Abstract
Genetic variability of trees influences the chemical composition of tissues. This determines herbivore impact and, consequently, herbivore performance. We evaluated the independent effects of plant genotype and provenance on the tannin content of holm oak (Quercus ilex) and their consequences for herbivory and performance of gypsy moth (Lymantria dispar) larvae. Oak seedlings of 48 open-pollinated families from six populations were grown in a common garden in central Spain. Half the plants were subjected to defoliation by gypsy moth larvae and the other half were destructively sampled for chemical analysis. Tannin content of leaves did not differ significantly among populations but differed significantly among families. Estimates of heritability (h 2) and quantitative genetic differentiation among populations for tannin content (Q ST) were 0.83 and 0.12, respectively. Defoliation was not related to the tannin content of plants nor to spine and trichome densities of leaves, although positive family–mean associations were observed between defoliation and both seed weight and plant height (P < 0.003). Among the oak populations, differential increase in larval weight gain with defoliation was observed. Leaf tannin content in Q. ilex is genetically controlled but does not influence defoliation or predict performance of the larvae. Different efficiencies of food utilisation depending on the oak genotypes indicate that other plant traits are influencing the feeding patterns and fitness of L. dispar and consequent population dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quantitative variation in secondary plant metabolites is largely responsible for patterns of herbivore performance and distribution across available hosts (Berenbaum 1995). Numerous studies have evaluated the independent effects of plant species (e.g. Mrdaković et al. 2011; Pearse 2011; Milanović et al. 2014) and environmental factors (e.g. Milanović et al. 2015; Rubert-Nason et al. 2015; Pollastrini et al. 2016) on herbivore damage to trees and performance of insects. The relative importance of plant genetic factors in tree defoliation and insect development has been studied (Osier and Lindroth 2001; Busby et al. 2015; Rubert-Nason et al. 2015) but in some tree systems remains poorly understood. We used an environmentally controlled experiment of holm oak (Quercus ilex L.) and the European gypsy moth (Lymantria dispar L.) to explore the effects of tree populations and families within populations on tannin content, plant damage and herbivore performance.
Tannins are widely occurring plant polyphenols of varying structure and molecular weight. Tannins from various botanical sources have extensive applications in the wine industry and are used worldwide as clarifying agents in alcoholic drinks and as aroma ingredients in alcoholic beverages, soft drinks and juices. Plant tannins and inorganic tanning agents are key compounds for the world’s leather production (Kite and Thomson 2006). In recent years, the effects of plant tannins on human health have gained attention, becoming topical in food science and disease prevention. Plant tannins are divided into two major groups: hydrolysable tannins and condensed tannins (also known as proanthocyanidins), which are both important for various biological processes, such as plant defence against herbivores (Barbehenn and Constabel 2011) and pathogens (Stong et al. 2013). Total tannins was chosen as a trait to study because tannins are among the most abundant secondary metabolites made by plants, commonly ranging from 5 to 10% dry weight of tree leaves (Barbehenn and Constabel 2011).
There are diverse perspectives on tannins in the context of their roles in plant defence to herbivores (Constabel et al. 2014). Tannins are known to bind proteins, and experiments performed by Feeny (1976) led to the widespread idea that tannins can act as general defences against insect pests by reducing the digestibility of proteins. Lepidopterans have a very basic gut pH, and tannins do not bind proteins under these conditions (reviewed in Barbehenn and Constabel 2011). While tannins can have negative effects on insects, according to recent literature (Barbehenn et al. 2009a, b), these appear not to be related to their protein-binding potential. Condensed tannins act as toxins and feeding deterrents causing oxidative damage within the epithelial cells of the midgut of insects (Barbehenn and Constabel 2011). Such effects depend in particular on the interaction between the plant-specific tannin and specific pH conditions in different parts of the digestive tract of the herbivore species (Barbehenn and Constabel 2011; Salminen and Karonen 2011; Büchel et al. 2016). However, in vertebrates, tannins can bind proteins in the acidic environment of the gut, and this reduction of digestibility can have significant impact on animal health and reproduction (Wallis et al. 2012).
Quercus ilex is one of the dominant tree species of Mediterranean forests and the most abundant forest tree species in Spain. The wide ecological amplitude of holm oak and its ecophysiological adaptability to hydric and thermal stress (Gimeno et al. 2009) accord with the high heterozygosity and allelic richness reported in this species (Michaud et al. 1995; Soto et al. 2007; Ortego et al. 2010; Vernesi et al. 2012; Guzmán et al. 2015). Intraspecific variation of morphological traits, reproductive biology and resistance to pathogens has also been reported (Michaud et al. 1992; Díaz et al. 2003; Tapias et al. 2008; Bonal et al. 2012; Galván et al. 2012; Caliskan 2014; Niinemets 2015; Corcobado et al. 2016). According to Baldantoni et al. (2013), chemical constituents of Q. ilex leaves (d.w.) are cellulose (330 mg g−1), lignin (310 mg g−1), starch (17 mg g−1) and soluble sugars (173 mg g−1), and main elements (d.w.) are C (480 mg g−1), N (16 mg g−1), Ca (49 mg g−1), Mg (17 mg g−1) and K (68 mg g−1). Leaves of Q. ilex have a dense layer of stellate, tufted non-glandular trichomes on the abaxial surface (Karioti et al. 2011b). Juvenile leaves in this species also have a spiny margin with very short spines 0.6 to 0.9 mm long. Although Q. ilex is one of the most studied tree species in southern Europe, information about genetic variability of structural (morphological and anatomical) traits and secondary metabolites related to plant defence is lacking.
