Abstract
We conducted an exhaustive study of gene expression in fig fruits to identify the gene complexes responsible for fundamental fruit physiology and phenotypic differences between ecotypes. We performed high-throughput pyrosequencing on cDNA libraries constructed from caprifig and common fig fruits and compared their transcriptomes by analyzing the expressed sequence tags obtained. We collected a total of 290,594 expressed sequence tag reads from the two fruit types and assembled them into 71,455 unigenes (19,166 contigs and 52,289 singletons). We identified many metabolic genes, including those encoding proteins in the ethylene, glucose, and anthocyanin synthesis pathways that are involved in fruit maturation. This set also contained unigenes with unidentified functions. We observed no significant differences between the fruit types with respect to Gene Ontology term representation. By reverse transcription polymerase chain reaction, however, we detected several polymorphisms at the level of individual genes. Inter-type variations with respect to the expression level or transcription product size were observed in B- and C-class MADS-box gene homologs and chalcone synthase homologs, which are believed to be involved in sexuality and parthenocarpy, respectively. Expression polymorphisms were also observed for other genes, including a gibberellin-regulated protein gene. Our data and results contribute to genetic research on fig fruits and will aid in the understanding of fruit physiology and mechanisms of phenotypic differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fig (Ficus carica L.) is classified in the Cronquist system as a genus of the family Moraceae in the order Utricales. More than half of the species in the Moraceae belong to the Ficus genus (Datwyler and Weiblen 2004), and fig is the most common species among them.
Fig is a gynodioecious plant with two major sex types. The caprifig (hermaphroditic) type, the presumptive ancestral species, has male flowers and long-style female flowers, whereas the fig type (female) has only short-style female flowers (Beck and Load 1988; Dellaporta and Calderon-Urrea 1993; Stover et al. 2007). The female fig type is further classified into three types according to its cultivation type: the Smyrna type is non-parthenocarpic, the San Pedro type is parthenocarpic in the first crop but not in the second crop, and the common type is parthenocarpic in both first and second crops (Storey 1975) (Fig. 1a). Every fig species thus falls into one of the four ecotypes: caprifig, Smyrna, San Pedro, or common.
Diagrammatic representation of gynodioecious fig (Ficus carica L.) fruit-type differentiation and analyzed fruits. a Fig taxonomy matrix based on parthenocarpy and sex traits. The thick double-headed arrow indicates the comparison undertaken in this study. The dotted double-headed arrow indicates the parthenocarpy range of caprifig type. b Left: Caprifig 6085 first crop (caprifig type), right: Houraishi second crop (common type). The maturity stage of the displayed fruits was between periods II and III. Bar = 2 cm
Phenotypic differentiation in traits associated with sexuality and parthenocarpy plays an important role in plant–insect ecosystems as well as in our agricultural history. The differentiation between the hermaphroditic and female strains forms a basis to maintain the close symbiotic relationship between Ficus plants and the Blastphaga wasp (Galil 1977; Wiebes 1979). The appearance of a trait for parthenocarpy suggests the possible first cultivation by humans; the fig may be the earliest cultivated plant (Kislev et al. 2006).
Economically, the fig is an important fruit tree grown mainly in Mediterranean countries, such as Turkey, Egypt, and Iran, but also elsewhere (FAO 2006). The fig plant is of value mainly for its edible fruit, particularly that of the sexual species (female fig type). This fruit has a unique morphology (hypanthodium) with countless small flowers contained within the fruit receptacle or syconium. The edible parts are the torus and the small flowers; these parts are consumed mainly in the dried form (as dried figs) or as processed or fresh fruit. Dried figs contain a high mineral and fiber content and are regarded as among the most convenient and nutritious preserved foods (Vinson et al. 2005). Recent studies have reported that anthocyanins such as cyanidin-3-rhamnoglucoside contained in fig fruits have antioxidant potential, possibly preventing fibroblast oxidation (Solomon et al. 2006; Duenas et al. 2008). The ripening process of fig fruits is climacteric (Watkins 2002), and, as in other climacteric fruits, ethylene hastens the ripening process (Owino et al. 2006). Because fig fruits dramatically increase their ripening speed in the brief period at the end of the growth stage, fruit quality control is a major issue in pre- and post-harvest management.
Fig fruits have many traits of physiological and economic importance. However, owing to the limited molecular details (in January 2012, the keyword “Ficus carica” retrieved 509 records in a National Center for Biotechnology Information (NCBI) “Nucleotide” search), the genetic structure underlying the traits of fig fruits is not well understood. In addition, there is no ongoing large-scale molecular research. Among the four fig ecotypes, the non-parthenocarpic caprifig type differs substantially from the common type, while the other types fall morphologically between these two types. For this reason, a large-scale comparative study of gene expression in the non-parthenocarpic caprifig and common strains should provide comprehensive data on gene expression in terms of fruit physiology and also elucidate the genetic factors underlying the traits involved in type differentiation.
The primary objective of this study was to obtain comprehensive and large-scale expressed sequence tag (EST) data to serve as a basis for the genetic understanding of fig fruit physiology. This was accomplished by 454 pyrosequencing, for the rapid generation of large genetic data sets, on non-parthenocarpic caprifig-type and parthenocarpic common-type fruits. As sequences generated from 454 pyrosequencing are highly accurate and longer than those from other platforms, 454 pyrosequencing has been utilized for the transcriptome analyses of non-model plant species, such as grapevine (Vitis vinifera L.) (Bellin et al. 2009), olive (Olea europaea L.) (Alagna et al. 2009), chestnut (Castanea spp.) (Barakat et al. 2009), and chickpea (Cicerarietinum L.) (Garg et al. 2011). Our second objective was to compare the transcriptomes of the two types to obtain molecular information on the genetics underlying the polymorphic traits, in particular the genes governing sexuality and parthenocarpy.
Material and methods
Plant materials and RNA extraction
We used a 15-year-old Caprifig 6085 (caprifig type; accession: JP number 113491) tree and a 24-year-old Houraishi (common type) tree for EST analyses.
