Abstract
In Neotropical humid forest, the majority of tree species have seeds dispersed by vertebrates. Seed deposition by vertebrates is often spatially aggregated and a low per capita survival for seeds and seedlings is predicted. However, mortality factors could be saturated by high densities. I evaluated whether recruitment of saplings of species dispersed by black and gold howlers (Alouatta caraya) in latrines is higher than at control sites: (1) below parent trees, (2) in trees not used by monkeys to sleep, (3) randomly chosen sites within the forest, and determined whether howlers may influence current floristic composition of the Paraná River flooded forest. I recorded saplings several years old in the territories of five monkey groups. In total, I found four times more saplings in latrines than in the other areas, and results suggest that latrines are recruitment foci for most species, though larger samples would be required to assess this for every species. Frequency distribution of the diameter of tallest saplings of more abundant species reflected recruitment over time. I found saplings of more species growing in latrines than outside of them. Saplings higher than 1 m of two species of laurels (Ocotea diospyrifolia and Nectandra megapotamica) and one species of Myrtaceae (Eugenia punicifolia) had higher densities in latrines than below parent trees. Results suggest that mortality factors were saturated in latrines and that sapling may grow at a higher rate in latrines. In relation to the influence on floristic composition E. burkartiana, an uncommon species in the forest, could increase in abundance as consequence of seed dispersal by howlers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spatial pattern of plant populations is governed by the pattern of seed deposition and post-seed dispersal processes that modify the original distribution of seeds (Schupp 1995). At the community level, seed dispersers are important in maintaining floristic heterogeneity in forest stands (Howe and Vande Kerckhove 1981; Bourliere 1985). In Neotropical humid forests, 50–90% of canopy tree species and up to 100% of sub-canopy species have fruits dispersed by vertebrates (Howe and Smallwood 1982; Terborgh et al. 2002). Seed deposition by vertebrates is often spatially aggregated (Schupp et al. 2002). Aggregation restricts the arrival of seeds to potential sites for recruitment (Howe and Smallwood 1982), and has consequences on recruitment at later life stages because survival can depend on the density of seeds, seedlings, or young saplings (Janzen 1970; Hubbell 1980). However, saturation of mortality factors can also have important consequences in microsites with a high density of seeds or seedlings, in which case a clumped distribution persists at all stages (Schupp et al. 2002; Russo and Augspurger 2004).
Primates are among the most frugivorous species of mammals and are among the most important taxonomic groups for seed dispersal in tropical forests (Corlett 1998; Poulsen et al. 2002; McConkey 2005). Studies with primates have revealed that clumps of seedlings of the more important species in their diets are linked to sleeping trees, the areas that received the highest frequency of seed deposition (Julliot 1997; Russo and Augspurger 2004), such that seed mortality factors are saturated. Nevertheless, these patterns do not necessarily imply a high recruitment linked to sleeping trees because seedlings can also suffer a high level of mortality (Janzen 1970; Jordano and Herrera 1995). Thus, in order to understand the importance of seed dispersal for plants at the population and community levels, it is necessary to link seed dispersal patterns among several life stages (Levine and Murrell 2003).
In some ecosystems, the importance of large primates as seed dispersers is supported by evidence at two levels: that of the tree population and that of the forest community. For example, around Manu National Park (Peru), local extinction of large-bodied primates brought about a reduction of 50% in species richness and 60% in abundance of juvenile trees (Nuñez-Iturri and Howe 2007; Nuñez-Iturri et al. 2008). As mentioned earlier, seedlings of species dispersed by large bodied-primates were found at higher densities around their sleeping trees (Julliot 1997; Russo and Augspurger 2004). However, all these studies focus on young juvenile trees so that it is not possible to assume a high recruitment at later stages linked to primate activities.
I evaluated the impact of seed dispersal by black and gold howler monkeys (Alouatta caraya) on the recruitment of young saplings and on the oldest saplings, at two levels, (1) populations of trees more frequently dispersed by them and, (2) the forest community. I selected a howler population inhabiting the Paraná River flooded forest in east-central Argentina. The black and gold howler is the most important arboreal frugivore in this ecosystem (Bravo and Sallenave 2003), and it disperses a great amount of seeds which are mostly deposited in latrines (Bravo 2009).
Under the hypothesis of saturation of mortality factors in latrines, I predict that saplings of different sizes/ages of species dispersed by black and gold howlers will be present in higher numbers in latrines than in other areas of the forest. My specific objectives were to: (1) determine whether recruitment of juveniles and saplings in latrines is higher than at control sites, such as below parent trees, in trees not used by monkeys to sleep, or at randomly chosen sites within the forest, and (2) determine whether seed dispersal by howlers may influence the current floristic composition of the forest, considering the particular dynamic of that forest.
Methods
Study site
The study was carried out on Brasilera Island (Argentina) from June to August of 2000. Brasilera Island is located at the confluence of the Paraná and Paraguay Rivers (27°30′S, 58°41′W). The climate is subtropical, with an annual average temperature of 21°C, and an annual thermal amplitude of 12°C. Annual precipitation is approximately 1,500 mm. Even though precipitation decreases during the winter, there is no marked dry season because evapotranspiration is lower during this period. The island is 280 ha in size, of which 141 ha are covered by flooded forests. This is one of the forest types that make up the Atlantic Forest further upstream, and at this latitude represents an intromission of the Atlantic Forest into the Humid Chaco along Paraná and Paraguay Rivers (Daly and Mitchell 2000).
