Abstract
The involvement of purinergic signalling in the physiology of erythrocytes, platelets and leukocytes was recognised early. The release of ATP and the expression of purinoceptors and ectonucleotidases on erythrocytes in health and disease are reviewed. The release of ATP and ADP from platelets and the expression and roles of P1, P2Y1, P2Y12 and P2X1 receptors on platelets are described. P2Y1 and P2X1 receptors mediate changes in platelet shape, while P2Y12 receptors mediate platelet aggregation. The changes in the role of purinergic signalling in a variety of disease conditions are considered. The successful use of P2Y12 receptor antagonists, such as clopidogrel and ticagrelor, for the treatment of thrombosis, myocardial infarction and stroke is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Erythrocytes
Extracellular actions of nucleotides
ATP release
Pathology
Platelets
Introduction
P2Y1 and P2Y12 receptors
P2X1 receptors
P1 (adenosine) receptors
ATP and ADP release
Ectonucleotidases
Thrombosis
Megakaryocytes
Leukocytes
Introduction
Purinergic signalling, ATP acting as an extracellular signalling molecule, was proposed in 1972 [1]. Separate families of receptors for adenosine (P1) and adenosine 5′-triphosphate (ATP) and adenosine 5′-diphosphate (ADP) (P2) were recognised in 1978 [2] and receptors for purines and pyrimidines cloned and characterised in the early 1990s (see [3]). Four P1 receptor subtypes (A1, A2A, A2B and A3), seven P2X ion channel receptor subtypes (P2X1–7) and eight P2Y G protein-coupled receptors (P2Y1, 2, 4, 6, 11, 12, 13 and 14) have been identified (see [4]).
The involvement of purinergic signalling in the biology of erythrocytes, platelets and leukocytes was recognised early, and this review aims to present an historical account leading to our current understanding of the various roles played by purine nucleotides and nucleosides in health and disease. A valuable earlier review was published about the roles of nucleotide receptors in blood cells [5].
Erythrocytes
Extracellular actions of nucleotides
Early papers were concerned with intracellular ATP levels (estimated at 107–284 μg/ml in a very early paper [6]) in erythrocytes and their relation to the cell shape [7–9] and storage ability [10]. There were also early papers concerned with the ectoenzymes involved in the metabolism of external adenine nucleotides [11–14]. A decrease in intracellular red cell ATP levels during aging was reported [15]. Early papers also showed that extracellular ATP increased Na+ and K+ permeability and altered the physical properties of mammalian red blood cells [16–19]. Erythrocyte membrane preparations (‘ghosts’) were used in numerous investigations of the actions of ATP (e.g. [20, 21]) and ATPases [22]. Using RT-PCR of red blood cell progenitor cells, messenger RNA (mRNA) expression of P2X1, P2X4 and P2X7, as well as P2Y1 receptors (but not for P2Y2, P2Y4 or P2Y6) was reported [23]. The turkey erythrocyte has also been utilised as a model for studies of purinergic signalling [24]. For example, P2Y receptors were identified and the kinetics of activation of phospholipase (PL) C by P2Y receptor agonists examined [25, 26]. Phosphatidylinositol 4,5-bisphosphate hydrolysis was shown to be regulated by P2Y receptors in turkey erythrocytes [27]. Later, this P2Y receptor was identified as the P2Y1 subtype [28, 29]. Extracellular ATP was reported to stimulate a volume decrease in red blood cells from Necturus [30] and activate a P2 receptor during hypotonic swelling [31].
Human erythrocytes were shown to express P2X7 receptors on all erythrocytes examined from eight subjects. P2X2 receptors were also identified, although they were at a far lower staining intensity in six of the eight subjects [32]. These studies also showed that purines increase cation fluxes in the potency order of 2′(3′)-O-(4-benzoylbenzoyl) adenosine 5′-triphosphate (BzATP) > ATP > 2-methythioATP > adenosine-5′-(γ-thio)-triphosphate, while ADP and uridine 5′-triphosphate (UTP) had no effect. A P2Y4-like receptor was claimed to increase [Ca2+]i in red blood cells of the lizard [33]. Elevated intracellular Ca2+ revealed a functional membrane nucleotide pool in intact human red blood cells [34]. P2X7 receptor activation caused phosphotidylserine exposure and cell shrinkage in human erythrocytes [35]. Erythrocytes are reservoirs of epoxyeicosatrienoic acids, which are vasodilators, anti-aggregatory and anti-inflammatory lipid mediators. Stimulation of rat erythrocyte P2X7 receptors induces the release of epoxyeicosatrienoic acids, arachidonic acid-derived lipid mediators that dilate arterioles [36, 37]. Canine erythrocytes express P2X7 receptors, which mediate a massive increase in cation permeability compared to human erythrocytes [38, 39]. 5-Nucleotidase activities were reported in human erythrocytes [40]. Activation of P2Y1 receptors triggers two calcium signalling pathways in bone marrow erythrocytes [41].
Extracellular adenosine was shown to significantly enhance glucose consumption and lactate production in washed human red blood cells [42]. The adenosine receptor, present on turkey erythrocytes, was shown to be coupled to adenylate cyclase [43]. Adenosine is rapidly taken up by erythrocytes [44, 45], which is critical since adenosine deaminase is localised in the plasma membranes of erythrocytes [46]. A2 receptors are present in embryonic red blood cells, but their numbers were reduced in later development [47]. Suicidal death of erythrocytes or eryptosis is characterised by cell shrinkage and cell membrane scrambling, and adenosine was shown to inhibit eryptosis [48]. It was reported that A2B receptors mediate regulatory volume decrease in mature human erythrocytes [49].
The level of intracellular ATP is crucial for maintaining the function and structural integrity of circulating red blood cells [50]. Elevated levels of ATP in red blood cells of patients with renal failure was reported, 4.88 μmol/gHb compared to control 3.64 μmol/gHb [51]. The loss of adenosine 5′-monophosphate deaminase activity in senescent erythrocytes may explain elevated ATP levels [52].
Ticagrelor, a P2Y12 receptor antagonist, reportedly inhibits adenosine uptake leading to augmentation of cardiac blood flow in a canine model of reactive hypoxia [53]. The authors suggest that ticagrelor may have additional benefits in patients with acute coronary syndrome beyond inhibition of platelet aggregation including the induction of ATP release, which was shown to occur in studies of human red blood cells [54]. The ticagrelor-induced adenosine increase may be beneficial by improving peripheral endothelial function [55] and also be cardioprotective by reducing myocardial infarct size [56].
Damage to healthy tissue is a major limitation of radiotherapy treatment of cancer patients, and radiation-induced release of pro-inflammatory cytokines may be involved in the side effects. In whole blood studies, ATP inhibited radiation-induced tumour necrosis factor-α release and increased interleukin (IL)-10 release, perhaps via P2Y11 receptors, and it was concluded that ATP alleviates radiation toxicity, mainly by inhibiting radiation-induced inflammation and DNA damage [57]. The ATP released from erythrocytes is anti-adhesive, and storage-induced deficiency in ATP release from transfused erythrocytes may promote microvascular pathophysiology in lung endothelial cells possibly via increased cell adhesion [58].
ATP release
Human erythrocytes release ATP upon exposure to mechanical deformation, β-adrenoceptor agonists, prostacyclin analogues, reduced O2 tension, acidosis or swelling [59]. Release of ATP from erythrocytes exposed to hypertonic solutions was described by Deyrup in 1951 [60], and aging ATP-depleted human erythrocytes were later shown to release vesicles [61, 62]. The release of ATP from human erythrocytes was shown to occur in response to a brief period of hypoxia in the presence of hypercapnia, such as would be found in exercising muscle [63] and was later demonstrated to similarly occur in response to low O2 in the absence of hypercapnia [64]. It has been proposed that red blood cells are not only O2 carriers but via ATP release have a direct role in regulation of vascular tone leading to the appropriate distribution of microvascular perfusion [64–67].
Erythrocytes release ATP in response to mechanical deformation as might occur when red cells are squeezed through small vessels or deformed in areas of high velocity [68–71] (Fig. 1). Increases in perfusate flow rate were a sufficient mechanical stimulus for ATP release from red blood cells in isolated rabbit lungs [72]. Studies with erythrocytes from individuals with cystic fibrosis suggested that this release required cystic fibrosis transmembrane conductance regulator (CFTR) [73] although they considered that it was unlikely that this is the channel by which ATP exits the red blood cell. Recent papers have reported that ATP release in response to reduced oxygen tension occurs partly via the hemichannel pannexin 1, which may also be the channel involved in ATP release in response to mechanical deformation above a specific stress threshold [74–76] (Fig. 2).
Microfluidic approach for shear-triggered release of ATP. a Schematic of the experimental apparatus (not to scale). A mixture of red blood cells (RBCs) and luciferase/luciferin solution are pumped through a microfluidic constriction. b Representative experimental measurements of the photon emission rate resulting from the reaction between luciferase/luciferin and ATP, measured versus position along the channel (ℓ c = 1600 μm and w c = 20 μm). The position x = 0 is defined as where the entrance to the constriction is located. The approximate ATP concentration (CATP) converted from the calibration curve is shown on the right axis. We focus here only on light collected outside of the constriction; no appreciable signal was measured inside the constriction. The error bars are reported as the standard error of the mean (n = 5 different measurements). Note that the photon emission rate increases far downstream from the constriction. (Reproduced from [103] with permission.) (Copyright note: Wan, J., Ristenpart, W.D. & Stone, H.A. (2008) Dynamics of shear-induced ATP release from red blood cells. Proc. Natl. Acad. Sci. U. S. A, 105, 16432-16437. Copyright (2008) National Academy of Sciences, USA)
ATP release from pannexin-1 wildtype (Panx1+/+) (white bars) and knockout (Panx1−/−) (grey bars) erythrocytes. ATP release, as determined with a luciferase assay, is stimulated by hypotonic K+ solution (K+) more profoundly in Panx1+/+ erythrocytes than in Panx1−/− erythrocytes. The Panx1 channel inhibitor probenecid (prob, 1 mM) attenuated ATP release in Panx1+/+ cells but not significantly (P > 0.05) in Panx1−/− cells. (Reproduced from [76] with permission from Elsevier.)
The release of ATP from erythrocytes in response to both low oxygen tension and mechanical deformation has been shown to require signal transduction pathways involving activation of pathway-specific membrane-bound adenylyl cyclase, cyclic adenosine monophosphate (cAMP), protein kinase (PK) A and CFTR; in addition, the direct stimulation of the G protein Gi also results in the release of ATP [77–80]. Although not conclusively established, evidence suggests that the release of ATP from erythrocytes in response to reduced oxygen tension is linked to the oxygenation state of the haemoglobin molecule via alterations in its confirmation [81, 82]. The release of ATP from erythrocytes in response to low O2 tension was demonstrated to occur in milliseconds making it a physiologically relevant mediator of microvascular blood flow [83]. A computational model to measure the dynamics of O2-dependent ATP release from erythrocytes confirmed this time course [84]. Data was subsequently presented from studies of the simultaneous effect of hypoxia and deformation on ATP release from erythrocytes to suggest that at an oxygen saturation point of around 25 % deformation contributes to ATP release, but beyond this saturation point, ATP release is largely due to hypoxia [85]. Data from in vivo and in vitro studies showed that significant amounts of ATP were released from erythrocytes on exposure to hypoxia and shear stress at the same time [86].
In addition to mechanical deformation and low oxygen tension, erythrocytes release ATP in response to prostacyclin analogs and β-adrenergic agonists. Although the physiological impact of the latter is unclear, prostacyclin is released from endothelial cells in response to shear stress and, although it clearly has direct vasodilatory effects, its capacity to release ATP would enhance its effectiveness. The release of ATP by prostacyclin analogs involves a distinct signal transduction pathway which is initiated by activation of Gs and involves distinct pools of cAMP which are regulated by pathway-specific phosphodiesterases [87]. Prostacyclin receptor-induced ATP release occurs via the voltage-dependent anion channel, suggesting the presence of yet another channel for ATP release from erythrocytes [88].
A number of factors affect erythrocyte ATP release. Nitric oxide (NO) was shown to inhibit the signal transduction pathway for ATP release from erythrocytes via its action on heteromeric G protein, Gi [89]. Statins increase erythrocyte deformability and reduce low O2-induced ATP release [90]. ATP was released in the presence of cell-free haemoglobin [91]. Fluoride causes ATP depletion and oxidative stress in rat erythrocytes in vitro [92]. Insulin inhibits low oxygen-induced ATP release from human erythrocytes [93, 94].
The ATP degradation product, ADP, inhibits ATP release by a negative feedback pathway mediated by P2Y13 receptors on human red blood cells [95]. Caffeine enhances ATP release from erythrocytes, most likely due to its effect on levels of cAMP [96], while lactate, in the absence of changes in pH, interferes with ATP release [97]. High blood lactate is a dangerous metabolic consequence of several common diseases, including septic shock and malaria. It has been proposed that nitrite-induced vasodilation is due to nitrite enhancement of release of ATP from erythrocytes, which then acts as a vasodilator [98, 99].
Human limb muscle and skin blood flow increases significantly with elevations of temperature. Erythrocytes from rabbits release ATP and dilate skeletal muscle arterioles in the presence of reduced oxygen tension [100]. Erythrocyte ATP release is sensitive to physiological increases in temperature, possibly via activation of CFTR channels [101]. The authors suggest that this raises the possibility of treatment of patients with peripheral vascular disease, by using local heating to stimulate erythrocyte ATP release to increase flow and oxygen to limbs.
It has been suggested that the shape changes of erythrocytes related to intracellular ATP concentrations can be explained in terms of ATP-induced cytoskeletal changes involved in binding of actin to spectrin filaments [102]. In a more recent paper, data was presented to suggest a model wherein the retraction of the spectrin-actin cytoskeleton network triggers the mechanosensitive release of ATP, while a shear-dependent membrane viscosity controls the rate of release [103].
Treatment of erythrocytes with diamide, a compound that decreases erythrocyte deformity, inhibits low O2 tension-induced ATP release [82, 104]. Hydroxyurea, a substance that affects erythrocyte deformability, stimulates the release of ATP from rabbit erythrocytes through an increase in calcium and NO production [105]. Reducing erythrocyte membrane cholesterol and simvastatin both increase cell deformability and therefore ATP release [106]. Hypoxia-induced ATP release from human erythrocytes is triggered through mechanisms involving haemoglobin [107]. Erythrocytes from older healthy humans fail to release ATP during haemoglobin deoxygenation [108]. Exchange proteins activated by cAMP inhibit ATP release via activation of PKC [109]. ATP release following complement receptor 1 ligation increased the mobility of the lipid fraction of erythrocyte membranes and had a stimulatory effect on phagocytosis of immune-adherent immune complexes [110].
It has been suggested that sensing of low blood O2 content may involve ATP release from red blood cells, leading to stimulation of sensory aortic body neurons via P2X2/3 receptors [111]. ATP released from erythrocytes incubated with hydroxyurea resulted in increased endothelium-derived NO production [112].
Pathology
An important role of ATP release from erythrocytes in vascular regulation has been suggested to have predictive value in disease processes. Infection with the malaria protozoan parasite, Plasmodium falciparum, induces osmolyte and anion channels in the host erythrocyte membranes involving ATP release and autocrine purinergic signalling [113]. Purinergic receptors are expressed in P. falciparum, where occupation by ATP triggers increase in [Ca2+]i, which is essential for the invasion of erythrocytes [114]. Hydrolysis of ATP with apyrase drastically reduced erythrocyte infection by the parasite. The effect of parasite infection on the kinetics of extracellular ATP accumulation was studied, using analysis of the rates of ATP release and extracellular hydrolysis at different stages of the infection cycle [115]. ATP depletion of erythrocytes stimulates the phenotype associated with pyruvate kinase deficiency and confers protection against Plasmodium in vitro [116]. Extracellular ATP did not induce osmolyte permeability in non-infected human erythrocytes but induced osmolyte permeability in malaria-infected erythrocytes [117]. They showed further that induction of osmolyte permeability in Plasmodium-infected erythrocytes involved autocrine purinoceptor signalling. In mouse erythrocytes harbouring the malaria parasite, P. yoelii, nucleoside transport had abnormally low sensitivity to nitrobenzylthioinosine [118]. In a more recent study, it was suggested that ATP released by the rupture of erythrocytes during the blood stage of Paramecium chabaudi malaria induced an increase in the expression P2X7 receptors in CD4+ T cells [119]. A review has been published concerned with malaria-infected erythrocytes and purinergic signalling [120].
Leukotoxin is a virulence factor secreted by some bacteria, which can cause localised aggressive periodontitis. Leukotoxin-mediated haemolysis is significantly potentiated by ATP release and P2X receptor activation of human erythrocytes [121] (Fig. 3). The bacterium Escherichia coli can produce virulence factors such as the exotoxin α-haemolysin (HlyA). HlyA is a protein that induces haemolysis by creating large pores in erythrocyte membranes, increasing permeability thereby producing cell swelling, which finally ruptures the erythrocyte. A study shows that this pore formation triggers purinergic receptor activation to mediate the full haemolytic action [122]. They showed that antagonists to P2X1 and P2X7 receptors and apyrase inhibited HlyA-induced lysis of erythrocytes and concluded that selective P2X receptor antagonists may ameliorate symptoms during sepsis with haemolytic bacteria. E. coli HlyA evoked ATP release and P2 receptor-mediated Ca2+ influx in human erythrocytes through the toxin pore [123, 124]. Another recent study proposed that erythrocytes damaged by HlyA insertion are effectively cleared from the blood stream, reducing the risk of intravascular haemolysis [125]. It was reported that, similar to haemolysis produced by HlyA, leukotoxin and α-toxin complement-induced haemolysis is amplified through ATP release and activation of P2 receptors [126]. Adenosine deaminase activity was altered in erythrocytes of dogs infected with Rangelia vitalii as well as the serum concentration of adenosine [127]. It was suggested that these changes may contribute to the pathogenesis of anaemia and immune response in infected dogs.