Across its range, Q. ilex is attacked by more than 100 species of insects, many of which are prone to population outbreaks. One of these is the European gypsy moth, which causes significant but patchy defoliation of Q. ilex (Ibáñez-Justicia et al. 2007). Taking advantage of an environmentally controlled experiment with plant material from six differentiated Q. ilex populations in Spain, in this study, we explored whether there is intraspecific among- and within-population variation of the tannin content of Q. ilex trees, whether herbivory of Q. ilex by L. dispar varies within and among tree populations and whether the performance of L. dispar larvae could be influenced by the genetic structure of populations. Through an ecological genetics approach, we addressed the following questions: (1) are tannins controlled genetically in Q. ilex trees? (2) Can herbivory and herbivore performance be influenced by tree origin? Because structural characteristics of leaves can act as mechanical plant defences against herbivory (Hanley et al. 2007; Pearse 2011), spines and trichomes of leaves in Q. ilex were assessed.
Materials and methods
Genetic material
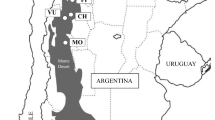
The genetic material studied comprised six Q. ilex ssp. ballota natural populations (Fig. 1), and seedlings grown from field-collected acorns were used. Sampling was performed in November and December 2013 and included trees from well-preserved forests. The sampling procedure comprised selecting randomly 10–12 fruit-bearing trees per population at minimum distances of 70 m apart to minimise the chances of intercrossing. About 100 acorns per tree were hand collected directly from the crown and stored in a cold chamber at 4 °C. In January 2014, the acorns were placed on trays with sterilised sand, moistened close to field capacity with deionised water, covered with transparent plastic film and left to germinate under laboratory conditions.
Distribution of Quercus ilex in Spain (green) and location (red dots) and climographs of the six populations used in this study. Black dots indicate additional populations sampled but with few or no viable acorns (S Somiedo Natural Park, PE Picos de Europa National Park, O Ordesa y Monte Perdido National Park, AT Aigües-Tortes y Lago de San Mauricio National Park, SG Sierra de Guadarrama National Park, SN Sierra Nevada National Park). Within the distribution of Q. ilex, grey lines delimit provenance regions according to Jiménez-Sancho et al. (1996)
Acorns with emerging radicles were individually weighed and planted in 48-cell rigid plastic root trainers (300 mL volume; 18 cm high, 5.3 × 5.3 cm upper surface) containing sand and peat (5:1, pH 5.5). Earlier research showed that this pot size would provide seedlings with unrestricted root growth during the experiment (Corcobado et al. unpublished results). The plants were kept in natural daylight under greenhouse shade that reduced solar radiation by 50% and were hand watered every 3–4 days to field capacity until they were well established.
Insect material
In October 2013, egg masses of L. dispar were collected from Quercus cerris L. trees in a forest (44° 20′ 37″ N, 22° 22′ 22″ E; 231 m a.s.l.) near Jabukovac, about 160 km from Belgrade, Serbia. Insect material was collected at considerable distances from the plant material to prevent herbivory variation among populations being biased by any interaction between the insect and the host (e.g. by coevolutionary relationships). Eggs were processed and hatched, and larvae were grown on an artificial medium (‘Gypsy moth diet’, MP Biomedicals, LLC) as described in Milanović et al. (2014). Freshly moulted third-instar larvae were used to cause defoliation and study the effect of Q. ilex provenance on L. dispar performance.
Experimental design and defoliation treatment
The study was conducted in a greenhouse at the Faculty of Forestry of Plasencia, University of Extremadura, Spain (40° 02′ 06″ N, 6° 04′ 59″ W; 374 m a.s.l.) under natural light at 17 to 26 °C. The plants were arranged following a split-plot design replicated in five blocks, with the two defoliation treatments acting as the main factor (two categories: defoliated and control; whole plots) and the six populations as the split factor (six categories: MO, CA, SN, PO, SM and GR as shown in Table S1 and Fig. 1; split plots). In all five blocks, each population was represented in each whole plot by two individuals from each of the eight open-pollinated families. Individuals were randomly positioned within each block. In total, there were 960 plants corresponding to 5 blocks × 2 defoliation treatments × 6 populations × 8 families × 2 individuals. The experiment therefore included 160 plants per population and 20 plants per family.
Half the seedlings were exposed to defoliation by larvae on 22 May 2014, coinciding with the expected period of attack in the field by outbreaking herbivores such as gypsy moth. On this date, about 1200 third-instar L. dispar larvae were kept under 21 °C in 12-cm-diameter Petri dishes. Two or three larvae per plant were used, depending on plant size. All plants were large enough to preclude larval starvation. The larvae were carefully placed with a soft brush on the upper leaves of plants. To stop the larvae from escaping, transparent rigid plastic tubes (one per plant), 5 cm in diameter, 15 cm high and 1 mm thick, were used (Fig. 2a). The tubes were individually covered with a plastic mosquito net attached by a rubber band (Fig. 2b). The average temperature of the plants covered with the plastic cages did not differ significantly from the average temperature of uncovered plants. Defoliation lasted 4 days.
Placement of individual plastic tubes around Quercus ilex plants before defoliation by Lymantria dispar (a); plant and L. dispar larvae in their L3 instar, before being covered with a mosquito net (b); plant with ca. 5 cm2 defoliation 4 days after exposure to larvae (c); stem heavily damaged but not girdled (d); main stem girdled (e)
Tissue sampling and chemical analyses
On 26 May 2014, leaves and roots from the other half of the plants (control) were collected manually and flash-frozen in liquid nitrogen. Fine roots were easily collected by immersion of the root ball in water then merged with the main root into one sample. Samples were immediately transported to the laboratory, lyophilised and ground into powder in a ball mill (Mixer Mill MM 400, Retsch GmbH, Haan, Germany). The milled samples were stored at −80 °C until analysis.