Caprifig 6085 is a hermaphroditic strain introduced to Japan in the mid-20th century. It shows little parthenocarpy at the first crop (approximately 10 % bearing), and at the second crop (approximately 0 % bearing) (Awamura et al. 1996). Houraishi is a highly productive cultivar and the oldest representative variety in Japan (Ikegami et al. 2009a). Both plants were provided by the former Fruit Tree Experiment Station (Tsukuba, Japan) and planted in the Fukuoka Agricultural Research Center, Buzen Branch (Fukuoka, Japan; Fig. 1b).
Sample fruits were harvested in 2009 and 2010. The first crop was harvested from the caprifig (non-parthenocarpy) type and the second from the common (parthenocarpy) type. To obtain gene expression data during the maturation period, fruits were harvested at the end of period II, when second rapid fruit growth and ethylene production start. In general, fig fruit development is divided into three periods based on changes in fruit size. In the first stage, intense cell division, differentiation and rapid growth occur (period I). A large period of stasis follows (period II) and a second phase of rapid growth occurs (period III), in which cell expansion and a change of color and texture are observed (Chessa 1997; Owino et al. 2006). Two harvested fruits for each type were sliced vertically and preserved at −80 °C following snap-freezing with liquid nitrogen.
Library preparation and 454 sequencing
The preserved fruits were ground in liquid nitrogen, and subjected to total RNA extraction, combining Fruit-mate (TakaraBio, Inc., Shiga, Japan) and an RNeasy Maxi kit (Qiagen, Hilden, Germany) (Ikegami et al. 2009b). Purified poly(A)-RNA was then obtained from the total RNA using a MicroPoly(A)Puristkit (Ambion, Carlsbad, CA). We used a cDNA Synthesis System kit (Roche, Penzberg, Germany), Roche “random primers” (Roche), and aGS FLX Titanium Rapid Library Preparation kit (Roche) to synthesize cDNA and prepare the library, which we then sequenced with a GS FLX Titanium Sequencer (Roche) following the manufacturer’s instructions.
Sequence quality controls and de novo assembly
Pre-processing and assembly were carried out as described by Habu et al. (2012). The raw reads obtained from 454 pyrosequencing were processed by Seqclean software (http://compbio.dfci.harvard.edu/tgi/software/) to trim low complexity [poly(A)] sequences. Then, the reads were further processed by RepeatMasker (Smit et al. 1996–2000) (http://www.repeatmasker.org) with RepBase (Jurka et al. 2005) to mask the repeat sequences to avoid mis-assembly. Finally, masked reads were processed by a Perl script as follows: (1) low-quality regions were masked, (2) masked regions of both ends were trimmed, (3) reads that were shorter than ten bases were removed, and (4) reads that contained more than 30 % of the masked regions were removed. We then ran MIRA v3.0.2 for sequence assembly, specifying the stringent parameter settings “de novo, accurate, EST, 454” with a minimum read length of 40 bases, minimum sequence overlap of 40 bases, and minimum percentage overlap identity of 95 %.
Sequence annotation and estimation
The assembled contigs and singletons were annotated with information from the NCBI non-redundant protein database and from the Arabidopsis Information Resource protein database (TAIR10) using the BLASTx program v2.2.24+ by cut-off E values of 1e−5, 1e−10 or 1e−100 with other default parameters (Altschul et al. 1990). The assembled unigene sequences were also annotated with functional Gene Ontology (GO) (The Gene Ontology Consortium 2000) using Blast2GO tool (http://www.blast2go.com/b2ghome). Then, the assigned GO terms were classified based on the GO slims (http://www.geneontology.org/GO.slims.shtml) by CateGOrizer (Hu et al. 2008). A statistical comparison of GO distributions in the two fruit types was performed with the R program using Fisher’s Exact Test. EC numbers were obtained from KEGG ENZYME and UniProt ENZYME.
To check whether each unigene was full length or not, ORFs of unigenes were predicted by ESTScan (Iseli et al. 1999) and the predicted ORF sequences that contained both start and stop codons were extracted as candidates for full-length sequences.
Identification of maturation-related gene pathways
We searched for metabolic pathway genes involved in maturation, including the ethylene, sugar, and anthocyanin synthetic pathways, using the local BLAST command of GENETYX (Genetyx Corporation, Tokyo, Japan) using Arabidopsis genes as queries and the unigene set as the target database.
Gene expression analysis by reverse transcription polymerase chain reaction
We extracted total RNA from fig fruits at stages I and II using Fruit-mate (Takara Bio, Inc.) and an RNeasy Plant Mini kit (Qiagen) with DNase I treatment (Takara Bio, Inc.). For reverse transcription polymerase chain reaction (RT-PCR) template synthesis, we used a SuperScriptIII First-Strand Synthesis System (Life Technologies, Inc., Carlsbad, CA) to generate cDNA from 80 ng of total RNA. The PCR reaction mixtures were prepared in a 12.5 μl volume containing 0.25 U AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA), 0.5 μM primers, 2.0 mM dNTPs, 1× PCR buffer and 1 μl of cDNA as template. The PCR amplification reaction was carried out as follows: 94 °C for 2 min followed by 38 to 40 cycles of 94 °C for 1 min, 68 °C for 2 min, and 72 °C for 2 min. The final extension was performed at 72 °C for 7 min. Gene-specific primers were designed using GENETYX based on the acquired EST sequences (Electronic supplementary material (ESM) Supplemental Tables 1 and 2). A primer pair specific for the F. carica β-actin gene (accession:AY487315.1) was used as the endogenous control.
Results and discussion
Pyrosequencing and de novo assembly
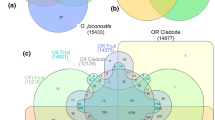
Using pyrosequencing, we generated 290,594 reads, with 165,442 from the caprifig and 125,152 from the common type (Table 1). Read quality was high, with an average read length of more than 300 bp and a QV40+ value of over 94 for each fruit type. After pre-processing, we obtained a total of 270,268 reads, with 155,391 from the caprifig and 114,877 from the common type. We first assembled these pre-processed reads for each type and obtained 62,420 unigenes (17,454 contigs and 44,966 singletons) for caprifig and 49,491 unigenes (12,771 contigs and 36,720 singletons) for the common type. We then assembled the combined set and obtained 19,166 contigs and 52,289 singletons for a total unigene number of 71,455 (Table 1, Fig. 2). The subsequent analysis used this combined unigene set.
