Abstract
Introduction
Liver cirrhosis (LC) is an advanced liver disease that can develop into hepatocellular carcinoma. Hepatitis B virus (HBV) infection is one of the main causes of LC. Therefore, there is an urgent need for developing a new method to monitor the progression of HBV-related LC (HBV-LC).
Objectives
In this study, we attempted to examine serum metabolic changes in healthy individuals as well as patients with HBV and HBV-LC. Furthermore, potential metabolite biomarkers were identified to evaluate patients progressed from health to HBV-LC.
Methods
Metabolic profiles in the serum of healthy individuals as well as patients with HBV and HBV-LC were detected using an NMR-based metabolomic approach. Univariate and multivariate analyses were conducted to analyze serum metabolic changes during HBV-LC progression. Moreover, potential metabolite biomarkers were explored by receiver operating characteristic curve analysis.
Results
Serum metabolic changes were closely associated with the progression of HBV-LC, mainly involving energy metabolism, protein metabolism, lipid metabolism and microbial metabolism. Serum histidine was identified as a potential biomarker for HBV patients. Acetate, formate, pyruvate and glutamine in the serum were identified as a potential biomarker panel for patients progressed from HBV to HBV-LC. In addition, phenylalanine, unsaturated lipid, n-acetylglycoprotein and acetone in the serum could be considered as a potential common biomarkers panel for these patients.
Conclusion
NMR-based serum metabolomic approach could be a promising tool to monitor the progression of liver disease. Different metabolites may reflect different stages of liver disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Liver cirrhosis (LC) as an advanced liver disease can develop into hepatocellular carcinoma (HCC) and seriously affect the public health (Mokdad et al. 2014). In general, LC can be caused by acute or chronic liver injury, such as alcohol abuse, hepatitis virus infection and hepatotoxic chemical exposure (Askgaard et al. 2015; Perz et al. 2006). Relative to other causes of LC, however, chronic infection with hepatitis B or C virus (HBV or HCV) has a higher risk of HCC (Fattovich et al. 2004; Bialecki and Bisceglie 2005). Perz et al. (2006) reported that 57% of cirrhosis and 78% of HCC were attributable to HBV or HCV infection. Currently, liver transplantation remains the only therapeutic approach for the treatment of LC, but it is only effective for a few patients (Tsochatzis et al. 2014). Thus, the best strategy for LC management should be prevention, early-diagnosis and early-intervention (Tsochatzis et al. 2014). The diagnosis of LC mainly includes liver transaminases test, ultrasonography, computerized tomography (CT) and magnetic resonance imaging (MRI) (Schuppan and Afdhal 2008). Yet, these methods are not sensitive and final diagnosis still needs liver biopsy. Therefore, it is of great interest and important to develop a new tool for monitoring the progression of LC.
Of note, omics-based approaches have shown great potential for disease diagnosis by identifying biological phenotypes at omics levels, including genomics, proteomics and metabolomics. For example, Callewaert et al. (2004) developed a clinical glycomics technique to distinguish compensated cirrhotic from noncirrhotic chronic liver disease patients with 79% sensitivity and 86% specificity. Using a genome-wide miRNA microarray, miR-106b and miR-181b were identified in the serum as specific biomarkers with an AUC of 0.774 for chronic HBV-LC and 0.915 for non-chronic HBV-LC (Chen et al. 2013). Moreover, Xie et al. (2014) reported that serum miR-101 can differentiate between HBV-HCC and HBV-LC with 95.5% sensitivity and 90.2% specificity. Serum microfibril-associated protein 4 (MFAP-4) was used for differentiating LC patients from individuals without liver disease (AUC value = 0.97) using a proteomic approach (Mölleken et al. 2009). In addition, Yin et al. (2009) used a mass spectrometry (MS)-based metabolomic approach to identify several potential serum biomarkers for LC patients such as glycocholic acid, glycochenodeoxycholic acid and taurocholic acid. Possible fecal biomarkers, including lysophosphatidylcholines, bile acids and bile pigments, were also detected in LC patients by MS-based metabolomics (Cao et al. 2011). Moreover, nuclear magnetic resonance (NMR)-based serum metabolomics was also successfully applied to characterize metabolic differences between healthy individuals and patients with LC or HCC (Gao et al. 2009). However, metabolomics-based diagnosis of liver disease is still in its infancy.
