Abstract
Prosopis chilensis (Molina) Stuntz (Leguminosae) is a valuable native species in Argentina that has been proposed to be used in reforestation, afforestation and restoration programmes. Natural provenances show important differentiation in height, shape, spine size, fruits and foliar traits throughout their distribution in the semiarid Monte ecoregion. The goal of this work was to characterize the genetic basis of the leaf variation in P. chilensis aiming to contribute to the improvement management program. We analyzed morphological variation and estimate narrow sense heritability for ten quantitative traits from a provenance-progeny trial founded from open pollinated families. We assessed the variance components by a generalized linear mixed model. Differences among provenances were quantified through univariate QST statistics and multivariate discriminant analysis of principal components. Finally, univariate and multivariate neutrality test were conducted to unveil the evolutionary forces that shape the variation. Univariate and multivariate analysis showed low genetic variation in foliar traits among provenances grown in the common garden. Consistently, the QST estimates for each trait were low. Both, the univariate (QST–FST comparison) and the multivariate neutrality test suggest that the leaf variation among provenances may be shaped by genetic drift rather than selective forces. Heritability estimates were significant only for leaflet apex and leaflet apex/leaflet area. Since genetic variation for most foliar traits among provenances estimated under controlled environmental conditions were very low or absent, the variation described in the wild would be explained merely by plastic response to varying environments. These results are discussed in terms of adaptive strategies and the use of different provenances as seed sources within the framework of the improvement program. It is expected that P. chilensis seeds or seedlings from trees selected under economical criteria will be able to develop in different areas thanks to the phenotypic plasticity of leaf traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forests in the semiarid Monte ecoregion in Argentina are dominated by species of the genus Prosopis (Leguminosae), mainly P. flexuosa DC. and P. chilensis (Molina) Stuntz (Roig 1993a; Villagra et al. 2004). The latter, is naturally distributed in the north and median area of the Monte province and is an important xerophytic species with the ability of living in highly saline soils, tolerating alkalinity, and growing in a wide variety of climatic conditions (Cony 1996a; Galera 2000; Roig 1993a, b). In the northern/median areas of P. chilensis distribution, mean temperature ranges between 11 and 20 °C according to altitude and extreme temperatures can be higher than 45 °C or as low as − 13 °C. Precipitations occur in summer and total annual precipitation ranges between ~ 100 and 350 mm (Cony 1996a, b; Labraga and Villaba 2009; Peri 2021). The growth habit of P. chilensis is highly variable, from shrub to arboreal individuals, and great variability was also observed in spine size and fruit number and shape. The highest trees can reach up to 18 m height and have a diameter at breast height of approximately 2 m (Burkart 1976; Karlin et al. 1997; Roig 1993a).
Based on leaf, fruit, and spine morphological variation, several varieties (P. chilensis var. chilensis, P. chilensis var. catamarcana, and P. chilensis var. riojana) and morphotypes have been described (Dalmasso 1993; Roig 1993a). Fruit shape variation was reported in the wild, showing a latitudinal gradient and explained mainly by mean temperatures (Fernandez 2020). Previous studies indicated that leaflets tend to be shorter and narrower in populations of the southern edge of its range in the Monte desert (San Juan and Mendoza provinces in Argentina) (Gil 2013; Roig 1993a). Fruit mesocarp varies from poor to very rich and pulpy in some northern populations of P. chilensis var. catamarcana (Roig 1993a). Phenology also varies in P. chilensis according to latitude and altitude, and variation was also described within and between natural populations (Gil 2013; Karlin et al. 1997).
In areas of sympatry, P. chilensis may hybridize with P. flexuosa (Hunziker et al. 1986; Verga 2000) and this mechanism has been postulated to increase their natural variability. Verga (1995) proposed that P. chilensis var. riojana is indeed a hybrid between P. chilensis var. chilensis and P. flexuosa var. flexuosa (Verga 1995) but Vázquez-Garcidueñas et al. (2003) suggested, based on morphological and genetic traits, that P. chilensis var. riojana is well differentiated from the other two taxa.
The success of reforestation and restoration programs depends on the genetic variation and the species ability to acclimate to the new environments and/or tolerate environmental stress. Cony (1993, 1995) proposed that the use of multipurpose trees, such as P. chilensis and P. flexuosa, should be considered in afforestation programs in the Monte ecoregion. According to Cony (1996a) outstanding traits such as height, straightness, lack of thorns, pod and biomass production and resiliency to different causes of stress are available in P. chilensis and P. flexuosa germplasm. In recent years in Argentina, a new law (N° 26.331) was established to promote environmental protection for enrichment, restoration, conservation, use and sustainable management of native forests and the environmental services forests provide to society (MAyDS 2017). Many products are obtained from P. chilensis, including wood, charcoal, forage, human food and pharmacological compounds, turning it a valuable multipurpose resource for the regional economy. Consequently, this species has been included in the programme “Conservation and Improvement of the native species of Prosopis” (Cony 1993, 1995, 1996a) and reforestation, afforestation and restoration plans have been raised and are in progress. The programme is expected to be successful provided that the high variability both at provenances and families’ levels (Bessega et al. 2019; Chequer-Charán et al. 2020; Cony 1996a, b; Mantován 2005) has a genetic basis.
A P. chilensis provenance-progeny trial was installed in 1991 in Argentina using seeds from mother plants chosen on the wild on the basis of height, basal diameter, straightness, branching and thorniness involving the latitudinal gradient within the Monte ecoregion (Cony 1993, 1996a, b). Tree provenance-progeny trials constitute expensive and time consuming to establish resources but are very important from economical and academic perspectives. Provenance-progeny trials allow to conduct valuable studies related to forest genetic improvement programs and also represent a seed transfer source. Typically, several potential seed sources (or provenances) are planted in trials established in different sites to evaluate provenance performance under different environmental conditions (Risk et al. 2021). The provenance-trials installed in a common environment, where the family structure is known, allows to make estimates of the (additive) genetic variance of each trait and thus contribute in obtaining a favorable selection response.
