Abstract
Nucleotides released from cells in response to mechanical stimulation or injury may serve as paracrine regulators of bone cell function. Extracellular nucleotides bind to multiple subtypes of P2 receptors on osteoblasts (the cells responsible for bone formation) and osteoclasts (cells with the unique ability to resorb mineralized tissues). Both cell lineages express the P2X7 receptor subtype. The skeletal phenotype of mice with targeted disruption of P2rx7 points to interesting roles for this receptor in the regulation of bone formation and resorption, as well as the response of the skeleton to mechanical stimulation. This paper reviews recent work on the expression of P2X7 receptors in bone, their associated signal transduction mechanisms and roles in regulating bone formation and resorption. Areas for future research in this field are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bone biology
Introduction
Bone is a specialized connective tissue composed of mineralized extracellular matrix and functionally distinct cell populations that include osteoblasts, osteocytes, and osteoclasts [1]. Among its many functions, the skeleton imparts mechanical stability and protection to vital organs and serves as a site for muscle attachment to support locomotion. Moreover, bone provides an environment for hematopoiesis and is a major repository for calcium and phosphate, thus contributing to ionic homeostasis. To maintain its mass and functionality throughout life, bone undergoes remodeling, a dynamic process that involves its coordinated resorption and formation. Remodeling occurs asynchronously at millions of sites throughout the skeleton, regulated primarily by local factors including autocrine/paracrine mediators and mechanical stimuli. In addition, systemic factors such as parathyroid hormone and estrogen modulate remodeling activity [2]. Under physiological conditions, resorption and formation are tightly coupled, and perturbations to this balance cause bone loss in metabolic diseases such as osteoporosis and inflammatory diseases such as rheumatoid arthritis and periodontitis [3].
Osteoblast origin, differentiation, and function
As the cells responsible for bone formation, osteoblasts play an essential role in skeletal development and remodeling (Fig. 1). Osteoblasts develop from mesenchymal stem cells that also give rise to other cell types including chondrocytes, adipocytes, and fibroblasts [4]. Commitment to the osteoblast lineage is controlled by a complex series of transcriptional events that are initiated and maintained by a number of extracellular stimuli, including bone morphogenetic proteins, transforming growth factor-β, Wnts, insulin-like growth factors, fibroblast growth factors, platelet-derived growth factors, parathyroid hormone and glucocorticoids [2, 5]. Runt-related transcription factor-2 (Runx2) is a key transcription factor controlling osteoblast differentiation, acting together with co-regulatory proteins to direct uncommitted progenitors towards the osteoblast lineage [5–8]. A second osteogenic transcription factor, Osterix (Osx) acts downstream of Runx2 and is specific for early and late stages of osteoblast differentiation [9]. The combined expression of Runx2 and Osx initiates development of the committed preosteoblast, which is characterized by expression of type I collagen and bone sialoprotein. Subsequent induction of activating transcription factor 4, together with Osx and various Wnt/β-catenin signaling components, leads to development of the mature osteoblast, which expresses type I collagen, alkaline phosphatase, and osteocalcin [2, 10].
The cells of bone. Osteoblasts and osteocytes arise from mesenchymal stem cells while osteoclasts are produced by fusion of precursors of the monocyte/macrophage lineage. Osteoblasts form bone through secretion of an organic matrix, termed osteoid, which is subsequently mineralized. During this process, a number of osteoblasts become embedded within the matrix and terminally differentiate into osteocytes, the most abundant cell type in bone. Other osteoblasts become “bone lining cells”, which cover the quiescent surfaces of bone. Osteoclasts are large, multinucleated cells that resorb mineralized matrix. A mature osteoclast is characterized by its attachment to the bone surface and subsequent formation of a specialized membrane structure termed the ruffled border. The transport of protons and secretion of hydrolytic enzymes across the ruffled border leads to dissolution of bone mineral and degradation of the organic matrix, respectively. This activity creates a resorption lacuna on the bone surface. Modified with permission from [1], copyright 1988, Liss
Mature osteoblasts secrete an organic matrix (termed osteoid) primarily composed of type I collagen intermixed with non-collagenous proteins and proteoglycans. Subsequent mineralization of the osteoid occurs through deposition of the calcium phosphate mineral hydroxyapatite. As bone formation proceeds, a number of osteoblasts become incorporated within the mineralized matrix and terminally differentiate to form osteocytes. Osteocytes maintain contact with each other as well as with osteoblasts and bone lining cells through an intricate array of cytoplasmic processes that interconnect via gap junctions. Through these cellular connections, osteocytes and osteoblasts are thought to detect and transmit signals arising from the mechanical stimulation of bone [11].
Osteoclast origin, differentiation, and function
Mononucleated precursors of the monocyte/macrophage lineage proliferate and fuse to form mature, multinucleated osteoclasts [3] (Fig. 1). The formation, resorptive activity and survival of osteoclasts are controlled by two key signaling molecules, macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor κB (RANK) ligand [12]. Marrow stromal cells and osteoblasts produce M-CSF and RANK ligand in membrane-bound and soluble forms, thereby regulating osteoclast development [13, 14].
In the early stages of differentiation, M-CSF acts on its receptor c-Fms to stimulate proliferation and survival of monocytes/macrophages. M-CSF also induces expression of RANK, leading to formation of preosteoclasts in the presence of RANK ligand. In both precursors and mature osteoclasts, binding of RANK ligand to RANK activates multiple signaling pathways, including mitogen-activated protein kinases, phosphatidylinositol 3-kinase, and Ca2+/calcineurin. In turn, these pathways activate several transcription factors including activator protein-1, nuclear factor-κB (NF-κB) and nuclear factor of activated T cells (NFAT) [13, 14]. Of these, NFATc1 is believed to be the master regulator of osteoclast terminal differentiation [15, 16]. On the other hand, osteoprotegerin (OPG), a soluble decoy receptor secreted by cells of the osteoblast lineage, can bind RANK ligand, thereby limiting RANK signaling and inhibiting osteoclast formation and function.
Resorption requires attachment of the osteoclast to the bone surface, forming a confined microenvironment termed the resorption lacuna. Subsequently, intracellular vesicles fuse with the plasma membrane creating the characteristic ruffled border. Vacuolar proton pumps in the ruffled border acidify the resorption lacuna leading to dissolution of bone mineral. Organic constituents exposed by the process of demineralization are then degraded by cathepsin K, matrix metalloproteinase 9, tartrate-resistant acid phosphatase, and other hydrolytic enzymes. After bone has been resorbed, osteoclasts either migrate to a new site on the bone surface or undergo apoptosis [3].
Regulation of bone remodeling
Remodeling provides a mechanism for preventive maintenance of the skeleton and the targeted replacement of fatigued or damaged bone. Each remodeling cycle is initiated by activation of osteoclast precursors, followed by bone resorption. After a reversal phase, preosteoblasts are recruited to the site, and bone is formed by the secretion of osteoid and its subsequent mineralization [17]. To maintain a precise balance between bone formation and resorption, this complex process is regulated by a network of systemic and local factors that include mechanical stimuli. In this regard, increased mechanical loading enhances bone formation resulting in improved skeletal strength. On the other hand, disuse suppresses formation and enhances the resorption of bone [10]. The process by which mechanical stimuli are translated into cellular responses, known as mechanotransduction, has been suggested to involve nucleotide release and subsequent P2 receptor activation [10, 18].
P2X7 in bone
Extracellular nucleotides signal through P2 receptors expressed in a variety of cell types, including osteoblasts and osteoclasts. These receptors are subdivided into two classes: the P2Y family of G protein-coupled receptors and the P2X family of ligand-gated cation channels [19]. Nucleotides released in response to mechanical stimulation or inflammation may serve as autocrine/paracrine regulators of both osteoblast and osteoclast function (reviewed in [18, 20–23]). Thus, nucleotides and the complex network of P2 receptors in bone may underlie the well-recognized responses of skeletal tissues to mechanical stimulation. In 1997, Collo et al. noted expression of P2X7 in the developing vertebrae and mandible of E19 rat embryos by in situ hybridization (Fig. 2) [24]. More recently, the phenotype of mice with targeted disruption of the gene encoding the P2X7 receptor (P2rx7) has been described, suggesting a role for this receptor in the regulation of bone formation and resorption [25] and in mechanotransduction [26].