In the present study, therefore, serum metabolic profiles in healthy individuals as well as patients with HBV and HBV-LC were detected using an NMR-based metabolomic approach. We then attempted to analyze serum metabolic changes from healthy individuals to HBV patients as well as from HBV patients to HBV-LC patients via univariate and multivariate analyses. Furthermore, possible metabolite biomarkers were explored by receiver operating characteristic curve analysis. Finally, this study will offer potential metabolic information for monitoring the progression of HBV-LC.
2 Materials and methods
2.1 Clinical sample collection
Seventy blood samples were collected from 25 healthy individuals (age = 46 ± 2 years; male/female = 15/10), 20 patients with hepatitis B virus (HBV: age = 45 ± 4 years; male/female = 13/7) and 25 patients with hepatitis B virus-related cirrhosis (HBV-LC: age = 48 ± 2 years; male/female = 16/9) from Lishui Central Hospital (Lishui, China). All healthy subjects without history of liver disease had a normal liver biochemistry. Patients with HBV were diagnosed by positive serum surface antigen of HBV for more than 6 months, but no sign of cirrhosis or any tumors. Patients with HBV-LC were diagnosed according to clinical biochemistry, imaging and histological examinations. Patients with other chronic liver diseases, including hepatitis C virus, hepatocellular carcinoma, autoimmune hepatitis, alcoholic liver disease and hepatic decompensation, were excluded in the present study. Blood samples were collected from the antecubital vein in the morning before breakfast. Serum sample was then separated by centrifugation at 1024 g for 10 min at 4 °C and stored at −80 °C until NMR analysis. Written informed consents were obtained from all participants. This study was approved by the Ethics Committee of Lishui Central Hospital in accordance with the Declaration of Helsinki.
2.2 NMR-based metabolomic analysis
Serum sample was thawed and vortexed, and 200 μL serum sample was mixed with 400 μL of 0.2 M phosphate buffer (pH = 7.4) to minimize pH variations. And then, the mixture was centrifuged at 12,000×g for 10 min at 4 °C. Supernatant (500 μL) was transferred to a 5 mm NMR tube and mixed with 100 μL D2O for NMR measurement. 1H NMR spectra were recorded using a Bruker AVANCE III 600 MHz NMR spectrometer with a 5-mm TXI probe (Bruker BioSpin, Rheinstetten, Germany) at 25 °C. In order to minimize the line-broadening effect of macromolecules such as protein and lipid, Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence with a fixed receiver-gain value was performed. The main acquisition parameters included: acquisition time, 1.64 s per scan; relaxation delay, 4 s; data points, 32K; spectral width, 10,000 Hz.
NMR spectra were automatically preprocessed using phase/baseline corrections and referenced to the methyl signal of lactate at 1.33 ppm in the Topspin 3.0 software (Bruker BioSpin, Rheinstetten, Germany). As shown in Fig. 1a, a total of 23 metabolite signals were assigned according to the reported data (Nicholson et al. 1995; Psychogios et al. 2011) and the human metabolome database (Wishart et al. 2012). Moreover, a representative sample was analyzed by two-dimensional 1H-1H COSY and TOCSY experiments to confirm the uncertain assignments. The relative level of each metabolite was calculated via its peak area by reference to the total spectrum area.