Strategy for the assembly and identification of type-specific transcripts in gynodioecious fig (Ficus carica L.) fruits. We first generated 290,594 total reads, with 165,442 from the caprifig and 125,152 from the common type using pyrosequencing. After pre-processing and assembly, we obtained a total of 270,268 reads and 71,455 unigenes with 19,166 contigs, and 52,289 singletons. We also assembled these pre-processed reads for each type and obtained 62,420 unigenes for caprifig and 49,491 unigenes for the common type. A statistical comparison of GO term distributions was conducted between each type’s unigene set. Functional annotations and extractions of type-specific expressed genes were performed using the total unigene set
Function and feature annotation
We functionally annotated the unigenes using three parameters: BLASTx match, GO term, and EC number. The hit rates of the BLAST search against the NCBI non-redundant and TAIR10 databases (at a threshold E value < 1e−5) were 60.6 and 57.8 %, respectively, while that for the EC number was 12.7 %. The GO slim terms for biological process, cellular component, and molecular function could be assigned to 34.9, 30.4, and 38.3 % of the unigene set, respectively. Of the annotated processes, for example, “metabolism” accounted for 15.09 %, and “biosynthesis” for 5.96 %. GO processes accounting for less than 5 % of the unigenes composed nearly half of the annotated set, while “biological process unclassified” composed 32.82 % (Fig. 3). These results suggest the expression of a wide range of genes with various processes and many unclassified genes in fig fruits. In total, 46.5 % of the unigene set were assigned at least one GO slim term. The number of unigenes annotated by BLAST, GO term or EC number was 44,070 and the total proportion annotated was 61.7 % (Table 2, Fig. 2).
By ESTScan, a total of 38,308 (53.6 %) unigenes could be predicted ORFs but only 1,303 (1.8 %) unigenes were candidates for full-length sequences. Among the candidates, 411 (0.6 %) unigenes were covered more than 80 % of the length of the most homologous Arabidopsis protein sequences (Table 2). Although our data contains few full-length sequences, many partial gene sequences obtained in this study will be useful for cloning full-length sequences and for expression analyses of unidentified genes in fig.
Major genes expressed in fig fruits
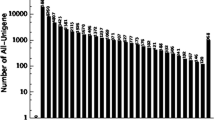
To identify the major genes expressed in fig fruits at the late period II, we extracted the 20 contigs that contained the largest number of reads in the unigenes (Table 3). The extracted list contained 1-aminocyclopropane-1-carboxylate oxidase (ethylene-forming enzyme), pectin lyase, beta-galactosidase, and expansin, and it confirmed the active expression of known maturation-related gene complexes in fruits. Highly expressed genes not related to maturation included the nucleotide-binding site leucine-rich repeat (NBS-LRR) family genes that function in DNA repair and disease resistance and the plasma membrane intrinsic protein 1C (PIP1C) genes that promote symplasticwater transport. These genes are also presumed to be required for fruit development.
Fruit ripening involves many biochemical events such as changes in, sugar content, acidity, color, texture, and aroma volatiles. These changes are controlled by the coordinated expression of maturation-related gene complexes (Bouzayen et al. 2010). Elucidation of the control mechanisms involved in individual events will be of great significance to the understanding of fig fruit physiology. We have summarized below our results obtained by extracting gene complexes for specific pathways related to fruit maturation utilizing gene conservation across fruit species.
Ethylene synthesis and signal transduction
The first stage of the fruit maturation process is ethylene synthesis. As recognition of synthesized ethylene by receptors is followed by many downstream maturation processes via the ethylene signaling pathway (Ohme-Takagi and Shinshi 1995; Solano et al. 1998; Riechmann et al. 2000; Klee 2004; Alba et al. 2005; Gupta et al. 2006; Kesari et al. 2007), ethylene synthesis and signaling are fundamental to the fruit maturation process. Accordingly, we first tried to detect the sequences of genes involved in the ethylene synthetic and signaling pathways in our unigene set. We were able to identify sequence fragments of all of the major genes ranging from SAMS at the beginning of the synthetic pathway down to ERF1 at the end of the signaling pathway (Fig. 4). Many reads were detected in this pathway as well as in the downstream glucose and anthocyanin synthesis pathways, suggesting that both early and late fruit maturation processes were simultaneously active in the sampled fruits.
Ethylene synthesis and signal transduction in F.carica fruit as inferred from Giovannori (2004) and Adams-Phillips (2004). The boxes show genes encoding enzymes that were isolated from fig fruit ESTs in this study (BLASTx, e–05 cut-off). The first and second numbers in the parentheses refer to the number of contigs and singletons, respectively. SAMS, S-adenosylmethionine synthase; ACS, 1-aminocyclopropane-1-carboxylate synthase; ACO, neutral invertase; SS, sucrose synthase; ETR1, ethylene response 1; ETR2, ethylene response 2; ERS1, ethylene response sensor 1; ERS2, ethylene response sensor 2; EIN4, ethylene insensitive 4; CTR1, constitutive triple response1; EIN2, ethylene insensitive 2; EIN3, ethylene insensitive 3; ERF1, ethylene response factor 1
Sugar synthesis pathway
Among downstream pathways, the understanding of the sugar, anthocyanin synthesis and cell-wall degradation pathways are of great importance for fruit quality management, because these pathways are directly linked to factors such as taste, appearance, and softening. Among the cell-wall degradation pathways, Owino et al. (2006) had already focused on cell wall modifying enzymes during fruit ripening. For these reasons, we extracted genes related to the sugar and anthocyanin synthesis pathways.
The major glucose content in fig fruits has been reported to comprise sucrose, glucose, and fructose (Yahata and Nogata. 1999). Our unigene set contained the synthase genes for all these saccharides (Fig. 5) plus the galactose synthase genes reported by Ersoy et al. (2007). Besides these saccharides synthases, we sought to extract genes encoding the sugar transporters known to be important in unloading from leaves to fruits and glucose storage in vacuoles (Yamaki 2010). We identified at least four homologous genes including SUT (a sucrose transporter), SORT (a sorbitol transporter), MANT (a mannitol transporter), and HEXT (a hexose transporter) (Fig. 5). In other fruit trees, translocated saccharides include sucrose, sorbitol, raffinose, stachyose, and mannitol (Ziegler 1975; Yamaki 2010). In the fig tree, the saccharide types translocated may correspond to the genes encoding the sugar transporters detected in this study.
