Abstract
With the continuing increase in human activities causing accelerating rates of anthropogenic nitrogen deposition inputs into forests, there is considerable interest in understanding the effects of nitrogen deposition on litter decomposition. Two dominant litters were chosen from Zijin Mountain in China: Quercus acutissima from a broad-leaved forest and Pinus massoniana from a coniferous forest. The litters were incubated in microcosms and treated with a gradient of nitrogen fertilization. During a 6-month incubation, changes in chemical composition (i.e., lignin, total carbohydrate, and nitrogen), litter mass losses, soil pH values, and the activities of degradative enzymes were determined. Results showed that medium-nitrogen and high-nitrogen fertilization significantly accelerated litter decomposition rates of leaves, while only the high-nitrogen fertilization significantly accelerated litter decomposition rates of needles. The results also showed that cellulase and nitrate reductase were primarily responsible for litter decomposition in the broad-leaved forest, while catalase, cellulase, and acid phosphatase were primarily responsible for litter decomposition in the coniferous forest under conditions of no N fertilization; catalase, cellulase, and acid phosphatase were primarily responsible for litter decomposition in the broad-leaved forest, while catalase, cellulase, invertase, and nitrate reductase were primarily responsible for litter decomposition in the coniferous forest under conditions of N fertilization. Nitrogen fertilization-stimulated litter decomposition was due to the fact that the activities of enzymes, particularly cellulase, were accelerated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic nitrogen (N) deposition is a global process that increases N inputs into terrestrial ecosystems (Lovett 1994). At present, atmospheric N deposition is a major source of anthropogenic N in the terrestrial ecosystems of the northeastern United States, Europe, and Asia (Galloway and Cowling 2002). With more and more anthropogenic N deposition input into the forest ecosystems, the effects on litter decomposition have recently received considerable interest, specifically in terms of the global N cycle and potential feedbacks to the carbon (C) cycle. Some studies reporting on the effects of N deposition on litter decomposition have used “N fertilization” methods and found neutral effects of N fertilization on litter decomposition (Johnson et al. 2000; Keller et al. 2005). This may be because (1) there is N saturation for the soil microorganisms, (2) C substrate quality was lower and the microbial communities did not shown an obvious response to N fertilization (Hobbie 2000), or (3) the forest was able to adapt to external N input due to a greater capacity for self-adjustment (Hobbie and Vitousek 2000). Other studies have found positive effects of N fertilization on litter decomposition (Sinsabaugh et al. 2002; Bragazza et al. 2006) due to the alleviated N limitations on microbial metabolism (Sinsabaugh et al. 2002). Others still have found negative effects of N fertilization on litter decomposition (Magill and Aber 1998; Micks et al. 2004). This may be due to the formation of more recalcitrant forest floor material after complexation between N and polyphenols and/or the inhibition of lignin-decomposing enzymes by low molecular N compounds (Carreiro et al. 2000). These results are obviously inconsistent. This may be due to the difference in the litter types used, the type or the level of N fertilization, or the time scale of the studies.
It is well known that exoenzymes can be used as an indicator of microbial decomposition (Moorhead and Sinsabaugh 2000), so changes in enzymatic activities under N fertilization could explain the effects of fertilization on litter decomposition. Some previous studies have shown that N fertilization accelerated the activities of degradative enzymes, such as cellulase (Waldrop et al. 2004; Sinsabaugh et al. 2005), phosphatase (Saiya-Cork et al. 2002; Sinsabaugh et al. 2005), polyphenol oxidase (Bragazza et al. 2006), and urease (Saiya-Cork et al. 2002). However, other studies have found that N fertilization restrained the activity of cellulase (Sinsabaugh et al. 2002; Deforest et al. 2004), urease (Ajwa et al. 1999), polyphenol oxidase (Gallo et al. 2004), peroxidase (DeForest et al. 2004), and oxidative enzymes (Waldrop et al. 2004; Gallo et al. 2004). This may be due to differences in the effects of N fertilization in the presence of different types or levels of different microorganism groups, which may secrete different exoenzymes to soils. Thus, to understand the role of exoenzymes during the litter decomposition process, changes in the activities of soil exoenzymes involved in litter decomposition were monitored in this study.