P2X7 receptor expression in bone revealed by in situ hybridization. Shown is a parasagittal section from an E19 rat embryo assessed for P2X7 mRNA using digoxigenin-labelled riboprobe hybridization. Positive staining for P2X7 receptor mRNA was seen in the developing vertebrae (dark staining, arrowheads) and mandible (arrow) providing evidence for expression of the P2X7 receptor in bone. Modified from [24], copyright 1997, with permission from Elsevier
P2X7 in cells of the osteoblast lineage
Expression of P2X7 in cells of the osteoblast lineage
There have been conflicting reports regarding expression of P2X7 receptors in cells of the osteoblast lineage. Expression of P2rx7 transcripts was initially demonstrated in the MG-63 human osteoblast-like cell line [27]. On the other hand, specific immunostaining for the receptor could not be detected in osteoblast-enriched cultures of rat calvarial cells [28]. A subsequent study conducted by Gartland et al. localized the P2X7 receptor to a subpopulation of human bone-derived cells in vitro using immunocytochemical analyses and pore-formation assays [29]. However, others reported that the P2X7 agonist 2′,3′-O-(4-benzoylbenzoyl)-ATP (BzATP) failed to elicit elevations of cytosolic free Ca2+ concentration ([Ca2+]i) or to induce pore formation in osteoblasts from cultures of adherent human bone marrow cells [30], leading these authors to conclude that functional P2X7 receptors are not expressed by osteoblasts.
More recent investigations have provided consistent evidence for the expression of P2X7 receptors in cells of the osteoblast lineage (Table 1). In 2003, the presence of P2rx7 transcripts was demonstrated in cultures of mouse calvarial cells (Fig. 3a) [25]. Moreover, BzATP induced pore formation in approximately 30% of calvarial osteoblasts from wild-type but not P2rx7 −/− mice (Fig. 3b), indicating that functional P2X7 receptors are expressed by only a subpopulation of calvarial cells. P2X7 protein expression was later confirmed in mouse calvarial cultures and MC3T3-E1 osteoblast-like cells by immunoblot analysis [26]. More recently, the expression of P2 receptors and nucleotide responses were examined during the differentiation of rat calvarial cells in vitro [31]. Using conventional RT-PCR, P2rx7 transcripts were found to be present at all time points examined in culture (from days 6 to 15).
Murine osteoblasts express functional P2X7 receptors. a RNA was isolated from wild-type (WT) and P2rx7 −/− calvarial osteoblast cultures and samples were divided into those that did (+) or did not (−) undergo reverse transcription. PCR revealed transcripts for P2X7 (expected size, 212 bp) in cells from wild-type but not P2rx7 −/− mice. There was no amplification product in samples that did not undergo reverse transcription or did not contain template (NT). b Fluorescence micrographs of ethidium bromide (EtBr) uptake by calvarial osteoblasts from wild-type and P2rx7 −/− mice. Cells were incubated with EtBr in nominally Ca2+- and Mg2+-free buffer in the presence of the P2X7 receptor agonist BzATP (300 μM). Nuclei were visualized using Hoechst 3342 and images were superimposed at right. BzATP induced pore formation resulting in EtBr uptake in a subpopulation of wild-type but not P2rx7 −/− osteoblasts. Scale bar 100 μm. Reproduced from [25], copyright 2003, with permission of The Endocrine Society
Membrane blebbing is a unique response exhibited by some cell types following activation of P2X7 receptors [35]. Panupinthu et al. characterized blebbing in cultured calvarial cells to verify expression of functional P2X7 receptors in vitro [32]. In these experiments, live cells were loaded with the fluorescent dye FM4-64 to label membranes and morphology was monitored by confocal microscopy (Fig. 4a). BzATP induced formation of multiple dynamic blebs, which enlarged and shrunk in an asynchronous manner, and did not contain FM4-64-stained intracellular membranes. Approximately 40% of cultured rat and murine calvarial cells exhibited dynamic membrane blebbing in response to BzATP or high concentrations of ATP. In contrast, BzATP did not induce blebbing of calvarial cells from P2rx7 −/− mice [32]. These findings provided further evidence for heterogeneous expression of functional P2X7 receptors in calvarial cells. At the same time, calvarial cultures contain cells at various stages of osteoblast differentiation, a fact that could explain the heterogeneous pattern of P2X7 expression. Thus, it would be of interest to determine quantitatively the stages of differentiation at which bone cells express P2X7 receptors, and how this expression is regulated.
BzATP acts through P2X7 receptors to induce dynamic membrane blebbing of osteoblasts. a Calvarial osteoblasts from wild-type mice were incubated with the fluorescent probe FM4-64 to stain membranes and observed using confocal microscopy. Cells were bathed in nominally Ca2+- and Mg2+-free buffer, and BzATP (300 μM) was added at time 0. Images show 1-μm-thick optical sections 10 μm above the substratum through a single cell at 7-min intervals (N nucleus, blue arrowhead enlarging bleb, yellow arrowhead shrinking bleb). Scale bar 10 μm. b Proposed mechanism of P2X7-induced blebbing in osteoblasts. ATP and BzATP act through the P2X7 receptor on osteoblasts to stimulate PLD and PLA2. These phospholipases catalyze the conversion of glycerophospholipid (PL) to phosphatidic acid (PA) and LPA. LPA acts on its receptor (LPAR) to stimulate Rho-associated kinase, which in turn causes membrane blebbing. Reproduced from [32] with permission of the American Society for Biochemistry and Molecular Biology
P2X7 receptor signaling in cells of the osteoblast lineage
ATP and BzATP induce opening of the P2X7 nonselective cation channel, which is permeable to Na+, K+, and Ca2+. This event leads to elevation of [Ca2+]i and depolarization of the plasma membrane. However, expression of multiple subtypes of P2X and calcium-mobilizing P2Y receptors on cells of the osteoblast lineage [31] makes it difficult to attribute responses to specific P2 receptors. Moreover, heterogeneity within calvarial cell cultures and other in vitro osteoblast models adds to the complexity of interpreting data from single-cell electrophysiology or calcium fluorescence studies. Thus, future work comparing responses of osteoblasts isolated from P2rx7 −/− mice and wild-type controls should prove informative.
In many cell types, decreasing divalent cation concentrations in the extracellular fluid potentiates the effects of P2X7 agonists, leading to formation of aqueous pores within the plasma membrane permeable to molecules as large as 900 Da [36]. Though its physiological relevance remains to be determined, this phenomenon has proven useful in characterizing P2X7 receptor expression. In this regard, high concentrations of ATP or BzATP induced uptake of ethidium bromide (394 Da) by cultured osteoblasts in divalent cation-free buffer [29]. In a separate study, cells on the ectocranial surfaces of calvariae, a site of active osteogenesis, exhibited pore formation in response to BzATP, thereby establishing that osteogenic cells express functional P2X7 receptors in situ [33]. This finding also confirmed that P2X7 expression in bone cells is not an artifact of in vitro culture, as has been found for some P2 receptors in other systems [37]. Moreover, P2X7 receptors mediated dye uptake by mouse calvarial cells in response to fluid shear stress [26], suggesting that pore formation may play a role in mechanotransduction.
In other cell types, P2X7 receptor activation leads to stimulation of phospholipase D (PLD) and A2 (PLA2) activity [38, 39], suggesting that P2X7 may couple to the production of bioactive lipids. In this regard, fluid shear stress stimulates production of prostaglandin E2 (PGE2) by osteoblasts in a manner dependent on P2X7 receptor signaling [26]. Panupinthu et al. have since identified a role for lipid-signaling pathways in mediating P2X7-induced blebbing in osteoblasts [32]. Specifically, activation of P2X7 receptors leads to stimulation of PLD and PLA2, resulting in production of the potent lipid mediator lysophosphatidic acid (LPA; Fig. 4b). LPA then acts through its G protein-coupled receptor to induce membrane blebbing via a pathway dependent on Rho-associated kinase. Thus, a number of the effects of P2X7 receptor activation in osteoblasts may be mediated by prostaglandins and LPA.