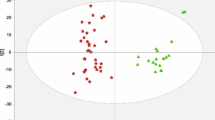
Serum metabolic characterizations during the progression of hepatitis B virus (HBV)-related liver cirrhosis (HBV-LC). a A typical 1H NMR spectrum of human serum and 23 metabolites assigned in this study. b Score plot and c loading plot of principal component analysis based on serum metabolomic data from healthy individuals (red color open triangle), HBV patients (green color plus symbol) and HBV-LC patients (blue color times symbol). Metabolite set enrichment analysis (MSEA) based on changes of 23 assigned metabolites from healthy individuals to HBV patients (d) as well as from HBV to HBV-LC patients (e). The red dots represent the pathways with a significant difference (P < 0.05) between two groups, and the numbers correspond to the metabolic pathways in Table S1
2.3 Data analysis
Metabolite data were Log-transformed and Auto-scaled prior to data analysis. Principal component analysis (PCA) was used to obtain an overview changes in metabolic patterns among healthy individuals, HBV and HBV-LC patients using the assigned metabolites on the MetaboAnalyst 3.0 (Xia et al. 2015). Partial least squares-discriminate analysis (PLS-DA) was carried out to compare the metabolic differences between healthy individuals and HBV patients as well as between HBV and HBV-LC patients. A leave-one-out cross-validation (LOO-CV) method was performed, where R2 and Q2 were calculated as goodness of fitness and predictability of PLS-DA, respectively. These two parameters close to 1.0 indicate an excellent PLS-DA model. Furthermore, a permutation test with 2000 permutations based on separation distance was employed to validate the model (Xia et al. 2015). Important metabolites for classification between two groups were identified by the variable importance in the projection (VIP) method. Metabolites with VIP values >1.0 were selected for further analysis. PLS-DA modeling was also conducted using the MetaboAnalyst 3.0 (Xia et al. 2015). Metabolite set enrichment analysis (MSEA) was performed to inspect changes of main metabolic pathways. Receiver operating characteristic (ROC) area under the curve (AUC) analysis was conducted to evaluate the classification ability of each metabolite using the MetaboAnalyst 3.0 (Xia et al. 2015). In the present study, metabolite with an AUC value >8.0 was considered as a good biomarker. The Venn diagram was drawn manually using Adobe Photoshop CS6 (Adobe Inc, San Jose CA). The difference of each metabolite between two groups was analyzed by Student’s t test with Bonferroni correction in SAS 9.2 (SAS Institute Inc, Cary, NC). In this study, the difference was considered significant if Bonferroni-adjusted P value <0.05.
3 Results
3.1 Serum metabolic characterizations in HBV and HBV-LC patients
Figure 1a illustrates a typical 1H NMR spectrum obtained from human serum. A total of 23 metabolite signals were identified, involving 3-hydroxybutyrate, acetate, acetoacetate, acetone, alanine, choline, citrate, creatine, formate, glucose, glutamine, glycine, histidine, isoleucine, lactate, LDL/VLDL, leucine, n-acetylglycoprotein, phenylalanine, pyruvate, tyrosine, unsaturated lipid and valine.
PCA score plot shows an obvious difference in serum metabolic characterizations among healthy individuals, HBV and HBV-LC patients (Fig. 1b). The corresponding loading plot reveals that most metabolites were presented in the HBV-LC region, as shown in Fig. 1c, mainly involving protein metabolism, energy metabolism and lipid metabolism. MSEA results were shown in Fig. 1d from healthy individuals to HBV patients as well as Fig. 1e from HBV to HBV-LC patients, and the detailed information were listed in Table S1. We found that the number of significantly changed pathways was obviously increased from HBV patients to HBV-LC patients (Fig. 1e) as compared with that from healthy individuals to HBV patients (Fig. 1d).
3.2 Serum metabolic changes during the progression of HBV-LC
We then analyzed changes in metabolic characterizations in the serum between healthy individuals and HBV patients as well as between patients with HBV and HBV-LC using PLS-DA. Clear and statistically significant separations were obtained between them as shown in Fig. 2a (H vs. HBV: PLS = 2; R2 = 0.61; Q2 = 0.50; P < 0.001) and Fig. 2c (HBV vs. HBV-LC: PLS = 2; R2 = 0.58; Q2 = 0.41; P < 0.001). Moreover, it can be seen from the corresponding VIP plot that histidine, n-acetylglycoprotein, phenylalanine, acetone, unsaturated lipid and citrate in the serum were mainly contributed to the metabolic difference between healthy individuals and HBV patients (VIP > 1.0, Fig. 2b). The change in metabolic pattern from HBV to HBV-LC shown in Fig. 2c was mainly attributed to unsaturated lipid, phenylalanine, acetone, n-acetylglycoprotein, glutamine, formate, acetate and pyruvate in the serum (VIP > 1.0, Fig. 2d). From Fig. 2e, we can see that serum histidine and citrate were only selected as important metabolites that contributed to the metabolic difference between healthy individuals and HBV patients. ANOVA results show that HBV patients had significantly higher levels of histidine and citrate than healthy individuals, as shown in Fig. 2f, g, respectively. However, we also found that their levels were significantly increased from HBV to HBV-LC. A Venn diagram exhibits the common metabolites including phenylalanine, unsaturated lipid, n-acetylglycoprotein and acetone (Fig. 2e). Serum phenylalanine (Fig. 2h) and unsaturated lipid (Fig. 2i) levels were significantly increased during the progression of HBV-LC, whereas significant reductions were observed in n-acetylglycoprotein (Fig. 2j) and acetone (Fig. 2k). In addition, pyruvate, glutamine, creatine, formate and acetate were only identified in the serum from the metabolic difference between HBV and HBV-LC (Fig. 2e). These findings were in line with ANOVA results. Compared with HBV patients, pyruvate (Fig. 2l), glutamine (Fig. 2m), creatine (Fig. 2n) and acetate (Fig. 2p) levels were significantly higher in HBV-LC patients, while serum formate level was significantly decreased (Fig. 2o). Yet, there was no significant difference between healthy individuals and HBV patients.
