Abstract
Genetic variation and relationships among 37 cultivars of Ziziphus mauritiana (Lamk.) native of India were analyzed using start codon targeted (SCoT), inter-simple sequence repeats (ISSR), and ribosomal DNA (rDNA) markers. High level of polymorphism among SCoT (61.6%) and ISSR (61%) primers with higher PIC values ranging from 63.1 to 90.4% of SCoT and 47.3 to 88.8% of ISSR primers was recorded. SCoT and ISSR dendrograms revealed similarity coefficients ranging from 0.80 to 0.92 and 0.79 to 0.96, respectively, and clearly delineated all the cultivars of Z. mauritiana into well-supported distinct clusters. Greater Gst signifies higher amount of differentiation observed over multiple loci among seven Z. mauritiana populations. On the other hand, higher gene flow demonstrating a very high migration rate between Z. mauritiana populations indicated higher rates of transfer of alleles or genes from one population to another. The genetic diversity of population 1 (Rajasthan) was the richest among all the seven populations. The largest genetic distance was measured between Maharashtra and West Bengal and the least between Rajasthan and Punjab cultivars. Most of the genetic diversity exists within population rather than among populations. Substantial variation in the ITS-1 region signifies its phylogenetic utility specifically in assessing genetic diversity in Z. mauritiana. The clustering patterns using three molecular marker systems vis-à-vis place of origin exhibited no consistency in grouping of Z. mauritiana cultivars as cultivars from the same place of origin were genetically cataloged into different SCoT, ISSR, and ITS phylogram clusters indicating wide genetic diversity and distribution across agro-climatic zones validating the robustness of marker systems tested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indian jujube (Ziziphus mauritiana Lamk.), commonly known as Ber, is mainly distributed in the tropic zone of southern Asia, Australia, and Africa including Taiwan and China (Pasternak et al. 2009; Qu and Wang 1993). Due to a high degree of climatic adaptability, it is an important multipurpose fruit tree of arid and semi-arid regions and plays a major role in preventing soil erosion and desertification (Pareek 2001). Z. mauritiana is tolerant to drought, flooding, salinity, and withering (Grice 1997; Hooda et al. 1990). It is well known for its nutritional and medicinal benefits. The fruits are rich in vitamins and are used as sedative, flavonoids, anticancer, tonic, and wound healer and against asthma (Ashraf et al. 2015; Hudina et al. 2008; Mishra et al. 2011; Nyanga et al. 2013). The extract from fruits (Okala et al. 2014–2015), leaves (Dahiru and Obidoa 2007), and seeds (Bhatia and Mishra 2009) have been reported to exhibit antioxidant activity. Most recently, Sadeghi (2015) demonstrated antibacterial activity and synthesis of gold nanoparticles from extracts of Z. mauritiana.
The lack of breakthrough has been due to underutilization of genetic variability for superior quality and high yield potential. The species demonstrates a rich genetic diversity mainly through natural cross-pollination due to self-incompatibility (Godara 1980). The elite plant types with desirable traits have been released as varieties, mass multiplied, and propagated through standard vegetative multiplication. However, the authenticity of cultivar identification in Z. mauritiana is unclear (Devanshi et al. 2007).
Different types of molecular markers based on DNA polymorphism have been used for the assessment of genetic diversity in the plant species. Recently, start codon targeted (SCoT) polymorphism has emerged as a new and promising marker technique for the genetic diversity assessment in plants (Collard and Mackill 2009) due to longer primer sequences and reproducibility. The SCoT marker is designed based on the conserved region surrounding the translation initiation codon, ATG (Sawant et al. 1999). It is used as a single primer for amplification of genomic DNA without prior genomic sequence information. Due to lesser recombination intensity between SCoT marker and the gene of interest, it can be used directly in marker-assisted breeding programs unlike random markers (Mulpuri et al. 2013). SCoT markers have been successfully used to assess genetic diversity and structure and identify cultivars and for quantitative trait loci (QTL) mapping and DNA fingerprinting in different plant species including orange (Jiang et al. 2011), date palm (Al-Qurainy et al. 2015), Pistacia species (Amirbakhtiar and Sorkheh 2015), mango (Leo et al. 2011), and jojoba (Heikrujam et al. 2015).
Inter-simple sequence repeats (ISSR) are inter-tandem repeats of short DNA sequences and are present within the microsatellite repeats. They exhibit specificity of sequence-tagged site markers showing the variation within unique regions of the genome at several loci. ISSR markers are an inexpensive and readily adaptable technique for routine germplasm fingerprinting and evaluation of the genetic relationship between accessions or genotypes (Agarwal et al. 2008) and the construction of genetic linkage maps (Sahu et al. 2015). The technique does not require sequence information for primer synthesis having the advantage of random markers. The ISSR markers have great potential to detect genetic polymorphism, determine intra- and inter-genomic diversity compared to other arbitrary primers, and simultaneously reveal variation within unique regions of the genome. The primers used in ISSR analysis are based on di-, tri-, tetra-, or penta-nucleotide motifs found at microsatellite loci and give a wide array of possible amplification products (Al-Turki and Basahi 2015). The technique is useful in examining genetic diversity, phylogenetic studies, gene tagging, genome mapping, and evolutionary biology studies in a wide range of crop species (Varshney et al. 2013). ISSR is a technique of choice for testing of genetic diversity in plant species for crop improvement (Dikshit et al. 2007; Pakseresht et al. 2013). Recently, its utility has been demonstrated in the genetic diversity analysis of Eucalyptus (Ballesta et al. 2015), chickpea (Bhagyawant et al. 2015), wild berry (Zoratti et al. 2015), Z. mauritiana (Singh et al. 2007), and rice (Al-Turki and Basahi 2015).