Model for leukotoxin from Aggregatibacter (LtxA)-induced haemolysis. 1 Interaction between LtxA and the erythrocyte membrane leads to an influx of ions and increase in [Ca2+]i. 2 The increase in [Ca2+]i stimulates a Ca2+-activated K+ efflux mediated by the Ca2+-activated K+ channel, KCa3.1. K+–Cl− co-transporters (KCCs) contribute to the K+ efflux. Initially, the K+ efflux exceeds the influx of ions leading to osmotically obliged H2O efflux and volume reduction. ATP is released from the cell through a yet unknown pathway and activates P2X receptors on the erythrocyte membrane, which is required for the full haemolytic effect of LtxA. In addition, activation of pannexin channels is also necessary for LtxA-induced haemolysis. 3 Later, the influx of ions exceeds the efflux of K+ resulting in osmotically obliged H2O influx and cell swelling. Finally, the erythrocyte lyses. (Reproduced from [121] with permission from John Wiley and Sons.)
Isoproterenol substantially altered cardiovascular haemodynamics and induced breakdown of ATP in erythrocytes to ADP and AMP, particularly in dying rats [128]. It was suggested that the relative concentrations of ATP, ADP and AMP in red blood cells may be used as a predictive biomarker for cardiovascular mortality. There is impaired release of ATP from red blood cells of humans with primary pulmonary hypertension [129]. Prostacyclin analogs and phosphodiesterase inhibitors had synergistic effects on ATP release from human erythrocytes, and it was suggested that that could influence the development of new therapeutic approaches for the treatment of pulmonary arterial hypertension [130].
It was proposed that reduced ATP release from erythrocytes contributes to vascular disease in type 2 diabetes [131, 132]. In type 2 diabetes, erythrocytes are under high oxidative stress and considered to be less deformable leading to lowered levels of deformation-induced ATP release [133]. The selective phosphodiesterase 3 inhibitor, cilostazol, facilitates PO2-induced ATP release from erythrocytes of humans with type 2 diabetes [134]. C-peptide and insulin were shown to have synergistic effects on low O2-induced ATP release from human erythrocytes, suggesting that administration of a combination of C-peptide and insulin could help in the prevention and treatment of peripheral vascular disease associated with diabetes [135, 136].
It has been suggested that adenosine is a potentially important therapeutic target for the treatment and prevention of sickle cell disease, a debilitating haemolytic genetic disorder where an abnormal type of haemoglobin precipitates in erythrocytes when blood is deprived of oxygen forming crystals that distort the cell (sickling) resulting in anaemia and jaundice [137–139]. However, complicating this approach, adenosine signalling also induces haemoglobin S polymerization, promoting sickling, vasoocclusion, haemolysis and organ damage [140, 141] (Fig. 4). Amyloid β peptide inhibits ATP release from deoxygenated erythrocytes by activating red cell caspase 3, suggesting a pathophysiologic role for vascular amyloid peptide in Alzheimer’s disease [142].
Adenosine worsens sickle cell disease (SCD) by increasing 2,3-diphosphoglycerate (2,3-DPG) in red blood cells through the A2B receptor. Increased amounts of ATP in circulation owing to chronic sickle red blood cell hemolysis and tissue damage from vasoocclusion are rapidly converted to adenosine. Activation of the A2A receptor on natural killer T (NKT) cells suppresses the innate immune response and limits inflammation and cellular injury during ischemia and reperfusion injury. Top, in contrast, Zhang et al. [140] show that activation of the A2B receptor by adenosine on erythrocytes increases 2,3-DPG levels through cAMP-dependent protein kinase A (PKA) activation, which reduces haemoglobin S (Hb S) oxygen affinity and promotes its polymerization and red blood cell sickling. Bottom, ‘crossroads’ of adenosine signaling determine positive or negative effects. The adenosine antagonist polyethylene glycol-modified adenosine deaminase (PEG-ADA) may be used to block adenosine signalling as a therapy for SCD; however, the development of specific agonists and inhibitors of these receptors may allow for selective inhibition of red blood cell A2B-dependent 2,3-DPG production and activation of A2A-dependent immune modulation to ease the disease more effectively. (Reproduced from [141] with permission from The Nature Publishing Group.)
Platelets
Introduction
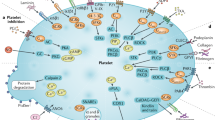
Platelets express P2Y1, P2Y12 and P2X1 receptor subtypes involved in platelet aggregation (see Fig. 5).
Three P2 receptor subtypes, P2X1, P2Y1 and P2Y12, are involved in ADP-induced platelet activation. Clopidogrel is a P2Y12 receptor blocker that inhibits platelet aggregation and is in highly successful use for the treatment of thrombosis and stroke. A P2Y1 receptor antagonist, MRS 2500, inhibits shape change. (Modified from [403] with permission from Elsevier.)
Review articles have been published on various aspects of purinergic signalling in platelets, including
-
Purinoceptor-evoked calcium signalling in human platelets [168]
-
P2Y receptor polymorphisms and disease [182]
P2Y1 and P2Y12 receptors
In 1956, it was shown that platelets contain very high concentrations of ATP [183] and that extracellular ATP is rapidly broken down to ADP [184]. It was shown by Hellem and coworkers that a factor derived from red blood cells was responsible for the adhesiveness of platelets to glass beads [185]. This factor was identified as ADP, and the ability of ADP to produce platelet aggregation was recognised early in two Nature papers ([186, 187] and see [188, 189]). Later, the possible mechanisms underlying ADP-induced platelet aggregation were explored [190–193]. ATP itself did not induce platelet aggregation but inhibited aggregation produced by ADP and platelet shape change [194, 195].
ADP was shown to be a potent inhibitor of human platelet plasma membrane adenylate cyclase [196], in retrospect an early indication that ADP was acting via G protein-coupled receptors, later identified as P2Y1 and P2Y12 receptors. ADP induced binding of von Willebrand factor to human platelets [197]. ATP analogues produced greater inhibition of aggregation induced by ADP than did AMP analogues [198], which in retrospect indicated inhibition via P2, rather than P1 receptors. ATP, UTP, guanosine-5′-triphosphate (GTP) and cytidine triphosphate inhibited platelet aggregation induced by collagen and epinephrine by acting as antagonists of the P2Y12 receptor [199, 200]. ADP produces an increase in [Ca2+]i in platelets [201]. Potentiation of ADP-induced platelet aggregation in platelet-rich plasma by 5-hydroxytryptamine (5-HT) and adrenaline was shown [202]. Diadenosine tetraphosphate (Ap4A) had anti-platelet aggregation activity [203]. ADP induced platelet α-granule release [204].
The non-selective P2 receptor antagonist, suramin, inhibited platelet aggregation induced by ADP [205]. It was claimed in 1993 that ADP-induced increase in [Ca2+]i in platelets was mediated by the P2T receptor (later identified as the P2Y12 receptor) [206, 207]. ADP-induced platelet aggregation was inhibited by the P2T receptor antagonists FPL 66096 [208] and FPL 67085 (also known as ARC 67085), both ATP variants [209]. The human platelet ADP receptor activates Gi2 proteins [210], another indication that P2Y12 receptors were involved. A radiolabelled selective antagonist, [3H]PSB-0413, was shown to be a tool for radioligand binding studies aimed at quantifying P2Y12 receptors to identify patients with P2Y12 receptor deficiencies and to quantify the effect of P2Y12 targeting drugs [211].
ADP inhibited 5-HT uptake into human platelets [212]. The aggregation behaviour of post-mortem platelets has been claimed to be a tool for estimating time of death [213]. The P2Y1 receptor was also shown to be expressed by platelets and megakaryocyte cell lines; it was antagonised by ATP [214, 215] and coupled to Gq [216]. Platelet shape change was identified as the main role of P2Y1 receptors [217, 218], although it also contributes to platelet aggregation [219]. Evidence for three P2 receptors on platelets was presented [220, 221]. The cloning of P2X1-specific complementary DNA (cDNA) from human platelets was achieved in 1998 [222]. BzATP was claimed to be an antagonist of rat and human P2Y1 receptors and of platelet aggregation [223]. It was confirmed that ADP can induce aggregation of human platelets via both P2Y1 and P2T (P2Y12) receptors [224]. The P2T receptor was identified as a P2Y12 receptor in 2001 (see [225–227]). Combinations of antagonists of P2Y1 and P2Y12 receptors were effective inhibitors of direct shear-induced platelet aggregation [228].
The chemokines, macrophage-derived chemokine, thymus activation-regulated chemokine and stromal cell-derived factor one, which may be produced during inflammatory responses, coupled with low levels of ADP or thrombin to serve as stimuli for activating platelet formation [229]. Evidence was presented to show that collagen required not only the thromboxane A2 (TxA2) receptor Tpα but also P2Y1 receptors, to induce platelet shape change [230]. Stimulation of the P2Y12 receptor is involved in platelet activation initiated by the binding of von Willebrand factor to platelet receptor protein GP Ibα induced by a high shear rate [231]. Quantitative RT-PCR studies showed that the order of expression of P2Y receptor mRNA was P2Y12 ≫ P2X1 > P2Y1 [232]. Depending on the experimental conditions, signalling from both fibrinogen and P2Y1 and P2Y12 receptors are necessary for PLA2 activation, resulting in arachidonic acid liberation and TxA2 generation [233]. Using a high-resolution channelyzer, it was concluded that P2Y12, as well as P2Y1 receptors, play a role in controlling shape change in human platelets [234], although this is controversial. Evidence has been presented to support the view that for thrombin-induced human platelet activation, the P2Y12 receptor is the drug target compared to the P2Y1 receptor [235]. However, a synergistic interaction was reported between antagonists of P2Y1- and P2Y12-mediated inhibition of ADP- and thrombin-induced human platelet activation [236].
In healthy subjects, it has been claimed that ADP-induced platelet aggregation is associated with a haplotype polymorphism of the P2Y12 receptor gene [237, 238]. Homozygosity for the P2Y1 1622G allele is associated with increased receptor signalling and platelet aggregation [239]. However, this P2Y12 receptor gene H2 haplotype was shown not to be associated with increased ADP-induced platelet aggregation in a separate study [240]. Furthermore, this genetic haplotype was not associated with the risk of myocardial infarction in a large study with more than 3000 patients [241]. Mutational analysis of the residues important for ligand interaction with the human P2Y12 receptor is available [242]. Interestingly, all cells contain an endogenous P2Y12 antagonist, farnesyl pyrophosphate, which acts as a traditional competitive antagonist to ADP [243].
The P2Y1 receptor antagonist, MRS2500, was shown to be the most potent inhibitor of P2Y1 receptor-mediated platelet shape change and aggregation [244]. There is a complex signalling interaction between P2Y1 and P2Y12 receptors; P2Y12 receptors positively regulate P2Y1 action, while P2Y1 receptors negatively regulate the action of P2Y12 receptors [245].
ADP caused desensitization of the P2Y1 receptor-driven calcium signal, but P2Y12 receptor-mediated inhibition of cAMP formation was not affected [246]. It was suggested that the absence of desensitization of the P2Y12 receptor-mediated platelet response could represent a mechanism to preserve the haemostatic properties of unresponsive platelets. In another paper, it was claimed that both P2Y1- and P2Y12-mediated platelet responses desensitise rapidly, but by different kinase-dependent mechanisms [247]. The desensitization of the P2Y receptor on platelets requires receptor internalization, and it was claimed that the GTP-binding protein ADP ribosylation factor 6 is required for P2Y receptor internalization [248].
ADP was shown to play a key role in irreversible platelet aggregation through the activation of phosphoinositide 3-kinase [249]. Platelet integrin αIIbβ3 plays a crucial role in platelet aggregation, and it was claimed that phosphatidylinositol 3-kinase is essential for ADP-stimulated αIIbβ3-mediated platelet activation and calcium oscillations [250]. Continuous interaction between ADP and P2Y12 receptors is critical for the maintenance of αIIbβ3 activation [251–253]. Evidence was presented that P2Y12 receptors potentiate platelet shape change induced by P2Y1 receptor activation by a Rho kinase-dependent mechanism [254]. The residual arachidonic acid-induced platelet activation in aspirin-treated patients is mediated in part by ADP-induced platelet activation [255]. It was suggested that the interaction of calmodulin with the P2Y1 receptor C-terminal tail may regulate P2Y1-dependent platelet aggregation [256].
P2Y14 receptor mRNA and protein were shown to be expressed by platelets, although the functional role of this receptor is not yet known [257, 258]. Involvement of basic amino acid residues in transmembrane regions 6 and 7 in agonist and antagonist recognition of the human platelet P2Y12 receptor has been reported [259]. A novel P2Y1 receptor radioligand has been synthesised, which is valuable for examining the expression of P2Y1 receptors on human and mouse platelets [260]. Resolvin E1, generated during acute inflammation, regulates ADP activation of human platelets [261]. It was suggested that cAMP regulates ADP-stimulated platelet activation due to inhibition of heat shock protein (HSP) 27 phosphorylation via p38 mitogen-activated protein MAP kinase [262]. 5-HT reuptake inhibitors reduce P2Y12 receptor-mediated amplification of platelet aggregation [263]. Inhibition of P2Y12 receptors potentiated the anti-platelet effect of prostacyclin [264]. Circulating platelets are exposed to NO released from endothelial cells, and NO reduces platelet aggregation and thrombus formation. Blockade of P2Y12 receptors significantly increased the platelet inhibitory actions of NO [265]. Platelet P2Y1 and P2Y12 and arachidonic acid receptor inhibition is a prominent early feature of coagulopathy in traumatic brain injury [266].
P2X1 receptors
ATP inhibited both collagen- and a thromboxane mimetic (U46619)-induced platelet aggregations via a P2X-like receptor [267], in retrospect by P2X1 receptors, since it was blocked by α,β-methylene ATP (α,β-meATP). It was suggested that human platelets express a P2X1 receptor, which mediates rapid Ca2+ entry, in contrast to the P2Y receptors which evoke release of calcium from intracellular stores [268]. Clopidogrel did not affect the binding of α,β-meATP to platelet P2X1 receptors [269]. The P2X1 receptor cDNA and protein was identified on human platelets, but not leukocytes [270–272]. It was reported that P2X1 receptors did not play a significant role in ADP-induced platelet shape change and aggregation [273]. ATP, but not ADP, is an agonist at P2X1 receptors on human platelets [274]. However, a later paper claimed that novel structurally altered P2X1 receptors on platelets and megakaryocytic cells were preferentially activated by ADP [275].
The P2Y1 receptor antagonist, adenosine-2′,5′-diphosphate, non-selectively antagonised the platelet P2X1 ion channel [276]. ATP, acting on P2X1 receptors, was claimed to contribute to platelet activation in addition to the earlier suggestion that ATP activation of P2X1 receptors had an inhibitory action at metabotropic platelet receptors [277]. During collagen-initiated platelet activation, the early secretion of ATP resulted in P2X1-mediated stimulation, which played a role as a positive regulator of further platelet responses [278]. From studies of P2X1 receptor knockout (KO) mice, it was shown that accumulation of P2X1 KO platelets on a collagen-coated surface was greatly reduced compared to wild-type (WT) platelets, suggesting a role of P2X1 receptors in platelet interaction with collagen [279].
The role of P2X1 receptors expressed by platelets has been difficult to assess, due to its rapid desensitization. However, P2X1 and P2Y1 receptor synergy was claimed in both murine megakaryocytes and human platelets [280]. From a study of P2X1 KO and WT mouse platelets treated with apyrase to prevent desensitization, it was shown that collagen-induced aggregation and secretion of P2X1-deficient platelets was decreased, as well as adhesion and thrombus growth on a collagen-coated surface [281]. The mortality of P2X1 KO mice in a model of systemic thromboembolism was reduced, and it was concluded that P2X1 receptors contribute to the formation of platelet thrombi, particularly in arteries in which shear forces are high [281]. In contrast, over-expression of P2X1 receptors in transgenic mice led to enhancement of platelet dense granule secretion and aggregation evoked by collagen or the TxA2 mimetic U46619; it also enhanced platelet responses under shear stress, but the responses to ADP or thrombin were normal [282]. The authors concluded that over-expression of P2X1 receptors on platelets generated a novel prothrombotic phenotype. It was reported that ADP did not contribute to the rapid ionotropic P2X1 receptor-mediated response in platelets but suggested that ATP plays a role during haemostasis and thrombosis [283]. Pharmacological inhibition of the P2X1 receptor using NF449 also resulted in thrombosis inhibition in vivo [284].
In an authoritative review of the emerging roles for P2X1 receptors in platelet activation, it was concluded that P2X1 receptors can mediate transient shape change and granule release, important early events in platelet activation, and that ATP acting on P2X1 receptors can synergise with ADP acting on platelet P2Y receptors to potentiate functional events, particularly under conditions of shear stress [149]. A detailed description of the intracellular pathways involved in P2X receptor stimulation is shown in Fig. 6. Subsequently, it was shown that ATP augments von Willebrand factor-dependent shear-induced platelet aggregation through Ca2+-calmodulin and myosin light chain kinase activation [285]. A major role for P2X1 receptors in early collagen-evoked intracellular Ca2+ responses of human platelets was reported, contributing to arterial thrombosis [286]. NF864, claimed to be the most potent platelet P2X1 receptor antagonist, blocked α,β-meATP-induced [Ca2+]i increases and shape change [287].