Serum metabolic changes during the progression of hepatitis B virus (HBV)-related liver cirrhosis (HBV-LC). a Score plot of partial least squares-discriminate analysis (PLS-DA) based on 23 assigned metabolites between healthy individuals and HBV patients (PLS = 2; R2 = 0.61; Q2 = 0.50; P < 0.001); b VIP plot between healthy individuals and HBV patients; c score plot of PLS-DA based on 23 assigned metabolites between HBV and HBV-LC patients (PLS = 2; R2 = 0.58; Q2 = 0.41; P < 0.001); d VIP plot between HBV and HBV-LC patients; e the Venn diagram showing metabolites with a VIP value >1.0. The change of metabolite level during the progression of HBV-LC: f histidine; g citrate; h phenylalanine; i unsaturated lipid; j n-acetylglycoprotein; k acetone; l pyruvate; m glutamine; n creatine; o formate; p acetate. r.u. relative unit, H healthy individuals, HBV patients with HBV, HBV-LC patients with HBV-related liver cirrhosis. Significant level: *P < 0.05; **P < 0.01; ***P < 0.001
Figure 3A shows the AUC value of each metabolite for distinguishing between healthy individuals and HBV patients as well as between HBV and HBV-LC patients. Metabolites with an AUC value >0.80 were summarized by a Venn diagram, as shown in Fig. 3b. Similar results were observed with PLS-DA results in Fig. 2e, except citrate and creatine. Serum histidine had an excellent AUC value of 0.91 for distinguishing between healthy individuals and HBV patients (H → HBV), but not for distinguishing between HBV and HBV-LC patients (HBV → HBV-LC, AUC value = 0.73). According to Fig. 3a, pyruvate, glutamine, formate and acetate in the serum were able to achieve AUC values >0.80 for HBV→HBV-LC, whereas AUC values <0.70 for H → HBV. In addition, phenylalanine, unsaturated lipid, n-acetylglycoprotein and acetone had AUC values of 0.87, 0.81, 0.86 and 0.87 for H → HBV as well as 0.89, 0.88, 0.87 and 0.87 for HBV → HBV-LC, respectively (Fig. 3a).
Receiver operating characteristic area under the curve (AUC) analysis. a AUC values of 23 assigned metabolites for distinguishing between healthy individuals and HBV patients (H → HBV) as well as between HBV and HBV-LC patients (HBV → HBV-LC). b The Venn diagram showing metabolites with an AUC value >8.0
4 Discussion
The liver is a central metabolic organ that maintains metabolic homeostasis in humans. Consequently, liver disease will certainly result in abnormal metabolism. In this study, we also found that patients with HBV or HBV-LC had a clear difference in serum metabolic pattern relative to healthy individuals. Also, metabolic difference in the serum was increasingly pronounced during the progression of HBV-LC. Our metabolomic results suggest that the altered metabolic pathways may mainly include energy metabolism, protein metabolism, lipid metabolism and even microbial metabolism.