Like other flooded forests associated with big rivers (e.g., várzea in Amazon), the flooded forests of the Paraná River islands are both geomorphologically and biogeographically influenced by fluvial dynamics (Daly and Mitchell 2000). Over time, the river course meanders across the floodplain creating a heterogeneous landscape with a diversity of fluvial elements such as oxbow lakes, lagoons, and point bars. The diversity of vegetation types (e.g., forest, grassland, savanna) found in this kind of ecosystem reflects this high landscape heterogeneity. Topographic, edaphic, and age factors define different forest types on islands, but islands in general tend to have fewer tree species than inland forests (Daly and Prance 1989; Daly and Mitchell 2000). It is an ideal forest in which to study regeneration because periodic floods favor vegetation with high recovery rates and resistance to inundation (Eskuche and Fontana 1996). During ENSO events, the islands of the Paraná and Paraguay Rivers suffer extraordinary floods. During particularly strong ENSO events, floods can last more than a year (Neiff et al. 2000; Camilloni and Barros 2003). A high proportion of trees (40–60%) and animals die during these extraordinary floods and in general all plant communities of the islands are destroyed, but after a few years the physiognomy of the forest and animal populations recover (Lewis et al. 1987; Neiff et al. 2000). The last of these floods recorded were in 1904 and 1982–1983 (Neiff et al. 2000; Camilloni and Barros 2003).
Mature forests on Brasilera Island, which occupy 66 ha at the center of the island, are surrounded by lagoons. The canopy is dominated by Ocotea diospyrifolia and Albizia inundata (Mart., Mimosaceae). The understory is dominated by Eugenia punicifolia, Psychotria carthagenensis, and O. diospyrifolia saplings (Bravo and Sallenave 2003).
There is no permanent human settlement on Brasilera Island, although it is impacted by surrounding human communities. The main human activities on Brasilera Island are selective logging, hunting, and cattle ranching. The frequency of those activities is variable, and they are greater in the periphery than at the center of the island. They generally increase when the water level is low, although hunting pressure is high when the river rises and there are only small areas free of water.
Characteristics of the black and gold howler’s seed dispersal pattern
The flooded forest of the Paraná River has the greatest density of howler monkeys within the genus’ distribution, from Mexico to Argentina (Crockett 1998), and Brasilera Island has the largest density (4.25 ind./ha) of black and gold howlers in mature forests (Kowalewski 2000). Black and gold howler monkeys defecate primarily at the end of a resting period and before or after a territorial encounter. Consequently, more than 65% of the seeds previously ingested by howlers are deposited in latrines located beneath sleeping trees and at the edges of the territories (Bravo 2009). Every group of monkeys uses a variable number of trees to sleep in (7–8), but they have a main sleeping tree where they rest approximately 50% of the nights and which in very few occasions is not used for more than two consecutive nights (Bravo 2009). This behavior results in the existence of a “big latrine” at these main sleeping trees. Big latrines can be used for more than 10 years (personal observation), have a diameter of approximately 20 m, and are characterized by several centimeters of fecal material and seeds (Bravo 2009). These big latrines are formed by the fusion of small latrines located around the main sleeping tree. Smaller latrines are those located at the edge of territories and are associated with trees used during confrontations between groups (vocalizations, fights, and chases between individuals), while other small latrines are associated with day-rest periods. Both types of small latrines are approximately 5 m in diameter (Bravo 2009).
Recruitment evaluation
I evaluated the recruitment of saplings of all tree species from which howlers were recorded eating fruits on the islands of Paraná River (Bravo 2003; Bravo and Sallenave 2003; Ludwig et al. 2008) (Table 1). Saplings of trees of different species were identified using a catalogue made during laboratory germination trials (Bravo 2003, 2009). I defined two kinds of saplings: short (≤25 cm, without considering seedlings), and tall saplings (>25 cm). I further classified tall saplings into three height classes (25–50, 51–100, and >101 cm). All saplings were lignified and some individuals in the last category were more than 10 years old (they were recorded and labeled in 1994 during a general survey of the vegetation). In order to obtain a better description of taller saplings and to determine if saplings were from the same or a different cohort, I measured the diameter of saplings of all species, for which I recorded at least 20 individuals taller than 1 m. If a sapling was shorter than 3 m, I measured the diameter at mid-trunk and if it was taller than 3 m, I measured the diameter at breast height.
For Psychotria carthagenensis (Jacq., Rubiaceae) saplings, I used only the first height class because taller individuals were adults. For the same reason, I counted Eugenia punicifolia (Kurth DC, Myrtaceae) saplings taller than 1 m only if they had a basal diameter of <3 cm.
On the island there are three species of laurels: Ocotea diospyrifolia (Meinz.) Mez., Nectandra angustifolia (Ness et Mart.) and Nectandra megapotamica (Spreng.) Mez. The first is the dominant species in mature forest and the second is less abundant than O. diospyrifolia, but easily identified. The third is the least abundant and is so similar to O. diospyrifolia that it is difficult to distinguish one from the other in the field, especially saplings. I therefore refer to O. diospyrifolia and N. megapotamica saplings as “laurels”.
