Abstract
Isovaleric acidemia (IVA, MIM 248600) can be a severe and potentially life-threatening disease in affected neonates, but with a positive prognosis on treatment for some phenotypes. This study presents the first application of metabolomics to evaluate the metabolite profiles derived from urine samples of untreated and treated IVA patients as well as of obligate heterozygotes. All IVA patients carried the same homozygous c.367 G > A nucleotide change in exon 4 of the IVD gene but manifested phenotypic diversity. Concurrent class analysis (CONCA) was used to compare all the metabolites from the original complete data set obtained from the three case and two control groups used in this investigation. This application of CONCA has not been reported previously, and is used here to compare four different modes of scaling of all metabolites. The variables important in discrimination from the CONCA thus enabled the recognition of different metabolic patterns encapsulated within the data sets that would not have been revealed by using only one mode of scaling. Application of multivariate and univariate analyses disclosed 11 important metabolites that distinguished untreated IVA from controls. These included well-established diagnostic biomarkers of IVA, endogenous detoxification markers, and 3-hydroxycaproic acid, an indicator of ketosis, but not reported previously for this disease. Nine metabolites were identified that reflected the effect of treatment of IVA. They included detoxification products and indicators related to the high carbohydrate and low protein diet which formed the hallmark of the treatment. This investigation also provides the first comparative metabolite profile for heterozygotes of this inherited metabolic disorder. The detection of informative metabolites in even very low concentrations in all three experimental groups highlights the potential advantage of the holistic mode of analysis of inherited metabolic diseases in a metabolomics investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metabolites are widely used as diagnostic and/or prognostic indicators of inherited metabolic diseases (IMDs). The prognosis for a number of treatable disorders has been shown to be increasingly encouraging, pending early detection, preferably by newborn screening. Isovaleric acidemia (IVA, MIM 248600) is one such example of a treatable condition, although it is still considered to be a severe and potentially life-threatening disorder in acutely affected neonates (Sweetman and Williams 2001). IVA is an autosomal recessive disorder of leucine catabolism caused by a deficiency of isovaleryl-CoA dehydrogenase (IVD, EC 1.3.99.10), which catalyses the conversion of isovaleryl-CoA to 3-methylcrotonyl-CoA. IVA is currently recognized as a disorder with a heterogeneous phenotype, ranging from acute neonatal metabolic acidosis (often fatal) to a mild, chronic disease (Vockley and Ensenauer 2006). The nature of the IVD gene mutations, located on chromosome 15q14-15, is likewise highly heterogeneous, showing many missense and frame-shift mutations in its coding region as well as splice site mutations (Ensenauer et al. 2004). IVA patients involved in the present metabolomics study were, however, genotypically homogeneous as all carried the same homozygous c.367 G > A nucleotide change in exon 4 of the IVD gene (Dercksen et al. 2012). Nevertheless, they did show the characteristic phenotypic diversity associated with IVA.
The first identification of IVA (Tanaka et al. 1966) was achieved by the analysis of urine organic acids using gas chromatography (GC). The subsequent introduction of mass spectrometry (MS) and application of GC–MS in the investigation of IMDs have greatly facilitated the recognition and metabolic description of novel IMDs (Scriver et al. 2001). These technological advances contributed to the identification of many secondary metabolites as well as conjugates of isovaleric acid (Sweetman and Williams 2001; Loots et al. 2005; Loots 2009) in the nearly fifty years since the first description of IVA. Apart from the routine identification of IMDs by chromatographic analysis of metabolites, the metabolomics analysis of IMDs are advocated to illuminate further the relationship between the biochemical aberrations and their resultant clinical conditions (Pan et al. 2007; Mamas et al. 2011; Knerr et al. 2011). This application may also benefit drug development, the treatment of these disorders as well as the understanding of drug toxicity associated with IMDs (Vangala and Tonelli 2007).
Many metabolomics investigations are based on only two experimental groups, namely, controls and patients. In this investigation, five experimental groups were selected to compare the metabolite profiles associated with different manifestations of IVA, as follows: (1) The first group consisted of children with the homozygous mutation of the IVD gene (homozygotes), but showing clinical variability (Dercksen et al. 2012). (2) The second group comprised the same patients, treated for variable lengths of time since their diagnosis of IVA, in order to alleviate the clinical manifestations of the disease. (3) The third group comprised obligate heterozygotes, with no apparent symptoms of IVA. Because age influences the urinary excretion profiles of endogenous metabolites (Guneral and Bachman 1994), we included two control groups: (1) infants and children under the age of 13 years as controls for the IVA homozygotes, and (2) an adult control group for the heterozygotes.