The sugar metabolism pathway in F. carica fruit. The boxes show genes encoding enzymes that were isolated from fig fruit ESTs in this study (BLASTx, e–05 cut-off). The first and second numbers in the parentheses refer to the number of contigs and singletons, respectively. NADP-SDH, NADP-dependent sorbitol dehydrogenase; NAD-SDH, NAD-dependent sorbitol dehydrogenase; NIN, neutral invertase; SS, sucrose synthase; VIN, vacuolar invertase; SUCT, sucrose transporter; SORT, sorbitol transporter; MANT, mannitol transporter; HEXT, hexose transporter
Anthocyanin synthesis pathway
Anthocyanins synthesized in fig fruits include cyanidin and pelargonidin, with a higher proportion of cyanidin in both the fruit skin and florets (Duenas et al. 2008). The cyanidin synthesis pathway genes identified in our study are shown in Fig. 6. We identified all the enzymatic genes, including phenylalaninecyanidin-3-rhamnoglucoside and cyanidin-3-glucoside. However, we did not identify flavonoid 3′ 5′ hydroxylase, which catalyzes the conversion of dihydroquercetin to dihydromyricetin in the delphinidin synthesis pathway. This supports the inactivity of the flavonoid 3′ 5′ hydroxylase pathway and is consistent with previous studies of fig fruit biochemical mechanisms (Solomon et al. 2006; Del Caro and Piga 2008; Duenas et al. 2008). Fig fruits have a wide variety of skin colors ranging from dark purple, purple, red, pink, and green to yellow. Further structural analyses and gene expression studies of the anthocyanin synthesis pathway genes such as those conducted in other studies (Kobayashi et al. 2004; Xie et al. 2011) may elucidate the formation mechanisms of these various skin colors.
Anthocyanin and anthocyanidin biosynthesis in F.carica fruit. The boxes show genes encoding enzymes that were isolated from fig fruit ESTs in this study (BLASTx, e–05 cut-off). The first and second numbers in the parentheses refer to the number of contigs and singletons, respectively. MYB, Myb transcription factors; PAL, phenylalanine ammonia lyase; 4CL, 4-coumarate-CoA ligase; C4H, cinnamate 4-monooxygenase (trans-cinnamate 4-monooxygenase); CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavonone 3-hydroxylase; F3′H, flavonoid 3′-hydroxylase; DFR, dihydroflavonol-4-reductase; LDOX, leucoanthocyanidin dioxygenase; 3GT, flavonoid 3-glycosyl-transferase; 3RT, anthocyanidin-3-glucoside rhamnosyltransferase
Comparison of GO term distribution
Classification of ecotypes such as the caprifig and common types is based mainly on sexuality and parthenocarpy. According to the segregation data obtained from inter-type hybridizations, the inheritance of sexuality and parthenocarpy has been explained by one or two genes (sexuality: GA/ga, with two closely linked pairs of alleles; parthenocarpy: P+, with a single pair of alleles) (Storey 1975; Saleeb 1965; Awamura 1996). Type differentiations can thus be considered to be controlled by this limited number of genes. However, specific differences between the caprifig and common type at the whole transcriptome level remain unknown. We thus compared GO terms representative of each type.
As a result, there was no significant difference observed in the distributions of GO terms (ESM Supplemental Fig. 1). This suggests that the period II fruits of the two types do not differ in their macro-level transcriptome expression patterns, but rather only in the expression of a relatively small set of genes. Therefore, we then studied the expressions of the sexuality and parthenocarpy related-genes by semi-quantitative RT-PCR.
Type-specific expression analysis of MADS-box genes
As stated above, two linked alleles (GA/ga) are involved in the sexual traits of fig fruits, where allele G is responsible for pistil length and allele A is responsible for the presence/absence of stamens (Storey 1975). However, the physiological identities of these genes are unknown. The ABCDE model describes a relationship between floral organ formation and gene expression (Ferrario et al. 2003; Theissen2001; Theissen and Saedler 2001), and its relevance to sexual traits has been examined in various plants (Kater et al. 2001; Sather et al. 2010; Park et al. 2003; Elo et al. 2001; Yu et al. 1999; Sheppard et al. 2000; Ainsworth et al. 1995; Hardenack et al. 1994; Heuer et al. 2001). We thus examined how well this model is conserved in the caprifig and common type fruits.
This was accomplished by extracting nine MADS genes corresponding to each class of the ABCDE model (ESM Supplemental Table 2) and analyzing their expression level by RT-PCR in stages I and II for each fruit type. While no polymorphic expression was observed for either stage or type for the genes in classes A, D, and E, there were clear differences in the expression levels of B- and C-class genes. The expression levels of the PISTILLATA homologs PI1 and PI2 (B class) in the caprifig type at stage II were 1.7 to 5.0 times higher than those in the common type. A polymorphism in the amplicon size was observed for the AGAMOUS homolog AG (C class), in which the common fig amplicon size was slightly smaller than caprifig amplicon size (Fig. 7).
RT-PCR analysis of ABCDE model MADS family genes in F.carica fruit (stages I and II). The abbreviations refer to fig homologs of each Arabidopsis MADS gene: AP1, APETALA1; AP2, APETALA2; AP3, APETALA3; PI1, PISTILLATA1; PI2, PISTILLATA2; AG, AGAMOUS; SHP, SHATTERPROOF; STK, SEEDSTICK; SEP1, SEPALLATA1; SEP3, SEPALLATA3; AGL6, AGAMOUS-like 6. Additional abbreviations: M, 100 bp molecular weight marker; Actin, β-actin. cp, caprifig type; cm, common type. The number under each lane indicates relative expression measured by AlphaEaseFC software v4.0.1 (Alpha Innotech, corp., SA)
Two alternative mechanisms account for the development of unisexuality: the degeneration of sexual organs or exclusive differentiation of only one type of sex organs (Heslop-Harrison 1964). Given that hermaphroditic fruits transition from the female period to the male period (Ramirez 1974), we suspected that figs fall into the former type and that different activities of the MADS box gene complexes are not the direct causes of sexual differentiation. These differences in activities are considered side effects of the sex determination process (Golenberg and Freeman 2006). This conjecture was supported by our finding that the expression level of the PI homologs between the two types was the same in period I, but different in period II.