The nuclear ribosomal RNA (rRNA) complex is a tandem repeat unit having one to several thousand copies of genes that evolve at varying rates and have different phylogenetic utilities (Singh et al. 2014). The nuclear ribosomal internal transcribed spacer (ITS) evolves relatively faster and has been successfully used to determine inter-species (Chen et al. 2001; Hsiao et al. 1994; Singh et al. 2005) and intra-species relationships (Kakani et al. 2011; Raturi et al. 2012; Singh et al. 2013). The polymorphism in the nuclear rDNA region is usually observed at different levels, i.e., genus, species, or individual, and make it an efficient tool for the assessment of phylogenetic evolution and bio-geographical diversity (Andrew and Kohn 2009; Carvalho et al. 2009). The phylogenetic utility of ITS polymorphism has been successfully exploited in several crops (Kakani et al. 2011; Pathak et al. 2010) and tree species (Xiao et al. 2010) including Z. mauritiana (Singh et al. 2014).
The characterization, evaluation, and improvement of Z. mauritiana germplasm are primarily based on morpho-physiological traits. Earlier researchers have used morphological descriptors, i.e., growth habit, leaf, shoot, flower, and fruit morphology, to classify and discriminate different varieties of Z. mauritiana (Gupta et al. 2003; Saran et al. 2006; Vashishtha 2001). These morphological descriptors are quite homogenous and often found insufficient to distinguish varieties within morphological groups. Consequently, the utility of morpho-physiological traits used to separate cultivars within Z. mauritiana as well as the phylogenetic relationships among cultivars are difficult to assess. Further, the true elements of hybridism cannot be validated during the long juvenile phase of Ziziphus, due to the absence of fruiting stages on the basis of morphological descriptors. Molecular marker technologies circumvent this problem and hasten the breeding process by detecting the coinheritance of characters of both the parents at very early juvenile stage.
The choice of suitable cultivars is of paramount importance for its success. There is an urgent need for improvement and establishment of Z. mauritiana under different agro-ecological environments. The objective of this study was to estimate genetic diversity and relationships among native Indian Jujube using SCoT, ISSR, and nuclear rDNA polymorphisms for crop improvement.
Methodology
Thirty-seven cultivars of Indian jujube representing seven Indian states, namely, Rajasthan, Maharashtra, Punjab, Haryana, Gujarat, Uttar Pradesh, and West Bengal, are being maintained in randomized block design in a horticulture block under field GenBank program at the Central Arid Zone Research Institute (CAZRI), Jodhpur, Rajasthan, India (Table 1). Fresh leaves of these Z. mauritiana cultivars were collected in the month of February 2016 for genomic DNA extraction.
DNA extraction
Total genomic DNA was extracted using the modified CTAB method (Doyle and Doyle 1990). The yield and purity of the extracted DNA was determined electrophoretically on 0.8% agarose gel and spectophotometrically at 260 and 280 nm wavelength using BioPhotometer (D30, Eppendorf, Germany).
SCoT and ISSR analysis
A set of 36 SCoT markers (Integrated DNA Technologies (IDT) designed by Collard and Mackill (2009) were screened, and 17 primers producing unambiguous and scorable fragments were selected for assessment of genetic diversity among 37 varieties of Z. mauritiana. Similarly, a set of 22 ISSR primers were initially tested and finally ISSR analysis was performed with 14 primers. Each PCR amplification was performed in a total reaction volume of 25 μl containing primer, 50 pmol; dNTP mix, 0.25 mM, Taq DNA polymerase, 1 U (Sigma Chemicals); 10× PCR buffer, 5.0 mM (Tris–HCl, pH 8.3, 15 mM MgCl2 250 mM KCl); and genomic DNA, 50 ng in dH2O. Amplicons were separated on 2% agarose gel pre-stained with ethidium bromide solution using 1× Tris–acetate–EDTA (TAE) buffer. The gels were run for 2 h at 110 V. The size of the amplified fragments was determined using 100 bp plus ladder (MBI Fermentas). All the reactions were performed twice to test the reproducibility of the amplicon profiles generated by both the marker systems.
SCoT–PCR amplification was performed in a gradient thermal cycler (Corbett Research, USA) with an initial denaturation step of 95 °C for 5 min followed by 40 amplification cycles of 95 °C for 45 s, annealing at 51 °C for 45 s and 72 °C for 1 min and 30 s, and final elongation at 72 °C for 10 min. On the other hand, the reaction mixture and PCR amplification conditions of ISSR were the same as that of SCoT amplification except the annealing temperature which was kept at 42 °C for ISSR.
Molecular analysis of SCoT and ISSR markers
Molecular data of SCoT and ISSR profiles were entered into a binomial matrix and were used to determine Jaccard’s similarity coefficient with NTSYSpc software (Rohlf 1997; Sneath and Sokal 1973). To perform molecular analysis, the 37 varieties of Z. mauritiana were divided into seven populations based on the state from which a variety was originally developed, viz., Pop 1 Rajasthan, Pop 2 Maharashtra, Pop 3 Punjab, Pop 4 Haryana, Pop 5 Gujarat, Pop 6 Uttar Pradesh, and Pop 7 West Bengal (Table 1). Analysis of molecular variance (AMOVA) and principal coordinate analysis via covariance matrix was calculated using GenAlEx 6 software (Peakall and Smouse 2006). On the other hand, diversity in the frequency of fragment size of SCoT and ISSR patterns was apportioned within and among Z. mauritiana varieties using Shannon’s information index (i) (Lewontin 1972) and gene diversity index (h) following Nei (1973) using PopGen 32 program. Mantel statistic was used to compare the dissimilarity matrices as well as the dendrograms produced by the SCoT and ISSR techniques using NTSYS software.