Sampling of big latrines
I found six big latrines by following six groups of black and gold howlers during 4 or 5 days from dawn to dusk. I sampled short saplings below the crown of the six sleeping trees using two transects 9 m long and 2 m wide, each. Transects were placed in opposite directions on each side of the trunk. For every tree, the initial direction was randomly selected and all short saplings of trees of different species were identified and counted. I sampled tall saplings in the same way and at the same site, but used 4-m-wide transects. Saplings of sleeping tree species were not considered, in part because sleeping trees were species whose seeds were not dispersed by howlers.
Sampling of small latrines
Twenty-seven small latrines were located by searching in the forest at random and during behavioral observations of the six groups described previously. I identified and counted all short saplings in 17 small latrines and counted and identified tall saplings in the rest of the small latrines (n = 10).
Impact at the population level
To evaluate the effect of seed dispersal by howlers on the population dynamics of different tree species, I compared recruitment of saplings in big latrines with recruitment below parent trees of the species dispersed more frequently by howlers: O. diospyrifolia, E. punicifolia, P. carthagenensis, Banara arguta (in order of relevance; Bravo 2003, 2009; Bravo and Sallenave 2003). I sampled short and tall saplings below the crown of the parent trees using transects similar to those used in big latrines. Psychotria carthagenensis and E. punicifolia are understory trees whose crowns have diameters of 1–2 m and which are distributed in high-density patches. I sampled species using the two 9-m-long transects in these patches.
Impact at the community level
To evaluate the effect at the community level and to discard any nurse effects on saplings by the trees found above the big latrines, I compared the recruitment of saplings in big latrines (below the main sleeping trees) with recruitment below six adult trees of the same species and size as the sleeping trees, but not known to be used by howler groups (non-sleeping trees). In order to evaluate the impact of small latrines on regeneration at the community level I compared the recruitment of saplings in small latrines with recruitment in control plots. Because small latrines had a mean diameter of 5 m, I selected at random 33 control plots with this diameter and with a vegetation cover similar to the 27 small latrines found. I identified and counted all short saplings in 17 control plots and tall saplings in 16 control plots. For evaluations of both big and small latrines, I sampled all plant species whose fruits are consumed by howlers (Bravo 2003; Bravo and Sallenave 2003; Ludwig et al. 2008).
To evaluate the impact of seed dispersal by howlers on floristic composition for big latrines, non-sleeping trees, small latrines and control plots, I calculated (1) mean richness: mean of number of species per latrine, non-sleeping tree or plot, and (2) cumulative richness: total number of species found in latrines, non-sleeping trees or plots.
Because small latrines are more abundant than big latrines, it was possible to also calculate (1) frequency: the percentage of latrines or control plots in which short and tall saplings of each species were present; and (2) abundance: mean number of individuals of each species per latrine or control plot. As a consequence of the small area of these latrines, the number of saplings growing in each is small and it was therefore impossible to do a detailed analysis of recruitment per height category as was done for big latrines.
In order to integrate community level results, I developed a conceptual model showing the richness and number of saplings censused in latrines (small and big) and in controls (control plots and non-sleeping trees) across the following height categories: short saplings (<25 cm), tall saplings between 25 cm and 1 m, and saplings taller than 1 m. I estimate transition probability in latrines and controls as the number of saplings censused in one height category divided the number of saplings censused in the previous height category.
Statistical analysis
In all analyses I considered trees or patches of trees as replicates and used the mean density of values obtained in the two transects. To evaluate differences in the density of tall saplings in big latrines, at latrines at non-sleeping trees and at parent trees (Laurels and E. punicifolia), I used a repeated-measures two factor analysis of variance (ANOVA). One factor was the site (big latrine/non-sleeping tree or parent tree) and the other was the height of saplings with three height classes within subjects (each tree). Comparisons of treatments after ANOVA were performed using Tukey’s test. Raw data of E. punicifolia were square root transformed (Zar 1996).
I used a two-sample t test to compare mean richness of tall saplings at big latrines, at non-sleeping trees, at small latrines, and at control plots. Because samples showed different variances, I used a two-sampled t′ test to compare mean richness and density of short saplings in big latrines and at non-sleeping trees, short sapling density of laurels and E. punicifolia in big latrines and parent trees, and mean richness and abundance of tall saplings in small latrines.
I used a U test to compare density of tall saplings of P. carthagenensis in big latrines and parent trees because data were not normally distributed.
Finally, B. arguta saplings had very low densities, thus I was not able to statistically analyze these data.
Results
Impact at the population level
Description of the tallest saplings
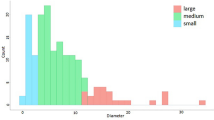
For four species, I found more than 20 individuals taller than 1 m, but only in latrines, the diameters of two laurels species (Fig. 1a), E. punicifolia (Fig. 1b), and E. burkartiana (Fig. 1c) show that saplings are several years old and not of the same age. Outside latrines (below parents trees, non-sleeping trees, or in control plots) I did not find more than 20 individuals of any species higher than 1 m. I found only two saplings of laurels taller than 1 m below parent trees and three below non-sleeping trees (Fig. 1a). I found two saplings of E. punicifolia taller than 1 m below parent trees and only one below non-sleeping trees (Fig. 1b). All saplings found in controls were associated with the two smallest diameter categories.