Microcosm experiments have been found to be useful for testing factors that influence decomposition under controlled conditions (Naeem et al. 2000). Thus, this study was carried out through a microcosm experiment to assess litter decomposition and the relationship with soil enzyme activities in a subtropical broad-leaved forest (Quercus acutissima) and a coniferous forest (Pinus massoniana) in Zijin Mountain in China, as well as the response to a gradient of N fertilization. The objectives of this study were (1) to determine the difference in litter decomposition between leaves and needles in response to a gradient of N fertilization, (2) to study the effects of external N fertilization on the activities of soil enzymes, and (3) to identify the major degradative enzyme contributors involved in litter decomposition in a subtropical forest after exposure to N fertilization.
Materials and methods
Site description
Three discrete sites were chosen in two types of forests, a broad-leaved forest (BF, dominated by Quercus acutissima) and a coniferous forest (CF, dominated by Pinus massoniana), located on Zijin Mountain (32°5′N, 118°48′E), Nanjing, China. The mountain has an area of 24 km2 and a peak of 447.1 m. The study areas have a subtropical humid climate. Annual mean temperature is 15.4°C with the monthly mean temperature reaching a maximum of 28.2°C in July and a minimum of 1.9°C in January. The average annual precipitation level is 1,106.5 mm, and the rainy season comes in June and July. The level of natural atmospheric N deposition is approximately 3 g N m−2 year−1. It is mainly in nitrate form (Wang et al. 2007).
Experimental design
Quercus acutissima leaves and Pinus massoniana needles were collected from the forest floor of the site in September 2008 (initial litter quality is shown in Table 1). All the litter samples were taken back to the laboratory and oven-dried at 70°C for 24 h to achieve constant weight for further study. Meanwhile, five soil samples from each forest type were collected from the top layers of the forest soil. All soil samples were kept in sealed bags and immediately (after ~2 h) taken back to the laboratory for further study. Soil samples were passed through a 2-mm sieve to remove leaves, plant roots, and gravel. All soil samples were then homogenized by thorough mixing and kept in a refrigerator at 4°C prior to incubation.
To study litter decomposition, 1 g litter was mixed with approximately 40 g soil in a 125-mL polypropylene specimen chamber. Soils of BF and CF were selected as the source of microorganisms. Leaves were incubated in the soil of BF, and needles were incubated in the soil of CF. The experimental design was full factorial, with two litters (leaves and needles) × four levels of N fertilization, i.e., low N (1 g N m−2 year−1, LN), medium N (2 g N m−2 year−1, MN), high N (3 g N m−2 year−1, HN), and control (no N fertilization, CK). As such, there were eight total treatment combinations: BF with low N (LN-BF), BF with medium N (MN-BF), BF with high N (HN-BF), BF with control (CK-BF), CF with low N (LN-CF), CF with medium N (MN-CF), CF with high N (HN-CF), and CF with control (CK-CF). N fertilization in the form of aqueous dissolved NH4NO3 was sprayed each week during the incubation. The samples were kept in the dark and were covered with plastic wrap to prevent evaporation. The length of the incubation was 6 months.
Three sample replicates per treatment were systematically harvested each month after incubation. The soil adhering to litter samples was carefully removed, and the litter samples were then oven-dried at 70°C for 24 h to achieve constant weight and used for chemical analyses and mass losses. Replicated soil samples were also harvested and mixed thoroughly per treatment. All replicated soil samples were kept in sealed bags and refrigerated at 4°C in preparation for pH and enzymatic activity measurements.