The concentration of total tannins present in the lyophilised leaves and roots was determined by radial diffusion in agarose gels, as described by Hagerman (1987). This method is based on the observation that tannins form a stable complex with bovine serum albumin (BSA), forming a clearly visible ring whose area is linearly proportional to the tannin level in samples. Petri dishes containing 1% agarose (Sigma-Aldrich) and a solution of 50 mM acetic acid, 60 μM ascorbic acid and 0.1% BSA were prepared. Fifty milligrams of milled tissue was extracted with 250 μL 50% (v/v) aqueous methanol for 60 min in an ultrasonic bath. The crude extracts were centrifuged at 5000g for 5 min, and the supernatant was collected and stored at −80 °C. Four uniform wells per Petri dish, 4 mm in diameter, had been made in the solidified agarose medium beforehand using a punch. Sixteen microlitres of each extract was inserted into each perforation via a micropipette. A calibration curve for tannic acid (0.1 to 1 mg) and a negative control (16 μL of 50% aqueous methanol) were used. Petri dishes were covered, sealed with parafilm and incubated at 30 °C for 120 h. The Petri dishes were then scanned, and ImageJ 1.44p software (NIH, Bethesda, MD, USA) was used to obtain the area of the rings. Tannin content was expressed as milligrams of tannic acid equivalent per 100 mg lyophilised sample.
Defoliation and larval assessment
Four days after plant exposure to larvae (Fig. 2c), plant defoliation was individually assessed by quantifying the defoliated areas of all leaves. Defoliated leaves were placed above a 0.25 × 0.25 cm grid, and their defoliated areas were estimated. The defoliated area per plant was obtained by the sum of defoliating values per leaf. Defoliation was expressed in square centimetres per number of larvae used. Stem damage was assessed as follows: 0 = no damage, 1 = some damage present, 2 = heavily damaged but not girdled (Fig. 2d) and 3 = main stem girdled (Fig. 2e). Caterpillars used to defoliate each plant were weighed together before and after plant exposure. Larval weight gain (growth rate) (mg day−1) was obtained using the following formula (modified from Milanović et al. 2015):
where WG t1 is the total weight of larvae at the beginning of the experiment, WG t2 the total weight of larvae at the end of the experiment, n the number of larvae used and t 2 − t 1 the number of days plants were exposed to defoliation (i.e. 4 days).
Assessment of structural traits of leaves
In 20 control seedlings per population, the spines of the leaf margins were counted. Trichome density was measured in three leaves per seedling using a pre-weighed self-adhesive tape (Karioti et al. 2011b). Trichomes were removed from the abaxial leaf surface. Trichome density was expressed as milligrams per square centimetre and as trichome dry weight per leaf dry weight (%) (Karioti et al. 2011b). Water content (WC) of leaves was expressed in grams per gram and determined as WC = (FW − DW) / DW, where FW is the fresh weight of leaves and DW is the dry weight of leaves after drying in an oven for 48 h at 60 °C. Succulence of leaves was expressed in grams per square centimetre and determined per control plant as (FW − DW) / LA where LA is the total leaf area. Leaf area was measured by the Image-Pro Plus v.4.5 software (Media Cybernetics, Inc., Silver Spring, MD, USA). Softness or specific leaf area (SLA) was calculated as LA / DW and expressed in square centimetres per gram dry weight (Hanley et al. 2007).
Data analysis
To ensure that plant development variation among populations was not biased by the environmental conditions of the experimental site, we compared the germination and growth rates of seedlings with the environmental distances between the experimental site and the conditions of origin of each population (for a similar procedure, see Hernández-Serrano et al. 2014). Climate data were obtained from www.opengis.uab.es/wms/iberia/mms/index.htm and www.climate-data.org (see Supplementary Data Table S1). We first calculated the Gower distance between the climate variables of each population and those of the experimental site, and then correlated the germination and growth rates of each provenance with its environmental (Gower) distance. Correlations were not significant (Pearson r < 0.17, P > 0.21), indicating that the particular environment of the experimental site did not differentially affect development of the seedlings evaluated.
To estimate the variation in tannin content among and within populations of Q. ilex, a general linear mixed (GLM) model was used. The tannin content (expressed in mg TA eq/100 mg) was used as the dependent variable; block was used as a fixed factor; population and mother tree (nested within the population) were used as random factors; and individual seed weight, time to germinate and plant height were used as covariates. The block × mother tree-within-population interaction was not significant (P > 0.05) and therefore was not included in the model. To estimate the variation in defoliation by L. dispar among and within populations, a similar GLM model was applied, this time using defoliated area (expressed in cm2) as the dependent variable, block as a fixed factor and population and mother tree (nested within the population) as random factors. The block × population interaction, and individual seed weight, time to germinate, plant height and initial larval weight covariates, were included in the model. With these models, we estimated variance components, and narrow-sense heritability across populations (h 2) was estimated as the additive genetic variance (V A) divided by the phenotypic variance (V P). To correct estimates of h 2 for a generalised selfing rate of about 1 to 3% in native Q. ilex stands (Ortego et al. 2014), an r coefficient of relatedness of 0.27 rather than 0.25 for true half-sibs was used (because full-sibs share half their genes and half-sibs share one quarter). In the numerator, the additive genetic variance was therefore calculated as 3.704 times the variance component among families (σ 2 f (pop)). Phenotypic variance was deemed to be the sum of σ 2 f (pop), the among-populations variance (σ 2 p) and the error variance (σ 2 e). Pooled within-populations narrow-sense heritability was then estimated as
Approximate standard errors of heritability estimates were obtained following Jayaraman (1999). In accordance with Leinonen et al. (2013), Q ST was calculated as
To check whether different populations and mother trees influence larval performance, the same GLM model was applied, this time with larval weight gain (expressed in mg day−1) as the dependent variable. During the 4 days of larval growth, larval weight gain was assumed to follow a linear function. Initial larval weight was included as a covariate. The residuals of the models were checked for normality and means were compared using the Tukey HSD test.
To estimate the variation of structural traits of leaves among populations, a GLM model was used. Spines per leaf, spines per leaf area, trichome density, trichome dry weight per leaf dry weight, water content, succulence and specific leaf area were used as the dependent variables, population was used as the random factor and individual seed weight, time to germinate and plant height were used as covariates.