Nevertheless, it is likely that the PI homologs are important genes in caprifig stamen formation because they showed distinct polymorphic expression. The relevance to the sexuality of the expression–product polymorphism of the AG homolog is unknown, but the variety of functional roles played by the C-class genes in floral-organ formation (Drews et al. 1991; Mizukami and Ma 1992; Busch et al. 1999; Lohmann et al. 2001) suggests many possibilities. To understand the physiological basis of the G and A genes, we need to examine the involvement of the PI and AG homologs further.
Type-specific expression analysis of the gibberellin and chalcone synthase genes
For the parthenocarpic traits of figs, the relevance of fruit auxin and gibberellin concentrations has been investigated previously (Crane et al. 1959; Lodhi et al. 1969). We accordingly focused on eight homologs (one GA20ox, two GID1, one GAMYB1, and four DELLAs) of the gibberellin synthesis genes, whose roles in the genetic pathway are well-known, and conducted inter-type comparative analyses of their respective gene-expression patterns in period II fruits. However, we did not observe any clear polymorphisms among these homologs (data not shown).
Previous studies have reported that RNA interference-mediated suppression of the chalcone synthase (CHS) genes induced parthenocarpy in tomatoes, and that fruits with RNA interference-mediated downregulation of CHS displayed impaired pollen tube growth (Schijlen et al. 2007). Moreover, overexpression of the grape-derived stilbene synthase (STS) gene induced male sterility and parthenocarpy in tomatoes due to the depletion of coumaric and ferulic acids, which are necessary for lignin and sporopollenin biosynthesis (Ingrosso et al. 2011). Interestingly, a CHS homologous gene is known to lie upstream of the genes that expressed specifically in the common type (ESM Supplemental Tables 3 and 4.). Our analysis of CHS homologs by RT-PCR detected no polymorphisms in period I fruits. However, in period II fruits, we detected a new smaller sized transcriptional product in the common type, and its transcripts had lower total expression levels (Fig. 8).
RT-PCR analysis of Arabidopsis chalcone synthase (CHS)-homologous genes in F.carica fruit (stages I and II). M, 100 bp molecular weight marker; Actin, β-actin; cp, caprifig type; cm, common type. The number under each lane indicates relative expression measured by AlphaEaseFC software v4.0.1 (Alpha Innotech, corp., SA)
CHS and STS are similar in their mechanistic and structural aspects (Yamaguchi et al. 1999) and they are believed to be involved in pollen development and plant reproduction through flavonoid-synthesis metabolism (Mo et al. 1992; Ylstra et al. 1994; Hanhineva et al. 2009). It is thus possible that the polymorphic expression that we observed triggers changes in flavonoid-synthesis metabolism and governs parthenocarpy. We are currently investing the causes of the polymorphism by screening for CHS gene clones in a genomic library derived from the common type “Houraishi”.
Screening for new type-specific transcripts
It is likely that genes not mentioned above are also important factors for sexual and parthenocarpic traits and that genes showing polymorphic expression between the types are not limited to those controlling sexuality and parthenocarpy. Such genes may be studied using known genetic information as well as by searching for genes showing type-specific expression. We extracted the contigs consisting of the reads derived from either caprifig or common type based on the count data, so that 4,844 contigs were specific to the caprifig type and 2,260 were specific to the common type (ESM Supplemental Tables 3 and 4).
To evaluate the validity of the extracted gene lists, we randomly selected 18 ESTs (ten caprifig-specific and seven common-specific) and investigated them by RT-PCR. We observed significant inter-type differences in five genes in either expression level or in transcription-product size (Fig. 9, ESM Supplemental Table 1). The size and amount of transcription products varied for homologs of the pectin lyase-like superfamily protein, heavy metal transport protein, plant cadmium resistance protein and gibberellin-regulated protein. Expression in only one type was observed in nucleotide-binding site-leucine-rich repeats. The fact that five of the 18 tested genes were confirmed to be type specific shows that the extracted gene lists are useful for finding genes differentially expressed between types.
RT-PCR analysis of 18 subtracted genes in caprifig and common fig fruits (stages I and II). The analyzed genes were preferentially selected from the subtraction lists on the basis of their read number ranking. The abbreviations refer to fig homologs of Arabidopsis genes: PL, pectin lyase-like superfamily protein; MFS-1, major facilitator superfamily-1; AT, HXXXD-type acyl-transferase family protein; PE, pectin methylesterase inhibitor superfamily; bHLH, basic helix-loop-helix DNA-binding superfamily protein; SKU5, SKU5 similar 13; HMT, heavy metal transport; PCR11, plant cadmium resistance 11; TPC, terpenoid cyclases; GR, gibberellin-regulated family protein; MFS2, major facilitator superfamily protein 2; PP2C, highly ABA-induced PP2C gene 1; PAR1, PAR1 protein; COP1, COP1-interactive protein 1; NBS-LRR, disease resistance protein (CC-NBS-LRR class) family; XYL1, beta-xylosidase 1; PR; pathogenesis-related family protein. Additional abbreviations: M, 100 bp molecular weight marker; cp, caprifig type; cm, common type. The number under each lane indicates relative expression measured by AlphaEaseFC software v4.0.1 (Alpha Innotech, corp., SA)
It is known that pectin lyase softens fruits by breaking down pectin, while the heavy metal transport protein plays important roles in homeostatic maintenance of essential trace elements and heavy metal detoxification (Nelson 1999; Thomine et al. 2000). With regard to the gibberellin-regulated protein, its target gene product gibberellin is known to widely control traits including intercalary elongation, vegetative growth, and reproductive growth. There could be variations in the homolog functions or the splicing processes of these three genes that lead to functional differences in fig fruits. The plant cadmium resistance protein, like the heavy metal transport protein, is related to heavy metal transportation. The polymorphic expression of these two genes with similar functions may suggest characteristics of metal transportation specific to each type. The nucleotide-binding site-leucine-rich repeat gene is believed to have disease-resistance functions such as pathogen recognition or host defense (DeYoung and Inne 2006). This polymorphic expression also suggests differences in the disease resistance of each type.
A comprehensive search for type-specific genes would require, in addition to RT-PCR, a large-scale screening method such as microarray analysis or deeper RNA-sequencing. In the present study, we analyzed only one strain for each type. Therefore, a confirmatory studies using other varieties would also be required to corroborate inter-type polymorphisms.