Chemical analysis and enzymatic activity determination
Litter samples were analyzed to determine lignin, total carbohydrate, and N concentrations, and litter mass losses. Lignin concentration was determined by a gravimeter using hot sulfuric acid digestion (Osono and Takeda 2002). Total carbohydrate in the filtrate was estimated by the phenol-sulfuric acid method (Osono and Takeda 2002). N concentration was determined with Kjeldahl digestion (Bremner 1996).
Soil samples were analyzed to determine pH values and the activities of soil enzymes. Soil pH values were measured using a glass electrode (1:2.5 soil-to-water ratio) after shaking the samples to equilibration for approximately 30 min (Dick et al. 2000). The activities of soil enzymes involved in litter decomposition were determined spectrophotometrically with little modification: cellulase (E.C. 3.2.1.4; Ghose 1987), invertase (E.C. 3.2.1.26; Ohshima et al. 2007), polyphenol oxidase (E.C. 1.10.3.1; Perucci et al. 2000), catalase (E.C. 1.11.1.6; Guan 1986), nitrate reductase (E.C. 1.7.99.4; Daniel and Curran 1981), urease (E.C. 3.5.1.5; Nannipieri et al. 1980), and acid phosphatase (E.C. 3.1.3.2; Kandeler et al. 1999). The activities of soil enzymes were assayed within 1 week after sampling.
Statistical analyses
The constant potential mass loss over time was calculated by the following exponential equation (Olson 1963):
where x 0 is the original mass of a litter sample, x t is the amount of litter remaining after time t, and k is the litter decomposition coefficient (month−1).
Data were checked for deviations from normality and homogeneity of variance before analysis. An analysis of variance was applied to assess significant differences between various treatments using SPSS (version 13.0). Correlations were determined using the simple Pearson product-moment correlation coefficient. Statistically significant differences were set with P values <0.05, unless otherwise stated.
Results
Litter decomposition
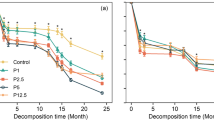
After a 6-month period of litter decomposition, the cumulative mass losses of LN-BF, MN-BF, HN-BF, CK-BF, LN-CF, MN-CF, HN-CF, and CK-CF were 20.41, 20.85, 22.40, 18.67, 16.98, 17.47, 18.54, and 16.39%, respectively (Fig. 1). Correspondingly, the litter decomposition coefficients (k values) were 0.0430 (HN-BF), 0.0399 (MN-BF), 0.0377 (LN-BF), 0.0350 (CK-BF), 0.0343 (HN-CF), 0.0325 (MN-CF), 0.0309 (LN-CF), and 0.0300 (CK-CF) (Table 2). N fertilization-accelerated litter decomposition rates were, on average, approximately 9.32 (LN-BF), 11.68 (MN-BF), 19.98 (HN-BF), 3.61 (LN-CF), 6.60 (MN-CF), and 13.15% (HN-CF) higher than those of CK, respectively. In contrast, MN and HN fertilization significantly accelerated the litter decomposition rates of leaves, while only HN fertilization significantly accelerated the litter decomposition rates of needles (Table 2, P < 0.05).
Lignin and total carbohydrate concentrations of the two litters under conditions of N fertilization and CK were not significantly changed during the litter decomposition process in all treatments (data not shown). N concentrations of the two litters showed similar trends, with a slight increase during the first 4 months and a slow decrease in the latter 2 months in all treatments, and there were significant differences between N fertilization and CK treatments during the latter 3 months of incubation (data not shown). The ratio of lignin:N and C:N of needles showed a trend of a slow decrease in the first 4 months and a slight increase in the latter 2 months, while those of leaves had no wide variation during the litter decomposition process (data not shown). Lignin:N of leaf litter was significantly correlated with mass losses during the litter decomposition process in MN and HN fertilization, while no significant relationships were found in LN fertilization or CK (Table 3, P < 0.05). N concentrations and the ratios of lignin:N and C:N of needle litter were significantly correlated with mass losses during the litter decomposition process in N fertilization, while no significant relationship was found in CK (Table 3, P < 0.05).