Most recently, P2X7 receptors have been shown to mediate extracellular signal-regulated kinase (ERK) 1/2 activation by fluid shear stress in an osteoblast-like cell line [34, 40]. The phosphorylation of ERK1/2 required ATP release, and appeared to be dependent on both elevation of [Ca2+]i and activation of protein kinase C (PKC) [34]. In summary, P2X7 receptors in cells of the osteoblast lineage couple to multiple signaling pathways. It will be of considerable interest to examine cross-talk among P2X7 receptor signaling and pathways that function downstream of other P2 receptors, as well as those activated by osteotropic hormones and cytokines.
Physiological function of P2X7 receptors in cells of the osteoblast lineage
Overall, P2rx7 −/− mice are viable and fertile and can not be distinguished from wild-type littermates by observation alone [41]. Nevertheless, defects have been described including impaired IL-1 release from P2rx7 −/− macrophages challenged with ATP [41]. Moreover, when arthritis is induced using a monoclonal anti-collagen antibody, P2rx7 −/− mice exhibit an attenuated inflammatory response compared to wild-type controls [42]. In 2003, Ke et al. characterized the effects of targeted disruption of P2rx7 on bone formation and remodeling [25]. Unexpectedly, this study identified a skeletal phenotype for the P2rx7 −/− mouse consistent with a role for this receptor in osteogenesis. Specifically, when compared to wild-type littermates, adult P2rx7 −/− mice displayed significant reduction in total and cortical bone content. Moreover, radiographs and peripheral quantitative computed tomography analyses demonstrated that femoral bone diameters were smaller in P2rx7 −/− mice compared with wild-type controls, consistent with impaired periosteal bone formation (Fig. 5a,b). In contrast, femur length did not differ, indicating that P2X7 receptors do not regulate the longitudinal growth of bones. Since longitudinal growth is mediated primarily by the cartilaginous growth plate, this finding is in keeping with the absence of P2X7 in cells of the chondrocyte lineage (unpublished observations).
The P2X7 receptor regulates periosteal bone formation. a Radiographs of femurs from adult (9 months) male wild-type and P2rx7 −/− mice. P2rx7 −/− mice had femurs of smaller diameter but similar length compared to wild-type controls. b Peripheral quantitative computerized tomography images of a femoral shaft from adult male wild-type and P2rx7 −/− mice. c Fluorescence images of sections of tibial shafts, from wild-type and P2rx7 −/− littermate mice at 2 months of age showing double calcein labels (green) at the periosteal surface. Calcein was administered 12 and 2 days before necropsy. The interlabeling distance is shorter in P2rx7 −/− mice compared with wild-type controls, indicating reduced rates of periosteal mineral apposition and bone formation. Modified from [25], copyright 2003, with permission of The Endocrine Society
When double calcein-labeling was used to reveal sites of active osteogenesis, reduced rates of bone formation were observed in P2rx7 −/− mice, as revealed by the shorter interlabeling distance at periosteal surfaces of the tibial shafts (Fig. 5c) [25]. The unique phenotype of the P2rx7 −/− mouse prompted Ke and colleagues to speculate that these mice may possess decreased sensitivity to mechanical loading [25]. This hypothesis was tested directly by Li et al., who used an in vivo ulnar loading system (Fig. 6a) to compare the anabolic effects of mechanical stimulation in P2rx7 −/− and control mice [26]. Interestingly, the sensitivity to mechanical loading was reduced by up to 73% in P2rx7 −/− mice as demonstrated by dual-fluorochrome labeling (Fig. 6b), establishing that the effects of mechanical stimulation on periosteal bone formation are dependent on the P2X7 receptor.
The P2X7 receptor is required for mechanically induced periosteal bone formation. a A schematic showing the apparatus used for axial loading of the mouse forelimb. Under anesthesia, the right forearm of the mouse was loaded cyclically (peak of 2,500 µstrain, stimulated at 2 Hz for 120 cycles per day for three consecutive days). The left forearm of the same mouse was not loaded and served as an internal control. Used with permission of A. G. Robling. b Fluorescence images of the periosteal surface of ulnar midshafts showing double fluorochrome labeling from wild-type (bi) and P2rx7 −/− (bii) mice. Calcein (green) and alizarin (red) were administered 5 and 11 days after the first loading day. Mechanical loading of the right ulna activated the formerly quiescent periosteal surface. The responses in wild-type mice were more robust (illustrated by increased fluorescence intensity and interlabeling distance) compared with P2rx7 −/− mice. Modified from [26], with permission of the American Society for Biochemistry and Molecular Biology
The skeletal phenotype of a second P2rx7 −/− mouse model has been described by Gartland et al. [43]. In contrast to the observations made by Ke et al. [25], these mice showed no overt skeletal phenotype with the exception of thicker cortical bones than their wild-type controls. This discrepancy could be due to the different strategies used to generate the mice or their different genetic backgrounds. In this regard, the P2rx7 −/− mice characterized by Gartland et al. were constructed by insertion of a lacZ gene at the beginning of exon 1 of P2rx7 [44], whereas the P2rx7 −/− mice described by Ke et al. carried a deletion of the region encoding C-terminal amino acids 506–532 [41]. Despite these differences, P2X7 receptor protein is not detectable in either model [44]. It is noteworthy that the decrease in bone density observed by Ke et al. was more pronounced in adult male mice, whereas Gartland et al. analyzed the bones of a relatively small number of animals, the gender of which was not specified.
Since a number of cell types express P2X7 receptors, it was not known until recently whether the phenotype of the P2rx7 −/− mouse was due to an intrinsic defect in osteoblast function or to an indirect effect mediated by other cell types. To address this question, Panupinthu et al. employed a well-characterized bone formation assay in which rat and murine calvarial osteoblasts differentiate and form bone-like nodules in vitro [33]. BzATP induced pore formation in cells within these nodules, indicating that the calvarial cells responsible for osteogenesis in vitro express functional P2X7 receptors. Moreover, activation of P2X7 receptors by exogenous nucleotides stimulated osteoblast differentiation and enhanced mineralization. On the other hand, the expression of osteoblast markers was suppressed in calvarial cells from P2rx7 −/− mice compared to wild-type controls. Interestingly, the stimulatory effects of P2X7 activation on bone formation in vitro were dependent on both LPA signaling and cyclooxygenase activity. Thus, P2X7 receptors enhance bone formation through an osteoblast-autonomous mechanism. Moreover, this study identified a novel signaling axis that links P2X7 receptors to production of LPA and cyclooxygenase metabolites, which in turn stimulate osteogenesis. The involvement of LPA, which signals in part through Rho, is consistent with the role of RhoA and Rho-associated kinase in driving the differentiation of osteoblasts from mesenchymal stem cells [45]. Moreover, the dependence of osteogenesis on cyclooxygenase activity is in keeping with the well-established role of prostaglandins in stimulating bone formation [46] and in mediating skeletal mechanotransduction in vivo [47].
It is possible that P2X7 may have other important functions in cells of the osteoblast lineage. For example, it has been suggested that ATP and other organic phosphates provide a source of inorganic phosphate, which is required for the formation of bone mineral crystals [48]. In this regard, P2X7 receptor activation triggers ATP efflux [49], providing a potential source of phosphate to support mineralization. It is also possible that P2X7 receptors on osteoblasts are involved in the processing and secretion of cytokines, as is well-established in leukocytes [50]. Lastly, activation of P2X7 receptors promotes apoptosis in a number of cell systems [51]. In fact, Gartland et al. interpreted P2X7-induced membrane blebbing of human bone-derived cells as reflecting apoptosis [29]. In addition, ATP and BzATP induced delayed release of lactate dehydrogenase from osteoblastic cells, indicating cell death. However, Panupinthu et al. found BzATP-induced membrane blebbing of murine calvarial cells was reversible upon removal of agonist, indicating that P2X7 receptors do not induce acute cell death [32]. Moreover, stimulation of P2X7 receptors in MC3T3-E1 osteoblastic cells does not activate caspase 3, a key mediator of apoptosis [26], arguing against a pro-apoptotic effect. Future studies are needed to clarify the roles of P2X7 receptors in regulating survival and other functions of osteoblasts.