Number of saplings taller than 1 m in each category of diameter for species with more than 20 individuals recorded. Data of big and small latrines were combined (black bars), data of all controls (parents trees, non-sleeping trees, control plots) were combined (white bars). a Laurels, b E. punicifolia, c E. burkartiana saplings
Big latrines versus parent trees
For three out of four species evaluated, the density of saplings was higher in big latrines than below parent trees, but not all were significant differences.
The density of short saplings of laurels was significantly higher in big latrines than below parent trees (t′ = 3.1; df = 18.6; p = 0.006; Fig. 2). Interaction between site and height classes of tall saplings was also significant (F = 44.42; df = 2; p < 0.00001), and the density of saplings taller than 1 m was higher in big latrines than below parent trees (Tukey test, p < 0.05; Fig. 2).
Mean density (+SE) of short and tall saplings of each height class in black and gold howler big latrines (black bars) and under parent trees (white bars) for Banara arguta, laurels (Ocotea diospyrifolia and Nectandra megapotamica), Eugenia punicifolia, and Psychotria carthagenensis. The density of tall saplings of P. carthagenensis is not distinguished by size class because I considered only individuals of the first class (i.e., between 25 and 50 cm of height). Note differences in the scale of each graph. Different letters above bars indicate significant differences. **p = 0.006, *p = 0.02
There was no significant difference in the density of E. punicifolia short saplings between big latrines and below parent trees (short saplings: t′ = 1.7; df = 5.3; p = 0.1; Fig. 2). Nevertheless, density of short and tall saplings in latrines was higher than below parent trees for all categories (Fig. 2). Interaction between site and height classes of tall saplings was significant (F = 5.76; df = 2; p < 0.01), and density of saplings taller than 1 m was significantly lower below parent trees than in big latrines (Tukey test, p < 0.05; Fig. 2).
The density of P. carthagenensis short saplings was significantly higher in big latrines than below parent trees (t′ = 3.28; df = 4.29; p = 0.02; Fig. 2), but was not significant for tall saplings (U = 3.0; n = 4; n = 5; p = 0.08; Fig. 2).
Short saplings of Banara arguta had very low densities (an average of <0.8 individuals per m2), with high variability in both big latrines and below parent trees, but with higher density below parent trees (Fig. 2). Tall saplings were rare in big latrines and below parent trees (on average <0.02 individuals per m2; Fig. 2).
Impact at the community level
Big latrines versus non-sleeping trees
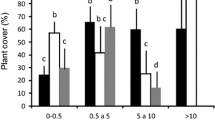
The density of short saplings in big latrines was greater than below non-sleeping trees (t′ = 2.7; df = 5.4; p = 0.04; Fig. 3a). Tall sapling densities showed significant differences between sites (F = 28.92; df = 1; p < 0.0003) but not between height classes (F = 3.32; df = 2; p = 0.06), and there was no interaction (F = 2.25; df = 2; p = 0.13, Fig. 3a). Cumulative richness was 13 species in big latrines and 10 species below non-sleeping trees (Table 1). Mean richness of short saplings was similar at both sites (t′ = 0.7; df = 6.1; p = 0.5; Fig. 3b), but mean richness of tall saplings was lower below non-sleeping trees than in big latrines (t = 4.1; df = 10; p = 0.002; Fig. 3b).
a Mean density (+SE) of short and tall saplings of each size class in black and gold howler’s big latrines (black bars) and under non-sleeping trees (white bars), considering all species together. *p < 0.05 for differences in the density of short saplings, tested by a two sample t′ test. Different letters above bars of tall saplings indicate significant differences tested by ANOVA. b Mean richness (+SE) of short and tall saplings in black and gold howler big latrines and under non-sleeping trees. *p < 0.05
Small latrines versus control plots
The abundance of short and tall saplings in small latrines was 139 ± 32 (mean ± SE) individuals per latrine and 25 ± 4 individuals per latrine, respectively. Mean abundances were lower in control plots than in small latrines, with 36 ± 4 individuals per plot for short saplings and 6 ± 2 individuals per plot for tall saplings (short saplings: t′ = 3.19; df = 16.60; p = 0.005; tall saplings: t′ = 5.26; df = 10.17; p = 0.0003). Cumulative richness was 13 species in small latrines and 11 species in control plots (Table 1). Mean richness of short and tall saplings was higher in small latrines than in control plots (short saplings: 4.4 ± 0.4 vs. 3 ± 0.5; t′ = 3.2; df = 21.79; p = 0.004; tall saplings: 4 ± 1 vs. 2 ± 0.5; t = 4.11; df = 23; p = 0.004). Considering both short and tall saplings together, nine species were present in more than 20% of latrines (Fig. 4a), whereas in control plots only four species surpassed this percentage, and their abundance was several times lower than in small latrines (Fig. 4b). Eugenia punicifolia and P. carthagenensis short saplings were found in 100% of small latrines (Fig. 4a) and control plots (Fig. 4b). Short saplings of laurels were present in 100% of small latrines (Fig. 4a), but in only 56.7% of control plots (Fig. 4b).