P2X1 receptor signalling and regulation in the platelet. Summary of the pathways whereby P2X1 receptors have been proposed to couple to functional responses in platelets, together with the mechanisms that regulate these ion channels. (Reproduced from [150] with permission from Springer.)
Evidence was presented to suggest that lipid rafts play a significant role in the regulation of P2X1, but not P2Y1 receptors in human platelets [288]. Another study concluded that ATP should be considered alongside ADP and TxA2 as a significant secondary platelet agonist [289]. Activation of P2X1 receptors with ATP had a dual effect, causing a significant concentration-dependent increase in platelet NO production and causing aggregation and adhesion, although platelet aggregation was initially decreased [290]. Ap4A, a constituent of platelet dense granules, is an antagonist of platelet P2Y1 receptors where it inhibits the effects of ADP and an agonist of platelet P2X1 and P2Y12 receptors [291]. Margatoxin, a voltage-dependent K+ channel inhibitor, reduced the P2X1- and TxA2 receptor-evoked [Ca2+]i increases [292]. P2X1 receptors are constitutively regulated by HSP90, and inhibitors of HSP90 reduce trafficking of ATP-gated P2X1 receptors and human platelet responsiveness [293].
P1 (adenosine) receptors
Adenosine was shown to be a competitive inhibitor of platelet aggregation by ADP [294]. A receptor for adenosine on platelets that mediated inhibition of platelet function via activation of adenylate cyclase was recognised early [295–297]. Aggregation of human platelets induced by ADP was inhibited by 2-azidoadenosine, a photolysable analogue of adenosine, and deamination of adenosine by adenosine deaminase was inhibited by 2-azidoadenosine [298]. Adenosine is taken up and deaminated by platelets [299]. Dipyridamole inhibited adenosine uptake into platelets [300] and potentiated the anti-platelet action of adenosine [301, 302].
5′-N-ethylcarboxamidoadenosine was shown to be a potent inhibitor of human platelet aggregation [303]. A xanthine amine congener was introduced as a radioligand for A2 receptors of human platelets [304]. Prostacyclin analogues diminished A2 receptor responsiveness of platelets [305]. The effects of adenosine derivatives confirmed that A2 receptors mediate inhibition of human and rabbit platelet aggregation [306], later identified as A2A receptors [307–309]. Synergistic inhibition of thrombin-induced platelet aggregation by an NO donor and adenosine was reported [310]. It was claimed that there was A2 receptor-mediated inhibition of platelet aggregation in humans, but not in canine models [311].
Treatment of mouse and human blood with 5′-nucleotidase (which led to increased extracellular adenosine) inhibited platelet aggregation [312]. Gene expression profiling led to the identification of functional A2B receptors on human platelets [313]. It was later shown that A2B receptors on mouse platelets were upregulated under stress in vivo and played a significant role in regulating ADP receptor expression [314]. ADP inhibited platelet aggregation in the presence of P2Y12 receptor antagonists due to its conversion to adenosine [315]. It has been claimed recently that adenosine may be the major active ingredient for anti-platelet activity of black soybean, used for the treatment of cardiovascular diseases [316].
In summary, platelets express pro-aggregatory P2Y1 and P2Y12 receptors, anti-aggregatory A2A and A2B receptors, as well as P2X1 receptors, which appear to have synergistic actions with the P2Y receptors.
ATP release
Upon stimulation, platelets secrete ATP and ADP, which evoke platelet aggregation (see [159]). ATP release from activated platelets was shown using cell surface-attached firefly luciferase [317]. Later, lumi-aggregometers were used as an ATP release assay for the assessment of platelet function disorders [318–320]. An HPLC assay has also been used to determine ATP and ADP secretion [321]. The vesicular nucleotide transporter, VNUT, has been claimed to be responsible for vesicular storage and release of nucleotides from platelets [322, 323] (Fig. 7).
a Transmission electron micrograph of an untreated rabbit platelet after the uranaffin reaction. Four 5-HT organelles (→) are selectively stained. One of these (asterisk) has apparently fused with the platelet membrane to release its contents (5-HT and ATP), possibly by the process of exocytosis. Note the difference in staining between the membrane of this organelle and the platelet plasma membrane. α-Granules (arrowhead). (Reproduced from [322] with permission; Prada, M., Lorez, H.P. & Richards, J.G. (1982) Platelet granules. In Poisner, A. M. & Trifaro, J. M. (eds), The Secretory Granule. Elsevier Biomedical, Amsterdam, pp. 279-316, Copyright Elsevier.) b Schematic diagram of nucleotide storage and release in platelets. Vesicular nucleotide transporter (VNUT) is associated with dense granules and transports nucleotides into granule using Δψ that is established by V‐ATPase. VNUT is thus involved in nucleotide release and that is inhibited by glyoxylate, a VNUT inhibitor. (Reproduced from [323] with permission from Wiley Periodicals, Inc.)
Ectonucleotidases
It was suggested that ADPase may act as a platelet aggregation inhibitor in the placental and foetal circulation [324]. The ecto-ATPase present on human platelets, responsible for breaking down ATP to ADP, was examined, and a direct role of ecto-ATPase activity on platelet aggregation was shown to be relatively small [325]. ATP diphosphohydrolase (apyrase) was later identified on rat platelets to hydrolyse ATP to ADP [326–328]. ATPDase/CD39 expression was described in human platelets and endothelial cells [329] to modulate platelet activation and thrombus formation [330]. Endothelial cells contribute to control of platelet reactivity via endothelial ectoNTPDase-1/CD39 [331, 332] by rapidly metabolizing ADP released from platelets, thereby preventing further platelet activation or recruitment. Acetylsalicylic acid inhibited ATP diphosphohydrolase activity by platelets from adult rats [333]. Extracellular hydrolysis of ATP by intact rat blood platelets is achieved by NTPDase 3 and 5′-nucleotidase, resulting in the production of adenosine [334]. Ebselen, which exhibits anti-oxidant, anti-inflammatory, anti-atherosclerotic and cytoprotective properties, inhibited the extracellular hydrolysis of ATP [334]. The possibility of inhibiting platelet P2X1 receptors or elevating CD39/NTPDase1 activity as novel therapeutic approaches to reduce platelet reactivity and recruitment of platelets at prothrombotic locations has been discussed [335]. Reduced degradation of ADP by ectonucleotidases contributes to the amplification of ADP-evoked aggregation [336].
After ovariectomy, there was a decrease in the ecto-hydrolysis of ATP by platelet NTPDase and 5′-nucleotidase, indicating that hormonal deprivation affects platelet aggregation [337]. Distinct roles for PKC isoforms have been described in the regulation of platelet P2Y receptor function and trafficking [338]. Plasma ectonucleotidases prevent desensitization of purinergic receptors on platelets [339]. Enhanced NTPDase and 5′-nucleotidase activities in platelets in human pregnancy suggests that these enzymes are involved in thromboregulation in pregnancy [340]. Intravascular ADP augments platelet activity during strenuous exercise, and these prothrombotic responses are counteracted by concurrent release of soluble nucleotide-inactivating enzymes [341].
Thrombosis
Antagonists to P2T (P2Y12) receptors were reported for use against ADP-induced arterial thrombosis [154]. Non-peptide glycoprotein inhibitors were reported to show anti-thrombotic efficacy against ADP-induced platelet aggregation [342]. An analogue of ATP, AR-C67085MX, was identified as a very potent antagonist at P2T platelet receptors [343]. Defective platelet aggregation and increased resistance to thrombosis were reported in P2Y1 receptor KO mice [344].
Percutaneous coronary interventions with metal stents initially had serious problems causing life-threatening stent thrombosis. It was not until the addition of a P2Y12 receptor antagonist (first ticlopidine, later clopidogrel) to aspirin that stent thrombosis could be prevented. A randomised, blinded, clinical trial of clopidogrel, a thienopyridine like the later drug prasugrel, versus aspirin showed improved protection against thrombosis, ischaemic stroke and myocardial infarction [345]. The CURE study demonstrated that addition of clopidogrel to aspirin reduced the incidence of myocardial infarction, which led to the worldwide adoption of this dual anti-platelet therapy for patients with acute coronary syndromes [346]. Inhibition of ADP-induced platelet aggregation by clopidogrel was reported [171, 347], and it was shown to act as an antagonist to P2YAC (later identified as P2Y12), but not P2Y1 and P2X1 receptors [348]. The anti-platelet activity of clopidogrel was shown early to be dependent on hepatic transformation to an active metabolite as an antagonist to P2Y12 receptors ([349, 350] and see [351]) (Fig. 8). A subpopulation of patients are not responsive to clopidogrel, due to polymorphisms of either the cytochrome P450 isoenzyme, CYP2C19 [352] or the P2Y12 receptor [353]. A new dysfunctional platelet P2Y12 receptor variant associated with bleeding diathesis has been identified [252].
Purinergic receptors and mechanism of action of clopidogrel. Clopidogrel is a pro-drug of which approximately 85 % is hydrolysed by esterases in the blood to inactive metabolites, and only 15 % is metabolised by the cytochrome P450 (CYP) system in the liver into an active metabolite. The active metabolite irreversibly inhibits the adenosine diphosphate (ADP) P2Y12 receptor. The P2X1 receptor, which uses adenosine triphosphate (ATP) as an agonist, is involved in platelet shape change through extracellular calcium influx and helps to amplify platelet responses mediated by other agonists. Activation of the P2Y1 receptor leads to alteration in shape and initiates a weak and transient phase of platelet aggregation. The binding of ADP to the Gq-coupled P2Y1 receptor activates phospholipase C (PLC), which generates diacylglycerol (DAG) and inositol triphosphate (IP3) from phosphatidylinositol bisphosphate (PIP2). Diacylglycerol activates protein kinase C (PKC) leading to phosphorylation of myosin light chain kinase (MLCK-P), and IP3 leads to mobilization of intracellular calcium. The P2Y1 receptor is coupled to another G-protein, G12, which activates the “Rho” protein and leads to the change in platelet shape. The binding of ADP to the Gi-coupled P2Y12 receptor liberates the Gi protein subunits αi and bγ, resulting in stabilization of platelet aggregation. The α1 subunit inhibits adenylyl cyclase (AC) and, thus, reduces cyclic adenosine monophosphate (cAMP) levels, which diminishes cAMP-mediated phosphorylation of vasodilator-stimulated phosphoprotein (VASP-P). The status of VASP-P modulates glycoprotein (GP) IIb/IIIa receptor activation. The subunit βγ activates the phosphatidylinositol 3-kinase (PI3K), which leads to GP IIb/IIIa receptor activation through activation of a serine-threonine protein kinase B (PKB/Akt) and of Rap1b GTP binding proteins. Prostaglandin E1 (PGE1) activates AC, which increases cAMP levels and status of VASP-P. Solid arrows indicate activation; dotted arrows indicate inhibition. (Reproduced from [404] with permission from Elsevier.)
Apyrase, an ectoenzyme that metabolises ATP and ADP released from platelets and endothelial cells, reduces platelet activation and was recommended for the treatment of platelet-mediated thrombosis [354]. Reversible P2Y12 receptor antagonists, BX 667, INS50589 and Arg256, were shown to be inhibitors of platelet aggregation and thrombus formation [355–357]. Unlike the irreversible action of the anti-thrombotic clopidogrel, an orally active, reversible direct P2Y12 receptor antagonist, AZD 6140 (ticagrelor), was introduced for the prevention of myocardial infarction [358]. Ticagrelor and prasugrel are more potent P2Y12 receptor inhibitors compared to clopidogrel and both have been shown to demonstrate improved clinical effects in preventing myocardial infarction [359, 360]. Additional inhibition by P2Y1 receptor antagonists of platelet aggregation produced by P2Y12 receptor antagonists has been considered [361, 362]. A complex of high molecular weight heparin and ATP prevented thrombus formation [363]. ADP-inducible platelet reactivity increased with age [364]. Various side effects have been reported for both clopidogrel (bleeding, liver and bone injury, enhanced lipopolysaccharide-induced inflammation) [365, 366] and ticagrelor (bleeding, dyspnoea) [367]. In more recent reports, it was suggested that there may be a role for endogenous adenosine in ticagrelor-induced dyspnoea [368, 369].
As well as the anti-thrombotic use of clopidogrel, other actions have been identified. For example, clopidogrel has been used for the prevention of cardiac ischaemic complications in percutaneous coronary intervention [370–372]. Clopidogrel has also been reported to enhance periodontal repair in rats through decreased inflammation [373]. P2Y12 receptor antagonists are widely used in combination with aspirin for the treatment of thrombosis, especially for patients with acute coronary syndrome and those undergoing percutaneous coronary intervention [374, 375]. Clopidogrel has also been reported to prevent endothelial dysfunction and vascular remodelling in aortas from hypertensive rats [376].
An important recent publication describes the crystal structure of the P2Y12 receptor complex with a non-nucleotide reversible P2Y12 receptor antagonist, AZD1283 [377] (Fig. 9). This will aid medicinal chemists to develop further new P2Y12 receptor antagonists. P2Y12 receptor antagonists are now one of the world’s most used medications and have saved many lives by preventing myocardial infarction and stroke. Recently, a fast acting P2Y12 receptor antagonist (Cangrelor), which is given as an infusion, was shown to prevent stent thrombosis and myocardial infarction. Since it is rapidly degraded, it reduces bleeding risk.
Cartoon representation of the P2Y12 receptor–AZD1283 complex structure. The P2Y12 receptor is coloured green and AZD1283 is shown as magenta spheres. Cholesterol and lipids have yellow carbons. The disulphide bridge is shown as lime sticks. Missing loops and membrane boundaries are indicated as black and blue dashed lines, respectively. (Reproduced from [377] with permission from The Nature Publishing Group.)
Megakaryocytes
Megakaryocytes are platelet precursor cells in bone marrow. ADP, as for platelets, raised [Ca2+]i and evoked release of granules from megakaryocytes and aggregation [378–380]. Release of ATP from megakaryocytes was reported [381]. Rat megakaryocytes responded to ATP, which mediated activation of K+ channels and oscillations of cytoplasmic calcium concentrations [382]. Adenine enhanced the ATP-induced Ca2+ oscillations [383] and suramin, and reactive blue 2 antagonised this action [384]. Patch clamp studies confirmed the presence of both ATP- and ADP-activated receptors in rat megakaryocytes [385]. A P2T (P2Y12) receptor was identified on the human megakaryocyte cell line, Dami [386]. The authors reported that this cell line also responded to ATP and UTP, suggesting the presence of P2U receptors, later identified as P2Y2 and/or P2Y4 receptors. The presence of these receptors was also described for the human megakarioblastic Meg-01 cell line [387]. ADP induced rapid inward currents through Ca2+ cation channels in mouse, rat and guinea pig megakaryocytes [388]. Functional expression of an ADP-activated receptor in Xenopus oocytes injected with megakaryocyte (CMK11-5) RNA was reported [389]. P2X1 receptor mRNA was identified in two megakarioblastic cell lines, Dami and CHRF-288 cells [270]. A study has characterised the functional P2X1 receptor in mouse megakaryocytes both pharmacologically and electrophysiologically [280, 390] (Fig. 10a). A P2Y1 receptor was also shown to be expressed by megakarioblastic cells [214, 391, 392]. Ca2+ signals evoked via P2Y1 receptors can be markedly potentiated by depolarisation or inhibited by hyperpolarisation [393] (Fig. 10b). As platelets have no nucleus, the level of P2X1 receptor expression depends on transcriptional regulation in megakaryocytes, the platelet precursor cell. It was shown that Sp1/3 and NF-1 mediate transcription of the human P2X1 receptor gene in megakarioblastic Meg-01 cells [394]. Acetylsalicylic acid, a cyclooxygenase-1 inhibitor and anti-thrombotic agent, enhanced P2Y receptor-mediated outward current in rat megakaryocytes [395]. ADP released by megakaryocytes regulates pro-platelet formation by human megakaryocytes via P2Y13 receptors [396, 397].
a P2X receptor-mediated inward currents are absent in P2X1 receptor-deficient mice megakaryocytes. α,β-Methylene ATP (α,β-meATP; 10 μM) evoked rapid transient inward currents in wild-type (+/+) megakaryocytes; these were absent in megakaryocytes from P2X1 receptor-deficient (−/−) mice. Bar indicates period of drug application. (Reproduced from [280] with permission from John Wiley and Sons.) b Effect of pulse amplitude on the depolarization-evoked [Ca2+]i increase during stimulation of P2Y receptors. [Ca2+]i responses of rat megakaryocytes to ADP (1 μM, horizontal bar) and step depolarizations from a holding potential of −75 mV. The effect of depolarization during ADP application was assessed after the agonist-evoked increase had settled to a raised plateau level. Effect of increasing the amplitude of the depolarizing step in 5 mV increments up to 75 mV. (Reproduced from [393] with permission from John Wiley and Sons.)
Leukocytes
Leukocytes are white blood cells. They consist largely of immune cells, which have been reviewed in detail in an associated article (see [398]). The different immune cells are all involved in protecting the body from foreign substances and in antibody production. In disease, a variety of the cell types may appear in the blood, notably immature forms of the normal red and/or white bold cells.