Disturbance of energy metabolism is a common metabolic feature in cirrhosis. In this study, our metabolomic data show that citrate and pyruvate, as a key substrate of Krebs cycle, were increased in the serum during the progression of HBV-LC, particularly in patients with HBV-LC. These findings indicate an increase in energy metabolism during HBV-LC progression. Moreover, relative to healthy individuals and HBV patients, serum creatine, as an important metabolite in the energy transfer process (Longo et al. 2011), was also significantly increased in HBV-LC patients. This result seems to further confirm an increased energy metabolism in HBV-LC patients. Increased levels of citrate, pyruvate and creatine in the serum of LC patients were also identified by Embade et al. (2016), Gao et al. (2009) and Qi et al. (2012) via an NMR-based metabolomic approach, respectively. Taken together, an increase in energy metabolism could be considered as an important characteristic during the progression of HBV-LC. Using indirect heat measurement, however, Meng et al. (2010) found that Chinese patients with chronic HBV and LC had a lower energy expenditure as well as respiratory quotient. We speculate that indicators based on indirect heat measurement were not correlated with serum metabolomic data for evaluating HBV-LC progression. In fact, Tajika et al. (2002) have reported that resting energy expenditure was associated with anthropometric parameters but not with blood biochemistry. They proposed that parameters measured by indirect calorimetry may be mainly affected by extrahepatic factors (Tajika et al. 2002). Therefore, in clinical practice, we should consider that different measurements may lead to different interpretations.
Abnormality in protein metabolism occurs commonly in liver disease, since the liver is a main organ for amino acid conversion. In our study, serum amino acid levels were disordered during the progression of HBV-LC, as indicated by higher levels of histidine, phenylalanine and glutamine in patients with HBV or HBV-LC than healthy individuals. Increased phenylalanine and glutamine levels in the serum of LC patients were also detected by Gao et al. (2009). We speculate that HBV or HBV-LC may be able to activate proteolysis. Meng et al. (2010) have reported that patients with chronic HBV and LC had a higher protein oxidation rate. Thus, enhanced hydrolysis/oxidation of protein may result in protein deficiency in HBV and HBV-LC patients. In fact, protein-energy malnutrition has been regarded as a common feature for LC patients (Huisman et al. 2011). Nevertheless, more attention is paid to a decreased level of branched-chain amino acids (BCAAs) in LC patients (Moriwaki et al. 2004; Holecek 2010). In the present study, serum BCAAs were not selected as potential biomarkers for HBV and HBV-LC. However, histidine, phenylalanine and glutamine in the serum were identified. Therefore, we suggest that other amino acids excepting BCAAs also need to be considered for the study of liver disease. For example, Yang et al. (2012) reported that the ratio of BCAAs to tyrosine could be used for the diagnosis, treatment selection and prognosis of patients with different stages of HBV infection. In addition, we also found that serum n-acetylglycoprotein level was significantly decreased during the progression of HBV-LC. Using an NMR-based metabolomic approach, Amathieu et al. (2011) and Embade et al. (2016) also observed a reduced level of serum n-acetylglycoprotein in patients with chronic liver failure and advanced liver fibrosis, respectively. Yet, serum n-acetylglycoprotein was not reported for monitoring patients with HBV and HBV-LC.
Liver disease is also associated with impaired lipid metabolism. Vere et al. (2012) found significantly lower levels of serum lipid and cholesterol in both HBV- and HCV-LC as compared with controls. Relative to healthy individuals, patients with non-alcoholic LC had an increased LDL-cholesterol but a decreased HDL-cholesterol (Chrostek et al. 2014). Moreover, serum triglycerides may be considered as a marker only for alcoholic cirrhosis (Chrostek et al. 2014). Arain et al. (2017) reported that patients with HBV-LC had a higher level of saturated/monounsaturated fatty acid and a lower level of polyunsaturated fatty acid in the serum than healthy controls. In the present study, we observed a significantly increased level of unsaturated lipid in the serum of patients with HBV and HBV-LC compared with healthy individuals. Acetone is mainly produced in the mitochondria of liver cells via lipolysis. The level of serum acetone was significantly decreased during the progression of HBV-LC, indicating an inhibition of lipolysis. Taken together, it should not be generalized that the reduction of lipid metabolism is correlated with HBV-LC progression. In addition, acetate and formate belong to short-chain fatty acids, which are derived from gut microflora fermentation. An alteration of gut microflora has been associated with liver cirrhosis in humans (Qin et al. 2014). Therefore, our results reveal that the increase in acetate and the decrease in formate may be attributed to changes of gut microflora.
