulation of short- and medium-chain acylcarnitines in HCC patients (Fig. 3), and supports the hypothesis that beta-oxidation of fatty acids is an important source of energy production in cancer cells, in which the relative accumulation of long-chain acylcarnitines would be detected in HCC compared with healthy controls. On the other hand such accumulation of long-chain acylcarnitines may inhibit the formation of short-chain acyl-CoA, which causes a relatively smaller generation of short-chain acylcarnitines in HCC patients (Xiao et al. 2012).
3.5 Differential metabolites in patients with and without liver cirrhosis
We next investigated the metabolic variations between these HCC patients with and without liver cirrhosis, by comparing the metabolic profiles of HCC patients with and without liver cirrhosis, as well as the healthy individuals. Although the PCA scores plot only showed a weak tendency of difference in metabolic profiles for patients with and without liver cirrhosis, along the direction of the second principal component (Supplementary Material Fig. S2), the OPLS-DA scores plot revealed a clear separation for HCC patients with versus without liver cirrhosis, and healthy individuals cluster tightly apart from HCC patients as well, with the performance of R2X (cum) = 0.311, R2Y (cum) = 0.887, and Q2 (cum) = 0.500 (Supplementary Material Fig. S3A). The validation plot strongly indicated the validity of the corresponding PLS-DA model without over-fitting, since the intercept of the Q2 regression line in blue was below zero and the permuted R2 values in green on the left were lower than the original point on the right (Supplementary Material Fig. S3B). The result showed a robust metabolic difference between HCC patients with and without liver cirrhosis. To further define effective biomarkers for HCC patients with liver cirrhosis, we compared the distribution of identified 45 metabolites in HCC patients with and without liver cirrhosis. Detailed statistical information is presented in Supplementary Material Table S5. The significance was expressed by using the Student’s t test. Finally, we found that levels of five long-chain acylcarnitines and three fatty acids in HCC patients with liver cirrhosis were significantly higher than in those without liver cirrhosis, whereas the concentrations of glucose were significantly lower in HCC patients with liver cirrhosis (Fig. 4).
Scatter dot plots show the differential levels of long-chain acylcarnitines, fatty acids, and glucose in healthy individuals (healthy, n = 19), HCC patients without liver cirrhosis (HCC, n = 14), and HCC patients with liver cirrhosis (HCC + LC, n = 22). The horizontal bar indicates the mean value. Y-axis represents peak areas of these metabolites. *P < 0.05, **P < 0.01, ***P < 0.001 compared to the HCC patients without liver cirrhosis; # P < 0.05, ## P < 0.01, ### P < 0.001 compared to the healthy individuals
3.6 Diagnostic potential of differential metabolites
The long-term survival remains dismal for HCC patients due to lack of effective diagnostic markers. AFP is the most widely used biomarker currently available for detection of HCC. However, as mentioned above, serum AFP levels are known to be of low sensitivity and specificity for HCC diagnosis. As an evidence, there are 16 out of the 46 HCC patients recruited in this study whose AFP level is less than the clinical cut-off value (20 mg/L) (Table 1). ALT and AST are often measured to identify liver disease, especially liver cirrhosis and hepatitis caused by alcohol, drugs, or viruses. When the liver is diseased or damaged, additional ALT and AST are released into the bloodstream, which make their levels go up. Recently, the AST/ALT ratio (>1) has been reported as an indicator of liver cirrhosis, which is frequently elevated in an alcoholic liver disease pattern in patients with liver cirrhosis. (Nyblom et al. 2006). However, no significant difference in AST/ALT ratio was found between HCC patients with and without liver cirrhosis in our study (p > 0.05), yet serum levels of ALT in 28 patients with liver cirrhosis were significant lower than those in 18 patients without liver cirrhosis (p < 0.05) (Table 1). In this study, therefore, we evaluated the diagnostic potential of these differential metabolites for HCC and liver cirrhosis.