Traits associated with plant response to ecological constraints are usually difficult to be quantified at large scale (Cornelissen et al. 2003). An indirect way to evaluate the effects of environmental factors on plant physiology is using leaf morphology traits because they are usually correlated with plant response to environmental stress and are easy to measure (Cornelissen et al. 2003). Leaves are organs of great taxonomical and ecological importance as they are related to grow and survival performance, they may be considered predictors of plant performance and act as resource-use strategies (Udayukumar and Sekar 2021). Foliar and wood traits had demonstrated to covary in Pericopsis elata (Kafuti et al. 2020) and leaf parameters, like leaf dry mass and leaf area, demonstrated to be directly related with plant relative growth rate (Poorter et al. 2009). Moreover, specific leaf area was inversely correlated with fine root diameter in in Populus tremula and P. tremuloides (Hajek et al. 2013). Previous studies have focused on the mechanisms involved in the diversity of leaf shape and size in species of “algarrobos” (Prosopis sect. Algarobia). In P. flexuosa Darquier et al. (2013) observed that the distribution of genetic variation for leaf traits does not agree with the expected under neutrality and that selection favors different optima in different populations. For P. alba Griseb., both diversifying and stabilizing selection evidences were detected over nine morphological traits (Bessega et al. 2015). Signals of directional selection was suspected for three life history traits (height, biomass and basal diameter) and seven foliar traits (leaflet total area, leaflet width, petiole length, number of pinnae, leaflet length, leaflet apex, and leaflet length/width ratio). For spine length and leaflet falcate the results suggested stabilizing or uniform selection. Additionally, Roser et al. (2014) recorded in P. alba highly significant differences in most of the studied morphological foliar traits between clones selected for increased or reduced salinity tolerance, which could not be explained by environmental causes. Leaflet length, leaflet width, leaflet apex, and number of pairs of leaflets per pinna were suggested to be useful as morphological markers for clonal identification and to make phenotypic selection for quantitative traits of adaptive significance. In P. juliflora mean leaf area, specific leaf area, leaf dry matter content and leaflet thickness varies between urban and rural areas and it was suggested that different acclimation strategies may be used in different areas to cope with contrasting environments (de Oliveira et al. 2021).
The geographical distribution of molecular locus polymorphisms is expected to reflect demographic processes dependent on population size and dispersal ability. The corresponding patterns observed for these supposedly neutral loci are frequently used as null hypothesis to test the effects local adaptation on quantitative trait genetic variation (Merila and Crnokrak 2001). The genetic differentiation of quantitative traits among sampling sites or provenances may be properly evaluated in common gardens where the effects of environment are minimized and/or randomized. Indeed, the use of a suitable statistical design allows to estimate genetic differentiation from the observed phenotypic variation among individuals. The phenotypic variation observed in the wild in P. chilensis might allow the selection of profitable characteristics under different environmental conditions. However, such variation may be at least partially the result of phenotypic plasticity, a feature that may condition the response of plants to abiotic and biotic factors (de Oliveira et al. 2021). The P. chilensis provenance-progeny trial installed in Mendoza (32° 51′ 14,89 S, 68° 43′ 49,85 W) constitute a valuable resource in order to deeply study the differentiation among possible seed sources (provenances), the hereditary components of each trait variation, and the evolutionary forces responsible for the observed variation.
The goal of this work was to characterize the genetic basis of the leaf variation in P. chilensis among provenances aiming to contribute to the improvement management programme. We analyzed morphological variation of ten quantitative leaf traits (petiole length, number of pairs of leaflets per pinna, pinna length, leaflet length, leaflet width, ratio leaflet length/width, leaflet falcate, leaflet total area, leaflet apex, and ratio leaflet apex/total area). The interest on these traits resides in a previous work on P. alba clones where they were shown to be associated with salt tolerance, suggesting some adaptative role in arid regions (Roser et al. 2014). We evaluated the genetic differentiation among provenances and estimate their heritability in the Mendoza provenance-progeny trial. We compare the genetic differentiation of each quantitative trait among provenances (estimated by QST) with the neutral variation previously quantified by SSR markers through FST (Chequer-Charán et al. 2020) to address the following questions: (1) is there significant genetic differentiation among provenances for quantitative leaf traits?, if so, (2) is this differentiation parallel to the molecular neutral variation?, (3) which leaf traits have significant h2? The rationale of these analyses is that even after 10 years of natural selection acting at the provenance progeny trial, the morphometric leaf differences among provenances will indicate a genetic basis behind these traits. The variation among families in the trial may be used to obtain heritability estimates and predict the effect of selection of seed sources within the trial to reforestation, afforestation and restoration programmes. Finally, the comparison among morphological and neutral differentiation contributes to unveil the evolutionary forces that shaped the observed variation.
Materials and methods
F1 trial sampling
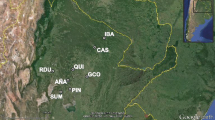
Prosopis chilensis individuals were sampled from a provenance-progeny trial installed in 1991 at the locality of El Sauce, Mendoza, Argentina. This trial was established from seeds collected in 1990 from 84 open pollinated mother plants from 9 Argentine provenances (Cony 1993). The sampling pattern was designed to represent a latitudinal collection of natural populations (provenances) from the Monte ecoregion. Usually, seeds collected from open pollinated trees are assumed to represent half-sibs arrays but mating system information can be valuable for the appropriate design to be applied. A P. chilensis mating system analysis in a wild population (Bessega et al. 2000) yielded a high multilocus outcrossing rate (tm = 0.81) and a high correlation of paternity (rp = 0.96) indicating the occurrence of low levels of selfing and suggesting that most of the individuals of the array may be considered full-sibs. The provenance-progeny trial consisted of a completely randomized block design with five replicates (blocks) where each family was represented by three trees per block planted in a 4.5 × 4.5 m grid design (1260 individuals in total). Unfortunately, in 2004, a natural fire partially destroyed block V. For this study, we randomly sampled 96 trees representing four provenances (Fiambalá, Chilecito, Mogna and Villa Unión) that are located along a latitudinal gradient between 28.2° and 30.5° (Fig. 1, Table 1). Parameters like altitude, temperature and precipitation varied among the sites where the P. chilensis seeds were collected to establish the provenance-progeny trial (Table 1). Aiming to obtain a balanced sampling, we sampled 4 families of each provenance (16 families) from blocks I to IV considering 6 individuals per family. During collection herbarium vouchers were obtained from each sampled tree for morphological analysis.