Genetic (r g) and phenotypic (r p) Pearson correlations among seed weight, time to germinate, plant height, leaf tannin content, defoliation and larval weight gain variables were obtained using family-mean and individual values, respectively. The Bonferroni correction was used, and significances were divided by the number of statistics involved (α/15). At the population level, relationships between defoliation (and larval weight gain) and structural traits of leaves were examined by means of pairwise Pearson correlations. To assess whether relationships between performance of L. dispar larvae and defoliation vary as a function of populations and mother trees, tests for homogeneity of slopes on covariates were performed. A GLM analysis included larval weight gain (mg day−1) as the dependent variable and the interaction between defoliation (cm2; continuous predictor) and population (categorical predictor) as variables. The same GLM was then performed using mother tree as the categorical predictor. All analyses were performed with STATISTICA v10 (Stat Software Inc., Tulsa, OK, USA).
Results
Tannin content variation
Leaf tannin content did not differ significantly among populations, but it differed significantly among families and covaried positively with plant height (Table 1). In some populations, mean values of tannins were significantly different among their mother trees (e.g. SN and GR) whereas in others not (Fig. 3). Within the SN population, for example, seedlings from tree 1 had 70% higher leaf tannin content than seedlings from tree 2 (P = 0.008; Fig. 3). Tannin content of leaves of 458 individuals ranged from 16.31 to 2.87 mg TAeq/100 mg and showed a mean value of 7.5 mg TAeq/100 mg (SD = 2.1). Leaf tannin content showed significant narrow-sense heritability across populations (ĥ 2) of 0.83 ± 0.23 and genetic differentiation (Q ST) of 0.12 (Table 2).
Tannin content of leaves of Quercus ilex families originating from Monfragüe National Park (MO), Cabañeros National Park (CA), Sierra Norte de Sevilla Natural Park (SN), Pollensa (PO), Sierra de María-Los Vélez Natural Park (SM) and Grazalema Natural Park (GR) populations. Eight families per population were analysed except for CA and SM, in which one family had less than three seedlings available (+). Vertical bars are standard errors and asterisks indicate significant differences of mean values within populations (asterisk P < 0.05) according to the Tukey HSD test
Root tannin content was not significantly variable at either the population or within-population level. Tannin content of roots of 460 individuals ranged from 7.65 to 0.09 mg TAeq/100 mg and showed a mean value of 1.9 mg TAeq/100 mg (SD = 0.9). Because of the lack of significance, ĥ 2 and Q ST of root tannin content are not shown.
Leaf tannin content of trees in the common garden was not related to root tannin content or the climate parameters of the provenance sites of trees. At the population level, despite the failure to detect statistical population differences, root tannin content was significantly related to summer rainfall of sites (r = 0.88; P = 0.018). A positive relation between leaf tannin content and time to germinate (r = 0.92; P = 0.010) was observed, indicating a higher tannin level in provenances germinating later. At the family level, time to germinate was also predictive of leaf tannin content (Table 3, above the diagonal). At the individual level, however, time to germinate was not related to leaf tannin content (Table 3, below the diagonal).
Herbivory variation
Defoliation was strongly dependent on population (P = 0.011) and family (P = 0.012) and was affected by plant height (Table 3). Narrow-sense heritability across populations and Q ST estimates of defoliation were 0.13 ± 0.17 and 0.36, respectively (Table 2). Defoliation was not related to leaf or root tannin content, or stem damage. Positive family-mean relations were observed between defoliation and seed weight and between defoliation and plant height (Table 3).
Herbivore performance variation
Larval weight gain differed significantly depending on which populations (Fig. 4b) and families of Q. ilex seedlings were defoliated. Interestingly, larval weight gain covaried positively with seed weight (P = 0.017). At the population level, larval weight gain was not related to plant tannin content, defoliation or stem damage. Other significant relations at the family and individual levels are shown in Table 3.
Defoliation rates (a) and larval weight gain (b) following herbivory by L. dispar of Q. ilex seedlings originating from Monfragüe National Park (MO), Cabañeros National Park (CA), Sierra Norte de Sevilla Natural Park (SN), Pollensa (PO), Sierra de María-Los Vélez Natural Park (SM) and Grazalema Natural Park (GR) populations. Values were not adjusted for covariation. Vertical bars are standard errors and different letters indicate significant differences of mean values between populations (P < 0.05) according to the Tukey HSD test
Depending on the tree population, differential increase in larval weight gain with defoliation was observed, as indicated by the significant population × defoliation interaction revealed in the homogeneity of slopes test (P = 0.001). Leaves of SM trees, for example, resulted in higher larval weight gain with defoliation than leaves of MO trees (Fig. 5). Differential increase in larval weight gain with defoliation was not dependent on the mother tree (P = 0.577). Differential increase in larval weight gain with seed weight was not dependent on the population, but was dependent on the mother tree (P = 0.236 and P = 0.029, respectively).
Variation of structural traits of leaves among populations
The number of spines per leaf did not differ significantly among populations (Table 4). However, trichome dry weight per leaf dry weight, water content and succulence of leaves varied among populations (Table 4). At the population level, defoliation and larval weight gain were not related to spine and trichome densities but larval weight gain was significantly related to water content of leaves (r = 0.92, P = 0.010).