Extracellular ATP and ADP at micromolar concentrations lead to impaired production of interferon-γ and IL-12 in leukocytes in the lipopolysaccharide-stimulated whole human blood model of sepsis [399]. ATP contributes to atherogenesis, via P2Y2, P2Y6, P2X4 and P2X7 receptors by inducing leukocyte recruitment in mice [400]. Abacavir is linked to cardiovascular disease, and ATP has been shown to play a role in leukocyte accumulation induced by abacavir, via P2X7 receptors [401]. P1 (adenosine) receptor mRNA expression has been described in human leukocytes of patients with valvular disease [402].
References
Burnstock G (1972) Purinergic nerves. Pharmacol Rev 24:509–581
Burnstock G (1978) A basis for distinguishing two types of purinergic receptor. In: Straub RW, Bolis L (eds) Cell membrane receptors for drugs and hormones: a multidisciplinary approach. Raven Press, New York, pp 107–118
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Burnstock G (2007) Purine and pyrimidine receptors. Cell Mol Life Sci 64:1471–1483
Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR (2001) Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 97:587–600
Wolff R (1947) Etude de lacide adenosine-triphosphorique dans le sang du lapin. C R Seances Soc Biol Fil 141:665–667
Nakao M, Nakao T, Yamazoe S, Yoshikawa H (1961) Adenosine triphosphate and shape of erythrocytes. J Biochem 49:487–492
Feo C, Mohandas N (1977) Clarification of role of ATP in red-cell morphology and function. Nature 265:166–168
Knull HR, Bronstein WW, Porter PJ (1978) Adenosine triphosphate and diphosphoglycerate levels in red blood cells from patients with Down’s syndrome. Experientia 34:1133–1134
Dern RJ, Brewer GJ, Wiorkowski JJ (1967) Studies on the preservation of human blood. II. The relationship of erythrocyte adenosine triphosphate levels and other in vitro measures to red cell storageability. J Lab Clin Med 69:968–978
Parker JC (1970) Metabolism of external adenine nucleotides by human red blood cells. Am J Physiol 218:1568–1574
Quist EE, Roufogalis BD (1977) Association of (Ca + Mg)-ATPase activity with ATP-dependent Ca uptake in vesicles prepared from human erythrocytes. J Supramol Struct 6:375–381
Katz S, Roufogalis BD, Landman AD, Ho L (1979) Properties of (Mg2+ + Ca2+)-ATPase of erythrocyte membranes prepared by different procedures: influence of Mg2+, Ca2+, ATP, and protein activator. J Supramol Struct 10:215–225
Maretzki D, Reimann B, Klatt D, Rapoport S (1980) A form of (Ca2+ + Mg2+)-ATPase of human red cell membranes with low affinity for Mg-ATP: a hypothesis for its function. FEBS Lett 111:269–271
Lichtman MA (1975) Does ATP decrease exponentially during red cell aging? Nouv Rev Fr Hematol 15:625–632
Parker JC, Snow RL (1972) Influence of external ATP on permeability and metabolism of dog red blood cells. Am J Physiol 223:888–893
Elford BC (1975) Interactions between temperature and tonicity on cation transport in dog red cells. J Physiol 246:371–395
Romualdez A, Volpi M, Sha’afi RI (1976) Effect of exogenous ATP on sodium transport in mammalian red cells. J Cell Physiol 87:297–305
Parker JC, Castranova V, Goldfinger JM (1977) Dog red blood cells: Na and K diffusion potentials with extracellular ATP. J Gen Physiol 69:417–430
Quist EE (1980) Regulation of the shape of unsealed erythrocyte membranes by Mg-ATP and Ca2+. Arch Biochem Biophys 203:123–133
Patel VP, Fairbanks G (1986) Relationship of major phosphorylation reactions and MgATPase activities to ATP-dependent shape change of human erythrocyte membranes. J Biol Chem 261:3170–3177
Beleznay Z, Zachowski A, Devaux PF, Ott P (1997) Characterization of the correlation between ATP-dependent aminophospholipid translocation and Mg2+-ATPase activity in red blood cell membranes. Eur J Biochem 243:58–65
Hoffman JF, Dodson A, Wickrema A, Dib-Hajj SD (2004) Tetrodotoxin-sensitive Na+ channels and muscarinic and purinergic receptors identified in human erythroid progenitor cells and red blood cell ghosts. Proc Natl Acad Sci U S A 101:12370–12374
Downes CP, Berrie CP, Hawkins PT, Stephens L, Boyer JL, Harden TK (1988) Receptor and G-protein-dependent regulation of turkey erythrocyte phosphoinositidase C. Philos Trans R Soc Lond B Biol Sci 320:267–280
Boyer JL, Downes CP, Harden TK (1989) Kinetics of activation of phospholipase C by P2Y purinergic receptor agonists and guanine nucleotides. J Biol Chem 264:884–890
Vaziri C, Downes CP (1992) G-protein-mediated activation of turkey erythrocyte phospholipase C by β-adrenergic and P2y-purinergic receptors. Biochem J 284:917–922
Berrie CP, Hawkins PT, Stephens LR, Harden TK, Downes CP (1989) Phosphatidylinositol 4,5-bisphosphate hydrolysis in turkey erythrocytes is regulated by P2y purinoceptors. Mol Pharmacol 35:526–532
Boyer JL, Schachter JB, Sromek SM, Palmer RK, Jacobson KA, Nicholas RA, Harden TK (1996) Avian and human homologues of the P2Y1 receptor: pharmacological, signaling, and molecular properties. Drug Dev Res 39:253–261
Sak K (2000) Are P2Y1 purinoceptors expressed in turkey erythrocytes? Neurosci Lett 293:78–80
Light DB, Capes TL, Gronau RT, Adler MR (1999) Extracellular ATP stimulates volume decrease in Necturus red blood cells. Am J Physiol 277:C480–C491
Light DB, Dahlstrom PK, Gronau RT, Baumann NL (2001) Extracellular ATP activates a P2 receptor in necturus erythrocytes during hypotonic swelling. J Membr Biol 182:193–202
Sluyter R, Shemon AN, Barden JA, Wiley JS (2004) Extracellular ATP increases cation fluxes in human erythrocytes by activation of the P2X7 receptor. J Biol Chem 279:44749–44755
Sartorello R, Garcia CR (2005) Activation of a P2Y4-like purinoceptor triggers an increase in cytosolic [Ca2+] in the red blood cells of the lizard Ameiva ameiva (Squamata, Teiidae). Braz J Med Biol Res 38:5–10
Tiffert T, Lew VL (2011) Elevated intracellular Ca 2+ reveals a functional membrane nucleotide pool in intact human red blood cells. J Gen Physiol 138:381–391
Sluyter R, Shemon AN, Wiley JS (2007) P2X7 receptor activation causes phosphatidylserine exposure in human erythrocytes. Biochem Biophys Res Commun 355:169–173
Jiang H, Zhu AG, Mamczur M, Falck JR, Lerea KM, McGiff JC (2007) Stimulation of rat erythrocyte P2X7 receptor induces the release of epoxyeicosatrienoic acids. Br J Pharmacol 151:1033–1040
Jiang H, Anderson GD, McGiff JC (2010) Red blood cells (RBCs), epoxyeicosatrienoic acids (EETs) and adenosine triphosphate (ATP). Pharmacol Rep 62:468–474
Sluyter R, Shemon AN, Hughes WE, Stevenson RO, Georgiou JG, Eslick GD, Taylor RM, Wiley JS (2007) Canine erythrocytes express the P2X7 receptor: greatly increased function compared with human erythrocytes. Am J Physiol Regul Integr Comp Physiol 293:R2090–R2098
Stevenson RO, Taylor RM, Wiley JS, Sluyter R (2009) The P2X7 receptor mediates the uptake of organic cations in canine erythrocytes and mononuclear leukocytes: comparison to equivalent human cell types. Purinergic Signal 5:385–394
Bontemps F, Van den Berghe G, Hers HG (1988) 5'-Nucleotidase activities in human erythrocytes. Identification of a purine 5'-nucleotidase stimulated by ATP and glycerate 2,3-bisphosphate. Biochem J 250:687–696
Paredes-Gamero EJ, Craveiro RB, Pesquero JB, França JP, Oshiro ME, Ferreira AT (2006) Activation of P2Y1 receptor triggers two calcium signaling pathways in bone marrow erythroblasts. Eur J Pharmacol 534:30–38
Kostic MM, Mojsilovic LP, Zivkovic RV (1981) Effect of adenosine on glycolysis in human red-blood-cells. Ircs Med Sci-Biochem 9:186–187
Braun S, Levitzki A (1979) Adenosine receptor permanently coupled to turkey erythrocyte adenylate cyclase. Biochemistry 18:2134–2138
Porsche E (1982) Effects of methylxanthine derivatives on the adenosine uptake in human-erythrocytes. Ircs Med Sci-Biochem 10:389
Plagemann PG, Wohlhueter RM, Kraupp M (1985) Adenosine uptake, transport, and metabolism in human erythrocytes. J Cell Physiol 125:330–336
Franco R, Aran JM, Colomer D, Matutes E, Vives-Corrons JL (1990) Association of adenosine deaminase with erythrocyte and platelet plasma membrane: an immunological study using light and electron microscopy. J Histochem Cytochem 38:653–658
Baumann R, Blass C, Götz R, Dragon S (1999) Ontogeny of catecholamine and adenosine receptor-mediated cAMP signaling of embryonic red blood cells: role of cGMP-inhibited phosphodiesterase 3 and hemoglobin. Blood 94:4314–4320
Niemoeller OM, Bentzen PJ, Lang E, Lang F (2007) Adenosine protects against suicidal erythrocyte death. Pflugers Arch 454:427–439
Pafundo DE, Alvarez CL, Krumschnabel G, Schwarzbaum PJ (2010) A volume regulatory response can be triggered by nucleosides in human erythrocytes, a perfect osmometer no longer. J Biol Chem 285:6134–6144
Nagy S, Paál M, Kõszegi T, Ludany A, Kellermayer M (1998) ATP and integrity of human red blood cells. Physiol Chem Phys Med NMR 30:141–148
Planker M, Schnurr E, Schneider W (1983) Elevated ATP levels in the red cells of patients with renal failure. Klin Wochenschr 61:709–713
Dale GL, Norenberg SL (1989) Time-dependent loss of adenosine 5'-monophosphate deaminase activity may explain elevated adenosine 5'-triphosphate levels in senescent erythrocytes. Blood 74:2157–2160
van Giezen JJ, Sidaway J, Glaves P, Kirk I, Björkman JA (2012) Ticagrelor inhibits adenosine uptake in vitro and enhances adenosine-mediated hyperemia responses in a canine model. J Cardiovasc Pharmacol Ther 17:164–172
Öhman J, Kudira R, Albinsson S, Olde B, Erlinge D (2012) Ticagrelor induces adenosine triphosphate release from human red blood cells. Biochem Biophys Res Commun 418:754–758
Torngren K, Ohman J, Salmi H, Larsson J, Erlinge D (2013) Ticagrelor improves peripheral arterial function in patients with a previous acute coronary syndrome. Cardiology 124:252–258
Ye Y, Birnbaum GD, Perez-Polo JR, Nanhwan MK, Nylander S, Birnbaum Y (2015) Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol Epub ahead of print 4/6/15
Swennen EL, Dagnelie PC, Van den Beucken T, Bast A (2008) Radioprotective effects of ATP in human blood ex vivo. Biochem Biophys Res Commun 367:383–387
Zhu H, Zennadi R, Xu BX, Eu JP, Torok JA, Telen MJ, McMahon TJ (2011) Impaired adenosine-5'-triphosphate release from red blood cells promotes their adhesion to endothelial cells: a mechanism of hypoxemia after transfusion. Crit Care Med 39:2478–2486
Leal Denis MF, Incicco JJ, Espelt MV, Verstraeten SV, Pignataro OP, Lazarowski ER, Schwarzbaum PJ (2013) Kinetics of extracellular ATP in mastoparan 7-activated human erythrocytes. Biochim Biophys Acta 1830:4692–4707
Deyrup IJ (1951) Release of adenine derivatives from mammalian erythrocytes following admixture of blood with strongly hypertonic solutions. Am J Physiol 167:749–755
Pessina GP, Bocci V, Paulesu L, Alessandrini C, Gerli R (1980) Sialocompounds-poor vesicles isolated from ATP-depleted human erythrocytes. J Submicrosc Cytol 12:311–314
Müller H, Schmidt U, Lutz HU (1981) On the mechanism of red blood cell shape change and release of spectrin-free vesicles. Acta Biol Med Ger 40:413–417
Bergfeld GR, Forrester T (1992) Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26:40–47
Ellsworth ML, Forrester T, Ellis CG, Dietrich HH (1995) The erythrocyte as a regulator of vascular tone. Am J Physiol 269:H2155–H2161
Sprague RS, Ellsworth ML, Detrich HH (2003) Nucleotide release and purinergic signaling in the vasculature driven by the red blood cell. Curr Top Membr 54:243–268
Sprague RS, Bowles EA, Achilleus D, Ellsworth ML (2011) Erythrocytes as controllers of perfusion distribution in the microvasculature of skeletal muscle. Acta Physiol (Oxf) 202:285–292
Ellis CG, Milkovich S, Goldman D (2012) What is the efficiency of ATP signaling from erythrocytes to regulate distribution of O2 supply within the microvasculature? Microcirculation 19:440–450
Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ (1996) ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol 271:H2717–H2722
Price AK, Fischer DJ, Martin RS, Spence DM (2004) Deformation-induced release of ATP from erythrocytes in a poly(dimethylsiloxane)-based microchip with channels that mimic resistance vessels. Anal Chem 76:4849–4855
Moehlenbrock MJ, Price AK, Martin RS (2006) Use of microchip-based hydrodynamic focusing to measure the deformation-induced release of ATP from erythrocytes. Analyst 131:930–937
Wan J, Forsyth AM, Stone HA (2011) Red blood cell dynamics: from cell deformation to ATP release. Integr Biol (Camb) 3:972–981
Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ (1998) Increases in perfusate flow rate stimulate ATP release from red blood cells in isolated rabbit lungs. Exp Clin Cardiol 3:73–77
Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ (1998) Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol 275:H1726–H1732
Forsyth AM, Wan J, Owrutsky PD, Abkarian M, Stone HA (2011) Multiscale approach to link red blood cell dynamics, shear viscosity, and ATP release. Proc Natl Acad Sci U S A 108:10986–10991
Montalbetti N, Leal Denis MF, Pignataro OP, Kobatake E, Lazarowski ER, Schwarzbaum PJ (2011) Homeostasis of extracellular ATP in human erythrocytes. J Biol Chem 286:38397–38407
Qiu F, Wang J, Spray DC, Scemes E, Dahl G (2011) Two non-vesicular ATP release pathways in the mouse erythrocyte membrane. FEBS Lett 585:3430–3435
Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS (2004) Heterotrimeric G protein Gi is involved in a signal transduction pathway for ATP release from erythrocytes. Am J Physiol Heart Circ Physiol 286:H940–H945
Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ (2001) Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol 281:C1158–C1164
Sprague R, Bowles E, Stumpf M, Ricketts G, Freidman A, Hou WH, Stephenson A, Lonigro A (2005) Rabbit erythrocytes possess adenylyl cyclase type II that is activated by the heterotrimeric G proteins Gs and Gi. Pharmacol Rep 57(Suppl):222–228
Montalbetti N, Lazarowski E, Schwarzbaum P (2010) Human erythrocytes release ATP in a cyclic AMP-regulated manner. Purinergic Signalling 6:S73
Jagger JE, Bateman RM, Ellsworth ML, Ellis CG (2001) Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280:H2833–H2839
Sridharan M, Sprague RS, Adderley SP, Bowles EA, Ellsworth ML, Stephenson AH (2010) Diamide decreases deformability of rabbit erythrocytes and attenuates low oxygen tension-induced ATP release. Exp Biol Med (Maywood) 235:1142–1148
Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG Jr (2000) Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol 278:H1294–H1298
Sove RJ, Ghonaim N, Goldman D, Ellis CG (2013) A computational model of a microfluidic device to measure the dynamics of oxygen-dependent ATP release from erythrocytes. PLoS One 8:e81537
Faris A, Spence DM (2008) Measuring the simultaneous effects of hypoxia and deformation on ATP release from erythrocytes. Analyst 133:678–682
Mairbäurl H, Ruppe FA, Bärtsch P (2013) Role of hemolysis in red cell adenosine triphosphate release in simulated exercise conditions in vitro. Med Sci Sports Exerc 45:1941–1947
Adderley SP, Sprague RS, Stephenson AH, Hanson MS (2010) Regulation of cAMP by phosphodiesterases in erythrocytes. Pharmacol Rep 62:475–482
Sridharan M, Bowles EA, Richards JP, Krantic M, Davis KL, Dietrich KA, Stephenson AH, Ellsworth ML, Sprague RS (2012) Prostacyclin receptor-mediated ATP release from erythrocytes requires the voltage-dependent anion channel. Am J Physiol Heart Circ Physiol 302:H553–H559
Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS (2004) NO inhibits signal transduction pathway for ATP release from erythrocytes via its action on heterotrimeric G protein Gi. Am J Physiol Heart Circ Physiol 287:H748–H754
Clapp KM, Ellsworth ML, Sprague RS, Stephenson AH (2013) Simvastatin and GGTI-2133, a geranylgeranyl transferase inhibitor, increase erythrocyte deformability but reduce low O2 tension-induced ATP release. Am J Physiol Heart Circ Physiol 304:H660–H666
Cole RH, Malavalli A, Vandegriff KD (2009) Erythrocytic ATP release in the presence of modified cell-free hemoglobin. Biophys Chem 144:119–122
Agalakova NI, Gusev GP (2012) Fluoride induces oxidative stress and ATP depletion in the rat erythrocytes in vitro. Environ Toxicol Pharmacol 34:334–337
Hanson MS, Ellsworth ML, Achilleus D, Stephenson AH, Bowles EA, Sridharan M, Adderley S, Sprague RS (2009) Insulin inhibits low oxygen-induced ATP release from human erythrocytes: implication for vascular control. Microcirculation 16:424–433
Hanson MS, Stephenson AH, Bowles EA, Sprague RS (2010) Insulin inhibits human erythrocyte cAMP accumulation and ATP release: role of phosphodiesterase 3 and phosphoinositide 3-kinase. Exp Biol Med (Maywood) 235:256–262
Wang L, Olivecrona G, Götberg M, Olsson ML, Winzell MS, Erlinge D (2005) ADP acting on P2Y13 receptors is a negative feedback pathway for ATP release from human red blood cells. Circ Res 96:189–196
Ellsworth ML, Chrzaszcz MA, O’Keefe E, Achilleus D, Bowles E, Sprague RS (2007) Caffeine enhances ATP release from erythrocytes: consequences for peripheral vascular perfusion. FASEB J 21:A479–A480
Rozier MD, Zata VJ, Ellsworth ML (2007) Lactate interferes with ATP release from red blood cells. Am J Physiol Heart Circ Physiol 292:H3038–H3042
Cao Z, Bell JB, Mohanty JG, Nagababu E, Rifkind JM (2009) Nitrite enhances RBC hypoxic ATP synthesis and the release of ATP into the vasculature: a new mechanism for nitrite-induced vasodilation. Am J Physiol Heart Circ Physiol 297:H1494–H1503
Garcia JI, Seabra AB, Kennedy R, English AM (2010) Nitrite and nitroglycerin induce rapid release of the vasodilator ATP from erythrocytes: Relevance to the chemical physiology of local vasodilation. J Inorg Biochem 104:289–296
Sprague RS, Hanson MS, Achilleus D, Bowles EA, Stephenson AH, Sridharan M, Adderley S, Procknow J, Ellsworth ML (2009) Rabbit erythrocytes release ATP and dilate skeletal muscle arterioles in the presence of reduced oxygen tension. Pharmacol Rep 61:183–190
Kalsi KK, González-Alonso J (2012) Temperature-dependent release of ATP from human erythrocytes: mechanism for the control of local tissue perfusion. Exp Physiol 97:419–432
Gov NS, Safran SA (2005) Red blood cell membrane fluctuations and shape controlled by ATP-induced cytoskeletal defects. Biophys J 88:1859–1874
Wan J, Ristenpart WD, Stone HA (2008) Dynamics of shear-induced ATP release from red blood cells. Proc Natl Acad Sci U S A 105:16432–16437
Thuet KM, Bowles EA, Ellsworth ML, Sprague RS, Stephenson AH (2011) The Rho kinase inhibitor Y-27632 increases erythrocyte deformability and low oxygen tension-induced ATP release. Am J Physiol Heart Circ Physiol 301:H1891–H1896
Raththagala M, Karunarathne W, Kryziniak M, McCracken J, Spence DM (2010) Hydroxyurea stimulates the release of ATP from rabbit erythrocytes through an increase in calcium and nitric oxide production. Eur J Pharmacol 645:32–38
Forsyth AM, Braunmüller S, Wan J, Franke T, Stone HA (2012) The effects of membrane cholesterol and simvastatin on red blood cell deformability and ATP release. Microvasc Res 83:347–351
Akatsu T, Tsukada K, Hishiki T, Suga-Numa K, Tanabe M, Shimazu M, Kitagawa Y, Yachie-Kinoshita A, Suematsu M (2010) T-state stabilization of hemoglobin by nitric oxide to form alpha-nitrosyl heme causes constitutive release of ATP from human erythrocytes. Adv Exp Med Biol 662:109–114
Kirby BS, Garcia LJ, Crecelius AR, Richards JC, Luckasen GJ, Larson DG, Dinenno FA (2012) Erythrocytes from older healthy humans fail to release ATP during hemoglobin deoxygenation. FASEB J 26:860.22
Adderley SP, Sridharan M, Bowles EA, Stephenson AH, Sprague RS, Ellsworth ML (2011) Inhibition of ATP release from erythrocytes: a role for EPACs and PKC. Microcirculation 18:128–135
Melhorn MI, Brodsky AS, Estanislau J, Khoory JA, Illigens B, Hamachi I, Kurishita Y, Fraser AD, Nicholson-Weller A, Dolmatova E, Duffy HS, Ghiran IC (2013) CR1-mediated ATP release by human red blood cells promotes CR1 clustering and modulates the immune transfer process. J Biol Chem 288:31139–31153
Piskuric NA, Zhang M, Vollmer C, Nurse CA (2014) Potential roles of ATP and local neurons in the monitoring of blood O2 content by rat aortic bodies. Exp Physiol 99:248–261
Lockwood SY, Erkal JL, Spence DM (2014) Endothelium-derived nitric oxide production is increased by ATP released from red blood cells incubated with hydroxyurea. Nitric Oxide 38:1–7
Akkaya C, Shumilina E, Bobballa D, Brand VB, Mahmud H, Lang F, Huber SM (2009) The Plasmodium falciparum-induced anion channel of human erythrocytes is an ATP-release pathway. Pflugers Arch 457:1035–1047
Levano-Garcia J, Dluzewski AR, Markus RP, Garcia CR (2010) Purinergic signalling is involved in the malaria parasite Plasmodium falciparum invasion to red blood cells. Purinergic Signal 6:365–372
Alvarez CL, Schachter J, de Sá Pinheiro AA, Silva LS, Verstraeten SV, Persechini PM, Schwarzbaum PJ (2014) Regulation of extracellular ATP in human erythrocytes infected with Plasmodium falciparum. PLoS One 9:e96216
Ayi K, Liles WC, Gros P, Kain KC (2009) Adenosine triphosphate depletion of erythrocytes simulates the phenotype associated with pyruvate kinase deficiency and confers protection against Plasmodium falciparum in vitro. J Infect Dis 200:1289–1299
Tanneur V, Duranton C, Brand VB, Sandu CD, Akkaya C, Kasinathan RS, Gachet C, Sluyter R, Barden JA, Wiley JS, Lang F, Huber SM (2006) Purinoceptors are involved in the induction of an osmolyte permeability in malaria-infected and oxidized human erythrocytes. FASEB J 20:133–135
Gati WP, Lin AN, Wang TI, Young JD, Paterson AR (1990) Parasite-induced processes for adenosine permeation in mouse erythrocytes infected with the malarial parasite Plasmodium yoelii. Biochem J 272:277–280
de Machado Salles É, Zago CA, da Borges Silva H, Mosig JM, Coutinho Silva R, Lima MR (2011) Role of ATP and P2X7 receptor in the CD4+ T cell response to blood-stage Paramecium chabaudi malaria. Purinergic Signalling 7:140
Huber SM (2012) Purinoceptor signaling in malaria-infected erythrocytes. Microbes Infect 14:779–786
Munksgaard PS, Vorup-Jensen T, Reinholdt J, Söderstrom CM, Poulsen K, Leipziger J, Praetorius HA, Skals M (2012) Leukotoxin from Aggregatibacter actinomycetemcomitans causes shrinkage and P2X receptor-dependent lysis of human erythrocytes. Cell Microbiol 14:1904–1920
Skals M, Jorgensen NR, Leipziger J, Praetorius HA (2009) α-Hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. Proc Natl Acad Sci U S A 106:4030–4035
Skals MG, Leipziger J, Praetorius H (2013) E. coli α-haemolysin leads to ATP release and P2-dependent calcium influx in human erythrocytes. FASEB J 27:lb721
Skals M, Bjaelde RG, Reinholdt J, Poulsen K, Vad BS, Otzen DE, Leipziger J, Praetorius HA (2014) Bacterial RTX toxins allow acute ATP release from human erythrocytes directly through the toxin pore. J Biol Chem 289:19098–19109
Fagerberg SK, Skals M, Leipziger J, Praetorius HA (2013) P2X receptor-dependent erythrocyte damage by α-hemolysin from Escherichia coli triggers phagocytosis by THP-1 cells. Toxins (Basel) 5:472–487
Hejl JL, Skals M, Leipziger J, Praetorius HA (2013) P2X receptor stimulation amplifies complement-induced haemolysis. Pflugers Arch 465:529–541
Da Silva AS, Franca RT, Costa MM, Paim FC, Pimentel VC, Schmatz R, Jaques JA, Schetinger MR, Mazzanti CM, Tonin AA, Monteiro SG, Lopes ST (2013) Adenosine levels in serum and adenosine deaminase activity in blood cells of dogs infected by Rangelia vitalii. J Parasitol 99:1125–1128
Yeung PK, Seto D (2013) A study of the effect of isoproterenol on red blood cell concentrations of adenine nucleotides in a freely moving rat model in vivo. Cardiol Pharmacol 2:102
Sprague RS, Stephenson AH, Ellsworth ML, Keller C, Lonigro AJ (2001) Impaired release of ATP from red blood cells of humans with primary pulmonary hypertension. Exp Biol Med (Maywood) 226:434–439
Knebel SM, Elrick MM, Bowles EA, Zdanovec AK, Stephenson AH, Ellsworth ML, Sprague RS (2013) Synergistic effects of prostacyclin analogs and phosphodiesterase inhibitors on cyclic adenosine 3',5' monophosphate accumulation and adenosine 3'5' triphosphate release from human erythrocytes. Exp Biol Med (Maywood) 238:1069–1074
Sprague RS, Stephenson AH, Bowles EA, Stumpf MS, Lonigro AJ (2006) Reduced expression of Gi in erythrocytes of humans with type 2 diabetes is associated with impairment of both cAMP generation and ATP release. Diabetes 55:3588–3593
Sprague RS, Stephenson AH, Ellsworth ML (2007) Red not dead: signaling in and from erythrocytes. Trends Endocrinol Metab 18:350–355
Subasinghe W, Spence DM (2008) Simultaneous determination of cell aging and ATP release from erythrocytes and its implications in type 2 diabetes. Anal Chim Acta 618:227–233
Sprague RS, Bowles EA, Achilleus D, Stephenson AH, Ellis CG, Ellsworth ML (2011) A selective phosphodiesterase 3 inhibitor rescues low PO2-induced ATP release from erythrocytes of humans with type 2 diabetes: implication for vascular control. Am J Physiol Heart Circ Physiol 301:H2466–H2472
Richards JP, Stephenson AH, Ellsworth ML, Sprague RS (2013) Synergistic effects of C-peptide and insulin on low O2-induced ATP release from human erythrocytes. Am J Physiol Regul Integr Comp Physiol 305:R1331–R1336
Richards JP, Bowles EA, Gordon WR, Ellsworth ML, Stephenson AH, Sprague RS (2015) Mechanisms of C-peptide-mediated rescue of low O2-induced ATP release from erythrocytes of humans with Type 2 diabetes. Am J Physiol Regul Integr Comp Physiol 308:R411–R418
Zhang Y, Xia Y (2012) Adenosine signaling in normal and sickle erythrocytes and beyond. Microbes Infect 14:863–873
Zhang Y, Liu H, Sun K, Song A, Karmouty-Quintana H, Chen NY, Grenz A, Kellems RE, Idowu M, Juneja HS, Roach R, Eltzschig H, Blackburn MR, Xia Y (2013) Adenosine is a common factor regulating erythrocyte 2,3-bisphosphate induction in normal individuals at high altitude and in patients with sickle cell disease. Blood 122:952
Field JJ, Nathan DG, Linden J (2014) The role of adenosine signaling in sickle cell therapeutics. Hematol Oncol Clin North Am 28:287–299
Zhang Y, Dai Y, Wen J, Zhang W, Grenz A, Sun H, Tao L, Lu G, Alexander DC, Milburn MV, Carter-Dawson L, Lewis DE, Zhang W, Eltzschig HK, Kellems RE, Blackburn MR, Juneja HS, Xia Y (2011) Detrimental effects of adenosine signaling in sickle cell disease. Nat Med 17:79–86
Gladwin MT (2011) Adenosine receptor crossroads in sickle cell disease. Nat Med 17:38–40
Misiti F, Orsini F, Clementi ME, Masala D, Tellone E, Galtieri A, Giardina B (2008) Amyloid peptide inhibits ATP release from human erythrocytes. Biochem Cell Biol 86:501–508
Haslam RJ, Davidson MML, Lemmex BWG, Desjardins JV, McCarry BE (1979) Adenosine receptors of the blood platelet: interactions with adenylate cyclase. In: Baer HP, Drummond GI (eds) Physiological and regulatory functions of adenosine and adenine nucleotides. Raven Press, New York, pp 189–204
Agarwal KC (1987) Adenosine and platelet function. In: Stefanovich V, Okyayuz-Baklouti I (eds) Adenosine. VNU Science Press, The Netherlands, pp 107–124
Johnston-Cox HA, Ravid K (2011) Adenosine and blood platelets. Purinergic Signal 7:357–365
Skaer RJ (1975) Elemental composition of platelet dense bodies. Ciba Found Symp 35:239–259
Zucker MB (1980) The functioning of blood platelets. Sci Am 242:86–103
Da Prada M, Richards JG, Kettler R (1981) Amine storage organelles in platelets. In: Gordon JL (ed) Platelets in biology and pathology 2, research monographs in cell and tissue physiology. Elsevier, Amsterdam, pp 105–145
Mahaut-Smith MP, Tolhurst G, Evans RJ (2004) Emerging roles for P2X1 receptors in platelet activation. Platelets 15:131–144
Mahaut-Smith MP, Jones S, Evans RJ (2011) The P2X1 receptor and platelet function. Purinergic Signal 7:341–356
Hu H, Hoylaerts MF (2010) The P2X1 ion channel in platelet function. Platelets 21:153–166
Gachet C, Cazenave JP (1991) ADP induced blood platelet activation: a review. Nouv Rev Fr Hematol 33:347–358
Hourani SM, Cusack NJ (1991) Pharmacological receptors on blood platelets. Pharmacol Rev 43:243–298
Humphries RG, Robertson MJ, Leff P (1995) A novel series of P2T purinoceptor antagonists: definition of the role of ADP in arterial thrombosis. Trends Pharmacol Sci 16:179–181
Gachet C, Hechler B, Léon C, Vial C, Leray C, Ohlmann P, Cazenave JP (1997) Activation of ADP receptors and platelet function. Thromb Haemost 78:271–275
Mills DC (1996) ADP receptors on platelets. Thromb Haemost 76:835–856
Kunapuli SP (1998) Functional characterization of platelet ADP receptors. Platelets 9:343–351
Cattaneo M, Gachet C (2001) The platelet ADP receptors. Haematologica 86:346–348
Gachet C (2006) Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol 46:277–300
Gachet C (2008) P2 receptors, platelet function and pharmacological implications. Thromb Haemost 99:466–472
Oury C, Toth-Zsamboki E, Vermylen J, Hoylaerts MF (2006) The platelet ATP and ADP receptors. Curr Pharm Des 12:859–875
Hechler B, Gachet C (2011) P2 receptors and platelet function. Purinergic Signal 7:293–303
Jacobson KA, Deflorian F, Mishra S, Costanzi S (2011) Pharmacochemistry of the platelet purinergic receptors. Purinergic Signal 7:305–324
Packham MA, Rand ML (2011) Historical perspective on ADP-induced platelet activation. Purinergic Signal 7:283–292
Oh EY, Abraham T, Saad N, Rapp JH, Vastey FL, Balmir E (2012) A comprehensive comparative review of adenosine diphosphate receptor antagonists. Expert Opin Pharmacother 13:175–191
Cunningham MR, Nisar SP, Mundell SJ (2013) Molecular mechanisms of platelet P2Y12 receptor regulation. Biochem Soc Trans 41:225–230
Ferri N, Corsini A, Bellosta S (2013) Pharmacology of the new P2Y12 receptor inhibitors: insights on pharmacokinetic and pharmacodynamic properties. Drugs 73:1681–1709
Sage SO, MacKenzie AB, Jenner S, Mahaut-Smith MP (1997) Purinoceptor-evoked calcium signalling in human platelets. Prostaglandins Leukot Essent Fatty Acids 57:435–438
Zimmermann H (1999) Nucleotides and cd39: principal modulatory players in hemostasis and thrombosis. Nat Med 5:987–988
Yegutkin GG, Gonzalez-Alonso J (2007) Circulating ATP and ADP: important regulators of blood flow and platelet reactivity during exercise. Physiol News 68:31–33
Herbert JM, Savi P, Maffrand JP (1999) Biochemical and pharmacological properties of clopidogrel: a new ADP receptor antagonist. Eur Heart J 20:A31–A40
Kunapuli SP, Ding Z, Dorsam RT, Kim S, Murugappan S, Quinton TM (2003) ADP receptors - targets for developing antithrombotic agents. Curr Pharm Des 9:2303–2316
Boeynaems JM, van Giezen H, Savi P, Herbert JM (2005) P2Y receptor antagonists in thrombosis. Curr Opin Investig Drugs 6:275–282