Morphological data
Ten morphological traits were registered for each of the 96 sampled trees. In order to measure leaf morphology traits samples from each tree were first mounted on specimen boards. The measured traits were petiole length (PEL), number of pairs of leaflets per pinna (NLP), pinna length (PIL), leaflet length (LEL), leaflet width (LEW), ratio leaflet length/width (LEL/LEW), leaflet falcate (LEF), leaflet total area (LEA), leaflet apex (LEX), and ratio leaflet apex/total area (LEX/LEA) (Fig. 2). In each individual, nine repeats of PEL, NLP, PIL, SPL, and NPI were obtained involving boards from three different canopy regions. For a good representation we consider the upper, medium and lower parts of the canopy. Ninety repeats were obtained of LEL, LEL/LEW, LEF, LEX, LEX/LEA, involving 10 leaflets from nine pinnae sampled from three different regions of the canopy. All leaflet measures were obtained with the software HOJA1.1 (available from the author upon request: A. Verga, INTA-IFFIVE, arverga@yahoo.com.ar).
Morphological traits measured over the herbarium specimens of P. chilensis: general aspect of leaves and details of measurements used to estimate leaflet falcate (LEF) and leaflet apex (LEX). PEL petiole length, NLP pairs of leaflets per pinna, PIL pinna length, LEL leaflet length, LEW leaflet width, LEA leaflet area, LEF leaflet falcate, LEX leaflet apex. l denotes distance from the base to the tip of the leaflet, f the length from the base to the tip of the leaflet following a curved line running along the middle of the leaflet, t the area of the upper leaflet third and s the area of a rectangle with the same dimensions as t
Data analysis
Morphological analysis of variance components and differentiation among provenances
An analysis of genetic variance components of morphological traits was conducted applying the unbalanced generalized linear mixed model:
where yijkl is an observation of the trait for an individual tree l belonging to the family k, sampled in the provenance j, in the block i, μ is the overall mean, bi represents the fixed block effect, pj is the provenance effect, fjk corresponds to the random family nested within provenance, and eijkl is the random residual error. Variance components as well as their confidence intervals were estimated using the package lme4 (Bates et al. 2015) of the software R (ver.4.1.0) (R Development Core Team 2021).
In order to compare the means of each trait, provenance was considered a fixed factor and the significance was obtained with the function Anova of package car (Fox and Weisberg 2011). When the test resulted significant, pairwise comparisons of means between provenances were performed by Tukey contrasts using the function glht of multcomp package (Hothorn et al. 2008).
In order to obtain variance components and estimate the QST (Spitze 1993) coefficients provenance was considered a random factor. The morphological differentiation was estimated according to the expression:
where VGb is the quantitative genetic variance among provenances and VGw is the quantitative genetic variance within provenance. While the observed phenotypic variance among provenances (σ2b) is a good estimator of VGb, the estimation of VGw relies on the actual relationship of family arrays. Assuming that open pollinated families are composed of half-sibs only, VGw is equivalent to the variance among families multiplied by 4 (VGw = 4 σ2w) (Eq. 1), but for family arrays composed of full-sibs only, VGw = 2 σ2w (Eq. 2):
\({Q}_{ST\left(HS\right)}=\frac{{\sigma }_{b}^{2}}{{8\sigma }_{w}^{2}+{\sigma }_{b}^{2}}\) (1)
\({Q}_{ST\left(FS\right)}=\frac{{\sigma }_{b}^{2}}{{4\sigma }_{w}^{2}+{\sigma }_{b}^{2}}\) (2)
As the actual proportion of half and full sibs in progeny arrays is unknown, both models were considered. In order to estimate confidence intervals (CI95%), a bootstrap method considering 1,000 pseudo-replicates was applied using the function sample of R software.
Multivariate morphological and molecular differentiation among provenances
Based on the 10 morphological traits, differentiation among populations was evaluated by discriminant analysis of principal components (DAPC) as described by Jombart et al. (2008), using the function dapc of the adegenet package (Jombart 2008; Jombart and Ahmed 2011) of R. This analysis was conducted with prior information on individual provenances.
Univariate and multivariate neutrality test
The molecular marker data are those from Chequer-Charán et al. (2020) and comprise the genotypes of the same individuals in the following loci: MO8, MO13, MO16 (Mottura et al. 2005) and GL8, GL12 and GL23 (Bessega et al. 2013). Molecular differentiation among provenances was quantified by Chequer-Charán et al. (2020) using the FST coefficient.
The neutrality tests were based in two approaches. The first implies the comparison of phenotypic differentiation (QST) of each trait and the neutral marker genetic differentiation (FST). The second one consisted in a multivariate test by evaluating whether the multivariate estimates of among-population (D) and within-population (G) genetic covariance matrices are proportional as suggested by Martin et al. (2008). D represents the covariance matrix of the means of each trait in different provenances, whereas G represents the average of the covariance matrices of the means of each trait in different families of each provenance. As this analysis is dependent on the assumption of normality distribution of morphological traits, traits that did not adjust to normality were previously transformed using the function bestNormalize from package bestNormalize (Peterson and Cavanaugh 2019) of the program R that implements and choose the best among six possible normalization methods. Furthermore, normalized data were rescaled to have in all cases mean 1. The multivariate estimates of D and G covariance matrices were obtained using the function cov.B of the package vcvComp (Le Mâitre and Mitteroecker 2019) of R, considering D and G respectively the among provenances and among families covariance matrices. The proportionality between the matrices was evaluated by Mantel test (with 2,000 permutations) and Random Skewers method (Cheverud et al. 1983, 1996) (with 2,000 random vectors) using the evolq (Melo et al. 2015) package of R.