ITS amplification
The genomic DNA of all the 37 varieties of Z. mauritiana was used for amplification of the 5.8S gene region. The universal primers ITS-1 (19 bp) and ITS-4 (20 bp) developed by White et al. (1990) were used to amplify the internal transcribed spacer (ITS) region of ribosomal DNA, which encompasses the 5.8S gene and both ITS-1 and ITS-2 regions. PCR amplification was performed in a total volume of 50 μl containing 1 U Taq DNA polymerase (Sigma Chemicals), 2.5 mM MgCl2, 160 mM dNTP mix (MBI, Fermentas), 50 pmol of each ITS-1 and ITS-4 primers, and 200 ng genomic DNA in dH2O. The PCR reactions were performed with 1 min. Denaturation was performed at 95 °C, 30 s annealing at 50 °C, and 1 min and 20 s elongation at 72 °C, for 36 cycles with a final elongation step of 72 °C for 7 min. The PCR products were run on 1.6% agarose gel pre-stained with ethidium bromide in 1× TAE buffer at 60 V for 100 min.
ITS sequence analysis
The PCR product of the ITS-amplified region containing ITS-1, 5.8S rDNA, and ITS-2 were directly sequenced using ITS-1 (forward) and ITS-4 (reverse) primers by the BigDye terminator method in an ABI PRISM DNA sequencer. Nucleotide sequence comparisons were performed by using the Basic Local Alignment Search Tool (BLAST) network services against the National Centre for Biotechnology Information (NCBI) databases. The molecular identification of 37 Z. mauritiana cultivars was done based on similarity with the best-aligned GenBank sequences. The complete rDNA sequences of ITS1 and ITS2 encompassing 5.8S of all the 37 varieties of Z. mauritiana have been assigned with GenBank accession numbers by the NCBI database, USA. The multiple sequence alignment of all the 37 cultivars using Clustal X 2.0.11 generated a phylogram depicting bootstrap values using the NJ plot software (Perrière and Gouy 1996). Based on single nucleotide polymorphisms (SNPs), insertions/deletions (INDELS), ITS length diversity, and/or 5.8S nuclear rDNA regions, phylogenetic relationships of Z. mauritiana cultivars were established.
Results
SCoT analysis
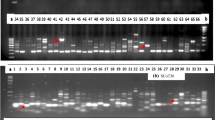
Out of 36 SCoT primers initially screened, 17 primers were finally used for assessment of genetic diversity based on reproducibility of scorable bands. Among the cultivars, 17 SCoT primers amplified a total of 125 clear and bright DNA fragments and their sizes ranged between 275 and 2500 bp. The number of amplified fragments varied from 1 (SCoT-23) to 12 (SCoT-13 and SCoT-31) with an average of 7.35 fragments per primer (Table 2). Of 125 amplified DNA fragments, 77 (61.6%) were found to be polymorphic. The detected polymorphism per primer among the tested cultivars ranged from 0 (SCoT-23) to 85.71% (SCoT-17). Except SCoT-23, all other primers exhibited higher polymorphic information content (PIC) ranging from 63.1 (SCoT-28) to 90.4% (SCoT-31). The profile generated by SCoT-13 is shown in Fig. 1 a, b.
All the 125 scored DNA fragments were used to calculate genetic similarity among cultivars of Z. mauritiana. A genetic similarity coefficient between pairs of accessions was obtained from the marker data based on similarity matrix coefficients. A dendrogram was constructed from cumulative cluster analysis of 17 SCoT primer scorable fragments which exhibited similarity of approximately 0.80 to 0.92 and clearly delineated all the test cultivars in to five well-supported distinct clusters and one outgroup (Fig. 2). Cluster I included five cultivars (BC-1, Seb, Vikas, S × K Hybrid, Sanaur-5). Cluster II contained 11 cultivars (Umran, Dandan, Jogia, Illaichi, Mundia, Gola, Banaras-Pebandi, CAZRI-Gola, Aliganj, Katha, Z-G-3). Cluster III had 10 cultivars (Tikadi, Kaithli, Babu, Banaras-Karaka, Narikeli, Thornless, Thar-Sevika, Sua, Wilayati, Chhuhara). Cluster IV grouped six cultivars (Kali, Ponda, Gola-Gurgaon, Popular-Gola, Laddu, Akrota). Cluster V comprised four (Bagwadi, Thar-Bhubraj, Maharwali, and Chonchal) cultivars whereas Reshmi did not cluster with other cultivars and was recorded as the most distinct outgroup. The highest genetic similarity value (0.923) was observed between Thar-Sevika and Sua.
ISSR analysis
Based on amplification of well-recorded, unambiguous, and reproducible DNA fragments, out of 22 ISSR primers tested, 14 were used for further analysis. Among the cultivars, 14 ISSR primers yielded a total of 100 clear and bright DNA fragments and their sizes ranged between 275 and 3000 bp. The number of amplified fragments varied from 2 (ISSR-42) to 10 (ISSR-12, ISSR-30, and ISSR-39) with an average of 7.14 amplicons per primer (Table 2). Of 100 fragments, 61 (61.0%) were polymorphic. The detected polymorphism per primer among the tested cultivars ranged from 33.3 (ISSR-40) to 100% (ISSR-3 and ISSR-50). All the primers exhibited higher PIC ranging from 47.3% (ISSR-42) to 88.8% (ISSR-5, ISSR-30). The profile generated by ISSR-50 exhibiting 100% polymorphism is shown in Fig. 3a, b.