a Percentage of small latrines in which short saplings (white bars) and tall saplings (black bars) of each species were present, and abundance of saplings of each species as the mean number (+SE) of saplings per small latrine. b Percentage of control plots in which short saplings (white bars) and tall saplings (black bars) of each species were present, and abundance of saplings of each species as mean number (+SE) of saplings per plot. Laurels refer to saplings of O. diospyrifolia and N. megapotamica. Note differences in the scale of abundance
Impact at the community level: integration model
Eighty percent of short saplings were found in big and small latrines (Fig. 5) and had a transition probability of becoming tall saplings similar to that of short saplings growing in control sites (non-sleeping trees and control plots). However, the number of tall saplings in latrines was five times higher than in controls because of differences in short sapling abundance (Fig. 5). Considering tall saplings shorter than 1 m, the transition probability of becoming taller than 1 m increases noticeably in latrines (0.7) but not in controls (0.13). The number of saplings taller than 1 m in latrines was 18 times higher than in controls and represented 95% of those saplings. Also notable was the reduction in richness in controls, from 13 to 6 species (Fig. 5).
Results at the community level. Number of saplings and richness are shown within circles. Arrows represent the transitions between sapling categories and numbers on arrows are the transition probabilities. Lines between latrines and controls are accompanied by a summary of the total number of saplings (controls plus latrines) and the percentage growing in each site
Discussion
Impact on recruitment
Short and tall saplings of studied species showed high abundance in latrines. In Brasilera Island, latrines appear to be important sites for the recruitment of at least the four most abundant species: the two laurels (O. diospyrifolia and N. megapotamica), E. punicifolia, and P. carthagenensis, and for one non-abundant species E. burkartiana. In several latrines, P. carthagenensis saplings reached the adult stage and produced fruits (unpublished data). In September 2004, 4 years after the present study was finished, I observed the flowering of one laurel sapling growing in a latrine. These observations, although anecdotal, suggest that the seed dispersal loop can be closed in black and gold howler latrines in the Paraná River flooded forest, at least for some species.
Previous studies reported clumps of seedlings or juvenile trees linked to primate latrines. For example, clumps of seedlings are linked to sleeping trees used by howler monkeys (Alouatta seniculus) in French Guiana (Julliot 1997), and the seedlings and saplings of Virola calophylla are found clumped under the sleeping trees of spider monkeys (Ateles paniscus) in Peru (Russo and Augspurger 2004). However, this is the first study to link primate latrines to the recruitment of saplings several years old. Tall saplings in particular were several years old and the last height class (>101 cm) likely experiences very low mortality. In big latrines, density of tall saplings was greater than under non-sleeping trees and parent trees for all the height classes. While the number of tall saplings decreased across height classes below parent trees, it increased or was constant in latrines, which implies that sampled saplings were the outcome of a continuous high recruitment over time in latrines. This recruitment over time is also reflected by the frequency distribution of the diameter of tallest saplings censused in big and small latrines.
Transition probabilities from short to tall saplings were similar in latrines and in controls; however, latrines had a larger number of tall saplings, exhibiting the potential for saturation. While transitional probabilities remain constant in controls, they increase noticeably in latrines from tall saplings shorter than 1 m to those taller than 1 m, denoting that another mechanism could be involved. For example, they could be due to a high growth rate because of repeated nutrient addition via feces. Nutrient levels (N, P, and C/N ratios) are high in howler latrines (even at different depths). Additionally, compared to outside of latrines, the micromorphology of the soil in latrines is more favorable for the development of roots (Feeley 2005; Dos Santos Neves et al. 2010).
Different factors, such as herbivory (Schupp 1988), pathogens (Augspurger 1984) or nutrients (Burslem et al. 1996) could determine seedling mortality and affect the number of saplings. All of these factors produce an increment in mortality when the density of seeds and seedlings increases, which in turn produces a low per capital survival at high densities. Any of these factors could be acting in black and gold howlers latrines in the Argentinean Paraná River flooded forest. Although the per capita survival in latrines is expected to be low, at the total forest scale, microsites receiving a large amount of seeds (i.e., latrines) are the sites with the higher recruitment of tall saplings. This persistence of spatial patterns of seed dispersion throughout recruitment stages occurs when mortality factors are saturated by a large amount of seeds and seedlings (Herrera et al. 1994; Rey and Alcantara 2000; Schupp et al. 2002; Russo and Augspurger 2004).
For laurels, another mechanism exists to promote recruitment from seeds dispersed by howlers. Laurels suffer an important pre-dispersal infestation, one individual tree can have up to 80% of their seeds infested by curculonidae larva, resulting in a lower potential to germinate. However, most of these larvae are killed during passage through the digestive tract of the howlers, and seeds arriving at latrines in good condition to germinate (Bravo 2008).