Heritability estimation
Heritabilities were estimated for each trait from the variance components corresponding to the following linear mixed model:
where yijk is an observation of the trait for an individual (ki) tree belonging to the family j, in the block i, μ is the overall mean, bi represents the fixed block effect, fj is the random family effect, and eijk is the random residual error.
The narrow sense heritability estimation was obtained by the following equations assuming half sibs, \({h}_{\left(HS\right)}^{2}\), and full sibs, \({h}_{\left(FS\right)}^{2}\), designs:
where \({\sigma }_{T}^{2}\) represents the total, and \({\sigma }_{F}^{2}\) is the between family variance. The significance of σ2F was obtained by the comparison of the mixed linear model including the random family effect (estimated with the function lmer of the package lme4) with the simple fixed model removing the family effect (estimated with the function lm of the package stats). This comparison was conducted with the function anova of the package stats of R. The heritability was considered significant when σ2F was significant.
Results
Morphometric analysis of variance components and differentiation among provenances
In the analysis of variance component considering the provenance as random factor, the highest components correspond to among-individual effects within family (Fig. 3). The proportion of variance represented at each level varied among traits. Percent of variance among provenances varied from 0 for NLP and LEL to 9.67 for LEW, and the family level varied from 0.94 for LEF to 30.03% for LEX, whereas PIL only showed variation at individual level.
Distribution of the variance component for each morphological trait considering provenances, families and individuals. PEL petiole length (mm), NLP pairs of leaflets per pinna (N°), PIL pinna length (mm), LEL leaflet length (mm), LEW leaflet width (mm), LEL/LEW ratio leaflet length/width, LEF leaflet falcate, LEA leaflet area (mm2), LEX leaflet apex (mm2), LEX/LEA ratio leaflet apex/total area
Boxplots represent median and quartiles of each trait in the provenances and suggests no significant differences among provenances for all the traits (Fig. 4). Deviance analysis revealed significant differences among provenances for 4 out of the 10 studied traits: LEW (X2 = 14.16, P = 0.003), LEA (X2 = 6.89, P = 0.075), LEX/LEA (X2 = 9.96 P = 0.019) and LEX (X2 = 7.73, P = 0.051). For these traits, the Tukey multiple contrasts were significant in 6 out of 24 pairwise comparisons, and all of them involved FI (Table 2). Although the number of families studied per provenance was low, most variance was represented by individuals within families (Fig. 3) and the differentiation among provenance is well supported.
Boxplots representing morphological variation among provenances for each trait analyzed. PEL petiole length (mm), NLP pairs of leaflets per pinna (N°), PIL pinna length (mm), LEL leaflet length (mm), LEW leaflet width (mm), LEL/LEW ratio leaflet length/width, LEF leaflet falcate, LEA leaflet area (mm2), LEX leaflet apex (mm2), LEX/LEA ratio leaflet apex/total area. Provenances are presented from North (N) to South (S) according to the sampling latitude: FI Fiambalá, CH Chilecito, VU Villa Unión, MO Mogna
The phenotypic differentiation (QST) ranged from zero to 0.068 and from zero to 0.138 considering the half- and full-sibs designs (Table 3 column 2 and 5 respectively).
Multivariate morphological and molecular differentiation among provenances
In the DAPC analysis based on the 10 leaf traits, three axes were retained that explained 99.40% of total variation (79.87, 19.00 and 0.53%, respectively). The individuals from the four provenances could not be differentiated (Fig. 5).
Scatterplot of individuals on the two principal components of the discriminant analysis of principal components (DAPC) based on the 10 morphological traits. The graph represents the individuals as dots and the groups as inertia ellipses. Eigenvalues of the analysis are displayed in inset with clusters defined a priori according to the provenance sampling site
Univariate and multivariate neutrality test
In order to detect signals of selection, the genetic differentiation estimated for each quantitative trait (QST) are usually compared with the global molecular differentiation coefficient (FST) and interpreted as follows (see Merila and Crnokrak 2001): FST = QST indicates that differentiation of the quantitative trait may be explained by gene flow–genetic drift interactions without the need to invoke natural selection; QST > FST, is evidence of positive directional natural selection favoring different optimal phenotypes in different populations; and QST < FST, is suggestive of uniform selection or stabilizing selection across the populations.
In this paper we compared the QST estimates with the FST (0.03) reported by Chequer-Charán et al. (2020) using the same individuals as those included in the present work. As expected, the QST estimates based on the half-sib design (QST (HS)) were lower than the ones obtained using the full-sib model (QST (FS)) but the neutrality tests were coincident between the two models (Table 3 columns 2 to 7). For 9 traits the difference among QST and FST was non-significant no matter the assumption about relationship within family arrays. Just in one case (NLP), the upper interval of the QST does not overlap with the FST estimate and was considered as suggestive of uniform selection (Table 3, column 4 and 7).
The among- (D) and within-population (G) covariance matrices resulted highly significantly correlated according to both Mantel (r = 0.81, P = 0.0005) and Random Skewers test (r = 0.75, P = 0.003) suggesting no significant departures of quantitative traits from the expectation for a neutral model.
Heritability estimation
Heritabilities (h2) were estimated considering half-sibs (HS) and full-sibs (FS) designs as the first may be overestimated according to the mating system information available of P. chilensis. Both models yielded similar results indicating that the estimates of heritability were variable among traits (Table 3 column 8 and 9). Under the assumption of a half sib model, h2 varies from 0 to more than 1 and for the assumption of full-sib design, h2 ranges from 0 to 0.726. For seven traits h2 was not significant; LEW showed a relatively high heritability (h2HS = 0.699, h2FS = 0.349) but only borderline significant and LEX and LEX/LEA were the only traits with highly significant heritability (Table 3 column 10).
Discussion
Based on the analysis of variance components conducted in the present paper in a common garden most variation occurs between individuals within families. Only four traits showed significant differences between provenances (LEW, LEX/LEA, LEX and LEA) and only one of them (LEA) might be associated with a north–south gradient. Based on pairwise Tukey contrasts, Fiambalá provenance (FI), the northernmost P. chilensis population studied in this paper was the most differentiated from the rest. Interestingly, Bessega et al. (2019) pointed in P. flexuosa that a provenance located in the same area (Fiambalá) was a valuable seed source for afforestation in the Monte ecoregion based on growing traits (height and stem diameter) in order to provide fast growing and larger individuals. However, these authors suggested an admixture provenance strategy representing different provenances according to short term expected phenotypes aiming to ensure evolutionary resilience.