Discussion
Tannins are genetically controlled in Q. ilex
Estimated within- and among-population genetic variances of leaf tannin content in Q. ilex were in the ratio 78:22%. The presence of substantial additive genetic variation within the species (ĥ 2 = 0.83) enables short-term evolutionary change in response to natural selection and makes future breeding initiatives designed to obtain tannin-rich plant material feasible. Although earlier studies explored the genetic basis of several adaptive traits in oak species (Ramírez-Valiente et al. 2014; Nisar et al. 2016), as far as we know, this study is the first to provide a quantitative estimation of heritability and genetic differentiation for a chemical trait in a Mediterranean oak species. Genetic control of condensed tannins in trees was reported only for Populus sp., with broad-sense heritability values ranging from 0.52 ± 0.42 to 0.90 ± 0.01 (Stevens and Lindroth 2005; Bailey et al. 2006; McKown et al. 2014). Genetic control of time to germinate (since sowing) and plant growth (≈increase of plant height since sowing) in Q. ilex is also reported here for the first time (ĥ 2 = 0.92 ± 0.18 and ĥ 2 = 0.41 ± 0.13, respectively; Table 2). Despite the small standard errors, values of ĥ 2 should be taken with caution given the relatively small number of families and individuals per family used.
Q ST is a standardised measure of genetic differentiation among populations displayed by quantitative traits. Comparative studies of the divergence of quantitative traits and neutral molecular markers, known as Q ST–F ST comparisons, provide a means for researchers to distinguish between natural selection and genetic drift as causes of population differentiation in traits (Leinonen et al. 2013). The use of Q ST–F ST comparisons has increased rapidly in the last few years (Hernández-Serrano et al. 2014; Santiso et al. 2015), enabling a wide range of questions relevant to evolutionary and ecological genetics to be addressed. However, estimates of Q ST and other traits, especially those related to plant defence, are lacking (Solla et al. 2015). The genetic differentiation of tannin content among Q. ilex populations observed (Q ST = 0.12) is a first step in providing some insight into the variation of a secondary plant metabolite throughout the vast heterogeneous area occupied by this oak species in Spain.
Variation of herbivory within and among Q. ilex populations
Herbivory is not distributed uniformly across available hosts among and within tree populations. Comparison of responses to defoliation in conspecific populations with different origins is a common experimental strategy for studying plant resistance to defoliation. The variation in the defoliation observed in seedlings from different mother trees and populations explains the non-uniform defoliation observed in Q. ilex forests when L. dispar outbreaks occur (Ibáñez-Justicia et al. 2007). Environmental factors and genetic differences among caterpillars (Mrdaković et al. 2013) may also explain non-uniform defoliation of Q. ilex forests.
Defoliation, larval weight gain and densities of spines and trichomes did not vary significantly between different populations from the same region of provenance, e.g. among MO, CA and SN (Fig. 4), from the ‘Extremadurense’ region (Fig. 1). However, earlier research in Q. ilex using enzyme polymorphism and studying acorn mass variation reported substantial genetic differentiation among nearby populations in the same region (Michaud et al. 1995; Bonal et al. 2012).
The southern populations of Q. ilex in Spain are currently suffering severe decline, mostly caused by episodes of drought and waterlogging and the presence of invasive Phytophthora species (Corcobado et al. 2014; Martín-García et al. 2015; Jung et al. 2016). Increasing temperatures have led to more abundant outbreaks of L. dispar (Spathelf et al. 2014), and it has recently been observed that L. dispar herbivory and larval growth rates are higher in declining oaks (Milanović et al. 2015). Thus, a positive feedback loop for Q. ilex decline is expected. This is the first study to report a heritability estimate for the level of herbivory in Q. ilex (ĥ 2 = 0.13). The intraspecific variation observed should be taken into account in conservation and breeding initiatives to preserve Q. ilex forests. Moreover, the low tannin content levels in roots of Q. ilex populations with greater exposure to summer drought could be relevant when explaining oak decline syndrome.
Tannin content in Q. ilex is not predictive of defoliation by L. dispar
That defoliation did not reflect tannin content is not surprising; in earlier studies, tannin content either predicted defoliation (Feeny 1976; Mrdaković et al. 2011) or not at all (Osier and Lindroth 2001; Barbehenn et al. 2009a, b; Milanović et al. 2015; Caldwell et al. 2016). Holm oak contains both hydrolysable (Gharzouli et al. 1999) and condensed (Estiarte et al. 2007) tannins, which probably have very different effects on insects. Among the hydrolysable tannins, ellagitannins are especially prone to oxidise in the midguts of several species of caterpillars (Barbehenn et al. 2009a, b; Barbehenn and Constabel 2011), forming reactive oxygen species able to cause lesions in the midgut epithelium. However, when L. dispar fed on Acer saccharum leaves coated with condensed tannins (15% d.w.), growth rates of larvae were unaffected and antioxidant effects were not observed (Barbehenn et al. 2009a). Although some classes of chemicals have been portrayed as having general effects on herbivores (e.g. condensed tannins; see Feeny 1976), other chemicals appear to be effective against specific insects (Johnson et al. 2014) or are most effective in combination with a specific mixture of other secondary metabolites. Moreover, specialist insect herbivores often evolve counter-adaptations to overcome or even benefit from specialised chemical defences (Johnson et al. 2014). Thus, correlations between herbivore susceptibility and genetic variation in the amount of a specific chemical can range anywhere from negative to positive and could therefore be non-significant, as observed here. Our results emphasise the need to measure tannin type and composition in leaves, rather than simply total tannins. Spines, trichomes, prickles and thorns on leaves and along stems can serve as defences against insects (Hanley et al. 2007; Karioti et al. 2011b; Pearse 2011). In Q. ilex, the number of spines and trichomes per leaf seems to have no effect on L. dispar attack. In consequence, other secondary metabolites (e.g. terpenoids, glucosinolates; Karioti et al. 2011a) may individually or synergistically influence L. dispar feeding patterns.
Performance of L. dispar larvae is influenced by the genetic structure and early-life traits of Q. ilex
Although the role of insects as a selective pressure shaping plant phenotypes has frequently been reported (e.g. Züst and Agrawal 2016), little is known about the genetic basis of hosts and early-life traits of plants influencing herbivore performance. As far as we know, variability of herbivory and insect performance has not been assessed in defoliated Q. ilex hosts. This lack of information limits our understanding of defoliation outbreaks as an evolutionary process influencing herbivores and trees. Severe defoliation of oaks by L. dispar results in tree mortality (Morin and Liebhold 2016), acorn loss during the outbreak (unpublished results) and lower acorn production the following year (Liebhold et al. 2000).