Gachet C (2005) The platelet P2 receptors as molecular targets for old and new antiplatelet drugs. Pharmacol Ther 108:180–192
Gachet C, Léon C, Hechler B (2006) The platelet P2 receptors in arterial thrombosis. Blood Cells Mol Dis 36:223–227
Cattaneo M (2010) New P2Y12 inhibitors. Circulation 121:171–179
O’Connor S, Montalescot G, Collet JP (2011) The P2Y12 receptor as a target of antithrombotic drugs. Purinergic Signal 7:325–332
Tello-Montoliu A, Jover E, Rivera J, Valdés M, Angiolillo DJ, Marín F (2012) New perspectives in antiplatelet therapy. Curr Med Chem 19:406–427
Gachet C, Hechler B (2013) The P2Y receptors and thrombosis. WIREs Membr Transp Signal 2:241–253
Nurden AT (2011) Platelets, inflammation and tissue regeneration. Thromb Haemost 105(Suppl 1):S13–S33
Gachet C (2012) P2Y12 receptors in platelets and other hematopoietic and non-hematopoietic cells. Purinergic Signal 8:609–619
Cattaneo M (2011) Molecular defects of the platelet P2 receptors. Purinergic Signal 7:333–339
Born GV (1956) Adenosinetriphosphate (ATP) in blood platelets. Biochem J 62:331
Born GV (1958) Changes in the distribution of phosphorus in platelet-rich plasma during clotting. Biochem J 68:695–704
Hellem AJ (1960) The adhesiveness of human blood platelets in vitro. Scand J Clin Lab Invest 12(Suppl):1–117
Gaarder A, Jonsen J, Laland S, Hellem A, Owren PA (1961) Adenosine diphosphate in red cells as a factor in the adhesiveness of human blood platelets. Nature 192:531–532
Born GV (1962) Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 194:927–929
Agarwal KC, Parks RE Jr, Townsend LB (1979) Adenosine analogs and human platelets - II. Inhibition of ADP-induced aggregation by carbocyclic adenosine and imidazole-ring modified analogs. Significance of alterations in the nucleotide pools. Biochem Pharmacol 28:501–510
Born GV (1984) Platelets and blood vessels. J Cardiovasc Pharmacol 6(Suppl 4):S706–S713
Born GV, Cross MJ (1963) The aggregation of blood platelets. J Physiol 168:178–195
Salzman EW, Chambers DA, Neri LL (1966) Possible mechanism of aggregation of blood platelets by adenosine diphosphate. Nature 210:167–169
Ardlie NG, Packham MA, Mustard JF (1970) Adenosine diphosphate-induced platelet aggregation in suspensions of washed rabbit platelets. Br J Haematol 19:7–17
Stafford NP, Pink AE, White AE, Glenn JR, Heptinstall S (2003) Mechanisms involved in adenosine triphosphate--induced platelet aggregation in whole blood. Arterioscler Thromb Vasc Biol 23:1928–1933
Macfarlane DE, Mills DC (1975) The effects of ATP on platelets: evidence against the central role of released ADP in primary aggregation. Blood 46:309–320
Wang TY, Hussey CV, Garancis JC (1977) Inhibitory effects of ATP on platelet aggregation: cation interaction and shape change. Ann Clin Lab Sci 7:88–92
Cooper DM, Rodbell M (1979) ADP is a potent inhibitor of human platelet plasma membrane adenylate cyclase. Nature 282:517–518
Fujimoto T, Hawiger J (1982) Adenosine diphosphate induces binding of von Willebrand factor to human platelets. Nature 297:154–156
Cusack NJ, Hourani SM (1982) Specific but noncompetitive inhibition by 2-alkylthio analogues of adenosine 5'-monophosphate and adenosine 5'-triphosphate of human platelet aggregation induced by adenosine 5'-diphosphate. Br J Pharmacol 75:397–400
Kauffenstein G, Hechler B, Cazenave JP, Gachet C (2004) Adenine triphosphate nucleotides are antagonists at the P2Y receptor. J Thromb Haemost 2:1980–1988
Aslam M, Sedding D, Koshty A, Santoso S, Schulz R, Hamm C, Gündüz D (2013) Nucleoside triphosphates inhibit ADP, collagen, and epinephrine-induced platelet aggregation: role of P2Y1 and P2Y12 receptors. Thromb Res 132:548–557
Hallam TJ, Rink TJ (1985) Responses to adenosine diphosphate in human platelets loaded with the fluorescent calcium indicator quin2. J Physiol 368:131–146
Vanags DM, Rodgers SE, Duncan EM, Lloyd JV, Bochner F (1992) Potentiation of ADP-induced aggregation in human platelet-rich plasma by 5-hydroxytryptamine and adrenaline. Br J Pharmacol 106:917–923
Zamecnik PC, Kim B, Gao MJ, Taylor G, Blackburn GM (1992) Analogues of diadenosine 5',5'''-P1, P4-tetraphosphate (Ap4A) as potential anti-platelet-aggregation agents. Proc Natl Acad Sci U S A 89:2370–2373
Rinder CS, Student LA, Bonan JL, Rinder HM, Smith BR (1993) Aspirin does not inhibit adenosine diphosphate-induced platelet α-granule release. Blood 82:505–512
Hourani SM, Hall DA, Nieman CJ (1992) Effects of the P2-purinoceptor antagonist, suramin, on human platelet aggregation induced by adenosine 5'-diphosphate. Br J Pharmacol 105:453–457
Hall DA, Hourani SM (1993) Effects of analogues of adenine nucleotides on increases in intracellular calcium mediated by P2T-purinoceptors on human blood platelets. Br J Pharmacol 108:728–733
Hourani SMO, Welford LA, Cusack NJ (1996) Effects of 2-methylthioadenosine 5'β,γ-methylenetriphosphonate and 2-ethylthioadenosine 5'-monophosphate on human platelet activation induced by adenosine 5'-diphosphate. Drug Dev Res 38:12–23
Humphries RG, Tomlinson W, Ingall AH, Cage PA, Leff P (1994) FPL 66096: a novel, highly potent and selective antagonist at human platelet P2T-purinoceptors. Br J Pharmacol 113:1057–1063
Humphries RG, Tomlinson W, Clegg JA, Ingall AH, Kindon ND, Leff P (1995) Pharmacological profile of the novel P2T-purinoceptor antagonist, FPL 67085 in vitro and in the anaesthetized rat in vivo. Br J Pharmacol 115:1110–1116
Ohlmann P, Laugwitz KL, Nürnberg B, Spicher K, Schultz G, Cazenave JP, Gachet C (1995) The human platelet ADP receptor activates Gi2 proteins. Biochem J 312:775–779
Ohlmann P, Lecchi A, El-Tayeb A, Muller CE, Cattaneo M, Gachet C (2013) The platelet P2Y12 receptor under normal and pathological conditions. Assessment with the radiolabeled selective antagonist [3H]PSB-0413. Purinergic Signal 9:59–66
Anderson GM, Hall LM, Horne WC, Yang JX (1996) Adenosine diphosphate inhibits the serotonin transporter. Biochim Biophys Acta 1283:14–20
Thomsen H, Schmidtke E (1997) Stimulation of postmortem platelets with adenosine-5-diphosphate and epinephrine. Forensic Sci Int 89:47–55
Léon C, Hechler B, Vial C, Leray C, Cazenave JP, Gachet C (1997) The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Lett 403:26–30
Hechler B, Vigne P, Léon C, Breittmayer JP, Gachet C, Frelin C (1998) ATP derivatives are antagonists of the P2Y1 receptor: similarities to the platelet ADP receptor. Mol Pharmacol 53:727–733
Jantzen HM, Gousset L, Bhaskar V, Vincent D, Tai A, Reynolds EE, Conley PB (1999) Evidence for two distinct G-protein-coupled ADP receptors mediating platelet activation. Thromb Haemost 81:111–117
Jin J, Daniel JL, Kunapuli SP (1998) Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem 273:2030–2034
Savi P, Beauverger P, Labouret C, Delfaud M, Salel V, Kaghad M, Herbert JM (1998) Role of P2Y1 purinoceptor in ADP-induced platelet activation. FEBS Lett 422:291–295
Hechler B, Léon C, Vial C, Vigne P, Frelin C, Cazenave JP, Gachet C (1998) The P2Y1 receptor is necessary for adenosine 5'-diphosphate-induced platelet aggregation. Blood 92:152–159
Daniel JL, Dangelmaier C, Jin J, Ashby B, Smith JB, Kunapuli SP (1998) Molecular basis for ADP-induced platelet activation. I. Evidence for three distinct ADP receptors on human platelets. J Biol Chem 273:2024–2029
Geiger J, Hönig-Liedl P, Schanzenbächer P, Walter U (1998) Ligand specificity and ticlopidine effects distinguish three human platelet ADP receptors. Eur J Pharmacol 351:235–246
Scase TJ, Heath MF, Allen JM, Sage SO, Evans RJ (1998) Identification of a P2X1 purinoceptor expressed on human platelets. Biochem Biophys Res Commun 242:525–528
Vigne P, Hechler B, Gachet C, Breittmayer JP, Frelin C (1999) Benzoyl ATP is an antagonist of rat and human P2Y1 receptors and of platelet aggregation. Biochem Biophys Res Commun 256:94–97
Jarvis GE, Humphries RG, Robertson MJ, Leff P (2000) ADP can induce aggregation of human platelets via both P2Y1 and P2T receptors. Br J Pharmacol 129:275–282
Barnard EA, Simon J (2001) An elusive receptor is finally caught: P2Y12, an important drug target in platelets. Trends Pharmacol Sci 22:388–391
Hollopeter G, Jantzen H-M, Vincent D, Li G, England L, Ramakrishnan V, Yang R-B, Nurden P, Nurden A, Julius D, Conley PB (2001) Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409:202–207
Zhang FL, Luo L, Gustafson E, Lachowicz J, Smith M, Qiao X, Liu YH, Chen G, Pramanik B, Laz TM, Palmer K, Bayne M, Monsma FJ Jr (2001) ADP is the cognate ligand for the orphan G protein-coupled receptor SP1999. J Biol Chem 276:8608–8615
Turner NA, Moake JL, McIntire LV (2001) Blockade of adenosine diphosphate receptors P2Y12 and P2Y1 is required to inhibit platelet aggregation in whole blood under flow. Blood 98:3340–3345
Gear AR, Suttitanamongkol S, Viisoreanu D, Polanowska-Grabowska RK, Raha S, Camerini D (2001) Adenosine diphosphate strongly potentiates the ability of the chemokines MDC, TARC, and SDF-1 to stimulate platelet function. Blood 97:937–945
Mangin P, Ohlmann P, Eckly A, Cazenave JP, Lanza F, Gachet C (2004) The P2Y1 receptor plays an essential role in the platelet shape change induced by collagen when TxA2 formation is prevented. J Thromb Haemost 2:969–977
Goto S, Tamura N, Eto K, Ikeda Y, Handa S (2002) Functional significance of adenosine 5'-diphosphate receptor (P2Y12) in platelet activation initiated by binding of von Willebrand factor to platelet GP Ibα induced by conditions of high shear rate. Circulation 105:2531–2536
Wang L, Östberg O, Wihlborg AK, Brogren H, Jern S, Erlinge D (2003) Quantification of ADP and ATP receptor expression in human platelets. J Thromb Haemost 1:330–336
Jin J, Quinton TM, Zhang J, Rittenhouse SE, Kunapuli SP (2002) Adenosine diphosphate (ADP)-induced thromboxane A2 generation in human platelets requires coordinated signaling through integrin αIIbβ3 and ADP receptors. Blood 99:193–198
Jagroop IA, Burnstock G, Mikhailidis DP (2003) Both the ADP receptors, P2Y1 and P2Y12 play a role in controlling shape change in human platelets. Platelets 14:15–20
Nylander S, Mattsson C, Ramstrom S, Lindahl TL (2003) The relative importance of the ADP receptors, P2Y12 and P2Y1, in thrombin-induced platelet activation. Thromb Res 111:65–73
Nylander S, Mattsson C, Ramström S, Lindahl TL (2004) Synergistic action between inhibition of P2Y12/P2Y1 and P2Y12/thrombin in ADP- and thrombin-induced human platelet activation. Br J Pharmacol 142:1325–1331
Fontana P, Dupont A, Gandrille S, Bachelot-Loza C, Reny JL, Aiach M, Gaussem P (2003) Adenosine diphosphate-induced platelet aggregation is associated with P2Y 12 gene sequence variations in healthy subjects. Circulation 108:989–995
Hetherington SL, Singh RK, Lodwick D, Thompson JR, Goodall AH, Samani NJ (2005) Dimorphism in the P2Y1 ADP receptor gene is associated with increased platelet activation response to ADP. Arterioscler Thromb Vasc Biol 25:252–257
Hetherington SL, Lodwick D, Goodall AH, Samani NJ (2005) Molecular basis for platelet hyper-reactivity to adenosine diphosphate (ADP) associated with the platelet P2Y1 receptor 1622 G allele. Heart 91:A60
von Beckerath N, von Beckerath O, Koch W, Eichinger M, Schomig A, Kastrati A (2005) P2Y12 gene H2 haplotype is not associated with increased adenosine diphosphate-induced platelet aggregation after initiation of clopidogrel therapy with a high loading dose. Blood Coagul Fibrinolysis 16:199–204
Amisten S, Braun OÖ, Johansson L, Ridderstrale M, Melander O, Erlinge D (2008) The P2Y13 Met-158-Thr polymorphism, which is in linkage disequilibrium with the P2Y12 locus, is not associated with acute myocardial infarction. PLoS One 3:e1462
Mao Y, Zhang L, Jin J, Ashby B, Kunapuli SP (2010) Mutational analysis of residues important for ligand interaction with the human P2Y12 receptor. Eur J Pharmacol 644:10–16
Högberg C, Gidlöf O, Deflorian F, Jacobson KA, Abdelrahman A, Müller CE, Olde B, Erlinge D (2012) Farnesyl pyrophosphate is an endogenous antagonist to ADP-stimulated P2Y12 receptor-mediated platelet aggregation. Thromb Haemost 108:119–132
Cattaneo M, Lecchi A, Ohno M, Joshi BV, Besada P, Tchilibon S, Lombardi R, Bischofberger N, Harden TK, Jacobson KA (2004) Antiaggregatory activity in human platelets of potent antagonists of the P2Y1 receptor. Biochem Pharmacol 68:1995–2002
Hardy AR, Jones ML, Mundell SJ, Poole AW (2004) Reciprocal cross-talk between P2Y1 and P2Y12 receptors at the level of calcium signaling in human platelets. Blood 104:1745–1752
Baurand A, Eckly A, Hechler B, Kauffenstein G, Galzi JL, Cazenave JP, Lén C, Gachet C (2005) Differential regulation and relocalization of the platelet P2Y receptors after activation: a way to avoid loss of hemostatic properties? Mol Pharmacol 67:721–733
Hardy AR, Conley PB, Luo J, Benovic JL, Poole AW, Mundell SJ (2005) P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood 105:3552–3560
Kanamarlapudi V, Owens SE, Saha K, Pope RJ, Mundell SJ (2012) ARF6-dependent regulation of P2Y receptor traffic and function in human platelets. PLoS One 7:e43532
Trumel C, Payrastre B, Plantavid M, Hechler B, Viala C, Presek P, Martinson EA, Cazenave JP, Chap H, Gachet C (1999) A key role of adenosine diphosphate in the irreversible platelet aggregation induced by the PAR1-activating peptide through the late activation of phosphoinositide 3-kinase. Blood 94:4156–4165
Sun DS, Lo SJ, Tsai WJ, Lin CH, Yu MS, Chen YF, Chang HH (2005) PI3-kinase is essential for ADP-stimulated integrin αIIbβ3-mediated platelet calcium oscillation, implications for P2Y receptor pathways in integrin αIIbβ3-initiated signaling cross-talks. J Biomed Sci 12:937–948
Kamae T, Shiraga M, Kashiwagi H, Kato H, Tadokoro S, Kurata Y, Tomiyama Y, Kanakura Y (2006) Critical role of ADP interaction with P2Y12 receptor in the maintenance of αIIbβ3 activation: association with Rap1B activation. J Thromb Haemost 4:1379–1387
Lecchi A, Razzari C, Paoletta S, Dupuis A, Nakamura L, Ohlmann P, Gachet C, Jacobson KA, Zieger B, Cattaneo M (2015) Identification of a new dysfunctional platelet P2Y12 receptor variant associated with bleeding diathesis. Blood 125:1006–1013
Nicholas RA (2015) Insights into platelet P2Y12 receptor activation. Blood 125:893–895
Hardy AR, Hill DJ, Poole AW (2005) Evidence that the purinergic receptor P2Y12 potentiates platelet shape change by a Rho kinase-dependent mechanism. Platelets 16:415–429
Frelinger AL III, Furman MI, Linden MD, Li Y, Fox ML, Barnard MR, Michelson AD (2006) Residual arachidonic acid-induced platelet activation via an adenosine diphosphate-dependent but cyclooxygenase-1- and cyclooxygenase-2-independent pathway: a 700-patient study of aspirin resistance. Circulation 113:2888–2896
Arthur JF, Shen Y, Mu FT, Leon C, Gachet C, Berndt MC, Andrews RK (2006) Calmodulin interacts with the platelet ADP receptor P2Y1. Biochem J 398:339–343
Moore DJ, Murdock PR, Watson JM, Faull RL, Waldvogel HJ, Szekeres PG, Wilson S, Freeman KB, Emson PC (2003) GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: possible role in neuroimmune function. Brain Res Mol Brain Res 118:10–23
Dovlatova N, Wijeyeratne YD, Fox SC, Manolopoulos P, Johnson AJ, White AE, Latif ML, Ralevic V, Heptinstall S (2008) Detection of P2Y14 protein in platelets and investigation of the role of P2Y14 in platelet function in comparison with the EP3 receptor. Thromb Haemost 100:261–270