All the 100 scored amplicons were used to calculate genetic similarity among the test cultivars of Z. mauritiana. A genetic similarity coefficient between pairs of accessions was obtained from the marker data based on similarity matrix coefficients. A dendrogram was constructed from cumulative cluster analysis of scorable fragments generated by 14 ISSR primers which exhibited a similarity coefficient of approximately 0.79 to 0.96 and clearly delineated all the 37 cultivars of Z. mauritiana in to six well-supported distinct clusters and one outgroup (Fig. 4). Cluster I included six cultivars (BC-1, Seb, Umran, Illaichi, Gola, CAZRI-Gola). Cluster II contained 11 cultivars (Banaras-Karaka, Z-G-3, Aliganj, Katha, Thornless, Ponda, Thar-Bhubraj, Mundia, Bagwadi, Maharwali, Banaras-Pebandi,). Cluster III had six cultivars (Gola-Gurgaon, Popular-Gola, Laddu, Jogia, Dandan, Thar-Sevika). Cluster IV grouped eight cultivars (Reshmi, S × K Hybrid, Chhuhara, Chonchal, Kali, Kaithli, Narikeli, Sua). Cluster V comprised three cultivars (Akrota, Vikas, Babu). Cluster VI separated two cultivars Tikadi and Sanaur-5 from others. The cultivar Wilayati did not cluster with any other cultivar and was recorded as the most distinct outgroup. The highest genetic similarity value (0.923) was recorded between Jogia and Dandan.
Correlation between SCoT and ISSR
The cophenetic coefficient was acceptable in both the molecular marker systems indicating good fit for clustering. The values of mantel test correlation showed a positive correlation between the SCoT and ISSR. The correlation coefficient (r) was 0.24 between SCoT and ISSR (significant P > 0.05) with an approximate t value of 3.57.
Genetic analysis of SCoT markers
Principal coordinate analysis (PCA) of all the seven Z. mauritiana populations revealed contribution of the first three eigen factors at 21.74, 20.26, and 16.36%, respectively, explaining a total of 58.36% variability (Fig. 5). The allocation pattern of seven populations across all the principal coordinates indicates their wide distribution except population 5 which had only one cultivar. The mean values of all the seven populations together for Nei’s gene diversity (h) was 0.2365, and the Shannon information index (i) was 0.3456 (Table 3). Results revealed that the genetic diversity of population 1 was the richest. To further explicate the gene differentiation among populations, Nei’s unbiased measure of genetic distance ranged from 0.0083 to 0.1812 and the genetic identity ranged from 0.8342 to 0.9931 (Table 4). The maximum genetic distance was recorded between populations 2 and 7 and the least between populations 1 and 3. The distance matrix for all the seven populations permitted a partitioning of the overall variations into two levels: among populations and within populations. The results showed that most of the genetic variations existed within populations. The proportion of variations attributed within populations was 99%, and the remaining variations (1%) occurred among populations (Table 5).
All the seven populations of Z. mauritiana representing different geographical areas showed total genetic diversity (HT) of 0.2295, genetic diversity within each population (Hs) was 0.1451, the coefficient of gene differentiation (Gst) was 0.3680, and the level of gene flow (Nm) was 0.8586 (Table 6).
Genetic analysis of ISSR markers
Seven Z. mauritiana populations were subjected to PCA (Fig. 6). The eigen factor analysis indicated that the contributions of the first three factors were 30.26, 20.90, and 17.55%, respectively, explaining a total of 68.71% variability. The distribution pattern of seven populations across principal coordinates indicates their wide distribution except population 5 which had only one cultivar. A summary of mean genetic variation statistics of all the seven populations along with the mean of all loci is presented in Table 3. The mean values of all the seven populations together for Nei’s gene diversity (h) was 0.2209, and the Shannon information index (i) was 0.3280. Results showed that the genetic diversity of Z. mauritiana cultivars of population 1 was the richest among all the seven populations. Nei’s unbiased measure of genetic distance was employed to further elucidate the gene differentiation among populations (Table 4). Nei’s genetic distance ranged from 0.0173 to 0.2189. The largest genetic distance occurred between populations 2 and 7 and the least between populations 1 and 3. The AMOVA of the distance matrix for all the seven populations permitted a partitioning of the overall variations into two levels: among populations and within populations. The results showed that most of the genetic variations existed within populations. The proportion of variations attributed within populations was 95%, and the remainder of variations (5%) occurred among populations. As a result, obvious genetic differentiation existed within the Z. mauritiana population (Table 5).
Genetic structure estimates for the seven populations of Z. mauritiana representing different geographical areas are presented in Table 6. The total genetic diversity (HT) was 0.2065, genetic diversity within each population (Hs) was 0.1293, the coefficient of gene differentiation (Gst) was 0.3741, and the level of gene flow (Nm) was 0.8365.
ITS amplification and sequencing
Upon PCR with universal primers ITS-1 and ITS-4, all the 37 cultivars of Z. mauritiana generated a single prominent amplified product (approx. 625 bp) of the 5.8S gene region. The amplified product included partial sequence of the 18S gene; complete sequence of ITS-1, 5.8S gene, and ITS-2; and partial sequence of the 28S gene. All the 37 novel gene sequences exhibiting nucleotide variations have been submitted to the NCBI database and assigned with GenBank accession numbers (Table 7). The conserved 5.8S rDNA portion was recorded with a uniform nucleotide length of 164 bp in all the 37 cultivars. The ITS-2 region was 179 bp long in all the test cultivars except Kaithli (180 bp). Substantial variation was recorded in the ITS-1 region ranging from 255 bp (Tikadi) to 263 bp (Gola-Gurgaon). The total length of the complete 5.8S gene region also varied from 598 to 603 bp.