Conclusion
Using high-throughput sequencing, we extracted and identified gene complexes including genes regulating maturation, expressed in fig fruits. Our GO term analysis did not detect a significant difference between the fruit types, suggesting that genetic differences between the types are not expressed over the entire transcriptome in the tested fruits. Polymorphic expression was detected for several genes including CHS, PI, AG homolog genes, and gibberellin-regulated protein. The CHS gene is of special interest in understanding the induction of parthenocarpy owing to its putative role in the origin of plant domestication.
Because there would be other genes that were not analyzed in the present study but that contribute to trait differentiation among the types and varieties, further studies using large-scale screening are recommended. However, the EST data that we generated in this study, our arrangement of maturation-related genes and our findings of inter-type polymorphism will contribute to the elucidation of the physiological traits of fig fruits and thus to the study of fig genetics.
References
Adams-Phillips L, Barry C, Giovannoni J (2004) Signal transduction systems regulating fruit ripening. Trends Plant Sci 9:331–338. doi:10.1016/j.tplants.2004.05.004
Ainsworth C, Crossley S, Buchanan-Wollaston V, Thangavelu M, Parker J (1995) Male and female flowers of the dioecious plant sorrel show different patterns of MADS box gene expression. Plant Cell 10:1583–1598. doi:10.1105/tpc.7.10.1583
Alagna F, D’Agostino N, Torchia L, Servili M, Rao R, Pietrella M, Giuliano G, Chiusano ML, Baldoni L, Perrotta G (2009) Comparative 454 pyrosequencing of transcripts from two olive genotypes during fruit development. BMC Genomics 10:399. doi:10.1186/1471-2164-10-399
Alba R, Payton P, Fei Z, McQuinn R, Debbie P, Martin GB, Tanksley SD, Giovannoni JJ (2005) Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. Plant Cell 17:2954–2965. doi:10.1105/tpc.105.036053
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Awamura M, Shoda K, Yahata D (1996) Effect of various seed parents on frequency distribution of parthenocarpy among seedling progenies of fig (Ficus carica L.). J Japan Soc Hort Sci 65:21–26. doi:10.2503/jjshs.65.21
Barakat A, DiLoreto DS, Zhang Y, Smith C, Baier K, Powell WA, Wheeler N, Sederoff R, Carlson JE (2009) Comparison of the transcriptomes of American chestnut (Castanea dentata) and Chinese chestnut (Castanea mollissima) in response to the chestnut blight infection. BMC Plant Biol 9:51. doi:10.1186/1471-2229-9-51
Beck NG, Load EM (1988) Breeding system in Ficus carica, the common fig. II. Pollination events. Am J Bot 75:1913–1922
Bellin D, Ferrarini A, Chimento A, Kaiser O, Levenkova N, Bouffard P, Delledonne M (2009) Combining next-generation pyrosequencing with microarray for large scale expression analysis in non-model species. BMC Genomics 10:555. doi:10.1186/1471-2164-10-555
Bouzayen M, Latché A, Nath P, Pech JC (2010) Mechanism of fruit ripening. In: Pua EC, Davey MR (eds) Plant developmental biology—biotechnological perspectives, 1st edn. Springer, Heidelberg, pp 319–339
Busch MA, Bomblies K, Weigel D (1999) Activation of a floral homeotic gene in Arabidopsis. Science 285:585–587. doi:10.1126/science.285.5427.585
Chessa I (1997) In: Mitra S (ed) Postharvest physiology and storage of tropical and subtropical fruits. CAB International, Wallingford, pp 245–268
Crane JC, Bradley MV, Luckwill LC (1959) Auxins in parthenocarpic and non-parthenocarpic figs. J HortSci 34:142–153
Datwyler SL, Weiblen GD (2004) On the origin of the fig: phylogenetic relationships of Moraceae from ndhF sequences. Am J Bot 91:767–777. doi:10.3732/ajb.91.5.767
Del Caro A, Piga A (2008) Polyphenol composition of peel and pulp of two Italian fresh fig fruits cultivars (Ficus carica L.). Eur Food Res Technol 226:715–719. doi:10.1007/s00217-007-0581-4
Dellaporta SL, Calderon-Urrea A (1993) Sex determination in flowering plants. Plant Cell 5:1241–1251. doi:10.1105/tpc.5.10.1241
DeYoung BJ, Innes RW (2006) Plant NBS-LRR proteins in pathogen sensing and host-defense. Nature Imm 7:1243–1249. doi:10.1038/ni1410
Drews GN, Bowman JL, Meyerowitz ΕΜ (1991) Negative regulation of the Arabidopsis homeotic gene by the apetala2 product. Cell 65:991–1002. doi:10.1016/0092-8674(91)90551-9
Duenas M, Perez-Alonso JJ, Santos-Buelga C, Escribano-Bailon T (2008) Anthocyanin composition in fig (Ficus carica L.). J Food Composit Anal 21:107–115. doi:10.1016/j.jfca.2007.09.002
Elo A, Lemmetyinen J, Turunen M, Tikka L, Sopanen T (2001) Three MADS-box genes similar to APETALA1 and FRUITFULL from silver birch (Betula pendula). Physiol Plant 112:95–103. doi:10.1034/j.1399-3054.2001.1120113.x
Ersoy N, GözlekçiŞ KL (2007) Changes in sugar contents of fig fruit (Ficus carica Cv. Bursa Siyahi) during development. Süleyman Demirel Üniversitesi Ziraat Fakültesi Dergisi 2:22–26
FAO: Food and Agriculture Organization of the United Nations (2006) FAOSTAT agricultural data. http://faostat.fao.org/site/408/default.aspx. Accessed 13 July 2012
Ferrario SIT, Immink RGH, Shchennikova A, Busscher-Lange J, Angenent GC (2003) The MADS box gene FBP2 is required for the SEPALLATA function in petunia. Plant Cell 15:914–925. doi:10.1105/tpc.010280
Galil J (1977) Fig biology. Endeavour 1:52–56
Garg R, Patel RK, Jhanwar S, Priya P, Bhattacharjee A, Yadav G, Bhatia S, Chattopadhyay D, Tyagi AK, Jain M (2011) Gene discovery and tissue-specific transcriptome analysis in chickpea with massively parallel pyrosequencing and web resource development. Plant Physiol 156:1661–1678. doi:10.1104/pp.111.178616
Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell 16:S170–S180. doi:10.1105/tpc.019158
Golenberg EM, Freeman DC (2006) Environmental sex expression, sexual lability, biased sex ratios and other X-rated stories from the far-red side of the garden. In: da Silva JA T (ed) Floriculture, ornamental and plant biotechnology: advances and topical issues. Global Science Books, Ikenobe, pp 280–291
Gupta SM, Srivastava S, Sane AP, Nath P (2006) Differential expression of genes during banana fruit development, ripening and 1-MCP treatment: presence of distinct fruit specific, ethylene induced and ethylene repressed expression. Postharvest BiolTechnol 42:16–22. doi:10.1016/j.postharvbio.2006.05.002
Habu T, Yamane H, Igarashi K, Hamada K, Yano K, Tao R (2012) 454-pyrosequencing of the transcriptome in leaf and flower buds of Japanese apricot (Prunus mume Sieb. et Zucc.) at different dormant stages. J Japan Soc Hort Sci 81:239–250
Hanhineva K, Kokko H, Siljanen H, Rogachev I, Aharoni A, Kärenlampi S (2009) Stilbene synthase gene transfer caused alterations in the phenylpropanoid metabolism of transgenic strawberry (Fragaria × ananassa). J Exp Bot 60:2093–2106. doi:10.1093/jxb/erp085
Hardenack S, Ye D, Saedler H, Grant S (1994) Comparison of MADS box gene expression in developing male and female flowers of the dioecious plant white campion. Plant Cell 6:1775–1787. doi:10.1105/tpc.6.12.1775
Heslop-Harrison J (1964) Sex expression in flowering plants. In: Brookhaven National Laboratory (ed) Brookhaven symposia in biology. Brookhaven National Laboratory, Upton, New York, pp 109–125
Heuer S, Hansen S, Bantin J, Brettschneider R, Kranz E, Lorz H, Dresselhaus T (2001) The maize MADS box gene ZmMADS3 affects node number and spikelet development and is co-expressed with ZmMADS1 during flower development, in egg cells, and early embryogenesis. Plant Physiol 127:33–45. doi:10.1104/pp.127.1.33
Hu ZL, Bao J, Reecy JM (2008) CateGOrizer: a web-based program to batch analyze gene ontology classification categories. Online Journal of Bioinformatics 9:108–112
Ikegami H, Nogata H, Hirashima K, Awamura M, Nakahara T (2009a) Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genet Resour Crop Evol 56:201–209. doi:10.1007/s10722-008-9355-5
Ikegami H, Koshita Y, Yakushiji H, Hirashima K, Hirata C, Nakahara T (2009b) Simple and efficient RNA extraction and gene analysis in vegetative organs of Japanese persimmon. Plant Biotechnol 26:427–429. doi:10.5511/plantbiotechnology.26.427
Ingrosso I, Bonsegna S, De Domenico S, Laddomada B, Blando F, Santino A, Giovinazzo G (2011) Over-expression of a grape stilbene synthase gene in tomato induces parthenocarpy and causes abnormal pollen development. Plant Physiol Biochem 49:1092–1099. doi:10.1016/j.plaphy.2011.07.012
Iseli C, Jongeneel CV, Bucher P (1999) ESTScan: a program for detecting, evaluating, and reconstructing potential coding regions in EST sequences. Proc Int Conf Intell Syst Mol Biol 138–148
Jurka J, Kapitonov VV, Pavlicek A, Klonowski P, Kohany O, Walichiewicz J (2005) Repbase Update, a database of eukaryotic repetitive elements. Cytogenetic and Genome Res 110:462–467. doi:10.1159/000084979
Kater M, Franken J, Carney K, Colombo L, Angenent G (2001) Sex determination in the monoecious species cucumber is confined to specific floral whorls. Plant Cell 13:481–493. doi:10.1105/tpc.13.3.481
Kesari R, Trivedi PK, Nath P (2007) Ethylene-induced ripening in banana evokes expression of defense and stress related genes in fruit tissue. Postharvest Biol Technol 6:136–143. doi:10.1016/j.postharvbio.2007.04.010
Kislev ME, Hartmann A, Bar-Yosef O (2006) Early domesticated fig in the Jordan Valley. Science 312:1372–1374. doi:10.1126/science.1125910
Klee HJ (2004) Ethylene signal transduction. Moving beyond Arabidopsis. Plant Physiol 135:660–667. doi:10.1104/pp.104.040998
Kobayashi S, Goto-yamamoto N, Hirochika H (2004) Retrotransposon-induced mutations in grape skin color. Science 304:98. doi:10.1126/science.1095011
Lodhi F, Bradley MV, Crane JC (1969) Auxins and gibberellin-like substances in parthenocarpic and non-parthenocarpic syconia of Ficus carica L., cv. King. Plant Physiol 44:555–561. doi:10.1104/pp.44.4.555
Lohmann JU, Hong RL, Hobe M, Busch MA, Parcy F, Simon R, Weigel D (2001) A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 105:793–803. doi:10.1016/S0092-8674(01)00384-1
Mizukami Y, Ma H (1992) Ectopic expression of the floral homeotic gene agamous in transgenic Arabidopsis plants alters floral organ identity. Cell 71:119–131. doi:10.1016/0092-8674(92)90271-D
Mo Y, Nagel C, Taylor LP (1992) Biochemical complementation of chalcone synthase mutants defines a role for flavonols in functional pollen. Proc Nat Acad Sci USA 89:7213–7217
Nelson N (1999) Metal ion transporters and homeostasis. EMBO J 18:4361–4371. doi:10.1093/emboj/18.16.4361
Ohme-Takagi M, Shinshi H (1995) Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 7:173–182
Owino WO, Manabe Y, Mathooko FM, Kubo Y, Inaba A (2006) Regulatory mechanisms of ethylene biosynthesis in response to various stimuli during maturation and ripening in fig fruit (Ficus carica L.). Plant Physiol Biochem 44:335–342. doi:10.1016/j.plaphy.2006.03.009
Park HH, Ishikawa Y, Yoshida R, Kanno A, Kameya T (2003) Expression of AODEF, a B-functional MADS-box gene, in stamens and inner sepals of the dioecious species Asparagus officinalis L. Plant MolBiol 51:867–875. doi:10.1023/A:1023097202885