We conducted three kinds of multivariate analysis in this investigation: (1) unsupervised principal component analysis (PCA, Jolliffe 2002), (2) supervised partial least squares discriminant analysis (PLS-DA, Barker and Rayens 2003), and (3) concurrent class analysis (CONCA, Koekemoer et al. 2012). A generally recognized limitation of PLS-DA is its tendency to over-fit the data (Van den Berg et al. 2006); the outcomes of PCA (the loadings) are often not intuitive, and the optimized function (capturing variance) is not necessarily ideal for the discovery of biomarkers (Styczynski et al. 2007). Several approaches have been made to overcome these limitations, including the development of a disjoint PCA (DPCA, Wold 1976) and CONCA (Koekemoer et al. 2012). CONCA was specifically devised to analyze simultaneously data arising from more than two groups, by providing a traditional PCA model with the added benefit of an ability to identify which variables are responsible for group separation. Hence it attempts to explore the natural separation of groups given the metabolomic profile. Using the CONCA model it was shown to be possible to rank a limited number of variables associated with three experimental groups according to their discrimination power [designated as variables important for discrimination (VIDs) (Koekemoer et al. 2012)]. It was also noted in the latter investigation that some metabolites of relatively low concentrations might be of significance in a metabolomics study of IVA. We thus did not eliminate any of the variables from the original data set and introduced four different modes of scaling of the data. In this study, a new application of CONCA was thus used in comparing different modes of data scaling. Furthermore, the significance of perturbations under consideration was evaluated by three univariate methods [effect size (ES), t tests and Mann–Whitney tests] applied to the components identified by PCA, PLS-DA and ES.
The comprehensive metabolomics approach presented here illustrates a further application of CONCA, which indicates the importance of using different modes of scaling of metabolomics data to improve the biological interpretation. This approach opened insightful perspectives on IVA and its treatment and it subsequently led to the identification of 3-hydroxycaproic acid, an indicator of ketosis, but not previously described for IVA. This paper also reports on the first description of the metabolite profile of heterozygotes for an IMD. Our investigation strengthens the argument for a metabolomics approach to study inherited metabolic disorders.
2 Methods
2.1 Sample selection: background, design and assumptions
Urine samples from patients with a suspected IMD were referred by paediatricians, over a period of time, for diagnostic metabolite analyses to the Potchefstroom Laboratory for Inborn Errors of Metabolism (PLIEM, see www.pliem.co.za). Ten Caucasian patients with grossly increased levels of N-isovalerylglycine and N-isovalerylcarnitine were diagnosed with IVA and the metadata on these cases are described in Dercksen et al. 2012. The initial urine samples from these patients were stored at –80 °C from the time they were received and analysed at PLIEM. The following considerations directed the design of the metabolomics investigation from a biological point of view: (1) The samples used for the IVA positive cases (indicated by Ho) were collected at the time of diagnosis, stored at −80 °C and assumed to still largely reflect the metabolic profiles present at the time of diagnosis. This assumption is based on results from repeated analysis of samples from several inborn errors of metabolism, stored in our biobank at PLIEM. (2) Samples from the IVA patients, treated for the disease (indicated by T), as well as samples from the obligate heterozygotes (indicated by He), were collected on the same day (see below). (3) Urine samples from children (indicated by W) were used as controls for the treated and untreated IVA patients and urine from adults (indicated by A) were used as controls for the heterozygotes. Samples from both control groups were standardized in previous metabolomics studies in our laboratory.
All patients received treatment for IVA according to consensus between the relevant professionals, and based on the biochemical and clinical information on each case (treated patients, designated as “T” in the tables). Treatment of the different cases was thus not identical, but generally consisted of glycine and carnitine supplements, as well as a low protein diet in some cases. Their clinical response to treatment was monitored over time. The so-called treated samples reported in this investigation were obtained on a single day during a patient/parent meeting, following dietary prescriptions for the days preceding sampling (Dercksen et al. 2012).
Nine of the treated patients were still children at the time of sampling for this investigation (ages ranged from 2 to 16 years, mean 6.4 years) and one case was a young adult, aged 24. Against this background we assumed that any dependency between the untreated and the treated samples should not be taken into account in the metabolomics analysis, given that (1) the period from first diagnosis to sampling to evaluate the effect of treatment ranged over several years; (2) the treatment regimens of the 10 patients were comparable but not identical, given their clinical diversity, which called for a personalized approach; and (3) it was not possible to obtain time-dependent information for the three other groups [children (C) and adult (A) controls and obligate heterozygotes (He)].
The parents or guardians of the treated patients gave informed consent to this investigation in accordance with the ethical requirements of our laboratory (www.pliem.co.za). All parents who were present on the day of sampling of the treated cases, volunteered to produce a urine sample for use in this investigation. Given the parental status of the adults, those present on the day of sampling were assumed to be obligate heterozygotes.
A total of 86 samples from the 5 groups included in this study were thus investigated: 10 untreated and treated IVA cases, 12 heterozygotes, 22 children controls and 32 adult controls. The samples used for children as controls were selected from those referred for identification of a possible IMD, but for whom no prevailing disorder was detected. This way of selecting comparative controls when investigating IMDs is generally recognized (Barshop 2004). All adults, who agreed to act as controls, were healthy and on a balanced diet.
2.2 GC–MS and ESI–MS-MS analysis of urinary organic acids
Urinary organic acids and conjugates were extracted, derivatized and analyzed by GC–MS as described previously (Reinecke et al. 2012). Automated Mass Spectral Deconvolution and Identification System software (AMDIS, version 2.66 from the National Institute for Standards and Technology) was used for spectral deconvolution and identification of variables. A data matrix was created by aligning all the metabolites against the samples using visual basic applications (VBA). The matrix consisted of 86 cases and 208 variables, excluding the internal standard. Additional target-based ESI–MS-MS data of isovalerylcarnitine was obtained from the preceding investigation (Dercksen et al. 2012) and the analysis was done according to the protocol described by Pitt et al. (2002).