3.6.1 HCC diagnosis
The use of ROC curves was first exploited based on the results of area under the curve (AUC), and then the prediction probability was evaluated on the basis of sensitivity and specificity. Based on ROC analysis, we found that six differential metabolites including linolenic acid, 9-HODE, palmitoylcarnitine, lysoPEp (16:0), linoleic acid, and arginine showed a high capability to diagnose HBV-related HCC patients from healthy controls, for which AUC scores were greater than 0.950 (Supplementary Material Table S6). Further, we performed binary logistic regression and Pearson correlation analyses to obtain the best combination of biomarkers, and found palmitoylcarnitine coupled with arginine was the best subset for HCC detection, which was termed as “combined maker I” (AUC = 0.988) (Fig. 5a). The prediction probability values of this “combined maker I” obtained from the binary logistic regression is shown in Fig. 5b. At the traditional cut-off value (i.e., 0.5) (Zeng et al. 2014), 36 out of 36 HCC patients and 18 out of 19 healthy individuals were correctly discriminated in the training set, with 97.3 % sensitivity and 100 % specificity. Importantly, we found that the level of palmitoylcarnitine significantly correlated with serum AFP level in HBV-related HCC patients (r = 0.314, p < 0.05) (Supplementary Material Table S7), indicating the complementary function to AFP for detection of HCC. This hypothesis was also proved by our result that the simplified “combined marker I” is highly effective for those AFP false-negative (AFP < 20 mg/L, n = 16) HCC subjects with a diagnostic accuracy of 100 %. Therefore, the combinational use of “combined marker I” and AFP has the promising clinical potential to improve the diagnostic accuracy of HCC.
a ROC curve of “combined marker I” for HCC diagnosis; b Discrimination of HBV-related HCC patients and healthy subjects in training and test sets by using “combined marker I” at a cut-off of prediction probability of 0.5; c ROC curve of “combined marker II” for liver cirrhosis detection; d discrimination of HCC patients with and without liver cirrhosis in training and test sets by using “combined marker II”
3.6.2 Liver cirrhosis detection
Similarly, we found that the combination of oleic acid and glucose was better to establish the discrimination model for the differentiation of HCC patients with and without liver cirrhosis, which was termed as “combined maker II” (AUC = 0.828) (Fig. 5c). This result is consistent with our findings in Fig. 4 that significant overexpression of fatty acids and down-regulation of glucose in HCC patients with versus without liver cirrhosis. At the cutoff value of 0.5, 86.4 % and 64.3 % of HCC patients with and without liver cirrhosis were correctly discriminated in the training set, indicating 79.2 % sensitivity and 75 % specificity (Fig. 5d). Our results suggested that the combination of these two metabolites was useful for liver cirrhosis detection in HCC patients.
3.7 Independent validation
The diagnostic capabilities of the two combined markers with respect to HCC and liver cirrhosis were further validated in an independent test set, including ten HBV-related HCC patients and five healthy individuals. As shown in the classification tree (Fig. 6), the 15 subjects were first classified based on “combined marker I” (palmitoylcarnitine and arginine), and a case went to group A if its prediction probability value was less than 0.5 (Fig. 5b), otherwise went to group B. As a result, all of the five healthy subjects were in group A and ten HBV-related HCC patients were in group B. The ten cases in group B were further classified using “combined marker II” (linoleic acid and glucose), and a case went to group C if its prediction probability value was less than 0.5 (Fig. 5d), otherwise went to group D. Finally, four HCC patients without liver cirrhosis and one patient with liver cirrhosis were in group C and five HCC patients with liver cirrhosis were in group D. In summary, the “combined marker I” correctly predicted all of the HCC patients (ten out of ten) and healthy subjects (five out of five), showing a 100 % sensitivity and specificity. HCC patients with and without liver cirrhosis were also correctly discriminated using “combined marker II”, with an 80 % accuracy rate. The results further demonstrated that palmitoylcarnitine together with arginine is an effective combined marker for HBV-related HCC detection, and oleic acid coupled with glucose is useful for monitoring liver cirrhosis appearance in HCC patients.