As discussed above, we can conclude that serum metabolomic analysis could be used as a potential tool for monitoring HBV-LC progression. However, we suggest that different metabolites might reflect different stages of liver disease. For example, serum histidine could be used as a potential biomarker for patients with HBV. Acetate, formate, pyruvate and glutamine in the serum could be considered as a potential biomarker panel for discriminating between patients with HBV and HBV-LC. Moreover, a potential biomarker panel including phenylalanine, unsaturated lipid, n-acetylglycoprotein and acetone was considered for monitoring the development of cirrhosis. The application of metabolomics in clinical practice is derived from the assumption that diseases cause disturbance of biochemical pathways, thereby resulting in a special metabolic characteristic in patients (Monteiro et al. 2013). Individual metabolite test, such as glucose, creatinine, bilirubin and ammonia, has been commonly used in the daily clinical test. However, this test is not effective in the diagnosis of most diseases. Since metabolomics aims to analyze a comprehensive set of metabolites in biological samples, it can provide a more precise assessment of patients’ physiological status than individual metabolite test (Holmes et al. 2008). In the present study, we also proposed a combination biomarker approach for monitoring the development of cirrhosis.
Metabolomics shows great potential in biomarker discovery (Monteiro et al. 2013) and disease diagnosis (Medina et al. 2014); however, its clinical application is still in an early stage. Various metabolite biomarkers have been reported in multiple diseases, but so far no one biomarker is used in clinical practice. Clinical metabolomics still remains a challenging area. On the one hand, metabolome is highly sensitive to both internal (e.g. age, gender and genetics) and external (e.g. diet, drug and lifestyle) factors. Thus, these confounding factors must be considered for clinical metabolomics. In this study, other influence factors, such as age, gender, diet and other diseases, were minimized as much as possible to make the metabolomic data interpretable. On the other hand, metabolome contains a huge amount of data. A series of data reduction strategies were developed to handle this type of data. For example, in the present study, important metabolites related to liver disease were identified by PLS-DA and ROC analysis. Therefore, to accelerate the development of clinical metabolomics, two aspects need to be further explored in the future: (1) building a comprehensive disease metabolite database that contains metabolic characterizations of various diseases under different conditions; (2) developing more advanced models to analyze and manage the metabolome data, such as artificial intelligence model. We believe that metabolomics-based approach will be developed as an auxiliary diagnostic tool for clinical diseases.
5 Conclusions
In the present study, we found that serum metabolic changes were closely associated with the progression of HBV-LC using an NMR-based metabolomic approach. Thus, metabolomics-based serum analysis could be used to monitor HBV-LC progression. Moreover, different metabolites may reflect different stages of liver disease. In this study, serum histidine was identified as a potential biomarker for patients with HBV. Acetate, formate, pyruvate and glutamine in the serum were considered as a potential biomarker panel for patients progressed from HBV to HBV-LC. In clinical practice, however, serum metabolome could be affected by many factors, such as age, gender and other diseases. Further studies need to discover more sensitive and specific serum-based biomarkers for monitoring the progression of liver disease.
References
Amathieu, R., Nahon, P., Triba, M., Bouchemal, N., Trinchet, J. C., Beaugrand, M., et al. (2011). Metabolomic approach by 1H NMR spectroscopy of serum for the assessment of chronic liver failure in patients with cirrhosis. Journal of Proteome Research, 10(7), 3239–3245.
Arain, S. Q., Talpur, F. N., Channa, N. A., Ali, M. S., & Afridi, H. I. (2017). Serum lipid profile as a marker of liver impairment in hepatitis B Cirrhosis patients. Lipids in Health and Disease, 16(1), 51.
Askgaard, G., Grønbæk, M., Kjær, M. S., Tjønneland, A., & Tolstrup, J. S. (2015). Alcohol drinking pattern and risk of alcoholic liver cirrhosis: A prospective cohort study. Journal of Hepatology, 62(5), 1061–1067.