Hoffmann K, Sixel U, Di Pasquale F, von Kügelgen I (2008) Involvement of basic amino acid residues in transmembrane regions 6 and 7 in agonist and antagonist recognition of the human platelet P2Y12-receptor. Biochem Pharmacol 76:1201–1213
Ohlmann P, de Castro S, Brown GG Jr, Gachet C, Jacobson KA, Harden TK (2010) Quantification of recombinant and platelet P2Y1 receptors utilizing a [125I]-labeled high-affinity antagonist 2-iodo-N 6-methyl-(N)-methanocarba-2'-deoxyadenosine-3',5'-bisphosphate ([125I]MRS2500). Pharmacol Res 62:344–351
Fredman G, Van Dyke TE, Serhan CN (2010) Resolvin E1 regulates ASP activation of human platelets. Arterioscler Thromb Vasc Biol 30:2005–2013
Enomoto Y, Adachi S, Doi T, Natsume H, Kato K, Matsushima-Nishiwaki R, Akamatsu S, Tokuda H, Yoshimura S, Otsuka T, Ogura S, Kozawa O, Iwama T (2011) cAMP regulates ADP-induced HSP27 phosphorylation in human platelets. Int J Mol Med 27:695–700
Tseng YL, Chiang ML, Lane HY, Su KP, Lai YC (2013) Selective serotonin reuptake inhibitors reduce P2Y12 receptor-mediated amplification of platelet aggregation. Thromb Res 131:325–332
Cattaneo M, Lecchi A (2007) Inhibition of the platelet P2Y12 receptor for adenosine diphosphate potentiates the antiplatelet effect of prostacyclin. J Thromb Haemost 5:577–582
Kirkby NS, Lundberg MH, Chan MV, Vojnovic I, Solomon AB, Emerson M, Mitchell JA, Warner TD (2013) Blockade of the purinergic P2Y12 receptor greatly increases the platelet inhibitory actions of nitric oxide. Proc Natl Acad Sci U S A 110:15782–15787
Castellino FJ, Chapman MP, Donahue DL, Thomas S, Moore EE, Wohlauer MV, Fritz B, Yount R, Ploplis V, Davis P, Evans E, Walsh M (2014) Traumatic brain injury causes platelet adenosine diphosphate and arachidonic acid receptor inhibition independent of hemorrhagic shock in humans and rats. J Trauma Acute Care Surg 76:1169–1176
Soslau G, Brodsky I, Parker J (1993) Occupancy of P2 purinoceptors with unique properties modulates the function of human platelets. Biochim Biophys Acta 1177:199–207
MacKenzie AB, Mahaut-Smith MP, Sage SO (1996) Activation of receptor-operated cation channels via P2X1 not P2T purinoceptors in human platelets. J Biol Chem 271:2879–2881
Savi P, Bornia J, Salel V, Delfaud M, Herbert JM (1997) Characterization of P2x1 purinoreceptors on rat platelets: effect of clopidogrel. Br J Haematol 98:880–886
Vial C, Hechler B, Léon C, Cazenave JP, Gachet C (1997) Presence of P2X1 purinoceptors in human platelets and megakaryoblastic cell lines. Thromb Haemost 78:1500–1504
Clifford EE, Parker K, Humphreys BD, Kertesy SB, Dubyak GR (1998) The P2X1 receptor, an adenosine triphosphate-gated cation channel, is expressed in human platelets but not in human blood leukocytes. Blood 91:3172–3181
Sun B, Li J, Okahara K, Kambayashi J (1998) P2X1 purinoceptor in human platelets. Molecular cloning and functional characterization after heterologous expression. J Biol Chem 273:11544–11547
Takano S, Kimura J, Matsuoka I, Ono T (1999) No requirement of P2X1 purinoceptors for platelet aggregation. Eur J Pharmacol 372:305–309
Mahaut-Smith MP, Ennion SJ, Rolf MG, Evans RJ (2000) ADP is not an agonist at P2X1 receptors: evidence for separate receptors stimulated by ATP and ADP on human platelets. Br J Pharmacol 131:108–114
Greco NJ, Tonon G, Chen W, Luo X, Dalal R, Jamieson GA (2001) Novel structurally altered P2X1 receptor is preferentially activated by adenosine diphosphate in platelets and megakaryocytic cells. Blood 98:100–107
Toth-Zsamboki E, Oury C, Tytgat J, Vermylen J, Hoylaerts MF (2001) The P2Y1 receptor antagonist adenosine-2',5'-diphosphate non-selectively antagonizes the platelet P2X1 ion channel. Thromb Haemost 86:1338–1339
Rolf MG, Brearley CA, Mahaut-Smith MP (2001) Platelet shape change evoked by selective activation of P2X1 purinoceptors with α, β-methylene ATP. Thromb Haemost 85:303–308
Oury C, Toth-Zsamboki E, Thys C, Tytgat J, Vermylen J, Hoylaerts MF (2001) The ATP-gated P2X1 ion channel acts as a positive regulator of platelet responses to collagen. Thromb Haemost 86:1264–1271
Hechler B, Vial C, Freund M, Cazenave JP, Evans R, Gachet C (2002) A role for the platelet P2X1 receptor in hemostasis. Studies in P2X1 knockout mice. 7th Int Symp on Adenosine and Adenine Nucleotides 79
Vial C, Rolf MG, Mahaut-Smith MP, Evans RJ (2002) A study of P2X1 receptor function in murine megakaryocytes and human platelets reveals synergy with P2Y receptors. Br J Pharmacol 135:363–372
Hechler B, Lenain N, Marchese P, Vial C, Heim V, Freund M, Cazenave JP, Cattaneo M, Ruggeri ZM, Evans R, Gachet C (2003) A role of the fast ATP-gated P2X1 cation channel in thrombosis of small arteries in vivo. J Exp Med 198:661–667
Oury C, Kuijpers MJ, Toth-Zsamboki E, Bonnefoy A, Danloy S, Vreys I, Feijge MA, De Vos R, Vermylen J, Heemskerk JW, Hoylaerts MF (2003) Overexpression of the platelet P2X1 ion channel in transgenic mice generates a novel prothrombotic phenotype. Blood 101:3969–3976
Vial C, Pitt SJ, Roberts J, Rolf MG, Mahaut-Smith MP, Evans RJ (2003) Lack of evidence for functional ADP-activated human P2X1 receptors supports a role for ATP during hemostasis and thrombosis. Blood 102:3646–3651
Hechler B, Magnenat S, Zighetti ML, Kassack MU, Ullmann H, Cazenave JP, Evans R, Cattaneo M, Gachet C (2005) Inhibition of platelet functions and thrombosis through selective or nonselective inhibition of the platelet P2 receptors with increasing doses of NF449 [4,4',4'',4'''-(carbonylbis(imino-5,1,3-benzenetriylbis-(carbonylimino)))tetrakis -benzene-1,3-disulfonic acid octasodium salt]. J Pharmacol Exp Ther 314:232–243
Oury C, Sticker E, Cornelissen H, De Vos R, Vermylen J, Hoylaerts MF (2004) ATP augments von Willebrand factor-dependent shear-induced platelet aggregation through Ca2+-calmodulin and myosin light chain kinase activation. J Biol Chem 279:26266–26273
Fung CY, Brearley CA, Farndale RW, Mahaut-Smith MP (2005) A major role for P2X1 receptors in the early collagen-evoked intracellular Ca2+ responses of human platelets. Thromb Haemost 94:37–40
Horner S, Menke K, Hildebrandt C, Kassack MU, Nickel P, Ullmann H, Mahaut-Smith MP, Lambrecht G (2005) The novel suramin analogue NF864 selectively blocks P2X1 receptors in human platelets with potency in the low nanomolar range. Naunyn Schmiedeberg’s Arch Pharmacol 372:1–13
Vial C, Fung CY, Goodall AH, Mahaut-Smith MP, Evans RJ (2006) Differential sensitivity of human platelet P2X1 and P2Y1 receptors to disruption of lipid rafts. Biochem Biophys Res Commun 343:415–419
Fung CY, Cendana C, Farndale RW, Mahaut-Smith MP (2007) Primary and secondary agonists can use P2X1 receptors as a major pathway to increase intracellular Ca2+ in the human platelet. J Thromb Haemost 5:910–917
Karunarathne W, Ku CJ, Spence DM (2009) The dual nature of extracellular ATP as a concentration-dependent platelet P2X1 agonist and antagonist. Integr Biol (Camb) 1:655–663
Chang H, Yanachkov IB, Michelson AD, Li Y, Barnard MR, Wright GE, Frelinger AL III (2010) Agonist and antagonist effects of diadenosine tetraphosphate, a platelet dense granule constituent, on platelet P2Y1, P2Y12 and P2X1 receptors. Thromb Res 125:159–165
McCloskey C, Jones S, Amisten S, Snowden RT, Kaczmarek LK, Erlinge D, Goodall AH, Forsythe ID, Mahaut-Smith MP (2010) Kv1.3 is the exclusive voltage-gated K+ channel of platelets and megakaryocytes: roles in membrane potential, Ca2+ signalling and platelet count. J Physiol 588:1399–1406
Lalo U, Jones S, Roberts JA, Mahaut-Smith MP, Evans RJ (2012) Heat shock protein 90 inhibitors reduce trafficking of ATP-gated P2X1 receptors and human platelet responsiveness. J Biol Chem 287:32747–32754
Born GV, Cross MJ (1962) Inhibition of the aggregation of blood platelets by substances related to adenosine diphosphate. J Physiol 166:29P–30P
Haslam RJ, Rosson GM (1975) Effects of adenosine on levels of adenosine cyclic 3',5'-monophosphate in human blood platelets in relation to adenosine incorporation and platelet aggregation. Mol Pharmacol 11:528–544
Cusack NJ, Hickman ME, Born GV (1979) Effects of D- and L- enantiomers of adenosine, AMP and ADP and their 2-chloro- and 2-azido- analogues on human platelets. Proc R Soc Lond B Biol Sci 206:139–144
Jakobs KH, Saur W, Johnson RA (1979) Regulation of platelet adenylate cyclase by adenosine. Biochim Biophys Acta 583:409–421
Cusack NJ, Born GV (1976) Inhibition of adenosine deaminase and of platelet aggregation by 2-azidoadenosine, a photolysable analogue of adenosine. Proc R Soc Lond B Biol Sci 193:307–311
Doni MG (1981) Adenosine uptake and deamination by blood platelets in different mammalian species. Haemostasis 10:79–88
Subbarao K, Rucinski B, Rausch MA, Schmid K, Niewiarowski S (1977) Binding of dipyridamole to human platelets and to α1 acid glycoprotein and its significance for the inhibition of adenosine uptake. J Clin Invest 60:936–943
Dawicki DD, Agarwal KC, Parks RE Jr (1986) Potentiation of the antiplatelet action of adenosine in whole blood by dipyridamole or dilazep and the cAMP phosphodiesterase inhibitor, RA 233. Thromb Res 43:161–175
Bult H, Fret HR, Jordaens FH, Herman AG (1991) Dipyridamole potentiates platelet inhibition by nitric oxide. Thromb Haemost 66:343–349
Cusack NJ, Hourani SM (1981) 5'-N-ethylcarboxamidoadenosine: a potent inhibitor of human platelet aggregation. Br J Pharmacol 72:443–447
Ukena D, Jacobson KA, Kirk KL, Daly JW (1986) A [3H]amine congener of 1,3-dipropyl-8-phenylxanthine. A new radioligand for A2 adenosine receptors of human platelets. FEBS Lett 199:269–274
Edwards RJ, MacDermot J, Wilkins AJ (1987) Prostacyclin analogues reduce ADP-ribosylation of the α-subunit of the regulatory Gs-protein and diminish adenosine (A2) responsiveness of platelets. Br J Pharmacol 90:501–510
Dionisotti S, Zocchi C, Varani K, Borea PA, Ongini E (1992) Effects of adenosine derivatives on human and rabbit platelet aggregation. Correlation of adenosine receptor affinities and antiaggregatory activity. Naunyn Schmiedebergs Arch Pharmacol 346:673–676
Cristalli G, Vittori S, Thompson RD, Padgett WL, Shi D, Daly JW, Olsson RA (1994) Inhibition of platelet aggregation by adenosine receptor agonists. Naunyn Schmiedebergs Arch Pharmacol 349:644–650
Varani K, Gessi S, Dalpiaz A, Borea PA (1996) Pharmacological and biochemical characterization of purified A2a adenosine receptors in human platelet membranes by [3H]-CGS 21680 binding. Br J Pharmacol 117:1693–1701
Gessi S, Varani K, Merighi S, Ongini E, Borea PA (2000) A2A adenosine receptors in human peripheral blood cells. Br J Pharmacol 129:2–11
Grenegård M, Gustafsson MC, Andersson RG, Bengtsson T (1996) Synergistic inhibition of thrombin-induced platelet aggregation by the novel nitric oxide-donor GEA 3175 and adenosine. Br J Pharmacol 118:2140–2144
Linden MD, Barnard MR, Frelinger AL, Michelson AD, Przyklenk K (2008) Effect of adenosine A2 receptor stimulation on platelet activation-aggregation: differences between canine and human models. Thromb Res 121:689–698
Hart ML, Köhler D, Eckle T, Kloor D, Stahl GL, Eltzschig HK (2008) Direct treatment of mouse or human blood with soluble 5'-nucleotidase inhibits platelet aggregation. Arterioscler Thromb Vasc Biol 28:1477–1483
Amisten S, Braun OÖ, Bengtsson A, Erlinge D (2008) Gene expression profiling for the identification of G-protein coupled receptors in human platelets. Thromb Res 122:47–57
Yang D, Chen H, Koupenova M, Carroll SH, Eliades A, Freedman JE, Toselli P, Ravid K (2010) A new role for the A2b adenosine receptor in regulating platelet function. J Thromb Haemost 8:817–827
Iyú D, Glenn JR, White AE, Fox SC, Heptinstall S (2011) Adenosine derived from ADP can contribute to inhibition of platelet aggregation in the presence of a P2Y12 antagonist. Arterioscler Thromb Vasc Biol 31:416–422
Kim K, Lim KM, Shin HJ, Seo DB, Noh JY, Kang S, Chung HY, Shin S, Chung JH, Bae ON (2013) Inhibitory effects of black soybean on platelet activation mediated through its active component of adenosine. Thromb Res 131:254–261
Beigi R, Kobatake E, Aizawa M, Dubyak GR (1999) Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol 276:C267–C278
Cattaneo M (2009) Light transmission aggregometry and ATP release for the diagnostic assessment of platelet function. Semin Thromb Hemost 35:158–167
Pai M, Wang G, Moffat KA, Liu Y, Seecharan J, Webert K, Heddle N, Hayward C (2011) Diagnostic usefulness of a lumi-aggregometer adenosine triphosphate release assay for the assessment of platelet function disorders. Am J Clin Pathol 136:350–358
Hayward CP, Moffat KA, Castilloux JF, Liu Y, Seecharan J, Tasneem S, Carlino S, Cormier A, Rivard GE (2012) Simultaneous measurement of adenosine triphosphate release and aggregation potentiates human platelet aggregation responses for some subjects, including persons with Quebec platelet disorder. Thromb Haemost 107:726–734
von Papen M, Gambaryan S, Schütz C, Geiger J (2013) Determination of ATP and ADP secretion from human and mouse platelets by an HPLC assay. Transfus Med Hemother 40:109–116
Da Prada M, Lorez HP, Richards JG (1982) Platelet granules. In: Poisner AM, Trifaro JM (eds) The secretory granule. Elsevier Biomedical, Amsterdam, pp 279–316
Hiasa M, Togawa N, Miyaji T, Omote H, Yamamoto A, Moriyama Y (2014) Essential role of vesicular nucleotide transporter in vesicular storage and release of nucleotides in platelets. Physiol Rep 2:e12034
Barradas MA, Mikhailidis DP, Dandona P (1990) ADPase activity in human maternal and cord blood: possible evidence for a placenta-specific vascular protective mechanism. Int J Gynaecol Obstet 31:15–20
Beukers MW, Pirovano IM, van Weert A, Kerkhof CJ, IJzerman AP, Soudijn W (1993) Characterization of ecto-ATPase on human blood cells. A physiological role in platelet aggregation? Biochem Pharmacol 46:1959–1966
Frassetto SS, Dias RD, Sarkis JJ (1993) Characterization of an ATP diphosphohydrolase activity (APYRASE, EC 3.6.1.5) in rat blood platelets. Mol Cell Biochem 129:47–55
Frassetto SS, Dias RD, Sarkis JJ (1995) Inhibition and kinetic alterations by excess free ATP and ADP of the ATP diphosphohydrolase activity (EC 3.6.1.5) from rat blood platelets. Biochem Mol Biol Int 35:499–506
Pilla C, Emanuelli T, Frassetto SS, Battastini AM, Dias RD, Sarkis JJ (1996) ATP diphosphohydrolase activity (apyrase, EC 3.6.1.5) in human blood platelets. Platelets 7:225–230
Koziak K, Séevigny J, Robson SC, Siegel JB, Kaczmarek E (1999) Analysis of CD39/ATP diphosphohydrolase (ATPDase) expression in endothelial cells, platelets and leukocytes. Thromb Haemost 82:1538–1544
Atkinson B, Dwyer K, Enjyoji K, Robson SC (2006) Ecto-nucleotidases of the CD39/NTPDase family modulate platelet activation and thrombus formation: potential as therapeutic targets. Blood Cells Mol Dis 36:217–222
Birk AV, Broekman MJ, Gladek EM, Robertson HD, Drosopoulos JH, Marcus AJ, Szeto HH (2002) Role of extracellular ATP metabolism in regulation of platelet reactivity. J Lab Clin Med 140:166–175
Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Pinsky DJ, Sesti C, Levi R (2003) Heterologous cell-cell interactions: thromboregulation, cerebroprotection and cardioprotection by CD39 (NTPDase-1). J Thromb Haemost 1:2497–2509