The QST estimates, estimated assuming both HS and FS families, showed low level of differentiation among provenances for all the traits evaluated. A similar trend for foliar traits was obtained in P. flexuosa (Darquier et al. 2013) and P. alba (Bessega et al. 2009; Roser 2015). The low morphological differentiation was also evidenced by the multivariate analysis (DAPC) as P. chilensis populations are overlapped in the scatterplot. This result differs from that expected according to molecular genetic differentiation and STRUCTURE results by Chequer-Charán et al. (2020). These authors performed the molecular analysis using 6 SSR in the same provenance-progeny trial, and although the non-hierarchical FST was low among these 4 provenances, it was highly significant and three clear genetic groups were found: Villa Unión, Fiambalá and Mogna-Chilecito.
The FST coefficient estimated from neutral loci is expected to reflect the distribution of genetic variation resulting from the interaction between gene flow and genetic drift. In the case of quantitative traits selective processes may be also involved in the resulting QST statistic. For this reason, the comparison between FST and QST is widely used to estimate the relative contribution of selection and genetic drift on the distribution of genetic variation of quantitative traits in forest species (Merila and Crnokrak 2001). A multivariate extension of the QST–FST neutrality test is based on empirical estimates of the among-populations (D) and within-populations (G) genetic covariance matrices (Martin et al. 2008). The rate and direction of phenotypic divergence among populations is highly dependent on G for both adaptive and neutral traits (Mc Guigan 2006). Here, the multivariate and univariate neutrality test indicated that the leaf variation among provenances may be shaped by genetic drift rather than selective forces. The matrices D and G were proportional and the comparison of FST and QST estimates suggested that phenotypic leaf variation among provenances may be explained by drift in 9 out of the 10 foliar traits evaluated. The only trait in which neutrality was rejected, NLP, the scarce genetic variation among provenances suggested uniform selection.
Given the low genetic variation among provenances the question remains about the heritability of foliar trait variation in the El Sauce provenance-progeny trial because h2 has a predictive role that express the reliability of the phenotypic value as a breeding value estimate. The heritability in this work was estimated considering two contrasting assumptions, one where all the siblings of a family share the mother and not the father (HS), and another where all the individuals share both parents (FS). As the covariance in each case may include different components, the estimation of h2 in each case shows different accuracy. The proportion of half and full sibs in an array may depends on the species mating system, but it also depends on particular population parameters under study. Among the factors that are highly relevant are population density and the availability of dispersing agents (Bessega et al. 2000, 2011, 2017). The mating system studies made in a wild population of P. chilensis in Patquía (La Rioja) suggested that it is mainly oucrosser and that the array was constituted mainly by full sibs because the correlated paternity estimate within family arrays was 0.96 (Bessega et al. 2000). As we do not know the actual proportion of full and half sibs in the families planted in the common garden, heritabilities here were estimated in both extreme situations. Beyond the differences in the estimated value the conclusions under both assumptions were that h2 were non-significant for seven traits (PEL, NLP, PIL, LEL, LEL/LEW, LEF, LEA), borderline in one case (LEW), and highly significant for two (LEX and LEX/LEA). Based on the presence of full sibs, the h2HS estimations could be partially overestimated in the present study and in particular for LEX/LEA and LEX the values obtained are higher than unity. However, the h2FS could also be somewhat overestimated as the covariance estimation for full sibs may include, in addition to the additive variance, dominance and interaction variance components (Falconer and Mackay 1996).
According to Falconer and Mackay (1996), in natural populations trait heritability is inversely related with their importance in fitness (fertility and survival). This low h2 may be explained by high residual variation in comparison to additive variance (Houle 1992). Indeed, in P. flexuosa, Darquier et al. (2013) described that the traits with highest heritability values were those related to leaflet size and shape, whereas traits as height and basal diameter did not show significant h2 values. In consistency with the observed in P. flexuosa, Chequer-Charán et al. (2020) obtained heritability estimates in economical important traits of P. chilensis (height, biomass, basal diameter, spine length, number of stems) relatively low, varying from 0 to around 0.3. However, we observed here low h2 values for most leaf traits; only apex shape traits (LEX and LEX/LEA) have high heritability suggesting that these traits in P. chilensis would not be target of natural selection being less relevant in the physiological adaptation. The differences among the two species may be related to different adaptation strategy.
The joint analysis of the results here obtained under common garden conditions, allow us to discuss different scenarios modulating foliar traits variation. It may be argued that the lack of differentiation among provenances is due to natural selection for leaf traits in the 20 years-old provenance-progeny trial. If this were the case, it should be expected a relationship between leaf traits and indicators of tree-health. However, no evidence of correlation between the 10 leaf traits and the biomass (estimated in Chequer Charán et al. 2020) was obtained (data not shown). This result turns non-plausible the possibility of selection in the orchard on all measured leaf traits erasing all differences among provenances.
Alternatively, the morphological differences in leaf traits described by several authors among natural populations of P. chilensis (Cony 1996a, b; Dalmasso 1993; Gil 2013; Roig 1993a; Teich et al. 2017; Vega et al. 2021) would not have a genetic basis and are not detectable in a uniform environment. The described differences among natural populations could be merely a plastic response to varying environmental conditions. Adaptive plasticity of leaf traits was evidenced in the shrub Vaccinium elliottii by Anderson et al. (2021) using a reciprocal transplant experiment. The identification of acclimation mediated by plasticity may become important at the moment of proposing the use of selected material as a source of seeds to afforestation/restoration of new areas. The ability to survive and development success of plants growing in new particular conditions may depends both on the environmental heterogeneity and the phenotypic plasticity in heterogeneous environments (Valladares and Gianoli 2007). Foliar anatomy and morphology are strongly related to physiological performance so plasticity in leaves can be related to growth rate and survival capacity (Ivancich et al. 2012). The low differentiation among provenances and the low h2 of the foliar traits suggests that the acclimation plays an important role in the ability of P. chilensis to survive in different areas within the Monte ecoregion. For P. chilensis, as genetic drift rather than local adaptation would be modelling most of the leaf variation, it can be suggested that it could be used efficiently as seed sources for restoration and afforestation. It is expected that phenotypic plasticity might allow seedling to quickly acclimate without difficulty to environmental heterogeny including new habitats and new silvicultural proposals as stated by Ivancich et al. (2012).