Larval performance was positively related to defoliation and significantly conditioned by the mother tree and the tree population. That mother trees had a large effect on larval weight gain is not surprising, as differences among Populus genotypes were found to have similar effects in other studies (Osier et al. 2000 and references therein). In defoliated Populus tremuloides, performance of L. dispar larvae was strongly influenced by plant genotype and much less so by defoliation (Osier and Lindroth 2001). There is little information on maternal effects in oaks, but it is widely known that these effects generally appear at early ontogenetic stages, affecting early-life traits such as seed mass, germination rate, seedling growth and resistance to pathogens (Díaz et al. 2003; Solla et al. 2011; Vivas et al. 2013, 2014; Corcobado et al. 2016). Larvae feeding on some tree populations were much less efficient in converting food to body mass than larvae feeding on other populations (Fig. 5). Moreover, larvae were more efficient at converting ingested food to biomass when foliage originated from heavy acorns and freshly formed foliage, probably because leaves contained higher concentrations of compounds (e.g. nitrogen, water) favourable for caterpillar growth.
Conclusions
Three main findings can be derived from the results of this study. Firstly, constitutive leaf tannin content is a heritable trait in Q. ilex. Variability of constitutive tannins among and within Q. ilex populations may be an inherent source of stress-tolerant ecotypes in the face of current global change conditions. Secondly, tannin content in leaves and roots, and spine and trichome densities in leaves of Q. ilex are not predictive of L. dispar defoliation or larval performance. Finally, the origin and genotype of Q. ilex trees play a major role in determining patterns of L. dispar defoliation and larval performance.
References
Bailey JK, Wooley SC, Lindroth RL, Whitham TG (2006) Importance of species interactions to community heritability: a genetic basis to trophic-level interactions. Ecol Lett 9:78–85
Baldantoni D, Bellino A, Manes F, Alfani A (2013) Ozone fumigation of Quercus ilex L. slows down leaf litter decomposition with no detectable change in leaf composition. Ann For Sci 70:571–578
Barbehenn RV, Constabel CP (2011) Tannins in plant–herbivore interactions. Phytochemistry 72:1551–1565
Barbehenn RV, Jaros A, Lee G, Mozola C, Weir Q, Salminen J-P (2009a) Tree resistance to Lymantria dispar caterpillars: importance and limitations of foliar tannin composition. Oecologia 159:777–788
Barbehenn RV, Jaros A, Lee G, Mozola C, Weir Q, Salminen JP (2009b) Hydrolyzable tannins as “quantitative defenses”: limited impact against Lymantria dispar caterpillars on hybrid poplar. J Insect Physiol 55:297–304
Berenbaum MR (1995) The chemistry of defense—theory and practice. Proc Natl Acad Sci 92:2–8
Bonal R, Hernández M, Ortego J, Muñoz A, Espelta JM (2012) Positive cascade effects of forest fragmentation on acorn weevils mediated by seed size enlargement. Insect Conserv Diver 5:381–388
Büchel K, Fenning T, Gershenzon J, Hilker M, Meiners T (2016) Elm defence against herbivores and pathogens: morphological, chemical and molecular regulation aspects. Phytochem Rev 15:961–983
Busby PE, Lamit LJ, Keith AR, Newcombe G, Gehring CA, Whitham TG, Dirzo R (2015) Genetics-based interactions among plants, pathogens, and herbivores define arthropod community structure. Ecology 96:1974–1984
Caldwell E, Read J, Sanson GD (2016) Which leaf mechanical traits correlate with insect herbivory among feeding guilds? Ann Bot 117:349–361
Caliskan S (2014) Germination and seedling growth of holm oak (Quercus ilex L.): effects of provenance, temperature, and radicle pruning. iForest Biogeosci For 7:103–109
Constabel CP, Yoshida K, Walker V (2014) Diverse ecological roles of plant tannins: plant defense and beyond. Recent Advances in Polyphenol Research 4:115–142
Corcobado T, Cubera E, Juarez E, Moreno G, Solla A (2014) Drought events determine performance of Quercus ilex seedlings and increase their susceptibility to Phytophthora cinnamomi. Agric For Meteorol 192–193:1–8
Corcobado T, Miranda-Torres JJ, Martín-García J, Jung T, Solla A (2016) Early survival of Quercus ilex subspecies from different populations after infections and co-infections by multiple Phytophthora species. Plant Pathol. doi:10.1111/ppa.12627
Díaz M, Møller AP, Pulido FJ (2003) Fruit abortion, developmental selection and developmental stability in Quercus ilex. Oecologia 135:378–385
Estiarte M, De Castro M, Espelta JM (2007) Effects of resource availability on condensed tannins and nitrogen in two Quercus species differing in leaf life span. Ann For Sci 64:439–445
Feeny PP (1976) Plant apparency and chemical defense. In: Wallace JW, Mansell RL (eds) Biochemical interaction between plants and insects. Plenum Press, New York, pp. 1–40
Galván JV, Novo JJJ, Cabrera AG, Ariza D, García-Olmo J, Cerrillo RMN (2012) Population variability based on the morphometry and chemical composition of the acorn in holm oak (Quercus ilex subsp. ballota [Desf.] Samp.). Eur J For Res 131:893–904
Gharzouli K, Khennouf S, Amira S, Gharzouli A (1999) Effects of aqueous extracts from Quercus ilex L. root bark, Punica granatum L. fruit peel and Artemisia herba-alba Asso leaves on ethanol-induced gastric damage in rats. Phytother Res 13:42–45