Impact on floristic composition
Black and gold howler latrines may help to maintain forest species diversity and the current forest physiognomy in mature Paraná River flooded forests because species that are more common in latrines are the most abundant in the forest. Additionally, latrines could affect the abundance of uncommon tree species, such as E. burkartiana, whose saplings were present in the 60% of small latrines and were abundant in big latrines.
Big and small latrines had higher numbers of short and tall saplings (indeed, 80% of total saplings recorded in this study were found in latrines), and species richness was also higher in these microsites, however differences were not statistically significant for short saplings. Mean richness of tall saplings was significantly higher in latrines than in other microsites, and in general, species were more frequently observed, and was represented by a higher number of individuals in latrines than in the other microsites evaluated. However larger samples are required to confirm this for all species.
Recruitment limitation is hypothesized for several tree genera (e.g. Ocotea, Nectandra, Eugenia, Paulinia, Guarea) in Amazonia and Guiana, because they are mainly dispersed by Ateles spp. and Lagothrix spp., and these species are being overhunted and locally extinct in some areas (Peres and Roosmalen 2002). Seeds of these genera in Amazonia and Guiana are also dispersed by howler monkeys; however, their relevance on sapling recruitment was discarded based upon low recruitment expectation due to a heavily clumped pattern of seed dispersal (Peres and Roosmalen 2002). On the contrary, the results of the present study indicate that latrines are good sites for recruitment of these species, since they were the only sites where tall saplings were abundant. As a consequence, the relevance of seed dispersal by howlers should be evaluated in overhunted areas where they are the only large-bodied primates present.
In more complex forests, such as the rest of the Atlantic forest, and Amazonian, African, or Asian forests, there are more fruiting species and the frugivore community is more diverse. In these forests different animal groups usually show distinct fruit preferences but there is some overlap (Clark et al. 2001; Russo 2003, Stevenson et al. 2005) that does not necessarily imply redundancy in seed dispersal. For example, in Cameroon, hornbill and monkey diets include several species in common, but in different proportions, such that the seed rain generated by both groups is also different. Even monkeys do not form a single assemblage, demonstrating that they are not redundant as seed dispersers (Holbrook and Smith 2000; Poulsen et al. 2002; Clark et al. 2004). As a result, in more species-rich forests there is likely to be a more varied dispersal pattern than in the relatively species-poor Brasilera Island, where howlers are the main dispersers of large seeds.
Clumped seed dispersal patterns generated by some vertebrates are one of three characteristics used by other studies to disqualify the effectiveness of any vertebrate as a seed disperser (Peres and Roosmalen 2002; Wehncke et al. 2003). These characteristics (no specialized frugivore diet, a strong clumped seed dispersal pattern with high densities of seeds, and seeds deposited with a large amount of dung) predict a low level of per capita survival for seeds and seedlings. Nevertheless, my results and those of other studies (Fragoso 1997; Julliot 1997; Giombini et al. 2009) show that, despite the low per capita survival predicted, the higher and constant number of seeds arriving produces a saturation of mortality factors, permitting the recruitment of saplings.
It may not be appropriate to disregard the relevance of any seed disperser for forest regeneration based only on the characteristics of the seed dispersal pattern that it generates, or because the species it consumes are also consumed by other frugivores, or because it is not a specialized frugivore. Doing so may under-value the impact of local extinction of these vertebrates on forest regeneration or their potential value in restoration programs. For example, while howler monkeys, unspecialized frugivores, favored the recruitment of at least the main fruit species in their diets, probably by saturation of mortality factors, Civets, which are basically carnivorous, are the most effective seed dispersers of some species of plants because, dispersed seeds are deposited in locations optimal for germination and growth (Nakashima et al. 2010). Similar are the situation reported for gulls which diet is based on fish and marine invertebrated however, more recent studies showed that are the most important seed dispersers to Rubia fruticosa (Rubiaceae) (Nogales et al. 2001) and to Corema album (Empetraceae) (Calviño-Cancela 2002).
Seed dispersal by gold and black howlers is likely more than only that at latrines because latrines represent only a fraction of total dispersal—35% of scats are deposited outside latrines (Bravo 2009). Therefore, gold and black howlers also deposit seeds in low-density conditions, increasing their potential as seed dispersers for species that do not recruit in latrines. Similar patterns of seed dispersal were described for other large vertebrates, such as hornbills (Kitamura et al. 2008), other howler species (Julliot 1997; Andresen 2002) and spider monkeys (Russo and Augspurger 2004).
In summary, black and gold howlers are effective seed dispersers for several tree species in Paraná River flooded forests, and their latrines represent important recruitment areas. Black and gold howler monkeys likely play an important role in the maintenance of the physiognomy of mature Paraná River flooded forests and, in concordance with results of this study, areas with high densities of howlers have been found to have a higher potential for natural restoration (Vulinec et al. 2006). As a result, howlers may have more potential than currently appreciated as catalyzers in forest restoration programs of all Neotropical humid forests.