Soil pH values and enzymatic activities
During the 6-month incubation period, soil pH values increased slightly during the litter decomposition process in all treatments, while there was no significant difference between N fertilization and CK treatments in the two forests (data not shown).
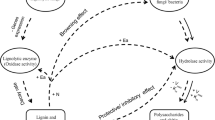
N fertilization accelerated the activities of the degradative enzymes, such as catalase, cellulase, invertase, acid phosphatase, polyphenol oxidase, and urease, of the two forests in most cases during the decomposition process (Fig. 2a–c, e–g). N fertilization restrained nitrate reductase activities in the two forests in most cases during the decomposition process (Fig. 2d). Under CK treatment, cellulase and nitrate reductase activities were positively correlated with litter mass losses in BF, while catalase, cellulase, and acid phosphatase activities were positively correlated with litter mass losses in CF (Table 4, P < 0.05). Under N fertilization, catalase, cellulase, and acid phosphatase activities were positively correlated with litter mass losses in BF, while catalase, cellulase, invertase, and nitrate reductase activities were positively correlated with litter mass losses in CF (Table 4, P < 0.05).
Changes in enzymatic activities of soils from the two forests during litter decomposition. a Catalase; b cellulase; c invertase; d nitrate reductase; e acid phosphatase; f polyphenol oxidase; g urease. Thin-lined bar LN; thick-lined bar MN; filled bar HN; open bar CK. Error bars indicate standard error (SE)
Discussion
Litter decomposition
Results of this study showed that decomposition rates of the two litters increased along the gradient of increasing N fertilization. This was consistent with the results of Kuperman (1999). N fertilization accelerated litter decomposition rates by enhancing the growth of microorganisms that decompose labile compounds of the litter (Sinsabaugh et al. 2002). However, our results showed that the responses of the decomposition for the two litters with different physicochemical properties were different. The litter mass losses of leaves were higher than those of needles under the same level of N fertilization, and the MN and HN fertilization significantly accelerated the litter decomposition rates for leaves, while only HN fertilization significantly accelerated the litter decomposition rates for needles. This may be because microbial communities in the broad-leaved forest are more responsive to N fertilization than those in the coniferous forest.
The level of atmospheric N deposition in the fast developing regions of China was approximately 3–5 g N m−2 year−1 in 2003 (Wang et al. 2007), thus, the most natural ecosystems in undeveloped regions are thought to be lower than this level. Therefore, according to the results of this study, litter decomposition rates in the broad-leaved forest would dramatically increase under increased atmospheric N deposition. At the same time, lower levels of atmospheric N deposition will not have significant effects on litter decomposition rates in the coniferous forest in most natural ecosystems in undeveloped regions.
Soil pH value and enzymatic activities
Haynes and Mokolobate (2001) have shown that soil pH values increased during litter decomposition. Results in this study were consistent with their results. This may be due to the production of ammonium during litter decomposition (Hoyt and Turner 1975) or the accumulation of organic matter in the soil (van Antwerpen and Meyer 1999). However, N fertilization had no significant effects on soil pH values in this study. We conjecture that this may be due to the metabolites produced by soil microorganisms during litter decomposition, which had a neutralizing effect on the acidity after NH4NO3 hydrolysis.
Investigators have revealed that cellulase and invertase are responsible for C mineralization (Romani et al. 2006; Antonious 2009), urease and nitrate reductase are responsible for N mineralization (Antonious 2009), and acid phosphatase is responsible for phosphorus (P) mineralization during litter decomposition (Dick et al. 2000), while polyphenol oxidase is responsible for the decomposition of recalcitrant polymers, such as lignin and humic acids (Allison et al. 2008). Catalase is susceptible to external disturbance (Bączek-Kwinta and Kościelniak 2009). Results in this study showed that N fertilization accelerated enzymatic activities, such as those of cellulase, invertase, acid phosphatase, polyphenol oxidase, and urease, and that the activities of those enzymes increased along the gradient of increasing N fertilization in most cases during the litter decomposition process. This suggested that the C, N, and P mineralization increased after N fertilization.