4 Concluding remarks
In summary, we characterized the serum metabolic profiles of HBV infected patients with HCC and liver cirrhosis by combining LC–MS and GC–MS analytical system. Our findings demonstrated that the differential metabolites revealed by the two techniques were complementary, which offered the potential to assess the perturbed metabolic pathways related to HBV-related HCC. Compared with the limited metabolomics studies about HBV-related HCC, the novel “combined marker I” consisting of palmitoylcarnitine and arginine was defined and evaluated as a remarkably effective tool for HCC diagnosis. Further, the “combined marker II” consisting of oleic acid and glucose was able to discriminate liver cirrhosis in HCC patients. Although the present data are promising, additional studies are needed in larger populations with and without HBV infection as well as with and without HCC and liver cirrhosis to examine the two combined markers are truly HCC and liver cirrhosis specific.
References
Baniasadi, H., Gowda, G. A., Gu, H., et al. (2013). Targeted metabolic profiling of hepatocellular carcinoma and hepatitis C using LC-MS/MS. Electrophoresis, 34, 2910–2917.
Beyoglu, D., Imbeaud, S., Maurhofer, O., et al. (2013). Tissue metabolomics of hepatocellular carcinoma: tumor energy metabolism and the role of transcriptomic classification. Hepatology, 58, 229–238.
Chaneton, B., Hillmann, P., Zheng, L., et al. (2012). Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature, 491, 458–462.
Chen, T., Xie, G., Wang, X., et al. (2011). Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Molecular and Cellular Proteomics, 10(M110), 004945.
Chi, H. C., Chen, C. Y., Tsai, M. M., Tsai, C. Y., & Lin, K. H. (2013). Molecular functions of thyroid hormones and their clinical significance in liver-related diseases. Biomed Research International, 601361.
Davis, G. L., Dempster, J., Meler, J. D., et al. (2008). Hepatocellular carcinoma: management of an increasingly common problem. Proc (Bayl Univ Med Cent), 21, 266–280.
Denkert, C., Budczies, J., Kind, T., et al. (2006). Mass spectrometry-based metabolic profiling reveals different metabolite patterns in invasive ovarian carcinomas and ovarian borderline tumors. Cancer Research, 66, 10795–10804.
El-Serag, H. B. (2011). Hepatocellular Carcinoma. New England Journal of Medicine, 365, 1118–1127.
Gao, Y. H., Lu, Y. H., Huang, S. M., et al. (2014). Identifying early urinary metabolic changes with long-term environmental exposure to cadmium by mass-spectrometry-based metabolomics. Environmental Science and Technology, 48, 6409–6418.
Gao, H., Lu, Q., Liu, X., et al. (2009). Application of 1H NMR-based metabonomics in the study of metabolic profiling of human hepatocellular carcinoma and liver cirrhosis. Cancer Science, 100, 782–785.
Gatenby, R. A., & Gillies, R. J. (2004). Why do cancers have high aerobic glycolysis? Nature Reviews Cancer, 4, 891–899.
Halket, J. M., Waterman, D., Przyborowska, A. M., et al. (2005). Chemical derivatization and mass spectral libraries in metabolic profiling by GC/MS and LC/MS/MS. Journal of Experimental Botany, 56, 219–243.
Jemal, A., Bray, F., Center, M. M., et al. (2011). Global cancer statistics. CA: A Cancer Journal for Clinicians, 61, 69–90.
Lai, H. S., Lee, J. C., Lee, P. H., Wang, S. T., & Chen, W. J. (2005). Plasma free amino acid profile in cancer patients. Seminars in Cancer Biology, 15, 267–276.