Gimeno T, Pías B, Lemos-Filho JP, Valladares F (2009) Plasticity and stress tolerance override local adaptation in the responses of Mediterranean holm oak seedlings to drought and cold. Tree Physiol 29:87–98
Guzmán B, Rodríguez-López CM, Forrest A, Cano E, Vargas P (2015) Protected areas of Spain preserve the neutral genetic diversity of Quercus ilex L. irrespective of glacial refugia. Tree Genet Genomes 11:124
Hagerman AE (1987) Radial diffusion method for determination tannin in plant extracts. J Chem Ecol 13:437–449
Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM (2007) Plant structural traits and their role in anti-herbivore defence. Persp Plant Ecol Evol System 8:157–178
Hernández-Serrano A, Verdú M, Santos-del-Blanco L, Climent J, González-Martínez SC, Pausas JG (2014) Heritability and quantitative genetic divergence of serotiny, a fire-persistence plant trait. Ann Bot 114:571–577
Ibáñez-Justicia A, Soto A, Martínez Gonzalvo M, Pérez-Laorga Arias E (2007) Distribución y abundancia de Lymantria dispar (Linnaeus, 1758) (Lepidoptera: Lymantriidae) en las principales masas de carrasca Quercus ilex (L.) subsp. rotundifolia (Lam.) y alcornoque Quercus suber (L.) de la Comunitat Valenciana. Bol San Veg Plagas 33:491–502
Jayaraman K (1999) A statistical manual for forestry research. FORSPA-FAO Publication, Bangkok
Jiménez-Sancho P, Díaz-Fernández PM, Iglesias-Sauce S, de Tuero M, Gil L (1996) Regiones de procedencia de Quercus ilex L. en España. ICONA, Madrid
Johnson MTJ, Ives AR, Ahern J, Salminen JP (2014) Macroevolution of plant defenses against herbivores in the evening primroses. New Phytol 203:267–279
Jung T, Orlikowski L, Henricot B et al (2016) Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. Forest Pathol 46:134–163
Karioti A, Sokovic M, Ciric A, Koukoulitsa C, Bilia AR, Skaltsa H (2011a) Antimicrobial properties of Quercus ilex L. proanthocyanidin dimers and simple phenolics: evaluation of their synergistic activity with conventional antimicrobials and prediction of their pharmacokinetic profile. J Agr Food Chem 59:6412–6422
Karioti A, Tooulakoc G, Bilia AR, Psaras GK, Karabourniotis G, Skaltsa H (2011b) Erinea formation on Quercus ilex leaves: anatomical, physiological and chemical responses of leaf trichomes against mite attack. Phytochemistry 72:230–237
Kite M, Thomson R (2006) Conservation of leather and related materials. Butterworth-Heinemann, Oxford
Leinonen T, McCairns RJS, O’Hara RB, Merilä J (2013) QST–FST comparisons: evolutionary and ecological insights from genomic heterogeneity. Nat Rev Genet 14:179–190
Liebhold A, Elkinton J, Williams D, Muzika RM (2000) What causes outbreaks of the gypsy moth in North America? Popul Ecol 42:257–266
Martín-García J, Solla A, Corcobado T, Siasou E, Woodward S, Belbahri L (2015) Influence of temperature on germination of Quercus ilex in Phytophthora cinnamomi, P. gonapodyides, P. quercina and P. psychrophila infested soils. For Pathol 45(3):215–223
McKown AD, Guy RD, Quamme L et al (2014) Association genetics, geography and ecophysiology link stomatal patterning in Populus trichocarpa with carbon gain and disease resistance trade-offs. Mol Ecol 23:5771–5790
Michaud H, Lumaret R, Romane F (1992) Variation in the genetic structure and reproductive biology of holm oak populations. Vegetatio 99–100:107–113
Michaud H, Toumi L, Lumaret R, Li TX, Romane F, Di Guisto F (1995) Effect of geographical discontinuity on genetic variation in Quercus ilex L. (holm oak): evidence from enzyme polymorphism. Heredity 74:590–606
Milanović S, Lazarević J, Popović Z, Miletić Z, Kostić M, Radulović Z, Karadžić D, Vuleta A (2014) Preference and performance of the larvae of Lymantria dispar (Lepidoptera: Lymantriidae) on three species of European oaks. Eur J Entomol 111:371–378
Milanović S, Lazarević J, Karadžić D, Milenković I, Jankovský L, Vuleta A, Solla A (2015) Belowground infections of the invasive Phytophthora plurivora pathogen enhance the suitability of red oak leaves to the generalist herbivore Lymantria dispar. Ecol Entomol 40:479–482
Morin RS, Liebhold AM (2016) Invasive forest defoliator contributes to the impending downward trend of oak dominance in eastern North America. Forestry 89:284–289
Mrdaković M, Perić-Mataruga V, Ilijin L, Vlahović M, Todorović D, Nenadović V, Lazarević J (2011) The effects of tannic acid on the fitness-related traits of Lymantria dispar L. larvae. Arch Biol Sci 63:1037–1045
Mrdaković M, Perić-Mataruga V, Ilijin L, Vlahović M, Todorović D, Nenadović V, Lazarević J (2013) Effects of tannic acid on trypsin and leucine aminopeptidase activities in gypsy moth larval midgut. Arch Biol Sci 65:1405–1413
Niinemets Ü (2015) Is there a species spectrum within the world-wide leaf economics spectrum? Major variations in leaf functional traits in the Mediterranean sclerophyll Quercus ilex. New Phytol 205:79–96
Nisar M, Ghafoor A, Wadood SF, Iqbal A (2016) Intra and inter specific profiling of Pakistani Quercus species growing in the hilly areas of District Dir Khyber Pakhtunkhwa, Pakistan. Pak J Bot 48:263–270
Ortego J, Bonal R, Muñoz A (2010) Genetic consequences of habitat fragmentation in long-lived tree species: the case of the Mediterranean holm oak (Quercus ilex L.). J Hered 101:717–726
Ortego J, Bonal R, Muñoz A, Aparicio JM (2014) Extensive pollen immigration and no evidence of disrupted mating patterns or reproduction in a highly fragmented holm oak stand. J Plant Ecol 7:384–395