2.3 Statistical analysis
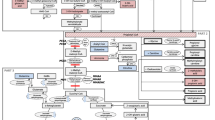
The sequence of statistical methods used, including data pre-treatment, multivariate and univariate analyses, is summarized in the flow diagram shown in Fig. 1. No reduction was applied to 208 variables of the original data matrix. To account for the large number of zeros observed in the data, all zeros were replaced by random numbers from a Beta (0.1,1) distribution bound between zero and the detection limit. The replacement values were extremely small and therefore did not influence the covariance structure of the data.
Schematic representation of the work flow following untargeted (GC–MS) and targeted (ESI–MS-MS) data generation. The data from the GC–MS analysis was generated as one batch, while the data from the ESI–MS-MS analysis was generated in the investigation of the untreated and treated IVA patients, reported in Dercksen et al. (2012). The workflow involved statistical pre-processing and data analysis in order to identify perturbation markers for untreated IVA, and comparison of the treated cases and heterozygotes. Abbreviations used for the cases are W for children controls, Ho for untreated IVA, T for treated IVA, A for adult controls, and He for heterozygotes
Four different non-linear methods of scaling were initially applied prior to the multivariate analyses. This was done to overcome the side effects of measurement error associated with very small values on the one hand, but also giving due consideration to metabolites with low concentrations which are relevant to IVD and its treatment, on the other hand. The scaling were: (1) a shifted logarithmic scaling with the shift parameter set at one; (2) a non-parametric transformation (Koekemoer and Swanepoel 2008); (3) a rank scaling (Becker et al. 1988) where the tied observations were replaced by the minimum rank, and (4) a second rank scaling where the tied ranks were replaced by the average rank of the tied observations. For rank minimum scaling, no zero replacement was performed prior to the ranking. This was regarded as essential so as not to diminish the effect of variables that were absent or present in any one or more of the five groups studied. Given the scaled data sets, the CONCA model was applied and VIDs across all five classes were obtained, supporting the selection of appropriate modes of scaling.
Once a relevant mode of scaling had been selected, multivariate analyses were used for dimensional reduction and identification of important variables. These were principal component analysis (PCA), as an unsupervised pattern recognition method (Johnson and Wichern 1998), and a partial least square discriminant analysis (PLS-DA) as a supervised method (Barker and Rayens 2003). Variables listed by the PCA with a modelling power greater than 0.5 were regarded as potential biomarkers (Brereton 2003); and for variables important in projection (VIPs) from PLS-DA, the ‘greater than one rule’ was used as the criterion for the selection of variables (Chong and Jun 2005).
Effect size (ES) was calculated from the scaled data in a univariate analysis [each IVD-deficient group (Ho, T and He), relative to its respective control group (W or A)] to ascertain the importance of a variable (Ellis and Steyn 2003). In addition, standard t-tests and the non-parametric Mann–Whitney tests were performed on the unscaled data to test the equality of group means and group medians, respectively. The outcomes of these analyses for the untreated and treated IVA homozygotes, as well as for the obligate IVA heterozygotes, were finally combined to inform on the biochemical information disclosed in this comprehensive metabolomics analysis.
3 Results and discussion
3.1 Variables important in discrimination between the IVA groups
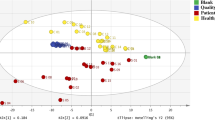
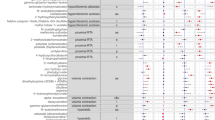
In the present study, the CONCA model was fitted to the complete set of data generated from five groups, aimed to compare four modes of scaling. Three-dimensional representations of the CONCA-based separation of the five groups using the different modes of scaling are shown in Fig. 2. These results clearly indicate the complete separation between all three IVA-related groups and their respective controls as well as the effect of different modes of scaling. Subsequently, the VIDs that discriminate among the five groups were identified using the four modes of scaling. For comparative purposes, the top ten VIDs for each mode of scaling are summarized in Table 1, indicating that some degree of discriminatory information was displayed by each of the modes of scaling. The outcomes of the shifted logarithmic and non-parametric modes were comparable, as both these methods of scaling illuminated the most important secondary metabolites that are formed as a consequence of the IVD-deficiency. However, the discriminating power from the shifted logarithmic scaling appeared to be superior: (1) the VID values of the top variables from the shifted logarithmic scaling (137.0; 133.3; 37.2 …) exceeded that of the non-parametric scaling (79.0; 78.0; 37.6 …), and (2) the three primary biomarkers (N-isovalerylglycine, N-isovalerylglutamic acid and 3-hydroxyisovaleric acid) and two minor indicators (N-isovalerylserine and N-isovalerylleucine) of IVA in the shifted logarithmic list exceeded those from the non-parametric scaling (only four indicators). The values of the VIDs from the rank minimum scaling (143.6; 124.4; 92.5 …) out-performed the VIDs of the rank average scaling (23.6, 20.9; 20.3 …). Both these modes of scaling disclosed the presence of indicators of treatment of IVA, e.g. 2,3,4-trihydroxybutyryllactone and gluco-6,1-lactone. However, the rank minimum scaling outperformed the rank average scaling (identification of three indicators relative to one, as shown in Table 1). It is also noteworthy from a biological point of view, that none of these metabolites observed in the treated IVA cases was observed amongst the top twenty metabolites disclosed by the shifted logarithmic or non-parametric modes of scaling. The CONCA procedure of data pre-treatment thus revealed the different metabolic patterns encapsulated in the global data set, which would not be disclosed by using only one mode of scaling (compare also Van den Berg et al. 2006). From these observations the shifted logarithmic and the rank minimum scaling were selected to further analyze the metabolomics information on the untreated and treated IVA cases, respectively. Identification of important variables associated with the three IVD-affected groups (Ho, T, He) was done through multivariate (PCA and PLS-DA) and applicable univariate methods as shown in the flow diagram of Fig. 1. VIDs derived from CONCA were not used in this process, as it could present a bias on the outcome, as CONCA was already applied in choosing the two modes of scaling used.