Bialecki, E. S., & Di Bisceglie, A. M. (2005). Clinical presentation and natural course of hepatocellular carcinoma. European Journal of Gastroenterology & Hepatology, 17(5), 485–489.
Callewaert, N., Van Vlierberghe, H., Van Hecke, A., Laroy, W., Delanghe, J., & Contreras, R. (2004). Noninvasive diagnosis of liver cirrhosis using DNA sequencer–based total serum protein glycomics. Nature Medicine, 10(4), 429–434.
Cao, H., Huang, H., Xu, W., Chen, D., Yu, J., Li, J., et al. (2011). Fecal metabolome profiling of liver cirrhosis and hepatocellular carcinoma patients by ultra performance liquid chromatography–mass spectrometry. Analytica Chimica Acta, 691(1), 68–75.
Chen, Y. J., Zhu, J. M., Wu, H., Fan, J., Zhou, J., Hu, J., et al. (2013). Circulating microRNAs as a fingerprint for liver cirrhosis. PLoS ONE, 8(6), e66577.
Chrostek, L., Supronowicz, L., Panasiuk, A., Cylwik, B., Gruszewska, E., & Flisiak, R. (2014). The effect of the severity of liver cirrhosis on the level of lipids and lipoproteins. Clinical and Experimental Medicine, 14(4), 417–421.
Embade, N., Mariño, Z., Diercks, T., Cano, A., Lens, S., Cabrera, D., et al. (2016). Metabolic characterization of advanced liver fibrosis in HCV patients as studied by serum 1H-NMR spectroscopy. PLoS ONE, 11(5), e0155094.
Fattovich, G., Stroffolini, T., Zagni, I., & Donato, F. (2004). Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology, 127(5), S35-S50.
Gao, H., Lu, Q., Liu, X., Cong, H., Zhao, L., Wang, H., et al. (2009). Application of 1H NMR-based metabonomics in the study of metabolic profiling of human hepatocellular carcinoma and liver cirrhosis. Cancer Science, 100(4), 782–785.
Holecek, M. (2010). Three targets of branched-chain amino acid supplementation in the treatment of liver disease. Nutrition (Burbank, Los Angeles County, Calif.), 26(5), 482–490.
Holmes, E., Wilson, I. D., & Nicholson, J. K. (2008). Metabolic phenotyping in health and disease. Cell, 134(5), 714–717.
Huisman, E. J., Trip, E. J., Siersema, P. D., van Hoek, B., & van Erpecum, K. J. (2011). Protein energy malnutrition predicts complications in liver cirrhosis. European Journal of Gastroenterology & Hepatology, 23(11), 982–989.
Longo, N., Ardon, O., Vanzo, R., Schwartz, E., & Pasquali, M. (2011). Disorders of creatine transport and metabolism. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 157(No. 1), 72–78.
Medina, S., Dominguez-Perles, R., Gil, J. I., Ferreres, F., & Gil-Izquierdo, A. (2014). Metabolomics and the diagnosis of human diseases-A guide to the markers and pathophysiological pathways affected. Current Medicinal Chemistry, 21(7), 823–848.
Meng, Q. H., Wang, J. H., Yu, H. W., Li, J., Feng, Y. M., Hou, W., et al. (2010). Resting energy expenditure and substrate metabolism in Chinese patients with acute or chronic hepatitis B or liver cirrhosis. Internal Medicine, 49(19), 2085–2091.
Mokdad, A. A., Lopez, A. D., Shahraz, S., Lozano, R., Mokdad, A. H., Stanaway, J., et al. (2014). Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Medicine, 12(1), 145.
Mölleken, C., Sitek, B., Henkel, C., Poschmann, G., Sipos, B., Wiese, S., et al. (2009). Detection of novel biomarkers of liver cirrhosis by proteomic analysis. Hepatology (Baltimore, Md.), 49(4), 1257–1266.
Monteiro, M. S., Carvalho, M., Bastos, M. L., & Guedes de Pinho, P. (2013). Metabolomics analysis for biomarker discovery: Advances and challenges. Current Medicinal Chemistry, 20(2), 257–271.