In the multiple sequence alignment of nucleotide sequences of all the 37 cultivars together, in addition to total length polymorphisms, we detected single nucleotide polymorphisms (SNPs) and insertion/deletions (INDELS) at several positions in ITS-1, 5.8S rRNA gene, and ITS-2 region. The multiple sequence alignment of all the varieties of Z. mauritiana generated a phylogram using Clustal X 2.0.22 and NJ plot (Fig. 7). The perusal of the phylogram further delineated Z. mauritiana varieties with each other with Illaichi as quite distinct outgroups.
Discussion
Indian jujube germplasm characterization, evaluation, and improvement are fundamentally based on morpho-physiological traits (Gupta et al. 2003; Pareek 2001; Saran et al. 2006; Vashishtha 2001). A high level of morphological variability exists, and similar genotypes are known by different names in different regions of cultivation; it is possibly due to the influence of environment on morphology (Singh et al. 2007), and therefore, morphological appearances are not reliable measures of diversity assessment among Z. mauritiana genotypes. Such morphological descriptors are quite homogeneous and often insufficient to distinguish genotypes within morphological groups. The lack of breakthrough has been due to underutilization of genetic variability, superior quality, and high yield potential.
The present study revealed that a high polymorphism could be detected among Z. mauritiana cultivars using SCoT and ISSR markers. High level of polymorphism among SCoT (61.6%) and ISSR (61%) primers with higher PIC values ranging from 63.1 to 90.4% of SCoT and 47.3 to 88.8% of ISSR primers are indicative of sufficient genetic diversity among 37 cultivars of Z. mauritiana. Nevertheless, no single SCoT or ISSR primer could distinguish all Ber cultivars independently. Perusal of the similarity coefficient presented in SCoT and ISSR dendrograms revealed similar values of similarity coefficients ranging from 0.80 to 0.92 and 0.79 to 0.96, respectively, and clearly delineated all the 37 cultivars of Z. mauritiana into well-supported distinct clusters which not only reveal appreciable genetic variation and relationships among the test cultivars but also validate the robustness of both the marker systems used. Previous studies on genetic diversity on Indian jujube polymorphism demonstrated similar ranges of genetic similarities using RAPD (Devanshi et al. 2007; Sehrawat et al. 2006; Singh et al. 2014), AFLP (Singh et al. 2006), and ISSR (Singh et al. 2007). Contrary to this, Obeed et al. (2008) using ISSR revealed 60 to 76% genetic similarity among five Ber cultivars from Pakistan. Fu et al. (2007) used ISSR and RAPD primer combinations for identification and classification of ber cultivars and reported a very narrow genetic background among Taiwan accessions. Liu et al. (2006) studied the genetic diversity in Ziziphus jujuba using RAPD and reported that the percentage of polymorphic loci in Zanhuangdazao population was lower (58%) than that in other Chinese jujube population (66%) and wild jujube population (89%).
It is evident from well-defined clusters of both the dendrograms that SCoT markers delineated Z. mauritiana cultivars better than ISSR markers with higher phylogenetic distances. In both the dendrograms, the grouping patterns of cultivars are more or less similar. For instance, grouping of BC-1 and Seb; Umran, Illaichi, Gola, Aliganj, and CAZRI-Gola; Banaras-Karaka and Thornless; Popular-Gola, Laddu, and Gola-Gurgaon; and Bagwadi, Thar-Bhubraj, and Maharwali in the same cluster in both the dendrograms validate reliable grouping and robustness of both the marker systems. The values of mantel test correlation showed a positive correlation between the SCoT and ISSR markers. A moderate correlation coefficient (r) of 0.24 is due to minor variations in the clustering patterns but eventually resulted in about 80% similarities in both the dendrograms indicating good fit for clustering. Pakseresht et al. (2013) performed comparative assessment of ISSR, DAMD, and SCoT markers for evaluation of genetic diversity and conservation of landrace chickpea genotypes and reported that the SCOT and DAMD markers are more effective in fingerprinting of chickpea genotypes. The cophenetic coefficient between the similarity values measured using ISSR and SCoT marker systems was acceptable indicating good fit for clustering.
Significantly higher contributions of the first three factors explaining a total of 58.36 and 68.71 variability using SCoT and ISSR marker systems resulting in wide distribution of Z. mauritiana cultivars across principal coordinates validate robustness and applicability of both the marker systems in analyzing the genetic diversity in Indian jujube germplasm. Further, mean genetic variation statistics of all the seven populations together revealed similar and significantly higher values of Nei’s gene diversity 0.2365 and 0.2209 together with Shannon information index of 0.3456 and 0.3280 in SCoT and ISSR marker systems, respectively. Higher Gst values signify higher amount of differentiation observed over multiple loci among Z. mauritiana populations. On the other hand, higher gene flow demonstrating a very high migration rate between Z. mauritiana populations indicates higher rates of transfer of alleles or genes from one population to another. Migration into or out of a population may be responsible for a marked change in allele frequencies which may result in the addition of new genetic variants to the established gene pool or its population (Whitlock and McCauley 1999).