Three-dimensional score plots for the CONCA model based on a four modes of scaling. a Shifted logarithmic scaling; b non-parametric scaling; c rank minimum scaling and d rank average scaling. In all four representations, the cases are (1) children controls (brown), (2) heterozygotes (blue), (3) untreated IVA cases (red), (4) treated IVA cases (green), and (5) adult controls (black)
3.2 Important variables in untreated IVA
A total of 208 different variables could be annotated from the GC–MS data extracted from the five experimental groups. No data reduction was applied to these variables, yielding a consolidated data matrix of 208 variables from 86 cases. Means and standard deviations of all variables for the children controls and untreated patients were calculated for the unscaled data, followed by t-test and Mann–Whitney analyses. The important metabolites associated with IVA were then identified from the original 208 variables by means of PCA, PLS-DA and ES analysis of the shifted logarithmic-scaled values. Nineteen variables from the log-scaled ES data had a d > 0.8 (practical highly significant), 21 from the PLS-DA had a VIP > 1.0 and 11 had a PCA power value greater than 0.5. Using a Venn diagram (included in Fig. 1), we found that the 11 variables from the PCA were common to the PLS-DA and ES analysis. These eleven variables were thus defined as the typical metabolite profile of untreated IVA, and are listed in Table 2, ranked according to the ES values.
Two detoxification products of IVA, N-isovalerylglycine, and N-isovalerylglutamic acid, are well-established diagnostic indicators of the disorder, and ranked highest among the 11 selected metabolites. N-isovalerylleucine and N-isovalerylserine, two rare detoxification products in IVA described by Loots et al. (2005), conspicuously also ranked high in this list, emphasizing the importance of acyl detoxification in untreated manifestations of this disease. Methylsuccinic, methylfumaric (mesaconic) and 3-hydroxyisovaleric acids were observed and have been described in the literature as secondary metabolites formed as a result of IVD deficiency (Truscott et al. 1981, Tanaka et al. 1988, Sweetman and Williams 2001). Glyceric acid was also present and is normally associated with defects of fructose metabolism at the level of d-glycerate kinase; increased urinary excretion has also been observed following an oral fructose challenge (Wang and Van Eys 1981). No satisfactory explanation for the occurrence of this acid in IVA is currently available. Finally, 3-hydroxybutyric acid may be regarded as a metabolite formed as a secondary clinical consequence of IVA, and normally observed during ketosis or ketoacidosis.
The last metabolite of the 10 listed in Table 2 is 3-hydroxycaproic acid, which has not been reported previously in IVA patients. It appeared as a minor peak on the GC chromatogram with a mass spectrum comparable with that of 3-hydroxycaproic acid in the AMDIS library (Fig. 3). The ion at m/z 261 was due to [M-CH3]+, whereas the fragments corresponding to m/z 233 and 145 were indicative of [C9H21O3Si2]+ and [C7H17OSi]+, respectively. This metabolite was originally identified and described as an abnormal constituent in urine of keto-acidotic diabetics (Niwa and Yamada 1985). We propose that 3-hydoxycaproic acid observed in IVA is produced by ketogenesis reactions starting from acetyl-CoA and butyryl-CoA as in diabetics. 3-Hydroxyisovaleric acid likewise elevates during episodes of metabolic decompensation together with ketone body production, as described by Sweetman and Williams (2001). The strong positive Pearson correlation of 0.69 which was observed between the values of 3-hydroxyisovaleric acid and 3-hydroxycaproic acid in the untreated cases, favours the hypothesis that both are associated with metabolic stress and ketosis. The related metabolic pathways are depicted in Fig. 4.
Proposed metabolic pathways and markers identified in this metabolomic study of IVA. Abbreviations: mBCAT mitochondrial branched-chain amino transferase, BCKAD branched-chain keto-acid dehydrogenase, IVD isovaleryl-CoA dehydrogenase, CytP450 cytochrome P450, CoA coenzyme-A, CRAT carnitine acetyltransferase, CACT carnitine-acylcarnitine transferase, CAT carnitine acyltransferase, GAT N-glycine-acyltransferase, NAGS N-acetylglutamate syntase, CPS carbamoyl phosphate synthetase, OTC ornitine transcarbamoylase, 3-MCC 3-methylcrotonyl carboxylase, TE thioesterase, EH enoyl-CoA hydratase, SDH succinate dehydrogenase, ACDHG acyl-CoA dehydrogenase
3.3 Important variables in treated IVA
The rank minimum mode of scaling was selected for the presentation of the data from the treated cases. The PCA, PLS-DA and ES modes of analysis were again used to select the common important variables using a Venn diagram (Fig. 1), indicating nine common variables, summarized in Table 3 with the applicable univariate data. The urinary excretions of N-isovalerylglycine were higher (1,195 mmol/mol creatinine; p = 0.000) in the treated IVA patients than in the untreated cases (1,033 mmol/mol creatinine; p = 0.000). This is in accordance with the detoxification treatment of these patients with glycine and carnitine supplementation. The detoxification effect of carnitine supplementation, indicated by the urinary excretion of isovalerylcarnitine, appeared to be highly effective as indicated by the increase of isovalerylcarnitine from 3.7 to 81.7 mmol/mol creatinine (p = 0.001) as well as the highly significant (p = 0.000) change in the isovalerylglycine to isovalerylcarnitine ratio from 276.9 to 14.6. Nine of the other metabolites important in untreated IVA no longer differed statistically from those of the controls which points to the efficacy of the detoxification treatment. An exception was the attenuated, but still high, concentration of N-isovalerylglutamic acid.