P2X7 in osteoclasts
Expression of P2X7 receptors in osteoclasts
In contrast to cells of the osteoblast lineage, there has been general agreement that osteoclasts express functional P2X7 receptors (Table 2). In 1994, Modderman et al. observed that, in the absence of divalent cations, ATP (2 mM) induced ethidium bromide uptake and increased the membrane conductance of murine osteoclasts [61]. P2rx7 transcripts have since been identified using RT-PCR in osteoclast-like cells derived from human bone marrow mononuclear cells [30] and peripheral blood monocytes [53, 54]. Moreover, mRNA encoding P2X7 was present throughout peripheral blood monocyte differentiation (0–21 days), suggesting that the receptor is expressed by both osteoclasts and their precursors [53, 54]. Immunocytochemical staining and pore-formation assays have also been used to establish the expression of functional receptors in authentic rat and murine osteoclasts, and in-vitro-derived murine and human osteoclasts [30, 54–56, 59, 62]. Furthermore, expression of P2X7 by human osteoclasts in vivo has been confirmed by labeling with a monoclonal antibody (Fig. 7) [54].
Human osteoclasts express P2X7 receptor protein in vivo. A section of human femoral head was immunostained with a monoclonal antibody raised against the extracellular domain of the P2X7 receptor. Positive staining of osteoclasts lining the surface of bone (brown staining, arrows) confirmed expression of the P2X7 receptor in vivo. Scale bar 50 μm. Modified from [54], Fig. 2b, copyright 2003, with kind permission from Springer Science and Business Media
P2X7 receptor signaling in osteoclasts
In cells expressing functional P2X7 receptors, ATP induces an activity-dependent, nonselective cation current. Whole-cell currents activated by P2X7 receptors in authentic osteoclasts were characterized by Naemsch et al. using patch-clamp techniques (Fig. 8) [56]. With K+ currents blocked by CsCl in the electrode solution, initial application of ATP (100 µM, sufficient to activate P2X4 and some P2Y receptors, but not P2X7) evoked inward P2X4 current. The P2X4 current activated rapidly and then declined, with successive applications of ATP eliciting little response (Fig. 8a). The current–voltage relationship revealed that the initial ATP-activated current was inwardly rectifying and reversed direction close to 0 mV (Fig. 8b). However, a different pattern was observed when BzATP was applied successively (Fig. 8c). The first application of BzATP evoked the same initial P2X4 current, but was followed by an inward current that increased in amplitude with successive stimulations. The later component of the BzATP-activated current was inwardly rectifying and also reversed close to 0 mV (Fig. 8d). Furthermore, the later component was activated only by BzATP or higher concentrations of ATP [56]. Thus, in contrast to P2X4 current which desensitizes with repeated agonist application, the later BzATP-induced current was activity-dependent, consistent with the behavior of P2X7 receptors in other systems [63].
Nucleotides activate two distinct P2X currents in osteoclasts: a transient P2X4 current followed by an activity-dependent P2X7 current that involves Ca2+ influx. Whole-cell currents were recorded from rabbit osteoclasts with CsCl in the electrode solution to block K+ currents. Voltage ramp commands were used to obtain I–V relationships at times indicated by numbers. Nucleotide-induced currents were revealed by subtraction of the control current. Symbols in a, c, and e represent current at −100 mV. Bars above the current traces represent the length of agonist applications. a ATP (100 μM) was applied to a single osteoclast at the times indicated, with initial application inducing an inward P2X4 current that desensitized with successive applications. b I–V relationships for the same cell as in panel a. The initial ATP stimulation (1) induced an inwardly rectifying current that reversed near 0 mV. Little current was elicited upon the 5th, 10th, or 15th stimulation. c A second osteoclast was stimulated with BzATP (100 μM). After the initial P2X4 response to BzATP, successive stimulations led to the progressive development of P2X7 current of increasing amplitude. d I–V relationships for the cell in panel c revealed that both early and developing BzATP-induced currents reversed close to 0 mV. e Whole-cell currents and [Ca2+]i were recorded simultaneously in a rabbit osteoclast. BzATP (100 μM) activated an inward current (bottom) that increased in amplitude with successive stimulations, accompanied by progressive and transient increases in [Ca2+]i (top trace). These data are consistent with an initial, transient activation of P2X4 channels followed by progressive activation of P2X7 channels and P2X7-mediated Ca2+ influx. Modified from [56], with permission of the American Society for Biochemistry and Molecular Biology
Although BzATP is a relatively potent agonist of P2X7, it activates a number of other P2 nucleotide receptors [35], including P2X4 (Fig. 8) [56]. Thus, investigators cannot rely on responses to BzATP alone to establish the involvement of P2X7 receptors. In this regard, functional characteristics of the BzATP-induced current have been examined in osteoclasts isolated from wild-type and P2rx7 −/− mice [25]. BzATP (300 µM) or a high concentration of ATP (1 mM) caused development of slowly deactivating inward current in wild-type, but not P2rx7 −/− osteoclasts, providing strong evidence that this inward current is mediated by P2X7 receptors.
In osteoclasts, voltage-gated Ca2+ channels are not expressed [64] and Ca2+ influx cannot be detected following stimulation of the P2X4 receptor [65]. To examine if the P2X7 receptor mediates Ca2+ influx in osteoclasts, [Ca2+]i changes in response to BzATP were assessed using combined patch-clamp and fluorescence techniques [56]. BzATP induced progressively increasing elevations of [Ca2+]i that were closely associated with the activity-dependent current (Fig. 8e). Moreover, removal of extracellular Ca2+ abolished the rise in [Ca2+]i, indicating that P2X7 receptors mediate influx of Ca2+ across the plasma membrane. A biphasic increase in [Ca2+]i has also been described in rabbit osteoclasts stimulated with BzATP (300 µM) [57]. This response consisted of an initial transient elevation of [Ca2+]i, due to activation of P2Y receptors, followed by progressively larger increases in [Ca2+]i dependent on Ca2+ influx through P2X7 receptors. In a separate study by Jorgensen et al., BzATP was shown to induce acute Ca2+ responses in in-vitro-derived human osteoclast-like cells [30]. These responses were inhibited by oxidized ATP and attributed to activation of P2X7 receptors. However, oxidized ATP is not a specific antagonist of P2X7 receptors [66, 67]. Moreover, in rabbit osteoclasts, the initial transient elevation of [Ca2+]i in response to BzATP is caused by P2Y receptor-mediated release of Ca2+ from intracellular stores [57].
Ca2+ influx through the P2X7 channel could couple to a number of intracellular signaling pathways activated by elevated [Ca2+]i. In this regard, Ca2+ entry mediated by the P2X7 receptor leads to NFAT activation in microglial-like cells [68] and enhances IL-1β secretion from monocyte and macrophage cell lines [69]. However, the role of P2X7-mediated Ca2+ influx in osteoclasts remains to be elucidated.
The conventional isoforms of protein kinase C (PKC), including PKCα and PKCβ1, are important intracellular signaling molecules, the activation of which is dependent on increases in [Ca2+]i. To determine whether P2X7 receptors activate PKC in osteoclasts, Armstrong et al. employed murine macrophage RAW 264.7 cells, which differentiate into multinucleated osteoclast-like cells in the presence of RANK ligand. Stimulation of differentiated RAW 264.7 cells with BzATP-induced transient translocation of PKCα to the plasma membrane [60]. In contrast, UTP and low concentrations of ATP, which activate P2 receptors other than P2X7, failed to elicit this response. Moreover, BzATP failed to induce PKC translocation in osteoclasts derived from the bone marrow of P2rx7 −/− mice, demonstrating specificity for P2X7. Translocation was temporally correlated with BzATP-induced elevations in [Ca2+]i and dependent on the presence of extracellular Ca2+, thereby implicating P2X7-mediated Ca2+ influx in PKC activation [60]. A recent study identified signaling through PKC as being essential for enhanced osteoclast survival in response to extracellular acidification [70]. However, the functions of PKC downstream of the P2X7 receptor remain to be identified. Overall, given the multitude of proteins and transcription factors regulated by Ca2+, the role for Ca2+ influx in P2X7 signaling in osteoclasts should prove to be a fruitful area for future research.