Ramirez BW (1974) Coevolution of Ficus and Agaonidae. Ann Mo Bot Gard 61:770–80
Riechmann JL, Heard J, Martin G, Reuber L, Jiang CZ, Keddie J, Adam L, Pineda O, Ratcliffe OJ, Samaha RR, Creelman R, Pilgrim M, Broun P, Zhang JZ, Ghandehari D, Sherman BK, Yu GL (2000) Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290:2105–2110. doi:10.1126/science.290.5499.2105
Saleeb WF (1965) Genetics and cytology of syconium persistence in Ficus carica. Unpublished PhD thesis. University of California
Sather DN, Jovanovic M, Golenberg EM (2010) Functional analysis of B and C class floral organ genes in spinach demonstrates their role in sexual dimorphism. BMC Plant Biol 10:46. doi:10.1186/1471-2229-10-46
Schijlen EG, de Vos CH, Martens S, Jonker HH, Rosin FM, Molthoff JW, Tikunov YM, Angenent GC, van Tunen AJ, Bovy AG (2007) RNA interference silencing of chalconesynthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpictomato fruits. Plant Physiol 144:1520–1530. doi:10.1104/pp.107.100305
Sheppard LA, Brunner A, Krutovskii K, Rottmann W, Skinner J, Vollmer S, Strauss SH (2000) A DEFICIENS homolog from the dioecious tree black cottonwood is expressed in female and male floral meristems of the two-whorled, unisexual flowers. Plant Physiol 124:627–640
Smit AFA, Hubley R, Green P (1996–2010) RepeatMasker Open-3.0. (http://www.repeatmasker.org). Accessed 10 Jan 2013
Solano R, Stepanova A, Chao QM, Ecker JR (1998) Nuclear events in ethylene signaling: atranscriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENERESPONSE-FACTOR1. Genes Dev 12:3703–3714
Solomon A, Golubowicz S, Yablowicz Z, Grossman S, Bergman M, Gottlieb HE, Altman A, Kerem Z, Flaishman MA (2006) Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J Agri Food Chem 54:7717–7723. doi:10.1021/jf060497h
Storey WB (1975) Figs. In: Janick J, Moore JN (eds) Advances in fruit breeding. Purdue University Press, West Lafayette, pp 568–589
Stover E, Aradhya M, Ferguson L, Crisosto CH (2007) The fig: overview of an ancient fruit. Hortscience 42:1083–1087
The Gene Ontology Consortium (2000) Gene Ontology: tool for the unification of biology. Nature Genet 25:25–29. doi:10.1038/75556
Theissen G (2001) Development of floral organ identity: stories from the MADS house. Curr Opin Plant Biol 4:75–85. doi:10.1016/S1369-5266(00)00139-4
Theissen G, Saedler H (2001) Plant biology: floral quartets. Nature 409:469–471. doi:10.1038/35054172
Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI (2000) Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc Nat Acad Sci USA 97:4991–4996. doi:10.1073/pnas.97.9.4991
Vinson JA, Zubik L, Bose P, Samman N, Proch J (2005) Dried fruits: excellent in vitro and in viva antioxidants. J Am Coll Nutr 24:44–50
Watkins CB (2002) Ethylene synthesis, mode of action, consequences and control. In: Knee M (ed) Fruit quality and its biological basis. Sheffield Academic Press, Sheffield, pp 180–224
Wiebes JT (1979) Co-evolution of figs and their insect pollinators. A Rev of Ecol Syst 10:1–12. doi:10.1146/annurev.es.10.110179.000245
Xie R, Zheng L, He S, Zheng Y, Yi S, Deng L (2011) Anthocyanin biosynthesis in fruit tree crops: genes and their regulation. African J Biotechnol 10:19890–19897. doi:10.5897/AJBX11.028
Yahata D, Nogata H (1999) Cultivar variations in sugar contents in fig syconia, their parts and nodal positions. J Japan Soc Hort Sci 68:987–992. doi:10.2503/jjshs.68.987
Yamaguchi T, Kurosaki F, Suh DY, Sankawa U, Nishioka M, Akiyama T, Shibuya M, Ebizuka Y (1999) Cross-reaction of chalcone synthase and stilbene synthase overexpressed in Escherichia coli. FEBS Lett 460:457–461
Yamaki S (2010) Metabolism and accumulation of sugars translocated to fruit and their regulation. J Japan Soc Hort Sci 79:1–15
Ylstra B, Busscher J, Franken J, Hollman PCH, Mol JNM, van Tunen AJ (1994) Flavonols and fertilization in Petunia hybrida: localization and mode of action during pollen tube growth. Plant J 6:201–6212. doi:10.1046/j.1365-313X.1994.6020201.x
Yu D, Kotilainen M, Pollanen E, Mehto M, Elomaa P, Helariutta Y, Albert V, Teeri T (1999) Organ identity genes and modified patterns of flower development in Gerbera hybrida (Asteraceae). Plant J 17:51–62. doi:10.1046/j.1365-313X.1999.00351.x
Ziegler H (1975) Nature of transported substances in the phloem. In: Zimmermann MH, Milburn JA (eds) Encyclopedia of plant physiology, NS Vol 1. Transport in Plants 1: Phloem Transport. Springer-Verlag, Berlin, pp 59–100
Acknowledgment
The authors would like to thank Edanz (http://www.edanzediting.co.jp/) for English language support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Wegrzyn
Data archiving statement
The sequence data generated in this study have been deposited at DDBJ in the Sequence Read Archive (DRA) and Transcriptome Shotgun Assembly (TSA) database under the accession number DRA000630 and FX376975-FX394131, respectively.
An erratum to this article is available at http://dx.doi.org/10.1007/s11295-015-0958-7.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PPT 208 kb)
Rights and permissions
About this article
Cite this article
Ikegami, H., Habu, T., Mori, K. et al. De novo sequencing and comparative analysis of expressed sequence tags from gynodioecious fig (Ficus carica L.) fruits: caprifig and common fig. Tree Genetics & Genomes 9, 1075–1088 (2013). https://doi.org/10.1007/s11295-013-0622-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11295-013-0622-z