Five polyhydroxy organic acids or their lactones were present at notably elevated levels relative to the paediatric controls. The latter substances were observed only by using the rank minimum mode of scaling. It has been suggested that the carbohydrate lactones may be explained as due to the treatment of IVA patients with a high synthetic carbohydrate and low protein diet (Chalmers et al. 1976; Koekemoer et al. 2012). The other polyhydroxy substances likewise seem to be derived from dietary substances (Sakata 1990; Ogura and Sakamoto 2012; Hegazi and Abd El-Hady 2009). The last metabolite related to IVA treatment listed in Table 3 is methylmalonic acid. Although the normal range for methylmalonic acid in the urine of children (1–13 years of age) was reported to be in the range from undetectable to 5 mmol/mol creatinine (Hoffman and Feyh 2002), this metabolite was not detected in the samples from the children controls and untreated IVA patients but was detected at very low concentrations in the heterozygotes and was present in the samples from nine of the 10 treated patients. For these cases the mean value was 2.31 mmol/mol creatinine [(SD = 2.54) and p = 0.017 relative to the children controls]. The natural source of vitamin B12 in the human diet is from animal protein (meat, poultry and fish) and methylmalonic acid is an excellent marker for vitamin B12 deficiency (Norman et al. 1982; Rasmunsen 1989). An increased risk of vitamin B12 deficiency has been reported for some patients with phenylketonuria who changed from a strict phenylalanine free diet, with additional vitamins and minerals, to an unrestricted or relaxed dietary regime. (Robinson et al. 2000). This may also hold true for the IVA patients on a low-protein or protein-restricted diet and represents important new information for nutritional scientists responsible for the treatment of IVA patients.
3.4 Important variables in IVA heterozygotes
Diagnostic biomarkers for IMDs have been detected at normal or slightly elevated baseline levels in urine samples from heterozygotes for these diseases (Bakker et al. 1978; Duran et al. 1979). The statistically significant (p < 0.05) presence of low levels of N-isovalerylglycine, 3-hydroxycaproic acid and 3-hydroxyisovaleric acid in the heterozygotes for IVA is noteworthy (Table 2). The observation of minor metabolites of an inherited metabolic disorder at such low levels has not been described previously for carriers of IVA and provides proof-of-concept of the original paradigm that an untargeted metabolomics investigation has the potential to reveal significant information on very low levels of metabolites (concentrations, not fluxes) present in biofluids (Kell 2004, 2007).
4 Pathophysiological interpretation of the IVA metabolite profile
The metabolite profile which we derived for untreated IVA patients (Fig. 4) is based on significant changes in their urinary organic acid excretion (Table 2). The primary perturbations corresponded to induced products resulting from the accumulation of isovaleryl-CoA. Reactions further downstream are secondary consequences of IVD deficiency, but nevertheless also important in the clinical assessment of IVA. Most of the IVA metabolites disclosed by the metabolomics analysis have been individually well researched and described previously. Observing these metabolites using an untargeted metabolomics approach, however, confirms that biologically well-defined samples are required for metabolomics investigations and illustrates the robustness of this technology. Furthermore, the comprehensive information disclosed by the metabolomics approach indicates that diverse metabolic pathways are affected as a consequence of the primary defect in IVA. In addition, our study serves to illustrate some pathophysiological consequences of IVA, as well as avenues for monitoring its treatment.
IVA is due to a perturbation of the mitochondrial pathway of leucine catabolism. The consequence of the inherited deficiency resulting in IVA is the inability to convert isovaleryl-CoA to 3-methylcrotonyl-CoA (Tanaka et al. 1966). A variety of amino acids can form conjugates with the accumulating isovaleryl-CoA. N-isovalerylglycine, N-isovalerylglutamic acid, N-isovalerylleucine and N-isovalerylserine have been shown to be the most significant in the present study. Also observed were N-isovalerylthreonine (p = 0.106) but conjugates of several other amino acids, described by Loots et al. (2005), were below the detection limit of our analytical approach. N-isovalerylglutamate is the product of the N-acetylglutamate synthase (NAGS, EC 2.3.1.1), which catalyzes the reaction between isovaleryl-CoA and glutamate. N-acetylglutamate, which also originates from glutamate, activates carbamoylphosphate synthetase required for the initial step in the urea cycle, and may be compromised, resulting in secondary hyperammonemia in IVA (Coude et al. 1979; Sweetman and Williams 2001). Treatment of IVA patients at the upper tolerable level of glycine supplementation supports the detoxification process through formation of N-isovalerylglycine at the expense of N-isovalerylglutamic acid, as shown for the treated patients (Table 3).