NF-κB is a transcription factor essential for osteoclast development [71, 72]. In resting cells, inactive NF-κB resides in the cytoplasm and upon activation translocates to the nucleus where it regulates transcription of a variety of genes involved in cell proliferation, apoptosis, and inflammation [73, 74]. Thus, NF-κB activation can be assessed indirectly by examining its subcellular localization [75]. Korcok et al. studied the effect of BzATP on NF-κB in osteoclasts isolated from wild-type and P2rx7 −/− mice [57]. Treatment of wild-type osteoclasts with BzATP, but not vehicle, increased nuclear localization of NF-κB (Fig. 9a,b). Importantly, P2rx7 −/− osteoclasts treated with BzATP did not show activation of NF-κB (Fig. 9c,e). NF-κB activation in wild-type osteoclasts was transient and reached a maximum 30 min following exposure to BzATP (Fig. 9d). Together, these data established that activation of the P2X7 receptor causes NF-κB translocation in osteoclasts. However, these studies employed authentic osteoclasts isolated from the marrow of rabbit and murine long bones. In addition to osteoclasts, these cultures contain marrow stromal cells and cells of the osteoblast lineage, which also express functional P2 receptors. Thus, extracellular nucleotides may act on osteoblasts to induce expression of RANK ligand [53], which could bind its receptor RANK on osteoclasts to activate NF-κB. To examine if BzATP activates NF-κB in osteoclasts indirectly by increasing RANK ligand expression, isolated cells were treated with the decoy receptor for RANK ligand, OPG. OPG did not inhibit the activation of NF-κB induced by BzATP [57], indicating that the P2X7 receptor-mediated activation of NF-κB is independent of RANK ligand. It remains to be determined whether P2X7 receptors in osteoclasts couple to activation of transcription factors in addition to NF-κB. For example, it is possible that P2X7-induced elevation of [Ca2+]i leads to activation of NFAT, as described in microglial cells [68].
BzATP signals through P2X7 receptors to induce nuclear translocation of NF-κB in murine osteoclasts. Osteoclasts isolated from wild-type (WT) and P2rx7 −/− mice were treated with BzATP (300 μM) or vehicle for 0–4 h. a–c The p65 subunit of NF-κB was visualized by immunofluorescence (green, left). All nuclei were stained with TOTO-3 (red, middle), with superimposed images to the right. a BzATP-treated wild-type osteoclasts showed nuclear localization of NF-κB at 30 min. b and c In contrast, vehicle-treated wild-type and BzATP-treated P2rx7 −/− osteoclasts showed cytoplasmic localization of NF-κB. d Wild-type osteoclasts treated with BzATP exhibited a significant increase in nuclear translocation of NF-κB at 30 min compared with time 0 (*p < 0.05). e In contrast, P2rx7 −/− osteoclasts did not show a significant change in NF-κB translocation at any time point after BzATP treatment. Data in d and e are the percentage of osteoclasts with nuclear localization of NF-κB. Reproduced from [57] with permission of the American Society for Bone and Mineral Research
Physiological function of P2X7 receptors in osteoclasts
In other cells of hematopoietic origin, the P2X7 receptor plays a role in a variety of processes such as giant-cell formation, posttranslational processing of IL-1, and cell death [50, 51, 76, 77]. The finding that the P2X7 receptor promotes giant-cell formation by macrophages [78] led to the proposal that it also plays a role in the fusion of osteoclast precursors, leading to formation of mature, multinucleated osteoclasts. In this regard, Gartland et al. reported that formation of osteoclast-like cells from human peripheral blood monocytes was inhibited by oxidized ATP or a blocking antibody directed against the P2X7 receptor [54]. Moreover, RAW 264.7 cells lacking functional P2X7 receptors failed to form multinucleated osteoclast-like cells in response to RANK ligand [59]. However, critical insights into the role of the P2X7 receptor in osteoclast formation have come from examination of P2rx7 −/− mice. In this regard, both P2rx7 −/− mouse models possess multinucleated osteoclasts, providing compelling evidence that the P2X7 receptor is not essential for either the fusion of osteoclast precursors or osteoclast differentiation [25, 43].
An important role for the P2X7 receptor in bone remodeling has been inferred from the phenotype of the P2rx7 −/− mouse [25]. In addition to the effects observed on osteoblast function and periosteal bone formation described above, P2rx7 −/− mice exhibited decreased trabecular bone volume accompanied by increased numbers of osteoclasts on the trabecular bone surface [25]. In this regard, P2X7 receptor signaling has been implicated in the regulation of resorption. There is a preliminary report that activation of P2X7 nucleotide receptors by BzATP inhibited bone resorption by human osteoclastoma-derived cells in vitro through initiation of apoptosis [52]. However, a later study from the same group found that P2X7 receptor antagonism led to decreased bone resorption and enhanced apoptosis of in vitro derived human osteoclasts [54]. These conflicting results led the authors to suggest that the timing and degree of receptor stimulation dictates the outcome of P2X7 activation in osteoclasts.
Jorgensen et al. have suggested that P2X7 receptors on osteoclasts play a role in mechanically induced intercellular signaling between osteoblasts and osteoclasts, and among osteoclasts [30]. It was proposed that mechanical stimulation of a cell causes ATP release, which leads to [Ca2+]i elevation in neighboring cells. Lack of desensitization and sensitivity of the response to oxidized ATP led the authors to suggest that this response is mediated by P2X7 receptors on osteoclasts. However, it is difficult to rule out the possible involvement of other calcium-mobilizing P2 receptor subtypes present in these cells [22].
Given the phenotype of the P2rx7 −/− mice [25], Korcok et al. proposed a model in which osteoclast survival is regulated by the P2X7 receptor [62]. Using authentic osteoclasts, it was found that the P2X7 receptor can regulate osteoclast survival by induction of acute cytolysis or apoptosis [62]. Osteoclast cytolysis results from activation of the receptor by high concentrations of exogenous nucleotides in the presence of low concentrations of extracellular Ca2+ and Mg2+. However, it is unlikely that these conditions occur in vivo. Thus, regulation of osteoclast survival was examined under more physiological conditions. Under such conditions, wild-type osteoclasts were more susceptible to apoptosis and showed lower survival rates than did osteoclasts isolated from P2rx7 −/− mice. A more recent study provided further evidence for a role of the P2X7 receptor in mediating apoptosis of osteoclasts [79] (see below).
It is interesting that stimulation of the P2X7 receptor can lead to diverse effects such as activation of NF-κB, an anti-apoptotic signaling factor, as well as apoptosis. However, it should be noted that these effects are achieved at different levels of P2X7 receptor stimulation. The presence of P2X7 receptors enhances osteoclast apoptosis even in the absence of exogenous nucleotides—a condition under which NF-κB is not activated. In contrast, NF-κB is activated by exogenous BzATP, with half-maximal effects observed at ~100 µM [57]. Thus, extracellular nucleotides can act through P2X7 receptors on osteoclasts to activate multiple signaling pathways and induce apoptosis, consistent with the increase in osteoclast numbers observed in P2rx7 −/− mice [25].