Osier T, Lindroth RL (2001) Effects of genotype, nutrient availability, and defoliation on aspen phytochemistry and insect performance. J Chem Ecol 27:1289–1313
Osier TL, Hwang SY, Lindroth RL (2000) Effects of phytochemical variation in quaking aspen Populus tremuloides clones on gypsy moth Lymantria dispar performance in the field and laboratory. Ecol Entomol 25:197–207
Pearse IS (2011) The role of leaf defensive traits in oaks on the preference and performance of a polyphagous herbivore, Orgyia vetusta. Ecol Entomol 36:635–642
Pollastrini M, Feducci M, Bonal D et al (2016) Physiological significance of forest tree defoliation: results from a survey in a mixed forest in Tuscany (central Italy). Forest Ecol Manag 361:170–178
Ramírez-Valiente JA, Valladares F, Aranda I (2014) Exploring the impact of neutral evolution on intrapopulation genetic differentiation in functional traits in a long-lived plant. Tree Genet Genomes 10:1181–1190
Rubert-Nason KF, Couture JJ, Major IT, Constabel CP, Lindroth RL (2015) Influence of genotype, environment, and gypsy moth herbivory on local and systemic chemical defenses in trembling aspen (Populus tremuloides). J Chem Ecol 41:651–661
Salminen JP, Karonen M (2011) Chemical ecology of tannins and other phenolics: we need a change in approach. Funct Ecol 25:325–338
Santiso X, López L, Gilbert KJ, Barreiro R, Whitlock MC, Retuerto R (2015) Patterns of genetic variation within and among populations in Arbutus unedo and its relation with selection and evolvability. Perspect Plant Ecol 17:185–192
Solla A, Aguín O, Cubera E, Sampedro L, Mansilla JP, Zas R (2011) Survival time analysis of Pinus pinaster inoculated with Armillaria ostoyae: genetic variation and relevance of seed and root traits. Eur J Plant Pathol 130:477–488
Solla A, López-Almansa JC, Martín JA, Gil L (2015) Genetic variation and heritability estimates of Ulmus minor and Ulmus pumila hybrids for budburst, growth and tolerance to Ophiostoma novo-ulmi. iForest Biogeosci For 8:422–430
Soto A, Lorenzo Z, Gil L (2007) Differences in fine-scale genetic structure and dispersal in Quercus ilex L. and Q. suber L.: consequences for regeneration of Mediterranean open woods. Heredity 99:601–607
Spathelf P, van der Maaten E, van der Maaten-Theunissen M, Campioli M, Dobrowolska D (2014) Climate change impacts in European forests: the expert views of local observers. Ann Forest Sci 71:131–137
Stevens MT, Lindroth RL (2005) Induced resistance in the indeterminate growth of aspen (Populus tremuloides). Oecologia 145:297–305
Stong RA, Kolodny E, Kelsey RG, González-Hernández MP, Vivanco JM, Manter DK (2013) Effect of plant sterols and tannins on Phytophthora ramorum growth and sporulation. J Chem Ecol 39:733–743
Tapias R, Moreira AC, Fernández M, Saenz A, Domingos AC, Melo E, Cravador A (2008) Variability in the tolerance/resistance of Quercus suber L. seedlings to Phytophthora cinnamomi Rands: evaluation of survival. In: Vázquez-Piqué J, Pereira H, González-Pérez A (eds) Suberwood: new challenges for the integration of cork oak forests and products. Universidad de Huelva, Huelva, pp. 237–246
Vernesi C, Rocchini D, Pecchioli E, Neteler M, Vendramin GG, Paffetti D (2012) A landscape genetics approach reveals ecological-based differentiation in populations of holm oak (Quercus ilex L.) at the northern limit of its range. Biol J Linn Soc 107:458–467
Vivas M, Zas R, Sampedro L, Solla A (2013) Environmental maternal effects mediate the resistance of maritime pine to biotic stress. PLoS One 8:e70148
Vivas M, Nunes C, Coimbra MA, Solla A (2014) Maternal effects and carbohydrate changes of Pinus pinaster after inoculation with Fusarium circinatum. Trees 28:373–379
Wallis IR, Edwards MJ, Windley H et al (2012) Food for folivores: nutritional explanations linking diets to population density. Oecologia 169:281–291
Züst T, Agrawal AA (2016) Mechanisms and evolution of plant resistance to aphids. Nat Plants 2:15206
Acknowledgments
We thank Adrián Mateos, Andrea Pérez and Marta Company for technical help, Dr. Rafael Zas (Misión Biológica, CSIC) for kindly helping us with the statistics, and Jane McGrath for correcting the manuscript. We thank Dr. Rowland Burdon and one anonymous reviewer for very useful comments on the manuscript. This study was funded by the Spanish Ministry of Agriculture, Food and Environment (Ministerio de Agricultura, Alimentación y Medio Ambiente) (project 956, ‘Determinants of biotic resistance in a model tree species: a new tool for adaptive management in national parks’) and the Ministry of Education, Science and Technological Development of the Republic of Serbia (project 43007, ‘Studying climate change and its influence on the environment: impacts, adaptation and mitigation’).
Data archiving statement
Tannin content, defoliation and plant and larval performance data may be requested from the corresponding author (asolla@unex.es).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Burdon
Rights and permissions
About this article
Cite this article
Solla, A., Milanović, S., Gallardo, A. et al. Genetic determination of tannins and herbivore resistance in Quercus ilex . Tree Genetics & Genomes 12, 117 (2016). https://doi.org/10.1007/s11295-016-1069-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-016-1069-9