Nei’s unbiased measure of genetic distance analyses of both the marker systems revealed that the genetic diversity of Z. mauritiana varieties of population 1 (Rajasthan) was the richest among all the seven populations. The largest genetic distance occurred between populations 2 (Maharashtra) and 7 (West Bengal) which was due to the largest variable climate and the least between populations 1 (Rajasthan) and 3 (Punjab) which is attributed to a similar genetic background. The AMOVA of the distance matrix for all the seven populations revealed higher genetic variations existed within populations, i.e., 99 and 95%, and among populations (1 and 5%) in SCoT and ISSR molecular analyses, respectively, demonstrating that most of the genetic diversity exists within population rather than among Indian jujube populations. Earlier, researchers have also reported greater genetic variations within populations of various plant species (Raturi et al. 2012; Kakani et al. 2011) including Z. mauritiana (Singh et al. 2014).
The appearance of a single prominent amplified product of the 5.8S gene region of approximately 625 bp and the uniform nucleotide length of 164 bp of 5.8S rDNA all the 37 varieties of Z. mauritiana demonstrates the conserved nature of rDNA region. Substantial variation in the ITS-1 region ranging from 255 to 263 bp signifies phylogenetic utility specifically in assessing genetic diversity and relationships of the Z. mauritiana germplasm. Upon BLAST search, all 37 of Z. mauritiana cultivars exhibited 98 to 99% identities with the best aligned GenBank reference sequences. The perusal of the phylogram revealed further delineated Z. mauritiana varieties into eight main clusters with Illaichi as quite distinct outgroups. A high degree of nucleotide sequence variation exhibited substantial intra-specific genetic diversity due to variations in the nucleotide sequences by way of SNPs, INDELS, and ITS length polymorphism with significant boot strap values and allowed separation of all the 37 varieties of Z. mauritiana in the present study. Bootstrap values are dependable measures of phylogenetic accuracy, and higher values are likely to indicate reliable group (Hillis and Bull 1993).
The perusal of clustering patterns using three molecular marker systems vis-à-vis place of origin exhibited no consistency in grouping of Z. mauritiana cultivars as cultivars from the same place of origin were genetically cataloged into different SCoT, ISSR, and ITS phylogram clusters. This indicates that Z. mauritiana has wide genetic diversity and is widely distributed across agro-climatic zones. For instance, studies on other plant species Trigonella foenum-graecum (Kakani et al. 2011), Vigna radiata (Raturi et al. 2012), Phaseolus vulgaris (Martins et al. 2006) have also been reported with low correlation between geographic distribution and genetic distances measured using molecular markers. Such clustering of genotypes representing different states ignored the influence of geographical variations within the genetic diversity of 37 Z. mauritiana cultivars tested. Such a lack of correlation between geographic and genetic diversity in Ziziphus species have also been reported by Devanshi et al. (2007) and Ma et al. (2011).
The genetic variation is of utmost need for designing further breeding strategies in this fruit crop. The present study has revealed a broad genetic base in Ziziphus species and suggests the taxonomist to review and resolve the mis-nomination of Ber genotypes using molecular markers. This study would help in the consideration of molecular fingerprinting for germplasm conservation, rectification, purification, and identification of genotypes especially using SCoT, ISSR informative markers, and rDNA polymorphism.
References
Agarwal M, Shrivastava N, Padh H (2008) Advances in molecular marker techniques and their applications in plant sciences. Plant Cell Rep 27(4):617–631
Al-Qurainy F, Khan S, Nadeem M, Tarroum M (2015) SCoT marker for the assessment of genetic diversity in saudi arabian date palm cultivars. Pak J Bot 47(2):637–643
Al-Turki TA, Basahi MA (2015) Assessment of ISSR based molecular genetic diversity of Hassawi rice in Saudi Arabia. Saudi J Biol Sci 22:591–599
Amirbakhtiar N, Sorkheh K (2015) Analysis of diversity and relationships among wild Pistacia species using Start Codon Targeted (SCoT) markers 1ST International and 9th International Congress of Islamic Republic of Iran, May 24–26, 2015, Shahid Beheshti University, Tehran, Iran pp 1–4
Andrew M, Kohn LM (2009) Single nucleotide polymorphism based diagnostic system for crop-associated Sclerotinia species. Appl Environ Microbiol 75:5600–5606
Ashraf A, Sarfraz RA, Anwar F, Shaukat Ali Shahid SA, Alkharfy KM (2015) Chemical composition and biological activities of leaves of Ziziphus mauritiana l. native to Pakistan. Pak J Bot 47(1):367–376
Ballesta P, Mora F, Contreras-Soto RI, Ruiz E, Perret S (2015) Analysis of the genetic diversity of Eucalyptus cladocalyx (sugar gum) using ISSR markers. Acta Scient 37:133–140
Bhagyawant SS, Gupta N, Gautam A, Chaturvedi SK, Shrivastava N (2015) Molecular diversity assessment in chickpea through RAPD and ISSR markers. World J Agric Res 3(6):192–197
Bhatia A, Mishra T (2009) Free radical scavenging and antioxidant potential of Ziziphus mauritiana (Lamk.) seed extract. J. Complement Integra Med 8:42–46
Carvalho A, Guedes-Pinto H, Lima BJ (2009) Genetic diversity among old Portuguese bread wheat cultivars and botanical varieties evaluated by ITS rDNA PCR-RFLP markers. J Genetics 88(3):363–367
Chen YC, Eisner JD, Kattar MM, Barrett SLR, Lafe K, Bui U, Limaye AP, Cookson BT (2001) Polymorphic internal transcribed spacer region 1 DNA sequences identify medically important yeasts. J Clin Microbiol 39(11):4042–4051
Collard BCY, Mackill DJ (2009) Start codon targeted (SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol Biol Rep 27(1):86–93
Dahiru D, Obidoa O (2007) Pretreatment of albino rats with aqueous leaf extract of Ziziphus mauritiana protects against alcohol induced liver damage. Trop J Pharma Res 6:705–710
Devanshi, Singh AK, Sharma P, Singh B, Singh R, Singh NK (2007) Molecular profiling and genetic relationship among ber (Ziziphus sp.) genotypes using RAPD markers. Indian J Genet 67(2):121–127
Dikshit HK, Jhang T, Singh NK, Koundal KR, Bansal KC, Chandra N, Tickoo JL, Sharma TR (2007) Genetic differentiation. Plantarum 3:451–457
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12(1):13
Fu JX, Liu CM, Xie JH (2007) Identification and classification of ber cultivars based on ISSR and RAPD analysis. Acta Hort 764:119–126
Godara NR (1980) Studies on floral biology and compatibility behavior in ber (Ziziphus mauritiana Lamk.) Ph.D. thesis, Haryana Agricultural University, Hisar, India.