Li, Y. H., Liu, Y., Li, Y. D., et al. (2012). GABA stimulates human hepatocellular carcinoma growth through overexpressed GABAA receptor theta subunit. World Journal of Gastroenterology, 18, 2704–2711.
Liu, Y., Hong, Z. Y., Tan, G. G., et al. (2014). NMR and LC/MS-based global metabolomics to identify serum biomarkers differentiating hepatocellular carcinoma from liver cirrhosis. International Journal of Cancer, 135, 658–668.
Liu, Y., Li, Y. H., Guo, F. J., et al. (2008). Gamma-aminobutyric acid promotes human hepatocellular carcinoma growth through overexpressed gamma-aminobutyric acid A receptor alpha 3 subunit. World Journal of Gastroenterology, 14, 7175–7182.
Liu, S. Y., Zhang, R. L., Kang, H., Fan, Z. J., & Du, Z. (2013). Human liver tissue metabolic profiling research on hepatitis B virus-related hepatocellular carcinoma. World Journal of Gastroenterology, 19, 3423–3432.
Lu, Y. H., Lam, H. M., Pi, E. X., et al. (2013). Comparative metabolomics in Glycine max and Glycine soja under salt stress to reveal the phenotypes of their offspring. Journal of Agricultural and Food Chemistry, 61, 8711–8721.
Matsumura, K., Opiekun, M., Oka, H., et al. (2010). Urinary volatile compounds as biomarkers for lung cancer: A proof of principle study using odor signatures in mouse models of lung cancer. PLoS One, 5.
Moco, S., Bino, R. J., De Vos, R. C. H., & Vervoort, J. (2007). Metabolomics technologies and metabolite identification. Trac-Trends in Analytical Chemistry, 26, 855–866.
Nicholson, J. K., & Lindon, J. C. (2008). Systems biology: Metabonomics. Nature, 455, 1054–1056.
Nyblom, H., Bjornsson, E., Simren, M., et al. (2006). The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver International, 26, 840–845.
Onodera, H., Ukai, K., & Minami, Y. (1995). Hepatocellular carcinoma cases with five-year survival and prognostic factors affecting the survival time. The Tohoku Journal of Experimental Medicine, 176, 203–211.
Patterson, A. D., Maurhofer, O., Beyoglu, D., et al. (2011). Aberrant lipid metabolism in hepatocellular carcinoma revealed by plasma metabolomics and lipid profiling. Cancer Research, 71, 6590–6600.
Ressom, H. W., Xiao, J. F., Tuli, L., et al. (2012). Utilization of metabolomics to identify serum biomarkers for hepatocellular carcinoma in patients with liver cirrhosis. Analytica Chimica Acta, 743, 90–100.
Santos, C. R., & Schulze, A. (2012). Lipid metabolism in cancer. FEBS Journal, 279, 2610–2623.
Shariff, M. I. F., Gomaa, A. I., Cox, I. J., et al. (2011). Urinary metabolic biomarkers of hepatocellular carcinoma in an Egyptian population: a validation study. Journal of Proteome Research, 10, 1828–1836.
Shariff, M. I. F., Ladep, N. G., Cox, I. J., et al. (2010). Characterization of urinary biomarkers of hepatocellular carcinoma using magnetic resonance spectroscopy in a Nigerian population. Journal of Proteome Research, 9, 1096–1103.
Sharma, S., & Black, S. M. (2009). Carnitine homeostasis, mitochondrial function and cardiovascular disease. Drug Discovery Today: Disease Mechanisms, 6, e31–e39.
Shen, Q. J., Fan, J., Yang, X. R., et al. (2012). Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncology, 13, 817–826.
Simonetti, R. G., Camma, C., Fiorello, F., et al. (1991). Hepatocellular carcinoma. A worldwide problem and the major risk factors. Digestive Diseases and Sciences, 36, 962–972.
Sreekumar, A., Poisson, L. M., Rajendiran, T. M., et al. (2009). Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature, 457, 910–914.