The extent at which different seed source may acclimate to changing environments should be evaluated by means of reciprocal transplants, although this constitutes a long-term experiment for a long-living forest species such as P. chilensis. Up to now the evidence suggests that the available material has plasticity and significant success would be expected when using it as a seed source. However, survival in arid zones may also depend on physiological traits whose plasticity has not been yet evaluated. As in other species of Prosopis (Bessega et al. 2009; Chequer-Charán et al. 2020; Darquier et al. 2013), the highest components of phenotypic genetic variation in P. chilensis corresponds to the among-individual level, followed in most cases by the variation between families. This suggests that at the time of collecting source material for restoration, reforestation and/or afforestation it is necessary to recover seeds not only from the different provenances but also to have special care in including different mother plants (families) in order to recover as much of the variation as possible.
Conclusion
This paper integrates the study of leaf morphology variation together with previous genetic studies in P. chilensis, a promising species for reforestation, afforestation and restoration programmes in Argentina. In a provenance-progeny trial we characterize the genetic basis of the leaf variation among provenances aiming to contribute in the improvement programme of this species. According to the sampling carried out here, genetic leaf morphology variation in the common garden would be lower than phenotypical variation described in the wild. The northernmost provenance, FI, was the more differentiated from the rest, however, most foliar variation may be explained by genetic drift. The low h2 estimates of most foliar traits suggests that phenotypic variation among natural populations could be explained by acclimation mediated by plasticity. The use of these native species for reforestation, afforestation and restoration may take advantage of this ability. It is expected that P. chilensis seeds or seedlings from trees selected under economical criteria (i.e. height, biomass, basal diameter) will be able to develop in different areas thanks to the phenotypic plasticity of leaf traits. However, it should be borne in mind that the movement of seeds and their use in reforestation, afforestation and restoration programs in different areas should not ignore the ecological consequences and the derangements that the movement of material from different sources can cause and should be studied before doing so. Future studies involving provenance-progeny trials installed in different areas in order to allow the evaluation of the GxE interactions are needed and would give support to the results presented.
References
Anderson JT, Jameel MI, Geber M (2021) Selection favors adaptive plasticity in a long-term reciprocal transplant experiment. Evolution 75:1711–1726. https://doi.org/10.1111/evo.14280
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–8. https://doi.org/10.18637/jss.v067.i01
Bessega C, Ferreyra LI, Julio N, Montoya S, Saidman BO, Vilardi JC (2000) Mating system parameters in species of genus Prosopis (Leguminosae). Hereditas 132:19–27
Bessega C, Saidman BO, Darquier MR, Ewens M, Sánchez L, Rozenberg P, Vilardi JC (2009) Consistency between marker- and genealogybased heritability estimates in an experimental stand of Prosopis alba (Leguminosae). Am J Bot 96:458–465
Bessega C, Pometti CL, Ewens M, Saidman BO, Vilardi JC (2011) Strategies for conservation for disturbed Prosopis alba (Leguminosae, Mimosoidae) forests based on mating system and pollen dispersal parameters. Tree Genet Genomes 8:277–288
Bessega C, Pometti C, Miller J, Watts R, Saidman B, Vilardi JC (2013) New microsatellite loci for Prosopis alba and P. chilensis (Fabaceae). Appl Plant Sci 5:1200324. https://doi.org/10.3732/apps.1200324
Bessega C, Pometti C, Ewens M, Saidman BO, Vilardi JC (2015) Evidences of local adaptation in quantitative traits in Prosopis alba (Leguminosae). Genetica 143:31–44
Bessega C, Pometti CL, Campos C, Saidman BO, Vilardi JC (2017) Implications of mating system and pollen dispersal indices for management and conservation of the semi-arid species Prosopis flexuosa (Leguminosae). For Ecol Manag 400:218–227
Bessega C, Cony MA, Saidman BO, Aguiló R, Villagra P, Alvarez J, Pometti C, Vilardi JC (2019) Genetic diversity and differentiation among provenances of Prosopis flexuosa DC (Leguminosae) in a progeny trial: implications for arid land restoration. For Ecol Manag 443:59–68. https://doi.org/10.1016/j.foreco.2019.04.016
Burkart A (1976) A monograph of the genus Prosopis (Leguminosae subfam. Mimosoideae). J Arnold Arboretum 57:219–240 (450–425)
Chequer-Charán D, Pometti C, Cony MA, Vilardi JC, Saidman BO, Bessega C (2020) Genetic variance distribution of SSR markers and economically important quantitative traits in a progeny trial of Prosopis chilensis (Leguminosae): implications for the ‘Algarrobo’ management programme. Forestry 94:204–218. https://doi.org/10.1093/forestry/cpaa026
Cheverud JM (1996) Quantitative genetic analysis of cranial morphology in the cotton-top (Saguinus oedipus) and saddle-back (S. fuscicollis) tamarins. J Evol Biol 9:5–42
Cheverud JM, Rutledge JJ, Atchley WR (1983) Quantitative genetics of development: genetic correlations among age-specific trait values and the evolution of ontogeny. Evolution 37:895–905