Genetic polymorphisms of the human P2X7 receptor
P2X7 is among the most polymorphic of P2 receptors [80]. Polymorphisms that have been identified within the coding region of the human P2X7 receptor have a wide range of outcomes, such as gain-of-function [81], loss-of-function [82, 83], impairment of cytokine release [84, 85], and altered cell death [86]. The relatively common Glu496Ala polymorphism results in reduced pore-forming ability [82] without altering channel function [87], whereas the Ile568Asn polymorphism prevents normal channel trafficking and function [88]. In this regard, Ohlendorff et al. found that Glu496Ala and Ile568Asn single-nucleotide polymorphisms in the human P2X7 receptor are associated with 10-year fracture risk in postmenopausal women [79]. Moreover, the Glu496Ala polymorphism resulted in decreased susceptibility to ATP-induced death of osteoclast-like cells derived from peripheral blood monocytes. Increased skeletal fragility in patients with loss-of-function polymorphisms in the P2X7 receptor is consistent with decreased susceptibility of osteoclasts to apoptosis [79]. As well, impaired osteoblast differentiation and bone formation may contribute to increased fracture risk. It is worth noting that the skeletal changes observed in postmenopausal women with loss-of-function polymorphisms of the P2X7 receptor [79] correspond to the phenotypic changes in the P2rx7 −/− mouse described by Ke et al. [25].
Prospects and conclusions
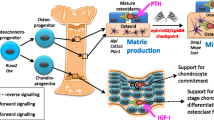
Nucleotides released into the extracellular environment by a variety of stimuli act through multiple P2 receptors on osteoblasts and osteoclasts to regulate the activity and interactions of these cells. Recent evidence reviewed here has provided insight into the importance of P2X7 receptor signaling in osteoblast and osteoclast function. The P2rx7 −/− mouse described by Ke et al. exhibits decreased periosteal bone formation, increased trabecular bone resorption [25], and impaired response to mechanical stimulation [26]. Moreover, P2X7 receptor activation in osteoblasts enhances differentiation and bone formation [33], whereas its activation in osteoclasts results in apoptosis [52, 62, 79] (Fig. 10). These remarkable differences in the effects of P2X7 receptor signaling in osteoblasts and osteoclasts may reflect a sophisticated mechanism through which the skeleton responds to mechanical stimulation by simultaneously increasing bone formation and suppressing its resorption. In this regard, the P2X7 receptor might be an ideal target for the development of drugs with combined anabolic and anti-resorptive actions for use in treatment of osteoporosis and the prevention of bone loss in microgravity.
Proposed role for P2X7 receptors in mechanotransduction in bone. Nucleotides are released from cells of the osteoblast lineage in response to mechanical stimulation. Once in the extracellular environment, ATP may act in an autocrine or paracrine manner to modulate bone remodeling. In this regard, P2X7 receptor signaling stimulates bone formation by osteoblasts, whereas its activation in osteoclasts induces apoptosis. Thus, the P2X7 receptor may be part of a sophisticated mechanism through which bone mass is regulated by mechanical stimulation
In vitro analyses have shown that P2X7 expression is restricted to an as-yet-unidentified subpopulation of osteoblastic cells. Osteoblast-enriched cultures encompass a number of cells at various stages of differentiation, a fact that might explain such heterogeneous patterns of expression. Additional studies are needed to examine whether expression of P2X7 is regulated and how such regulation might contribute to bone cell function. In this regard, exposure to certain stimuli has been found to enhance P2X7 expression in various cell types [89–91]. On the other hand, high concentrations of ATP appear to mediate internalization of P2X7 in osteoclast-like cells [59]. Modulation of P2X7 receptor expression could play an important role in regulating the responses of bone cells to extracellular ATP.
It is well-established that mechanical stimulation of various cell types including osteoblasts induces release of ATP [92, 93]. Moreover, osteoblast-like cells constitutively release nucleotides into the extracellular environment [94]. Since intracellular concentrations of ATP are ~5 mM, it is conceivable that ATP released into a confined extracellular compartment within the bone would produce levels sufficient to activate P2X7 receptors on osteoblasts and osteoclasts, consistent with the P2rx7 −/− phenotype described by Ke et al. [25]. In this regard, pores formed in response to activation of P2X7 permit additional ATP release [49]. While the physiological importance of this phenomenon remains unknown, mechanical stimulation of osteoblasts in vitro leads to pore formation under physiological conditions via a mechanism dependent on P2 receptor signaling [26], suggesting a role for pore formation in mediating P2X7 receptor activation in vivo. Alternatively, a second pathway for P2X7 activation has been identified. Seman et al. demonstrated that the P2X7 receptor on T cells can be activated following its ADP ribosylation by a cell surface ADP-ribosyltransferase utilizing NAD as a substrate [95, 96]. It would be of interest to determine whether a similar pathway is involved in activating P2X7 receptors on osteoblasts and osteoclasts.
The P2X7 receptor has been described classically as unique among other P2X receptors in that it forms only homomultimers within the plasma membrane [97]. However, a recent study has identified structural and functional interactions between P2X4 and P2X7 subunits, providing the first evidence for existence of functional P2X4/P2X7 heteromeric receptors [98, 99]. Since P2X4 is expressed in osteoclasts [28, 100] and in cells of the osteoblast lineage (unpublished observations), future studies should examine the presence and potential function of P2X4/P2X7 heteromers in these cells.
In summary, nucleotides released into the extracellular environment of bone during inflammation and in response to mechanical stimulation could regulate the differentiation and function of osteoblasts and osteoclasts by acting on P2 receptors. Immediately following nucleotide release, bone resorption could be inhibited and formation enhanced via ATP acting on P2X7 receptors to induce osteoclast apoptosis and to promote differentiation of osteoblasts. Since complex interactions likely occur among the pathways activated by different P2 receptors, the role of P2X7 and other nucleotide receptors in the sensing of mechanical stimuli by osteoblasts and osteoclasts will be a fertile ground for future research.
Abbreviations
- BzATP:
-
2′,3′-O-(4-benzoylbenzoyl)-ATP
- [Ca2+]i :
-
cytosolic free Ca2+ concentration
- ERK:
-
extracellular signal-regulated kinase
- LPA:
-
lysophosphatidic acid
- M-CSF:
-
macrophage colony-stimulating factor
- NFAT:
-
nuclear factor of activated T cells
- NF-κB:
-
nuclear factor κB
- OPG:
-
osteoprotegerin
- Osx:
-
Osterix
- PLA2 :
-
phospholipase A2
- PLD:
-
phospholipase D
- PGE2 :
-
prostaglandin E2
- PKC:
-
protein kinase C
- RANK:
-
receptor activator of NF-κB
- Runx2:
-
runt-related transcription factor-2
References
Marks SC Jr, Popoff SN (1988) Bone cell biology: the regulation of development, structure, and function in the skeleton. Am J Anat 183:1–44
Harada S, Rodan GA (2003) Control of osteoblast function and regulation of bone mass. Nature 423:349–355
Novack DV, Teitelbaum SL (2008) The osteoclast: friend or foe? Annu Rev Pathol 3:457–484
Minguell JJ, Erices A, Conget P (2001) Mesenchymal stem cells. Exp Biol Med 226:507–520
Huang W, Yang S, Shao J, Li YP (2007) Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci 12:3068–3092
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764
Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ (1997) Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89:765–771
Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW (2006) Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord 7:1–16
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108:17–29
Robling AG, Castillo AB, Turner CH (2006) Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 8:455–498
Bonewald LF (2006) Mechanosensation and transduction in osteocytes. Bonekey Osteovision 3:7–15
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Teitelbaum SL, Ross FP (2003) Genetic regulation of osteoclast development and function. Nat Rev Genet 4:638–649
Ishida N, Hayashi K, Hoshijima M, Ogawa T, Koga S, Miyatake Y, Kumegawa M, Kimura T, Takeya T (2002) Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J Biol Chem 277:41147–41156
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3:889–901
Dempster DW, Lian JB, Goldring SR (2006) Anatomy and functions of the adult skeleton. In: Favus MJ (ed) Primer on the metabolic bone diseases and disorders of mineral metabolism, 6th edn. American Society for Bone and Mineral Research, Washington, pp 7–11
Dixon SJ, Sims SM (2000) P2 purinergic receptors on osteoblasts and osteoclasts: Potential targets for drug development. Drug Dev Res 49:187–200
Burnstock G (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797
Hoebertz A, Arnett TR, Burnstock G (2003) Regulation of bone resorption and formation by purines and pyrimidines. Trends Pharmacol Sci 24:290–297
Jorgensen NR, Steinberg TH (2007) Purinergic signaling in osteoblasts. In: Burnstock G, Arnett TR (eds) Nucleotides and regulation of bone cell function. CRC, Boca Raton, pp 1–24
Korcok J, Sims SM, Dixon SJ (2007) P2 nucleotide receptor signaling in osteoclasts. In: Burnstock G, Arnett TR (eds) Nucleotides and regulation of bone cell function. CRC, Boca Raton, pp 25–59
Buckley KA, Gartland A, Gallagher JA (2007) The role of purinergic signaling in the interactions between skeletal cells. In: Burnstock G, Arnett TR (eds) Nucleotides and regulation of bone cell function. CRC, Boca Raton, pp 61–74
Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G (1997) Tissue distribution of the P2X7 receptor. Neuropharmacology 36:1277–1283
Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, Grasser WA, Paralkar VM, Li M, Audoly LP, Gabel CA, Jee WS, Dixon SJ, Sims SM, Thompson DD (2003) Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol 17:1356–1367
Li J, Liu D, Ke HZ, Duncan RL, Turner CH (2005) The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem 280:42952–42959
Nakamura E, Uezono Y, Narusawa K, Shibuya I, Oishi Y, Tanaka M, Yanagihara N, Nakamura T, Izumi F (2000) ATP activates DNA synthesis by acting on P2X receptors in human osteoblast-like MG-63 cells. Am J Physiol Cell Physiol 279:C510–C519
Hoebertz A, Townsend-Nicholson A, Glass R, Burnstock G, Arnett TR (2000) Expression of P2 receptors in bone and cultured bone cells. Bone 27:503–510
Gartland A, Hipskind RA, Gallagher JA, Bowler WB (2001) Expression of a P2X7 receptor by a subpopulation of human osteoblasts. J Bone Miner Res 16:846–856
Jorgensen NR, Henriksen Z, Sorensen OH, Eriksen EF, Civitelli R, Steinberg TH (2002) Intercellular calcium signaling occurs between human osteoblasts and osteoclasts and requires activation of osteoclast P2X7 receptors. J Biol Chem 277:7574–7580
Orriss IR, Knight GE, Ranasinghe S, Burnstock G, Arnett TR (2006) Osteoblast responses to nucleotides increase during differentiation. Bone 39:300–309
Panupinthu N, Zhao L, Possmayer F, Ke HZ, Sims SM, Dixon SJ (2007) P2X7 nucleotide receptors mediate blebbing in osteoblasts through a pathway involving lysophosphatidic acid. J Biol Chem 282:3403–3412
Panupinthu N, Rogers JT, Zhao L, Solano-Flores LP, Possmayer F, Sims SM, Dixon SJ (2008) P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: a signaling axis promoting osteogenesis. J Cell Biol 181:859–871
Liu D, Genetos DC, Shao Y, Geist DJ, Li J, Ke HZ, Turner CH, Duncan RL (2008) Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca2+- and ATP-dependent in MC3T3-E1 osteoblasts. Bone 42:644–652
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067
Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082
Turner JT, Weisman GA, Camden JM (1997) Upregulation of P2Y2 nucleotide receptors in rat salivary gland cells during short-term culture. Am J Physiol 273:C1100–C1107
Humphreys BD, Dubyak GR (1996) Induction of the P2z/P2X7 nucleotide receptor and associated phospholipase D activity by lipopolysaccharide and IFN-γ in the human THP-1 monocytic cell line. J Immunol 157:5627–5637
Alzola E, Perez-Etxebarria A, Kabre E, Fogarty DJ, Metioui M, Chaib N, Macarulla JM, Matute C, Dehaye JP, Marino A (1998) Activation by P2X7 agonists of two phospholipases A2 (PLA2) in ductal cells of rat submandibular gland. Coupling of the calcium-independent PLA2 with kallikrein secretion. J Biol Chem 273:30208–30217
Okumura H, Shiba D, Kubo T, Yokoyama T (2008) P2X7 receptor as sensitive flow sensor for ERK activation in osteoblasts. Biochem Biophys Res Commun 372:486–490
Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA (2001) Altered cytokine production in mice lacking P2X7 receptors. J Biol Chem 276:125–132
Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA (2002) Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol 168:6436–6445
Gartland A, Buckley KA, Hipskind RA, Perry MJ, Tobias JH, Buell G, Chessell I, Bowler WB, Gallagher JA (2003) Multinucleated osteoclast formation in vivo and in vitro by P2X7 receptor-deficient mice. Crit Rev Eukaryot Gene Expr 13:243–253
Sim JA, Young MT, Sung HY, North RA, Surprenant A (2004) Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci 24:6307–6314
McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS (2004) Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6:483–495
Ma YF, Li XJ, Jee WS, McOsker J, Liang XG, Setterberg R, Chow SY (1995) Effects of prostaglandin E2 and F 2α on the skeleton of osteopenic ovariectomized rats. Bone 17:549–554
Ehrlich PJ, Lanyon LE (2002) Mechanical strain and bone cell function: a review. Osteoporos Int 13:688–700
Nakano Y, Addison WN, Kaartinen MT (2007) ATP-mediated mineralization of MC3T3-E1 osteoblast cultures. Bone 41:549–561
Pellegatti P, Falzoni S, Pinton P, Rizzuto R, Di Virgilio F (2005) A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol Biol Cell 16:3659–3665
Gabel CA (2007) P2 purinergic receptor modulation of cytokine production. Purinergic Signal 3:27–38
Adinolfi E, Pizzirani C, Idzko M, Panther E, Norgauer J, Di Virgilio F, Ferrari D (2005) P2X7 receptor: Death or life? Purinergic Signal 1:219–227
Gartland A, Ginty AF, Gallagher JA, Bowler WB (1999) Activation of P2X7 receptors expressed by human osteoclastoma modulates bone resorption. Calci Tissue Int 64:S56, abstract
Buckley KA, Hipskind RA, Gartland A, Bowler WB, Gallagher JA (2002) Adenosine triphosphate stimulates human osteoclast activity via upregulation of osteoblast-expressed receptor activator of nuclear factor-κB ligand. Bone 31:582–590
Gartland A, Buckley KA, Bowler WB, Gallagher JA (2003) Blockade of the pore-forming P2X7 receptor inhibits formation of multinucleated human osteoclasts in vitro. Calcif Tissue Int 73:361–369
Penolazzi L, Bianchini E, Lambertini E, Baraldi PG, Romagnoli R, Piva R, Gambari R (2005) N-Arylpiperazine modified analogues of the P2X7 receptor KN-62 antagonist are potent inducers of apoptosis of human primary osteoclasts. J Biomed Sci 12:1013–1020
Naemsch LN, Dixon SJ, Sims SM (2001) Activity-dependent development of P2X7 current and Ca2+ entry in rabbit osteoclasts. J Biol Chem 276:39107–39114
Korcok J, Raimundo LN, Ke HZ, Sims SM, Dixon SJ (2004) Extracellular nucleotides act through P2X7 receptors to activate NF-κB in osteoclasts. J Bone Miner Res 19:642–651
Steinberg TH, Jørgensen NR, Bong JS, Henriksen Z, Atal N, Lin GC, Bennett BD, Eriksen EF, Sørensen OH, Civitelli R (2001) P2-mediated responses in osteoclasts and osteoclast-like cells. Drug Dev Res 53:126–129