Two alternative pathways with isovaleryl-CoA as substrate can be envisaged, giving rise to further important metabolites listed in Table 2: (1) Peroxisomal α-oxidation (recently reviewed by Wanders and Waterham 2006) of isovaleryl-CoA is proposed to result in the formation of 2-hydroxyisovaleric acid seen in low concentrations (4.81 mmol/mol creatinine; p = 0.121) in untreated IVA (Mukherji et al. 2002). (2) Free isovaleric acid, the original metabolite that led to the discovery of the first two cases of IVA (Tanaka et al. 1966), is proposed to be formed from isovaleryl-CoA, catalyzed by one of the many thioesterases. Subsequently, isovaleric acid can act as substrate for the cytochrome P450-related omega-oxidation system of the endoplasmic reticulum, resulting in the formation of methylsuccinic acid (35.71 mmol/mol creatinine; p = 0.040) from which methylfumaric acid (17.99 mmol/mol creatinine; p = 0.018) is subsequently formed, catalyzed by succinate dehydrogenase. Both these metabolites were significantly increased in our untreated IVA group.
The remaining important metabolite, showing high concentrations in untreated IVA, is 3-hydroxyisovaleric acid (273 mmol/mol creatinine; p = 0.023, see Table 2). It is easy to relate the occurrence of this acid to the ketosis as has been discussed before. However, it is also feasible that 3-hydroxyisovaleric acid is formed by (ω-1)-hydroxylation of isovaleric acid, another of the cytochrome P450-mediated reactions mentioned earlier (Sweetman and Williams 2001). Finally, it cannot be excluded that a small part of the endogenous isovaleryl-CoA is actually converted to 3-methylcrotonyl-CoA. The latter is expected to be carboxylated, but this carboxylation can be inhibited by short-chain acyl-CoA esters as has been shown recently (Luís et al. 2012).
Assessment of the clinical potential of a novel biomarker that emerges from metabolomics investigations of disease as outlined above can be critically appraised by asking three basic questions: (1) Can the clinical laboratory measure the biomarker? (2) Does it add new information? (3) Does it help the clinician to manage patients? (Morrow and de Lemos 2007). Today, numerous metabolites are routinely measured in clinical laboratories by using sophisticated equipment, which basically provides a positive inclination towards the first question. The second question encompasses a complex scenario. Metabolomics investigations of inherited diseases are mostly based on retrospective investigations whereas well-designed prospective studies, linked to rigorous validation, would be the benchmark to implement information from research to application in clinical practice (Mamas et al. 2011). A paediatrician specializing in IMDs will regularly be confronted with patients with metabolic diseases not previously encountered in the practice due to the rarity of the individual disease. However, a personalized approach is required in the treatment of these patients who often present with clinical heterogeneity as the IVA patients described by Dercksen et al. 2012. Metabolomics investigations might contribute to alleviating this requirement as was recently well articulated in a commentary by Italian paediatricians: Metabolomics is a novel area that promises to contribute significantly to the characterization of various disease phenotypes and to the identification of personal metabolic features that can predict response to therapies, enabling a way to distinguish between different disease phenotypes and to predict a drug’s effectiveness and/or toxicity. (Baraldi et al. 2009). It thus seems reasonable to consider a qualified affirmative answer to the question whether metabolomics studies will provide an aid in designing the clinical treatment of patients with IMDs.
5 Conclusions
In summary, using the expanded metabolomics approach presented in this paper, we succeeded in producing a comprehensive profile of metabolites of practical significance in cases of IVA, derived from an unreduced original set of variables. CONCA enabled the identification of VIDs across all five classes of experimental groups through the application of four modes of scaling, which produced different ranks of VIDs. This enabled the recognition of different metabolic patterns encapsulated in the data sets, which would not be revealed by using only one mode of scaling. In addition, this is the first report of such an investigation based on a genetically homogeneous group of IVA patients, including the metabolomics of urinary samples from their untreated and treated status as well as from obligate heterozygotes. This investigation substantiated the presence of known metabolites in cases of IVA and revealed the presence of 3-hydroxycaproic acid not previously reported for this disorder. The metabolite profile of treated IVA patients differed substantially from the untreated cases, and may be of value in monitoring the effect of treatment. In addition this investigation provides the first comparative metabolite profile for heterozygotes of an inborn error of metabolism, indicating the presence of minor metabolites present in very low concentrations in these individuals. The detection of several informative metabolites with very low concentrations highlights the potential advantage of the holistic mode of analysis in metabolomics investigations.
References
Bakker, H. D., Van Gennip, A. H., Duran, M., & Wadman, S. K. (1978). Methylmalonate excretion in a pregnancy at risk for methylmalonic acidaemia. Clinica Chimica Acta, 86, 349–352.
Baraldi, E., Carraro, S., Giordano, G., Reniero, F., Perilongo, G., & Zacchello, F. (2009). Metabolomics: moving towards personalized medicine. Italian Journal of Pediatrics, 35, 30–34.
Barker, M., & Rayens, W. (2003). Partial least squares for discrimination. Journal of Chemometrics, 17, 166–173.
Barshop, B. A. (2004). Metabolomic approaches to mitochondrial disease: Correlation of urine organic acids. Mitochondrion, 4, 521–527.