Cony MA (1993) Programa de conservación y mejoramiento de especies del género Prosopis en la Provincia Fitogeográfica del Monte, Argentina, pp 34–71. In: Unidades de Botánica y Fisiología Vegetal (IADIZA) (ed) Quinta Reunión Regional para América Latina y el Caribe de la Red de Forestación CIID, Conservación y Mejoramiento del Género Prosopis. IADIZA-CRICYT-CIID, Mendoza, Argentina
Cony MA (1995) Reforestación racional de zonas áridas y semiáridas con árboles de múltiples propósitos. Interciencia 20:249–253
Cony MA (1996a) Genetic potential of Prosopis in Argentina for its use in other countries. In: Felker P, Moss J (eds) Prosopis: semiarid fuelwood and forage tree building consensus for the disenfranchised. A workshop, U.S. Academy of Science Building, Washington D.C., pp 6.3–6.24
Cony MA (1996b) Genetic variability in Prosopis flexuosa DC, a native tree of the Monte phytogeographic province, Argentina. For Ecol Manag 87:41–49
Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380. https://doi.org/10.1071/BT02124
Dalmasso A (1993) Selección de formas de una población de Prosopis chilensis para ensayos de forestación. Conservación y mejoramiento de especies del género Prosopis. IADIZA-CRICYT-CIID, p 79
Darquier MR, Bessega C, Cony MA, Vilardi JC, Saidman BO (2013) Evidence of heterogeneous selection on quantitative traits of Prosopis flexuosa (Leguminosae) from multivariate QST–FST test. Tree Genet Genomes 9:307–320. https://doi.org/10.1007/s11295-012-0556-x
de Oliveira BO, da Costa Correia Araújo R, Barbosa da Silva L, D’Ávilla Fernandes Lopes Gomes R, de Oliveira Souza MI, de Souza Tavares RL, Ramosde Almeida G, de Faria Lopes S (2021) Variação nos traços funcionais foliares de Prosopis juliflora (sw.) dc. (Fabaceae) em diferentes ambientes no semiárido do brasil, Brazil. J Dev. https://doi.org/10.34117/bjdv7n3-598
Dray S, Dufour A (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22:1–20. https://doi.org/10.18637/jss.v022.i04
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, 4th edn. Addison Wesley Longman, Harlow
Fernández MC (2020). Variabilidad morfológica de las vainas y propiedades nutricionales de la harina de Prosopis flexuosa DC y Prosopis chilensis (Mol.) Stuntz, comparación entre diferentes procedencias de la provincia fitogeográfica del Monte. Tesina, Universidad Nacional de Cuyo, Argentina
Fox J, Weisberg S (2011) An {R} Companion to applied regression, 2nd edn. Sage. http://socserv.socsci.mcmaster.ca/jfox/Books/Companion. Accessed 30 Aug 2021
Galera F (2000) Food and Agriculture Organization (FAO). Las especies del género Prosopis (Algorrobos) de América Latina con especial énfasis en aquellos de interés económico, http://www.fao.org/3/ad314s/ad314s00.htm. Accessed 25 Aug 2021
Gil AR (2013) Estructura forestal y estado de conservación de los bosques de Prosopis chilensis y Prosopis flexuosa (algarrobales) de la Depresión del Río Bermejo, noreste de San Juan. Tesina de Grado, Ingeniería en Recursos Naturales Renovables Facultad de Cs. Agrarias—Universidad Nacional de Cuyo, Argentina
Hajek P, Hertel D, Leuschner C (2013) Intraspecific variation in root and leaf traits and leaf-root trait linkages in eight aspen demes (Populus tremula and P. tremuloides). Front Plant Sci. https://doi.org/10.3389/fpls.2013.00415
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Houle D (1992) Comparing evolvability and variability of quantitative traits. Genetics 130:195–204
Hunziker JH, Saidman BO, Naranjo CA, Palacios RA, Poggio L, Burghardt AD (1986) Hybridization and genetic variation of Argentine species of Prosopis. For Ecol Manag 16:301–315
Ivancich HS, Lencinas MV, Martínez Pastur GJ, Soler Esteban RM, Hernández L, Lindstrom I (2012) Foliar anatomical and morphological variation in Nothofagus pumilio seedlings under controlled irradiance and soil moisture levels. Tree Physiol 32:554–564. https://doi.org/10.1093/treephys/tps024
Jombart T (2008) adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24:1403–1405. https://doi.org/10.1093/bioinformatics/btn129
Jombart T, Ahmed I (2011) adegenet 1.3-1: new tools for the analysis of genome wide SNP data. Bioinformatics 11:1403–1405. https://doi.org/10.1093/bioinformatics/btr521
Kafuti C, Bourland N, De Mil T, Meeus S, Rousseau M, Toirambe B, Bolaluembe P, Ndjele L, Beeckman H (2020) Foliar and wood traits covary along a vertical gradient within the crown of long-lived light-demanding species of the Congo basin Semi-deciduous forest. Forests 11:35. https://doi.org/10.3390/f11010035
Karlin UO, Coirini RO, Catalan L, Zapata R (1997) Especies arbóreas y arbustivas para las zonas áridas y semiáridas de América Latina. Serie: Zonas áridas y semiáridas N°12. Organización de las Naciones Unidas para la Agricultura y la Alimentación. Santiago, Chile, pp 3–63
Labraga JC, Villalba R (2009) Climate in the Monte Desert: past trends, present conditions, and future projections. J Arid Environ 73:154–163
Le Mâitre A, Mitteroecker P (2019) Multivariate comparison of variance in R. Methods Ecol Evol 10:1380–1392
Mantovan NG (2005) Variabilidad intraespecífica de Prosopis flexuosa DC. var. flexuosa en el monte: Estudio morfo-fisiológico. Dissertation. Universidad Nacional de Cuyo, Mendoza Argentina
Martin G, Chapuis E, Goudet J (2008) Multivariate QST–FST comparisons: a neutrality test for the evolution of the G matrix in structured populations. Genetics 180:2135–2149
MAyDS (Ministerio de Ambiente y Desarrollo Sostenible) (2017) Ley 26331 de Presupuestos Mínimos de Protección Ambiental de los Bosques Nativos. Informe de estado de implementación 2010–2015. Ministerio de Ambiente y Desarrollo Sostenible, Presidencia de la Nación, Bs. As., Argentina, p 37
Mc Guigan K (2006) Studying phenotypic evolution using multivariate quantitative genetics. Mol Ecol 15:883–896