Grice AC (1997) Post-fire regrowth and survival of the invasive tropical shrubs Cryptostegia grandiflora and Ziziphus mauritiana Australian. J Ecol 22(1):49–55
Gupta RB, Sharma S, Sharma JR, Panwar RD (2003) Study on vegetative characters of some cultivated and wild forms of ber (Ziziphus spp.). Haryana J Hort Sci 32(1/2):15–18
Heikrujam M, Kumar J, Agrawal V (2015) Genetic diversity analysis among male and female Jojoba genotypes employing gene targetedmolecular markers, start codon targeted (SCoT) polymorphism and CAAT box-derived polymorphism (CBDP) markers. Meta Gene 5:90–97
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analyses. Syst Biol 42:182–192
Hooda PS, Sindhu SS, Mehta PK, Ahlawat VP (1990) Growth, yield and quality of ber (Zizyphus mauritiana Lamk.) as affected by soil salinity. J Hort Sci Biotechnol 65(5):589–593
Hsiao C, Chatterton NJ, Asay KH, Jensen KB (1994) Phylogenetic relationships of 10 grass species: an assessment of phylogenetic utility of the internal transcribed spacer region in nuclear ribosomal DNA in monocots. Genome 37:112–120
Hudina M, Liu M, Veberic R, Stampar F, Colaric M (2008) Phenolic compounds in the fruit of different varieties of Chinese jujube (Ziziphus jujuba Mill.). J Hort Sci Biotechnol 83:305–308
Jiang QQ, Long GY, Li WW, Deng ZN (2011) Identification of genetic variation in Citrus sinensis from Hunan based on start codon targeted polymorphism. Agric Sci Technol 12(11):1594–1599
Kakani RK, Singh SK, Pancholy A, Meena RS, Pathak R, Raturi A (2011) Assessment of genetic diversity in Trigonella foenum-graecum based on nuclear ribosomal DNA, Internal Transcribed Spacer and RAPD analysis. Plant Mol Biol Rep 29:315–323
Leo C, He XH, Chen H, SJ O, Gao MP, Brown JS, Tondo CT, Schnell RJ (2011) Genetic diversity of mango cultivars estimated using SCoT and ISSR markers. Biochem Syst Ecol 39:676–684
Lewontin RC (1972) The apportionment of human diversity. Evol Biol 6:391–398
Liu P, Peng JY, Peng SQ, Ji Z (2006) Genetic diversity evaluation of Ziziphus jujuba cv. Zanhuangdazao. J Fruit Sci 23(5):685–689
Ma QH, Wang GX, Liang LS (2011) Development and characterization of SSR markers in Chinese jujube (Ziziphus jujuba Mill.) and its related species. Sci Hort 129:597–602
Martins SR, Vences FJ, Saenz de Miera LE, Barrosa MR, Carnide V (2006) RAPD analysis of genetic diversity among and within Portuguese landraces of common white bean (Phaseolus vulgaris L.). Sci Hort 108:133–142
Mishra T, Khullar M, Bhatia A (2011) Anticancer potential of aqueous ethanol seed extract of Ziziphus mauritiana against cancer cell lines and ehrlich ascites carcinoma. Evidence based Complementary and Alternative Medicine Vol Article ID:765029 . doi:10.1155/2011/76502911 pages
Mulpuri S, Muddanuru T, Francis G (2013) Start codon targeted (SCoT) polymorphism in toxic and non-toxic accessions of Jatropha curcas L. and development of a codominant SCAR marker. Plant Sci 207:117–127
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Aca Sci USA 70:3321–3323
Nyanga LK, Gadaga TH, Nout MJR, Smid EJ, Boekhout T, Zwietering MH (2013) Nutritive value of masau (Ziziphus mauritiana) fruits from Zambezi Valley in Zimbabwe. Food Chem 138(1):168–172
Obeed RS, Harhash MM, Abdel-Mawgood AL (2008) Fruit properties and genetic diversity of five ber (Ziziphus mauritiana Lamk.) cultivars. Pak J Biol Sci 11(6):888–893
Okala A, Ladan MJ, Wasagu RSU, Shehu K (2014) Phytochemical studies and in vitro antioxidant properties of Ziziphus mauritiana fruit extract. Intl J Pharma Phytochem Res 6(4):885–888
Pakseresht F, Talebi R, Karami E (2013) Comparative assessment of ISSR, DAMD and SCoT markers for evaluation of genetic diversity and conservation of landrace Chickpea (Cicer arietinum L.) genotypes collected from North-West of Iran. Physiol Mol Biol Plants 19(4):563–574
Pareek OP (2001) Fruits for the future 2: Ber, International Centre for Underutilized Crop. Redwood Books, Wiltshire, p. 290
Pasternak D, Senbeto D, Nikiema A, Kumar S, Fatondji D, Woltering L, Ratnadass A, Ndjeunga J (2009) Bioreclamation of degraded African lands with women empowerment. Chronica Horti 49(2):24–27
Pathak R, Singh SK, Singh M, Henry A (2010) Molecular assessment of genetic diversity in clusterbean (Cyamopsis tetragonoloba) genotypes. J Genet 89(2):243–246
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population software for teaching and research. Mol Ecol Notes 6:288–295
Perrière G, Gouy M (1996) WWW-Query: an on-line retrieval system for biological sequence banks. Biochimie 78:364–369