References
Andresen E (2002) Primary seed dispersal by red howler monkeys and the effect of defecation patterns on the fate of dispersed seeds. Biotropica 34:261–272
Augspurger CK (1984) Seedling survival of tropical tree species: Interaction of dispersal distance, light-gap, and pathogens. Ecology 65:1705–1712
Bourliere F (1985) Primate communities: their structure and role in tropical ecosystems. Int J Primatol 6:1–26
Bravo SP (2003) Efecto de Carayá (Alouatta caraya) en la Dinámica y Regeneración de las Selvas de Inundación del Paraná Medio. Doctoral thesis, Universidad de Buenos Aires
Bravo SP (2008) Seed dispersal and ingestion of infested seeds by Black Howler monkeys in flooded forest of the Paraná River, Argentina. Biotropica 40:471–476
Bravo SP (2009) Implications of behavior and gut passage for seed dispersal quality: the case of black and gold howler monkeys. Biotropica 41:751–758
Bravo SP, Sallenave A (2003) Foraging behavior and activity patterns of Alouatta caraya in the northeastern Argentinean flooded forest. Int J Primatol 54:825–846
Burslem DFRPP, Grubb PJ, Turner IM (1996) Responses to simulated drought and elevated nutrients supply among shade-tolerant tree seedlings of lowland tropical forest in Singapore. Biotropica 28:639–648
Calviño-Cancela M (2002) Spatial patterns of seed dispersal and seedling recruitment in Corema album (Empetraceae): the importance of unspecialized dispersers for regeneration. J Ecol 90:775–784
Camilloni IA, Barros VR (2003) Extreme discharge events in the Paraná River and their climate forcing. J Hydrol 278:94–106
Clark CJ, Poulsen JR, Parker VT (2001) The role of arboreal seed dispersal groups on the seed rain of a lowland tropical forest. Biotropica 33:606–620
Clark CJ, Poulsen JR, Connor EF, Parker VT (2004) Fruiting trees as dispersal foci in a semi-deciduous tropical forest. Oecologia 139:66–75
Corlett RT (1998) Frugivory and seed dispersal by vertebrates in the oriental (Indomalayan) region. Biol Rev Camb Philos Soc 73:413–448
Crockett CM (1998) Conservation biology of genus Alouatta. Int J Primatol 19:549–576
Daly DC, Mitchell JD (2000) Lowland vegetation of tropical South America: an overview. In: Lentz D (ed) Imperfect balance: landscape transformations in the pre-Columbian Americas. Columbia University Press, New York, pp 391–454
Daly DC, Prance GT (1989) Brazilian Amazon. In: Campbell DG, Hammond HD (eds) Floristic inventory of tropical countries. The New York Botanical Garden, New York, pp 401–426
Dos Santos Neves N, Feer F, Salmon S, Chateil C, Ponge J-F (2010) The impact of red howler monkey latrines on the distribution of main nutrients and topsoil components in tropical rain forests. Austral Ecol 35:545–559
Eskuche U, Fontana JL (1996) La vegetación de las islas argentinas del Alto Paraná. F Bot Geobotan Corrent 11:1–15
Feeley K (2005) The role of clumped defecation in the spatial distribution of soil nutrients and the availability of nutrients for plant uptake. J Trop Ecol 21:99–102
Fragoso JM (1997) Tapir-generated seed shadows: scale-dependent patchiness in the Amazon rain forest. J Ecol 85:519–529
Giombini M, Bravo SP, Martinez M (2009) Seed dispersal of the palm Syagrus romanzoffiana by tapirs in the semi-deciduous Atlantic forest of Argentina. Biotropica 41:408–413
Herrera CM, Jordano P, Lopez-Soria L, Amat JA (1994) Recruitment of a mast-fruiting, bird-dispersed tree: bridging frugivore activity and seedling establishment. Ecol Monogr 64:315–344
Holbrook KM, Smith TB (2000) Seed dispersal and movement patterns in two species of Ceratogymna hornbills in a West African tropical lowland forest. Oecologia 125:249–257
Howe HF, Smallwood J (1982) Ecology of seed dispersal. Annu Rev Ecol Syst 13:201–228
Howe HF, Vande Kerckhove GA (1981) Removal of wild nutmeg (Virola surinamensis) crops by birds. Ecology 62:1093–1106
Hubbell SP (1980) Seed predation and the coexistence of tree species in tropical forests. Oikos 35:214–229
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:501–528
Jordano P, Herrera M (1995) Shuffling the offspring: uncoupling and spatial discordance of multiple stage in vertebrate seed dispersal. Ecoscience 2:230–237
Julliot C (1997) Impact of seed dispersal by red howler monkeys Alouatta seniculus on the seedling population in the understorey of tropical rainforest. J Ecol 85:431–440
Kitamura S, Yumoto T, Noma N, Chuailua P, Wohandee TMP, Poonswad P (2008) Aggregated seed dispersal by wreathed hornbills at a roost site in a moist evergreen forest of Thailand. Ecol Res 23:943–952
Kowalewski MM (2000) Birth seasonality in black howler monkeys (Alouatta caraya) in an island system in northern Argentina. Master’s thesis, State University of New York at Stony Brook, USA
Levine MJ, Murrell DJ (2003) The community-level consequences of seed dispersal patterns. Annu Rev Ecol Syst 34:549–574