Melo D, Garcia G, Hubbe A, Assis AP, Marroig G (2015) EvolQG—an R package for evolutionary quantitative genetics. F1000Research 4:925. https://doi.org/10.12688/f1000research.7082.3
Merila J, Crnokrak P (2001) Comparison of genetic differentiation at marker loci and quantitative traits. J Evol Biol 14:892–903
Mottura M, Finkeldey R, Verga A, Gailing O (2005) Development and characterization of microsatellite markers for Prosopis chilensis and Prosopis flexuosa and cross-species amplification. Mol Ecol Notes 5:487–489
Peri PL (2021) Uso sostenible del bosque: Aportes desde la Silvicultura Argentina. Pablo Luis Peri, Guillermo Martínez Pastur, Tomás Schlichter. 1a edición especial. Ciudad Autónoma de Buenos Aires. ISBN 978-987-46815-4-6 1. Bosques Nativos. 2. Silvicultura. 3. Desarrollo Sustentable. CDD 577.30982
Peterson RA, Cavanaugh JE (2019) Ordered quantile normalization: a semiparametric transformation built for the cross-validation era. J Appl Stat. https://doi.org/10.1080/02664763.2019.1630372
Poorter H, Niinemets U, Poorter K, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588. https://doi.org/10.1111/j.1469-8137.2009.02830.x
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, https://www.R-project.org/. Accessed 25 Aug 2021
Risk C, McKenney DW, Pedlar J, Lu P (2021) A compilation of North American tree provenance trials and relevant historical climate data for seven species. Sci Data 8:29. https://doi.org/10.1038/s41597-021-00820-2
Roig FA (1993a) Informe nacional para la selección de germoplasma de especies de Prosopis de la República Argentina. In: Unidades de Botánica y Fisiología Vegetal (IADIZA) (ed) Conservación y mejoramiento del género Prosopis. Contribuciones mendocinas a la quinta reunión regional para América Latina y el Caribe de la red de forestación CIID. Mendoza, Argentina: IADIZA-CRICYT-CIID, pp 1–36
Roig FA (1993b) Aportes a la etnobotánica del Género Prosopis. In: Unidades de Botánica y Fisiología Vegetal (IADIZA) (ed) Conservación y mejoramiento del género Prosopis. Contribuciones mendocinas a la quinta reunión regional para América Latina y el Caribe de la red de forestación CIID. Mendoza, Argentina: IADIZA-CRICYT-CIID, pp 99–119
Roser LG (2015) Genética del paisaje en poblaciones de Prosopis alba de la provincia de Santiago del Estero. Dissertation, Universidad de Buenos Aires, Argentina
Roser LG, Ferreyra LI, Ewens M, Vilardi JC, Saidman BO (2014) Genetic and morphometric characterization of clones of Prosopis alba, Algarobia, selected for salt tolerance. Tree Genet Genomes. https://doi.org/10.1007/s11295-013-0693-x
Spitze K (1993) Population structure in Daphnia obtuse: quantitative genetic and allozymic variation. Genetics 135:367–374
Teich I, Cosacov A, Lopez Lauenstein D, Vega C, Sersci A (2017) Variabilidad morfológica y diferenciación de entidades en un complejo de especies de Prosopis en el Gran Chaco Americano. Actas II Reunión Argentina Biol Evol 76
Udayakumar M, Sekar T (2021) Leaf traits of trees in tropical dry evergreen forests of Peninsular India. Ecologies 2:268–284. https://doi.org/10.3390/ecologies2030015
Valladares F, Gianoli E (2007) How much ecology do we need to know to restore mediterranean ecosystems? Restor Ecol 15:363–368
Vázquez-Garcidueñas S, Palacios RA, Segovia-Quiroz J, Frías-Hernández JT, Olalde-Portugal V, Martínez-De La Vega O, Mollard FPO, Vázquez-Marrufo G (2003) Morphological and molecular data to determine the origin and taxonomic status of Prosopis chilensis var. riojana (Fabaceae, Mimosoideae). Can J Bot 81:905–917
Verga A (1995) Genetische Untersuchungen an Prosopis chilensis und P. flexuosa (Mimosaceae) im trockenen Chaco Argentiniens. Gottingen Research Notes in Forest Genetics. Abteilung fur Forstgenetik und Forstpflanzenzuchtung der Universitat Gottingen. ISSN 0940-7103, Nro. 19
Verga A (2000) Clave para la identificación de híbridos entre Prosopis chilensis y P. flexuosa sobre la base de caracteres cuantitativos. Multequina 9:17–22
Vega CD, Aguilar D, Bessega C, Acosta MC, Cosacov A, Ewens M, Vilardi JC, Sérsic AN, Teich A, Verga A (2021) Genetic Variation Patterns of “Algarrobos” from the “Great American Chaco” (Prosopis alba, P. nigra, P. hassleri, P. fiebrigii, P. ruscifolia, P. chilensis, and P. flexuosa). In: Low intensity breeding of native forest trees in Argentina. Springer, pp 245–269
Villagra PE, Cony MA, Mantován NG, Rossi BE, González Loyarte MM, Villalba R, Marone L (2004) Ecología y manejo de los algarrobales de la Provincia Fitogeográfica del Monte. Editorial de la Universidad Nacional de La Plata (EDULP)
Acknowledgements
This research was funded by UNIVERSIDAD DE BUENOS AIRES, UBACYT 20020190200106BA given to C. Bessega. We thank Gualberto Zalazar for his valuable help during sampling in El Sauce progeny trial, Mendoza, Argentina.
Funding
This work was supported by Universidad of Buenos Aires (UBACYT 20020190200106BA) given to Cecilia Bessega.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Sampling and material preparation were performed by CB and MC. Data collection and data analysis were performed by CB, JCV, BS and CP. Funding acquisition was done by CB. The first draft of the manuscript was written by CB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The authors guarantee compliance with ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bessega, C., Vilardi, J.C., Cony, M. et al. Low genetic variation of foliar traits among Prosopis chilensis (Leguminosae) provenances. J Plant Res 135, 221–234 (2022). https://doi.org/10.1007/s10265-022-01378-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-022-01378-9