Qu ZZ, Wang YH (1993) Chinese fruit trees record-Chinese jujube. China forestry publishing house Beijing:33–37
Raturi A, Singh SK, Sharma V, Pathak R (2012) Molecular characterization of Vigna radiata (L.) Wilczek genotypes based on nuclear ribosomal DNA and RAPD polymorphism. Mol Biol Rep 39(3):2455–2465
Rohlf FJ (1997) NTSYS pc: numerical taxonomy and multivariate analysis system Version 2.02h. Exeter software, New York
Sadeghi B (2015) Zizyphus mauritiana extract-mediated green and rapid synthesis of gold nanoparticles and its antibacterial activity. J Nanostruct Chem 5:265–273
Sahu AR, Mishra RR, Rath SC, Panigrah J (2015) Construction of interspecific genetic linkage map of pigeon pea using SCoT, RAPD, ISSR markers and simple inherited trait loci. Nucleus 58(1):23–31
Saran PL, Godara AK, Sehrawat SK (2006) Characterization of ber (Ziziphus mauritiana Lamk.) genotypes. Haryana J Hort Sci 35(3/4):215–218
Sawant SV, Singh PK, Gupta SK, Madnala R, Tuli R (1999) Conserved nucleotide sequences in highly expressed genes in plants. J Genet 78:123–131
Sehrawat SK, Boora KS, Dahiya DS, Ahlawat VP, Goyal RK (2006) DNA finger printing of ber (Ziziphus mauritiana Lamk) germplasm. Haryana J Hort Sci 35(3/4):213–214
Singh SK, Tiwari M, Kamal S, Yadav MC (2005) Morel phylogeny and diagnostics based on restriction fragment length polymorphism analysis of ITS region of 5.8S ribosomal DNA. J Biochem Biotech 14:179–183
Singh AK, Sharma RK, Singh NK, Bansal KC, Koundal KR, Mohapatra T (2006) Genetic diversity in ber (Ziziphus sp.) revealed by AFLP markers. J Hort Sci Biotechnol 81:205–210
Singh AK, Devanshi SP, Singh R, Singh B, Koundal KR, Singh NK (2007) Assessment of genetic diversity in Ziziphus mauritiana using inter-simple sequence repeat markers. J Plant Biochem Biotechnol 16(1):35–40
Singh SK, Meghwal PR, Pathak R, Gautam R, Kumar S (2013) Genetic diversity in Punica granatum revealed by nuclear rRNA, internal transcribed spacer and RAPD polymorphism. Natl Acad Sci Letters 36(2):115–124
Singh SK, Meghwal PR, Pathak R, Bhatt RK, Gautam R (2014) Assessment of genetic diversity among Indian jujube varieties based on nuclear ribosomal DNA and RAPD polymorphism. Agric Res 3(3):218–228
Sneath PHA, Sokal RR (1973) Numerical taxonomy. W.H. Freeman & Co., San Francisco, California.
Varshney RK, Song C, Saxena RK, Azam S, Yu S, Sharpe AG, Cannon S, Baek J, Rosen BD, Tar’an B (2013) Draft genome sequence of chickpea (Cicer arietinum) provides a resource trait improvement. Nat Biotechnol 31(3):240–246
Vashishtha BB (2001) Ber varieties: a monograph. Agrobios (India), Agro House, Behind Nasrani Cinema, Chopasani Road, Jodhpur, Rajasthan, India. p 97
White TJ, Bruns SL, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innes MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols, a guide to methods and applications. Academic Press, San Diego, pp 315–322
Whitlock MC, McCauley DE (1999) Indirect measures of gene flow and migration: FST≠1/(4Nm+1). Heredity 82:117–125
Xiao LQ, Möller M, Zhu H (2010) High nrDNA ITS polymorphism in the ancient extant seed plant Cycas: incomplete concerted evolution and the origin of pseudogenes. Mol Phylogenet Evol 55:168–177
Zoratti L, Palmieri L, Jaakola L, Häggman H (2015) Genetic diversity and population structure of an important wild berry crop. AoB Plants 7:plv117. doi:10.1093/aobpla/plv117
Acknowledgements
Authors are thankful to Protection of Plant Varieties & Farmers’ Rights Authority (PPV&FRA), India, for the financial support to carry out this study. Authors also appreciate the support of Director, Central Arid Zone Research Institute, Jodhpur, for encouragement and for providing necessary research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Data archiving statement
All the 37 nuclear rDNA sequences have been deposited in NCBI, USA, and are available in public domain. Their GenBank accession numbers are KX688495–KX688507, JQ627029–JQ627050, and KC155274–KC155275. The details of the Ziziphus mauritiana cultivars and their sequences are presented in Table 7 of the manuscript.
Additional information
Communicated by W. Ratnam
Rights and permissions
About this article
Cite this article
Singh, S.K., Chhajer, S., Pathak, R. et al. Genetic diversity of Indian jujube cultivars using SCoT, ISSR, and rDNA markers. Tree Genetics & Genomes 13, 12 (2017). https://doi.org/10.1007/s11295-016-1092-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-016-1092-x