Moriwaki, H., Miwa, Y., Tajika, M., Kato, M., Fukushima, H., & Shiraki, M. (2004). Branched-chain amino acids as a protein-and energy-source in liver cirrhosis. Biochemical and Biophysical Research Communications, 313(2), 405–409.
Nicholson, J. K., Foxall, P. J., Spraul, M., Farrant, R. D., & Lindon, J. C. (1995). 750 MHz 1H and 1H-13C NMR spectroscopy of human blood plasma. Analytical Chemistry, 67, 793–811.
Perz, J. F., Armstrong, G. L., Farrington, L. A., Hutin, Y. J., & Bell, B. P. (2006). The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of Hepatology, 45(4), 529–538.
Psychogios, N., Hau, D. D., Peng, J., Guo, A. C., Mandal, R., Bouatra, S., et al. (2011). The human serum metabolome. PLoS ONE, 6(2), e16957.
Qi, S., Tu, Z., Ouyang, X., Wang, L., Peng, W., Cai, A., et al. (2012). Comparison of the metabolic profiling of hepatitis B virus-infected cirrhosis and alcoholic cirrhosis patients by using 1H NMR-based metabonomics. Hepatology Research, 42(7), 677–685.
Qin, N., Yang, F., Li, A., Prifti, E., Chen, Y., Shao, L., et al. (2014). Alterations of the human gut microbiome in liver cirrhosis. Nature, 513(7516), 59–64.
Schuppan, D., & Afdhal, N. H. (2008). Liver cirrhosis. Lancet, 371(9615), 838–851.
Tajika, M., Kato, M., Mohri, H., Miwa, Y., Kato, T., Ohnishi, H., & Moriwaki, H. (2002). Prognostic value of energy metabolism in patients with viral liver cirrhosis. Nutrition (Burbank, Los Angeles County, Calif.), 18(3), 229–234.
Tsochatzis, E. A., Bosch, J., & Burroughs, A. K. (2014). Liver cirrhosis. Lancet, 383(9930), 1749–1761.
Vere, C. C., Streba, C. T., Streba, L., & Rogoveanu, I. (2012). Lipid serum profile in patients with viral liver cirrhosis. Medical Principles and Practice, 21(6), 566–568.
Wishart, D. S., Jewison, T., Guo, A. C., Wilson, M., Knox, C., Liu, Y., et al. (2012). HMDB 3.0-the human metabolome database in 2013. Nucleic Acids Research, 41, 801–807.
Xia, J., Sinelnikov, I. V., Han, B., & Wishart, D. S. (2015). MetaboAnalyst 3.0-making metabolomics more meaningful. Nucleic Acids Research, 43(W1), W251–W257.
Xie, Y., Yao, Q., Butt, A. M., Guo, J., Tian, Z., Bao, X., et al. (2014). Expression profiling of serum microRNA-101 in HBV-associated chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Cancer Biology & Therapy, 15(9), 1248–1255.
Yang, J., He, J., Cao, H., Zhao, X., Fu, S., Lu, H., et al. (2012). Correlation between plasma amino acid profiles and the various stages of hepatitis B infection. European Journal of Clinical Microbiology & Infectious Diseases, 31(8), 2045–2052.
Yin, P., Wan, D., Zhao, C., Chen, J., Zhao, X., Wang, W., et al. (2009). A metabonomic study of hepatitis B-induced liver cirrhosis and hepatocellular carcinoma by using RP-LC and HILIC coupled with mass spectrometry. Molecular BioSystems, 5(8), 868–876.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos.: 21605115, 21575105, 81573657) and the Public Welfare Technology Application Research Foundation of Zhejiang Province (No.: 2017C33066).
Author information
Authors and Affiliations
Contributions
HCG, JSJ and HZ contributed to experimental design. MJC, SML and LCZ contributed to sample collection and NMR metabolomic analysis. HZ and HCG contributed to data analysis, result interpretation and writing. All authors have read, revised and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Lishui Central Hospital.
Informed consent
Written informed consents were obtained from all participants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, H., Chen, M., Lu, S. et al. Metabolic characterization of hepatitis B virus-related liver cirrhosis using NMR-based serum metabolomics. Metabolomics 13, 121 (2017). https://doi.org/10.1007/s11306-017-1260-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-017-1260-5