Becker, R. A., Chalmers, J. M., & Wilks, A. R. (1988). The new S-language. Pacific Grove: Wadsworth & Brooks Cole.
Brereton, R. G. (2003). Chemometrics—data analysis for the laboratory and chemical plant. West Sussex: John Wiley & Sons Ltd.
Chalmers, R. A., Healy, M. J. R., Lawson, A. M., & Watts, R. W. E. (1976). Urinary organic acid in man II. Effects of individual variation on diet on the urinary excretion of acidic metabolites. Clinical Chemistry, 22, 1288–1291.
Chong, I. G., & Jun, C. H. (2005). Performance of some variable selection methods when multicollinearity is present. Chemometrics and Intelligent Laboratory Systems, 78, 103–112.
Coude, F. X., Sweetman, L., & Nyhan, W. L. (1979). Inhibition by propionyl-coenzyme A of N-acetylglutamate synthase in rat liver mitochondria. A possible explanation for hyperammonemia in propionic and methylmalonic acidemia. Journal of Clinical Investigation, 64, 1544–1551.
Dercksen, M., Duran, M., Ijlst, L., Mienie, L. J., Reinecke, C. J., Ruiter, J. P. N., et al. (2012). Clinical variability of isovaleric acidimia in a genetically homogeneous population. Journal of Inherited Metabolic Diseases, 35, 1021–1029.
Duran, M., Schutgens, R. B. H., Ketel, A., et al. (1979). 3-Hydroxy-3-methylglutaryl coenzyme A lyase deficiency: Postnatal management following prenatal diagnosis by analysis of maternal urine. The Journal of Pediatrics, 95, 1004–1007.
Ellis, S. M., & Steyn, H. S. (2003). Practical significance (effect size) versus or in combination with statistical significance (p values). Management Dynamics, 12, 51–53.
Ensenauer, R., Vockley, J., Willard, J., et al. (2004). A common mutation is associated with a mild, potentially asymptomatic phenotype in patients with isovaleric acidemia diagnosed by newborn screening. American Journal of Human Genetics, 75, 1136–1142.
Guneral, F., & Bachman, C. (1994). Age-related reference values for urinary organic acids in a healthy Turkish pediatric population. Clinical Chemistry, 40, 862–868.
Hegazi, A. G., & Abd El-Hady, F. K. (2009). Influence of honey on suppression of human low density lipoprotein (LDL) peroxidation (in vitro). Evidence-Based Complementary and Alternative Medicine, 6, 113–121.
Hoffman, G. F., & Feyh, P. (2002). Organic acid analysis. In N. Blau, M. Duran, & M. E. Blaskovic (Eds.), Physician’s guide to the laboratory diagnosis of metabolic diseases (pp. 27–44). Berlin: Springer.
Johnson, R. A., & Wichern, D. W. (1998). Applied multivariate statistical analysis (4th ed.). NJ: Upper Saddle River.
Jolliffe, I. T. (2002). Principal component analysis (2nd ed.). New York: Springer.
Kell, D. B. (2004). Metabolomics and systems biology: making sense of the soup. Current Opinion in Microbiology, 7, 296–307.
Kell, D. B. (2007). Metabolomic biomarkers: search, discovery and validation. Expert Review of Molecular Diagnostics, 7, 329–333.
Knerr, I., Weinhold, N., Vockley, J., & Gibson, K. M. (2012). Advances and challenges in the treatment of branched-chain amino/keto acid metabolic defects. Journal of Inherited Metabolic Diseases, 35, 29–40.
Koekemoer, G., & Swanepoel, J. W. H. (2008). A semi-parametric method for transforming data to normality. Statistics and Computing, 18, 241–257.
Koekemoer, G., Dercksen, M., Allison, J., Santana, L., & Reinecke, C. J. (2012). Concurrent class analysis identifies discriminatory variables from metabolomics data on isovaleric acidemia. Metabolomics, 8, S17–S28.
Loots, D. T. (2009). Abnormal tricarboxylic acid cycle metabolites in isovaleric acidemia. Journal of Inherited Metabolic Diseases, 3, 402–411.
Loots, D. T., Erasmus, E., & Mienie, L. J. (2005). Identification of 19 new metabolites induced by abnormal amino acid conjugation in isovaleric acidemia. Clinical Chemistry, 51, 1510–1512.
Luís, P. B., Ruiter, J. P., Ijlst, L., Diogo, L., Garcia, P., de Almeida, I. T., et al. (2012). Inhibition of 3-methylcrotonyl-CoA carboxylase explains the increased excretion of 3-hydroxyisovaleric acid in valproate-treated patients. Journal of Inherited Metabolic Disease, 35, 443–449.
Mamas, M., Dunn, W. B., Neyses, L., & Goodacre, R. (2011). The role of metabolites and metabolomics in clinical applicable biomarkers of disease. Archives of Toxicology, 85, 5–17.
Morrow, D. A., & de Lemos, J. A. (2007). Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation, 115, 949–952.
Mukherji, M., Kershaw, N. J., Schofield, C. J., Wierzbicki, A. S., & Lloyd, M. D. (2002). Utilization of sterol carrier protein-2 by phytanoyl-CoA 2-hydroxylase in the peroxisomal α-oxidation of phytanic acid. Chemistry & Biology, 9, 597–605.