Lewis JP, Franceschi EA, Prado DE (1987) Effects of extraordinary floods on the dynamics of tall grasslands of the River Parana valley. Phytocoenologia 15:235–251
Ludwig G, Aguiar LM, Svoboda WK, Hilst CLS, Navarro IT, Vitule JRS, Passos FC (2008) Comparison of diet of Alouatta caraya (Primates: Atelidae) between a riparian island and mainland on the Upper Parana River, southern Brazil. Rev Bras Zoo 25:419–426
McConkey KR (2005) The influence of gibbon primary seed shadows on post-dispersal seed fate in a lowland dipterocarp forest in Central Borneo. J Trop Ecol 21:255–262
Nakashima Y, Inoue E, Inoue-Murayama M, Sukor JRA (2010) Functional uniqueness of a small carnivore as seed dispersal agents: a case study of the common palm civets in the Tabin Wildlife Reserve, Sabah, Malaysia. Oecologia 164:721–730
Neiff J, Mendiondo E, Depettris C (2000) ENSO floods on river ecosystems: catastrophes or myths? In: Toenmsnann F, Koch M (eds) River flood defence, kassel reports of hydraulic engineering No. 9/2000 Verlag, Kassel, vol I, Section F: flood risk, floodplain and floodplain management, pp F141–F152
Nogales M, Medina FM, Quilis V, González-Rodríguez M (2001) Ecological and biogeographical implications of Yellow-Legged Gulls (Larus cachinnans Pallas) as seed dispersers of Rubia fruticosa Ait. (Rubiaceae) in the Canary Islands. J Biogeogr 28:1137–1145
Nuñez-Iturri G, Howe HF (2007) Bushmeat and the fate of trees with seeds dispersed by large primates in a lowland rain forest in western Amazonia. Biotropica 39:348–394
Nuñez-Iturri G, Olsson O, Howe HF (2008) Hunting reduces recruitment of primate-dispersed trees in Amazonian Peru. Biol Conserv 141:1536–1546
Peres CA, Roosmalen MGMV (2002) Primate frugivory two species-rich—Neotropical forests: implications for the demography of large-seeded plants in overhunted areas. In: Levey DJ, Silva WR, Galetti M (eds) Seed dispersal and frugivory: ecology, evolution and conservation. CABI Publishing, Wallingford, pp 407–421
Poulsen JR, Clark CJ, Connor EF, Smith TB (2002) Differential resource use by primates and hornbills: implications for seed dispersal. Ecology 83:228–240
Rey PJ, Alcantara JM (2000) Recruitment dynamics of a fleshy-fruited plant (Olea europaea): connecting patterns of seed dispersal to seedling establishment. J Ecol 88:622–633
Russo SE (2003) Responses of dispersal agents to tree and fruit traits in Virola calophylla (Myristicaceae): implications for selection. Oecologia 136:80–87
Russo SE, Augspurger CK (2004) Aggregated seed dispersal by spider monkeys limits recruitment to clumped patterns in Virola calophylla. Ecol Lett 7:1058–1067
Schupp EW (1988) Seed and early seedling predation in the forest understory and in treefall gaps. Oikos 51:71–78
Schupp EW (1995) Seed-seedling conflicts, habitat choice, and patterns of plant recruitment. Am J Bot 82:399–409
Schupp EW, Milleron T, Russo SE (2002) Dissemination limitation and the origin and maintenance of species-rich tropical forest. In: Levey DJ, Silva WR, Galetti M (eds) Seed dispersal and frugivory: ecology, evolution and conservation. CABI Publishing, Wallingford, pp 305–321
Stevenson PR, Pineda M, Samper T (2005) Fruit choice by woolly monkeys in Tinigua National Park, Colombia. Int J Primatol 25:367–381
Terborgh J, Pitmann N, Silman M, Schichter H, Nuñez V (2002) Maintenance of tree diversity in tropical forests. In: Levey DJ, Silva WR, Galetti M (eds) Seed dispersal and frugivory: ecology, evolution and conservation. CABI Publishing, Wallingford, pp 1–19
Vulinec K, Lambert JE, Mellow DJ (2006) Primate and dung beetle communities in secondary growth rain forests: implications for conservation of seed dispersal systems. Int J Primatol 27:855–879
Wehncke EV, Hubbell SP, Foster RB, Dalling JW (2003) Seed dispersal patterns produced by white-faced monkeys: implications for the dispersal limitation of neotropical tree species. J Trop Ecol 91:677–685
Zar JH (1996) Biostatistical analysis, 3rd edn. Prentice Hall, New Jersey
Acknowledgments
I thank V. Cueto, G. Goldstein, M. Norconk, J. Lopez de Casenave and Alex Jahn for their valuable comments on earlier versions of the manuscript and on the English revision. I am especially grateful to Doug Levey for his support, and two anonymous reviewers for their valuable comments. I thank several students and A Pas de Loup volunteers who worked as field assistants. I am especially grateful to Mr. Gallo, his wife Titina, and the Cao family for their logistic support in the field. This study was funded by CONICET, IFS (International Foundation for Science D/2686-1, and D/2686-2).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bravo, S.P. The impact of seed dispersal by black and gold howler monkeys on forest regeneration. Ecol Res 27, 311–321 (2012). https://doi.org/10.1007/s11284-011-0904-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-011-0904-6