Hiken JF, Steinberg TH (2004) ATP downregulates P2X7 and inhibits osteoclast formation in RAW cells. Am J Physiol Cell Physiol 287:C403–C412
Armstrong S, Pereverzev A, Dixon SJ, Sims SM (2009) Activation of P2X7 receptors causes isoform-specific translocation of protein kinase C in osteoclasts. J Cell Sci 122:136–144
Modderman WE, Weidema AF, Vrijheid-Lammers T, Wassenaar AM, Nijweide PJ (1994) Permeabilization of cells of hemopoietic origin by extracellular ATP4−: elimination of osteoclasts, macrophages, and their precursors from isolated bone cell populations and fetal bone rudiments. Calcif Tissue Int 55:141–150
Korcok J, Sims SM, Dixon SJ (2004) P2X7 nucleotide receptors act through two distinct mechanisms to regulate osteoclast survival. J Bone Miner Res 19(Suppl. 1):S418–S419, abstract
Surprenant A, Rassendren F, Kawashima E, North RA, Buell G (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272:735–738
Sims SM, Kelly ME, Dixon SJ (1991) K+ and Cl− currents in freshly isolated rat osteoclasts. Pflügers Arch 419:358–370
Weidema AF, Dixon SJ, Sims SM (2001) Activation of P2Y but not P2X4 nucleotide receptors causes elevation of [Ca2+]i in mammalian osteoclasts. Am J Physiol Cell Physiol 280:C1531–C1539
Beigi RD, Kertesy SB, Aquilina G, Dubyak GR (2003) Oxidized ATP (oATP) attenuates proinflammatory signaling via P2 receptor-independent mechanisms. Br J Pharmacol 140:507–519
Di Virgilio F (2003) Novel data point to a broader mechanism of action of oxidized ATP: the P2X7 receptor is not the only target. Br J Pharmacol 140:441–443
Ferrari D, Stroh C, Schulze-Osthoff K (1999) P2X7/P2Z purinoreceptor-mediated activation of transcription factor NFAT in microglial cells. J Biol Chem 274:13205–13210
Gudipaty L, Munetz J, Verhoef PA, Dubyak GR (2003) Essential role for Ca2+ in regulation of IL-1β secretion by P2X7 nucleotide receptor in monocytes, macrophages, and HEK-293 cells. Am J Physiol Cell Physiol 285:C286–C299
Pereverzev A, Komarova SV, Korcok J, Armstrong S, Tremblay GB, Dixon SJ, Sims SM (2008) Extracellular acidification enhances osteoclast survival through an NFAT-independent, protein kinase C-dependent pathway. Bone 42:150–161
Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U (1997) Requirement for NF-κB in osteoclast and B-cell development. Genes Dev 11:3482–3496
Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R (1997) Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med 3:1285–1289
Li Q, Verma IM (2002) NF-κB regulation in the immune system. Nat Rev Immunol 2:725–734
Hayden MS, Ghosh S (2004) Signaling to NF-κB. Genes Dev 18:2195–2224
Armstrong S, Korcok J, Sims SM, Dixon SJ (2007) Activation of transcription factors by extracellular nucleotides in immune and related cell types. Purinergic Signal 3:59–69
Lemaire I, Falzoni S, Leduc N, Zhang B, Pellegatti P, Adinolfi E, Chiozzi P, Di Virgilio F (2006) Involvement of the purinergic P2X7 receptor in the formation of multinucleated giant cells. J Immunol 177:7257–7265
Steinberg TH, Hiken JF (2007) P2 receptors in macrophage fusion and osteoclast formation. Purinergic Signal 3:53–57
Chiozzi P, Sanz JM, Ferrari D, Falzoni S, Aleotti A, Buell GN, Collo G, Di Virgilio F (1997) Spontaneous cell fusion in macrophage cultures expressing high levels of the P2Z/P2X7 receptor. J Cell Biol 138:697–706
Ohlendorff SD, Tofteng CL, Jensen JE, Petersen S, Civitelli R, Fenger M, Abrahamsen B, Hermann AP, Eiken P, Jorgensen NR (2007) Single nucleotide polymorphisms in the P2X7 gene are associated to fracture risk and to effect of estrogen treatment. Pharmacogenet Genomics 17:555–567
Di Virgilio F, Wiley JS (2002) The P2X7 receptor of CLL lymphocytes—a molecule with a split personality. Lancet 360:1898–1899
Cabrini G, Falzoni S, Forchap SL, Pellegatti P, Balboni A, Agostini P, Cuneo A, Castoldi G, Baricordi OR, Di Virgilio F (2005) A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J Immunol 175:82–89
Gu BJ, Zhang W, Worthington RA, Sluyter R, Dao-Ung P, Petrou S, Barden JA, Wiley JS (2001) A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem 276:11135–11142
Dao-Ung LP, Gu BJ, Sluyter R, Shemon AN, Li C, Taper J, Gallo J, Manoharan A (2002) A loss-of-function polymorphic mutation in the cytolytic P2X7 receptor gene and chronic lymphocytic leukaemia: a molecular study. Lancet 359:1114–1119
Sluyter R, Dalitz JG, Wiley JS (2004) P2X7 receptor polymorphism impairs extracellular adenosine 5′-triphosphate-induced interleukin-18 release from human monocytes. Genes Immun 5:588–591
Sluyter R, Shemon AN, Wiley JS (2004) Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1β release from human monocytes. J Immunol 172:3399–3405
Le Stunff H, Auger R, Kanellopoulos J, Raymond MN (2004) The Pro-451 to Leu polymorphism within the C-terminal tail of P2X7 receptor impairs cell death but not phospholipase D activation in murine thymocytes. J Biol Chem 279:16918–16926
Boldt W, Klapperstuck M, Buttner C, Sadtler S, Schmalzing G, Markwardt F (2003) Glu496Ala polymorphism of human P2X7 receptor does not affect its electrophysiological phenotype. Am J Physiol Cell Physiol 284:C749–C756
Wiley JS, Dao-Ung LP, Li C, Shemon AN, Gu BJ, Smart ML, Fuller SJ, Barden JA, Petrou S, Sluyter R (2003) An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem 278:17108–17113
Gudipaty L, Humphreys BD, Buell G, Dubyak GR (2001) Regulation of P2X7 nucleotide receptor function in human monocytes by extracellular ions and receptor density. Am J Physiol Cell Physiol 280:C943–C953
Humphreys BD, Dubyak GR (1998) Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol 64:265–273
Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF (2005) The cytokine IL-1β transiently enhances P2X7 receptor expression and function in human astrocytes. Glia 49:245–258
Lazarowski ER, Boucher RC, Harden TK (2003) Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64:785–795
Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL (2005) Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res 20:41–49
Buckley KA, Golding SL, Rice JM, Dillon JP, Gallagher JA (2003) Release and interconversion of P2 receptor agonists by human osteoblast-like cells. FASEB J 17:1401–1410
Seman M, Adriouch S, Scheuplein F, Krebs C, Freese D, Glowacki G, Deterre P, Haag F, Koch-Nolte F (2003) NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity 19:571–582
Adriouch S, Bannas P, Schwarz N, Fliegert R, Guse AH, Seman M, Haag F, Koch-Nolte F (2008) ADP-ribosylation at R125 gates the P2X7 ion channel by presenting a covalent ligand to its nucleotide binding site. FASEB J 22:861–869
Torres GE, Egan TM, Voigt MM (1999) Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J Biol Chem 274:6653–6659
Dubyak GR (2007) Go it alone no more—P2X7 joins the society of heteromeric ATP-gated receptor channels. Mol Pharmacol 72:1402–1405
Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD (2007) Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol 72:1447–1456
Naemsch LN, Weidema AF, Sims SM, Underhill TM, Dixon SJ (1999) P2X4 purinoceptors mediate an ATP-activated, non-selective cation current in rabbit osteoclasts. J Cell Sci 112:4425–4435
Acknowledgments
We thank investigators in this rapidly advancing field for permission to reproduce illustrative material from their previously published work. We thank Frank Beier, Graeme Hunter, Dale Laird and Danielle Lapierre for constructive comments on the manuscript. Studies from the authors’ laboratories that were reviewed in this chapter were supported by the Canadian Institutes of Health Research (CIHR). M. Grol is supported by a CIHR Canada Graduate Scholarship and J. Korcok by the CIHR Network for Oral Research Training and Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grol, M.W., Panupinthu, N., Korcok, J. et al. Expression, signaling, and function of P2X7 receptors in bone. Purinergic Signalling 5, 205–221 (2009). https://doi.org/10.1007/s11302-009-9139-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-009-9139-1