Takehara, A., Hosokawa, M., Eguchi, H., et al. (2007). Gamma-aminobutyric acid (GABA) stimulates pancreatic cancer growth through overexpressing GABAA receptor pi subunit. Cancer Research, 67, 9704–9712.
Tan, B. B., Qiu, Y. P., Zou, X., et al. (2013). Metabonomics identifies serum metabolite markers of colorectal cancer. Journal of Proteome Research, 12, 3000–3009.
Veenstra, T. D. (2012). Metabolomics: The final frontier? Genome Medicine, 4, 40.
Wang, X. J., Zhang, A. H., & Sun, H. (2013). Power of metabolomics in diagnosis and biomarker discovery of hepatocellular carcinoma. Hepatology, 57, 2072–2077.
Wen, T., Gao, L., Wen, Z. M., et al. (2013). Exploratory investigation of plasma metabolomics in human lung adenocarcinoma. Molecular BioSystems, 9, 2370–2378.
Xiao, J. F., Varghese, R. S., Zhou, B., et al. (2012). LC-MS based serum metabolomics for identification of hepatocellular carcinoma biomarkers in Egyptian cohort. Journal of Proteome Research, 11, 5914–5923.
Yin, P. Y., Wan, D. F., Zhao, C. X., et al. (2009). A metabonomic study of hepatitis B-induced liver cirrhosis and hepatocellular carcinoma by using RP-LC and HILIC coupled with mass spectrometry. Molecular BioSystems, 5, 868–876.
Zeng, J., Yin, P., Tan, Y., et al. (2014). Metabolomics study of hepatocellular carcinoma: discovery and validation of serum potential biomarkers by using capillary electrophoresis-mass spectrometry. Journal of Proteome Research, 13, 3420–3431.
Zhang, T., Wu, X. Y., Ke, C. F., et al. (2013). Identification of potential biomarkers for ovarian cancer by urinary metabolomic profiling. Journal of Proteome Research, 12, 505–512.
Zhou, L., Ding, L., Yin, P., et al. (2012a). Serum metabolic profiling study of hepatocellular carcinoma infected with hepatitis B or hepatitis C virus by using liquid chromatography-mass spectrometry. Journal of Proteome Research, 11, 5433–5442.
Zhou, M., McDonald, J. F., & Fernandez, F. M. (2010). Optimization of a direct analysis in real time/time-of-flight mass spectrometry method for rapid serum metabolomic fingerprinting. Journal of the American Society for Mass Spectrometry, 21, 68–75.
Zhou, L., Wang, Q., Yin, P., et al. (2012b). Serum metabolomics reveals the deregulation of fatty acids metabolism in hepatocellular carcinoma and chronic liver diseases. Analytical and Bioanalytical Chemistry, 403, 203–213.
Zhu, J. J., Djukovic, D., Deng, L. L., et al. (2014). Colorectal cancer detection using targeted serum metabolic profiling. Journal of Proteome Research, 13, 4120–4130.
Acknowledgments
This study was supported in part by the grant of Natural Science Fund of The Science and Technology Commission of Shanghai, China (No. 12ZR1404300), Singapore Medical Research Council (No. NMRC/1242/2009), the NUS secondment Funds to CNO and the NUS Environmental Research Institute (NERI).
Conflict of Interest
All authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study. All participants voluntarily joined this study, gave written informed consent, and completed a questionnaire that provided demographical information including age, gender, lifestyle factors, and medical family history.
Compliance with Ethical Requirements
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was approved by the Ethics Committees of Shanghai Huashan Hospital and National University of Singapore.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yonghai Lu and Chong Huang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, Y., Huang, C., Gao, L. et al. Identification of serum biomarkers associated with hepatitis B virus-related hepatocellular carcinoma and liver cirrhosis using mass-spectrometry-based metabolomics. Metabolomics 11, 1526–1538 (2015). https://doi.org/10.1007/s11306-015-0804-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-015-0804-9