Niwa, T., & Yamada, K. (1985). 3-Hydroxyhexanoic acid: An abnormal metabolite in urine and serum of diabetic ketoacidotic patients. Journal of Chromatography, 337, 1–7.
Norman, E. L., Martelo, O. J., & Denton, M. D. (1982). Cobalamin (vitamin B12) deficiency detected by urinary methylmalonic acid quantitation. Blood, 59, 1128–1131.
Ogura, T. & Sakamoto, Y. Application of metabolomics techniques using LC/MS and GC/MS profiling analysis of green tea leaves. SHIMADZU Corporation, application note No. 10. http://www2.shimadzu.com/applications/GCMS,LCMS/LAANCXXE011.pdf. Accessed 7 Mar 2012.
Pan, Z., Gu, H., Talaty, N., Chen, H., Shanaiah, N., Hainline, B. E., et al. (2007). Principal component analysis of urine metabolites detected by NMR and DESI-MS in patients with inborn errors of metabolism. Analytical and Bioanalytical Chemistry, 387, 539–549.
Pitt, J. J., Eggington, M., & Kahler, S. G. (2002). Comprehensive screening of urine samples for inborn errors of metabolism by electrospray tandem mass spectrometry. Clinical Chemistry, 48, 1970–1980.
Rasmunsen, K. (1989). Studies on methylmalonic acid in humans. I. Concentrations in serum and urinary excretion in normal subjects after feeding and during fasting, and after loading with protein, fat, sugar, isoleucine and valine. Clinical Chemistry, 35, 2271–2276.
Reinecke, C. J., Koekemoer, G., van der Westhuizen, F. H., et al. (2012). Metabolomics of urinary organic acids in disorders of the respiratory chain. Metabolomics, 8, 264–283.
Robinson, M., White, F. J., Cleary, M. A., Wraith, E., Lam, W. K., & Walter, J. H. (2000). Increased risk of vitamin B12 deficiency in patients with phenylketonuria on an unrestricted or relaxed diet. The Journal of Pediatrics, 136, 545–547.
Sakata, T. (1990). Structural and stereoisometric specificity of serum-borne sugar acids related to feeding control in rats. Brain Research Bulletin, 25, 969–974.
Scriver, C. R., Beaudet, A. L., Sly, W. S., Valle, D., Childs, K., Kinzler, K. W. E., et al. (2001). The metabolic and molecular basis of inherited disease. New York: McGraw-Hill.
Styczynski, M. P., Moxley, J. F., Tong, L. V., Walther, J. L., Jensen, K. L., & Stephanopoulos, G. N. (2007). Systematic identification of conserved metabolites in GC/MS data for metabolomics and biomarker discovery. Analytical Chemistry, 79, 966–973.
Sweetman, L., & Williams, J. C. (2001) Branched chain organic acidurias. In C. R. Scriver, A. L. Beaudet, D. Valle, W. S. Sly, B. Childs, K. Kinzler, K. W. E. (Eds.) The metabolic and molecular basis of inherited disease (pp. 2125–2163). McGraw-Hill, New York.
Tanaka, K., Budd, M. A., Efron, M. L., & Isselbacher, K. J. (1966). Isovaleric acidemia: a new genetic defect of the leucine metabolism. Proceedings of the National Academy of Sciences of the United States of America, 56, 236–242.
Tanaka, K., Ikeda, Y., Matsubara, Y., & Hyman, D. (1988). Molecular basis of isovaleric acidemia in the study of the acyl-CoA dehydrogenase family. Advanced Neurology, 48, 107–131.
Truscott, R. J., Malegan, D., McCairns, E., Burke, D., Hick, L., Sims, P., et al. (1981). New metabolites in isovaleric acidemia. Clinica Chimica Acta, 110, 187–203.
Van den Berg, R. A., Hoefsloot, H. C. J., Westerhuis, J. A., Smilde, A. K., & Van der Werf, M. J. (2006). Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics, 7, 141–157.
Vangala, S., & Tonelli, A. (2007). Biomarkers, metabonomics, and drug development: Can inborn errors of metabolism help in understanding drug toxicity? AAPS Journal, 9, E284–E297.
Vockley, J., & Ensenauer, R. (2006). Isovaleric acidemia: New aspects of genetic and phenotypic heterogeneity. American Journal of Medical Genetics Part C, 142C, 95–103.
Wanders, R. J. A., & Waterham, H. R. (2006). Biochemistry of mammalian peroxisomes revisited. Annual Review of Biochemistry, 75, 295–332.
Wang, Y., & Van Eys, J. (1981). Nutritional significance of fructose and sugar alcohols. Annual Review of Nutrition, 1, 437–475.
Wold, S. (1976). Pattern recognition by means of disjoint principal component models. Pattern Recognition, 8, 127–139.
Acknowledgments
M. Dercksen is a recipient of a PhD scholarship from the Carolina MacGillavry Foundation awarded by the “Koninklijke Nederlandse Akademie van Wetenschappen”. Funding for the project from the Technological Innovation Agency of the South African Department of Science and Technology and from North-West University is likewise acknowledged. We also thank the IVA families for their participation in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dercksen, M., Koekemoer, G., Duran, M. et al. Organic acid profile of isovaleric acidemia: a comprehensive metabolomics approach. Metabolomics 9, 765–777 (2013). https://doi.org/10.1007/s11306-013-0501-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-013-0501-5