Buffon A, Ribeiro VB, Fürstenau CR, Battastini AM, Sarkis JJ (2004) Acetylsalicylic acid inhibits ATP diphosphohydrolase activity by platelets from adult rats. Clin Chim Acta 349:53–60
Fürstenau CR, Spier AP, Rücker B, Luisa Berti S, Battastini AM, Sarkis JJ (2004) The effect of ebselen on adenine nucleotide hydrolysis by platelets from adult rats. Chem Biol Interact 148:93–99
Fung CY, Marcus AJ, Broekman MJ, Mahaut-Smith MP (2009) P2X1 receptor inhibition and soluble CD39 administration as novel approaches to widen the cardiovascular therapeutic window. Trends Cardiovasc Med 19:1–5
Jones S, Evans RJ, Mahaut-Smith MP (2011) Extracellular Ca2+ modulates ADP-evoked aggregation through altered agonist degradation: implications for conditions used to study P2Y receptor activation. Br J Haematol 153:83–91
Pochmann D, Bohmer AE, Nejar Bruno A, Sarkis JJ (2005) Ecto-hydrolysis of adenine nucleotides in rat blood platelets are altered by ovariectomy. Platelets 16:334–339
Mundell SJ, Jones ML, Hardy AR, Barton JF, Beaucourt SM, Conley PB, Poole AW (2006) Distinct roles for protein kinase C isoforms in regulating platelet purinergic receptor function. Mol Pharmacol 70:1132–1142
Cauwenberghs S, Feijge MA, Hageman G, Hoylaerts M, Akkerman JW, Curvers J, Heemskerk JW (2006) Plasma ectonucleotidases prevent desensitization of purinergic receptors in stored platelets: importance for platelet activity during thrombus formation. Transfusion 46:1018–1028
Leal CA, Schetinger MR, Leal DB, Bauchspiess K, Schrekker CM, Maldonado PA, Morsch VM, da Silva JE (2007) NTPDase and 5'-nucleotidase activities in platelets of human pregnants with a normal or high risk for thrombosis. Mol Cell Biochem 304:325–330
Yegutkin GG, Samburski SS, Mortensen SP, Jalkanen S, Gonzélez-Alonso J (2007) Intravascular ADP and soluble nucleotidases contribute to acute prothrombotic state during vigorous exercise in humans. J Physiol 579:553–564
Cook JJ, Sitko GR, Holahan MA, Stranieri MT, Glass JD, Askew BC, McIntyre CJ, Claremon DA, Baldwin JJ, Hartman GD, Gould RJ, Lynch JJ Jr (1997) Nonpeptide glycoprotein IIb/IIIa inhibitors. 15. Antithrombotic efficacy of L-738,167, a long-acting GPIIb/IIIa antagonist, correlates with inhibition of adenosine diphosphate-induced platelet aggregation but not with bleeding time prolongation. J Pharmacol Exp Ther 281:677–689
Ingall AH, Dixon J, Bailey A, Coombs ME, Cox D, McInally JI, Hunt SF, Kindon ND, Teobald BJ, Willis PA, Humphries RG, Leff P, Clegg JA, Smith JA, Tomlinson W (1999) Antagonists of the platelet P 2T receptor: a novel approach to antithrombotic therapy. J Med Chem 42:213–220
Léon C, Hechler B, Freund M, Eckly A, Vial C, Ohlmann P, Dierich A, LeMeur M, Cazenave JP, Gachet C (1999) Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y1 receptor-null mice. J Clin Invest 104:1731–1737
Steering Committee CAPRIE (1996) A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events. Lancet 348:1329–1339
Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK (2001) Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 345:494–502
Weber AA, Reimann S, Schrör K (1999) Specific inhibition of ADP-induced platelet aggregation by clopidogrel in vitro. Br J Pharmacol 126:415–420
Geiger J, Brich J, Hönig-Liedl P, Eigenthaler M, Schanzenbächer P, Herbert JM, Walter U (1999) Specific impairment of human platelet P2YAC ADP receptor-mediated signaling by the antiplatelet drug clopidogrel. Arterioscler Thromb Vasc Biol 19:2007–2011
Savi P, Herbert JM, Pflieger AM, Dol F, Delebassee D, Combalbert J, Defreyn G, Maffrand JP (1992) Importance of hepatic metabolism in the antiaggregating activity of the thienopyridine clopidogrel. Biochem Pharmacol 44:527–532
Sugidachi A, Ogawa T, Kurihara A, Hagihara K, Jakubowski JA, Hashimoto M, Niitsu Y, Asai F (2007) The greater in vivo antiplatelet effects of prasugrel as compared to clopidogrel reflect more efficient generation of its active metabolite with similar antiplatelet activity to that of clopidogrel’s active metabolite. J Thromb Haemost 5:1545–1551
Angiolillo DJ, Ferreiro JL (2010) Platelet adenosine diphosphate P2Y12 receptor antagonism: benefits and limitations of current treatment strategies and future directions. Rev Esp Cardiol 63:60–76
Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS, Lachno DR, Salazar D, Winters KJ (2007) Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost 5:2429–2436
Staritz P, Kurz K, Stoll M, Giannitsis E, Katus HA, Ivandic BT (2009) Platelet reactivity and clopidogrel resistance are associated with the H2 haplotype of the P2Y12-ADP receptor gene. Int J Cardiol 133:341–345
Sun D, McNicol A, James AA, Peng Z (2006) Expression of functional recombinant mosquito salivary apyrase: a potential therapeutic platelet aggregation inhibitor. Platelets 17:178–184
Johnson FL, Boyer JL, Leese PT, Crean C, Krishnamoorthy R, Durham T, Fox AW, Kellerman DJ (2007) Rapid and reversible modulation of platelet function in man by a novel P2Y12 ADP-receptor antagonist, INS50589. Platelets 18:346–356
Wang YX, Vincelette J, da Cunha V, Martin-McNulty B, Mallari C, Fitch RM, Alexander S, Islam I, Buckman BO, Yuan S, Post JM, Subramanyam B, Vergona R, Sullivan ME, Dole WP, Morser J, Bryant J (2007) A novel P2Y12 adenosine diphosphate receptor antagonist that inhibits platelet aggregation and thrombus formation in rat and dog models. Thromb Haemost 97:847–855
Hoffmann K, Baqi Y, Morena MS, Glänzel M, Müller CE, von Kügelgen I (2009) Interaction of new, very potent non-nucleotide antagonists with Arg256 of the human platelet P2Y12 receptor. J Pharmacol Exp Ther 331:648–655
Springthorpe B, Bailey A, Barton P, Birkinshaw TN, Bonnert RV, Brown RC, Chapman D, Dixon J, Guile SD, Humphries RG, Hunt SF, Ince F, Ingall AH, Kirk IP, Leeson PD, Leff P, Lewis RJ, Martin BP, McGinnity DF, Mortimore MP, Paine SW, Pairaudeau G, Patel A, Rigby AJ, Riley RJ, Teobald BJ, Tomlinson W, Webborn PJ, Willis PA (2007) From ATP to AZD6140: the discovery of an orally active reversible P2Y12 receptor antagonist for the prevention of thrombosis. Bioorg Med Chem Lett 17:6013–6018
Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De SS, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM (2007) Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 357:2001–2015
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsen M (2009) Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 361:1045–1057
Braun OÖ, Amisten S, Wihlborg AK, Hunting K, Nilsson D, Erlinge D (2007) Residual platelet ADP reactivity after clopidogrel treatment is dependent on activation of both the unblocked P2Y1 and the P2Y12 receptor and is correlated with protein expression of P2Y12. Purinergic Signal 3:195–201
Pi Z, Sutton J, Lloyd J, Hua J, Price L, Wu Q, Chang M, Zheng J, Rehfuss R, Huang CS, Wexler RR, Lam PY (2013) 2-Aminothiazole based P2Y1 antagonists as novel antiplatelet agents. Bioorg Med Chem Lett 23:4206–4209
Obergan TY, Lyapina LA, Pastorova VE (2007) Antithrombotic activity of heparin-ATP complex. Bull Exp Biol Med 143:299–301
Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW (2010) Adenosine diphosphate-inducible platelet reactivity shows a pronounced age dependency in the initial phase of antiplatelet therapy with clopidogrel. J Thromb Haemost 8:37–42
Cattaneo M (2011) Bleeding manifestations of congenital and drug-induced defects of the platelet P2Y12 receptor for adenosine diphosphate. Thromb Haemost 105(Suppl 1):S67–S74
Liverani E, Rico MC, Tsygankov A, Kilpatrick LE, Kunapuli SP (2013) Clopidogrel modulates LPS-induced systemic inflammation through a P2Y12 receptor independent pathway. Blood 122:2309
Kastalli S, El AS, Zaiem A, Ben Abdallah H, Daghfous R (2010) Fatal liver injury associated with clopidogrel. Fundam Clin Pharmacol 24:433–435
Belchikov YG, Koenig SJ, Dipasquale EM (2013) Potential role of endogenous adenosine in ticagrelor-induced dyspnea. Pharmacotherapy 33:882–887
Nylander S, Femia EA, Scavone M, Berntsson P, Asztély AK, Nelander K, Löfgren L, Nilsson RG, Cattaneo M (2013) Ticagrelor inhibits human platelet aggregation via adenosine in addition to P2Y12 antagonism. J Thromb Haemost 11:1867–1876
Michelson AD (2011) Advances in antiplatelet therapy. Hematology Am Soc Hematol Educ Program 2011:62–69
Winchester DE, Brearley WD, Wen X, Park KE, Bavry AA (2012) Efficacy and safety of unfractionated heparin plus glycoprotein IIb/IIIa inhibitors during revascularization for an acute coronary syndrome: a meta-analysis of randomized trials performed with stents and thienopyridines. Clin Cardiol 35:93–100
Siller-Matula JM, Trenk D, Schrör K, Gawaz M, Kristensen SD, Storey RF, Huber K (2013) Response variability to P2Y12 receptor inhibitors: expectations and reality. JACC Cardiovasc Interv 6:1111–1128
Coimbra LS, Steffens JP, Rossa C Jr, Graves DT, Spolidorio LC (2014) Clopidogrel enhances periodontal repair in rats through decreased inflammation. J Clin Periodontol 41:295–302
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH (2011) 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines and the society for cardiovascular angiography and interventions. J Am Coll Cardiol 58:e44–122
Tello-Montoliu A, Thano E, Rollini F, Patel R, Wilson RE, Müniz-Lozano A, Franchi F, Darlington A, Desai B, Guzman LA, Bass TA, Angiolillo DJ (2013) Impact of aspirin dose on adenosine diphosphate-mediated platelet activities. Results of an in vitro pilot investigation. Thromb Haemost 110:777–784
Giachini FR, Leite R, Osmond DA, Lima VV, Inscho EW, Webb RC, Tostes RC (2014) Anti-platelet therapy with clopidogrel prevents endothelial dysfunction and vascular remodeling in aortas from hypertensive rats. PLoS One 9:e91890
Zhang K, Zhang J, Gao ZG, Zhang D, Zhu L, Han GW, Moss SM, Paoletta S, Kiselev E, Lu W, Fenalti G, Zhang W, Muller CE, Yang H, Jiang H, Cherezov V, Katritch V, Jacobson KA, Stevens RC, Wu B, Zhao Q (2014) Structure of the human P2Y12 receptor in complex with an antithrombotic drug. Nature 509:115–118
Leven RM, Nachmias VT (1982) Cultured megakaryocytes: changes in the cytoskeleton after ADP-induced spreading. J Cell Biol 92:313–323
Leven RM, Mullikin WH, Nachmias VT (1983) Role of sodium in ADP- and thrombin-induced megakaryocyte spreading. J Cell Biol 96:1234–1240
Kawa K (1990) Guinea-pig megakaryocytes can respond to external ADP by activating Ca2+-dependent potassium conductance. J Physiol 431:207–224
Miller JL (1983) Characterization of the megakaryocyte secretory response: studies of continuously monitored release of endogenous ATP. Blood 61:967–972
Uneyama C, Uneyama H, Takahashi M, Akaike N (1993) Cytoplasmic pH regulates ATP-induced Ca2+-dependent K+-current oscillation in rat megakaryocytes. Biochem J 295:317–320
Uneyama C, Uneyama H, Takahashi M, Akaike N (1994) Biological actions of purines on rat megakaryocytes: potentiation by adenine of the purinoceptor-operated cytoplasmic Ca2+ oscillation. Br J Pharmacol 112:349–351
Uneyama H, Uneyama C, Ebihara S, Akaike N (1994) Suramin and reactive blue 2 are antagonists for a newly identified purinoceptor on rat megakaryocyte. Br J Pharmacol 111:245–249
Somasundaram B, Mahaut-Smith MP (1994) Three cation influx currents activated by purinergic receptor stimulation in rat megakaryocytes. J Physiol 480:225–231
Murgo AJ, Contrera JG, Sistare FD (1994) Evidence for separate calcium-signaling P2T and P2U purinoceptors in human megakaryocytic Dami cells. Blood 83:1258–1267
Hechler B, Cazenave JP, Hanau D, Gachet C (1995) Presence of functional P2T and P2U purinoceptors on the human megakaryoblastic cell line, Meg-01. Characterization by functional and binding studies. Nouv Rev Fr Hematol 37:231–240
Kawa K (1996) ADP-induced rapid inward currents through Ca(2+)-permeable cation channels in mouse, rat and guinea-pig megakaryocytes: a patch-clamp study. J Physiol 495:339–352
Greco NJ (1997) Functional expression of a P2T ADP receptor in Xenopus oocytes injected with megakaryocyte (CMK 11-5) RNA. Arterioscler Thromb Vasc Biol 17:769–777
Ikeda M (2007) Characterization of functional P2X1 receptors in mouse megakaryocytes. Thromb Res 119:343–353
Hechler B, Toselli P, Ravanat C, Gachet C, Ravid K (2001) Mpl ligand increases P2Y1 receptor gene expression in megakaryocytes with no concomitant change in platelet response to ADP. Mol Pharmacol 60:1112–1120
Gurung IS, Martinez-Pinna J, Mahaut-Smith MP (2008) Novel consequences of voltage-dependence to G-protein-coupled P2Y1 receptors. Br J Pharmacol 154:882–889
Martinez-Pinna J, Tolhurst G, Gurung IS, Vandenberg JI, Mahaut-Smith MP (2004) Sensitivity limits for voltage control of P2Y receptor-evoked Ca2+ mobilization in the rat megakaryocyte. J Physiol 555:61–70
Zhao J, Ennion SJ (2006) Sp1/3 and NF-1 mediate basal transcription of the human P2X1 gene in megakaryoblastic MEG-01 cells. BMC Mol Biol 7:10
Young JP, Beckerman J, Vicini S, Myers A (2010) Acetylsalicylic acid enhances purinergic receptor-mediated outward currents in rat megakaryocytes. Am J Physiol Cell Physiol 298:C602–C610
Balduini A, Di Buduo CA, Malara A, Lecchi A, Rebuzzini P, Currao M, Pallotta I, Jakubowski JA, Cattaneo M (2012) Constitutively released adenosine diphosphate regulates proplatelet formation by human megakaryocytes. Haematologica 97:1657–1665
Di Buduo C, Malara A, Pallotta I, Lecchi A, Balduini A, Cattaneo M (2013) The interaction of adenosine diphosphate with P2Y13 receptors in vitro proplatelet formation from human megakaryocytes. J Thromb Haemost 9:73
Burnstock G, Boeynaems J-M (2014) Purinergic signalling and immune cells. Purinergic Signalling 10:529–564
Nalos M, Huang S, Sluyter R, Khan A, Santner-Nanan B, Nanan R, McLean AS (2008) "Host tissue damage" signal ATP impairs IL-12 and IFNγ secretion in LPS stimulated whole human blood. Intensive Care Med 34:1891–1897
Stachon P, Hergeth SH, Anto Michel NM, Dufner B, Rodriguez AR, Hoppe N, Wolf DW, Bode CB, Idzko MI, Zirlik AZ (2013) Extracellular atp contributes to atherogenesis via purinergic receptors by inducing leukocyte recruitment in mice. Eur Heart J 34:442
De Pablo C, Orden S, Esplugues JV, Álvarez A (2013) ATP plays a role in leukocyte accumulation induced by ABC through its P2X7 receptors. Basic Clin Pharmacol Toxicol 113:18
Cabiati M, Caruso R, Verde A, Sabatino L, Morales MA, Del Ry S (2013) Transcriptomic profiling of the four adenosine receptors in human leukocytes of heart failure patients. Biomed Res Int 2013:569438
Kunapuli SP (1998) Multiple P2 receptor subtypes on platelets: a new interpretation of their function. Trends Pharmacol Sci 19:391–394
Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, Costa MA (2007) Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol 49:1505–1516
Acknowledgments
The author thanks Professor Howard A. Stone, Professor David Erlinge, Professor Randy S. Sprague and Dr. Vera Ralevic for their valuable and constructive comments on this manuscript and Dr. Gillian E. Knight for her excellent editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burnstock, G. Blood cells: an historical account of the roles of purinergic signalling. Purinergic Signalling 11, 411–434 (2015). https://doi.org/10.1007/s11302-015-9462-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-015-9462-7