Some investigators have suggested that soil microorganisms, especially fungi, usually dominate at N-limited sites (Tietema 1998). Thus, the accelerated enzymatic activities in this study may be attributed to the alleviation of N limitation on soil microorganisms (Sinsabaugh et al. 2002). It could be expected that nitrate reductase activities would be increased when nitrogen was added directly. On the contrary, however, nitrate reductase activities decreased unexpectedly after N fertilization in this study. The reason may be that denitrification accelerated after nitrate inputs (Rudd et al. 1988). The inconsistencies in the effects of N fertilization on the activities of soil enzymes may be attributed to differences in the response of the metabolic activities of different microorganism groups, which may secrete different enzymes into the forest soils for litter decomposition under different levels of N fertilization.
Results in this study show that cellulase and nitrate reductase were primarily responsible for litter decomposition in the broad-leaved forest, while catalase, cellulase, and acid phosphatase were primarily responsible for litter decomposition in the coniferous forest under conditions of no N fertilization; catalase, cellulase, and acid phosphatase were primarily responsible for litter decomposition in the broad-leaved forest, while catalase, cellulase, invertase, and nitrate reductase were primarily responsible for litter decomposition in the coniferous forest under conditions of N fertilization. The results suggest that the type and quantity of the major degradative enzymes involved in litter decomposition differed in different forests under different treatment. This may be due to the difference in the physicochemical properties of the two litters or the two forest soils under different treatments, which, in turn, may have effects on the activities of microbial metabolism, particularly on the enzymatic secretion. The results also suggest that the accelerated litter decomposition rates under external N fertilization may be due to the increased activities of those enzymes, particularly cellulase. Some studies have also suggested that a major effect of N fertilization is to stimulate litter decomposition through an increase in cellulase activities (Sinsabaugh et al. 2002).
Results in this study showed that N fertilization accelerated the litter decomposition in a subtropical forest. The higher litter decomposition rates may cause an increase in the greenhouse gas CO2 in the atmosphere (Jenkinson et al. 1991). Elevated atmospheric CO2 might also lead to a warmer climate (Trenberth 1999), which could enhance the rates of litter decomposition in forest ecosystems (Hobbie 1996). Therefore, anthropogenic N deposition can lead to related changes in climatic factors, such as increasing atmospheric CO2 concentration and temperature, which, in turn, generally have multiple effects on forest ecosystems. Some studies have reported that the effects of N fertilization on early decomposition stages and later stages may be totally different (Moran et al. 2005). Thus, further studies, especially field studies, are required to characterize the long-term effects of N fertilization on litter decomposition in forest ecosystems.
References
Ajwa HA, Dell CJ, Rice CW (1999) Changes in enzyme activities and microbial biomass of tall grass prairie soil as related to burning and nitrogen fertilization. Soil Biol Biochem 31:769–777
Allison SD, Czimczik CI, Treseder KK (2008) Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Global Change Biol 14:1156–1168
Antonious GF (2009) Enzyme activities and heavy metals concentration in soil amended with sewage sludge. J Environ Sci Health A 44:1019–1024
Bączek-Kwinta R, Kościelniak J (2009) The mitigating role of environmental factors in seedling injury and chill-dependent depression of catalase activity in maize leaves. Biol Plantarum 53:278–284
Bragazza L, Freeman C, Jones T, Rydin H, Limpens J, Fenner N, Ellis T, Gerdol R, Hájek M, Hájek T, Iacumin P, Kutnar L, Tahvanainen T, Toberman H (2006) Atmospheric nitrogen deposition promotes carbon loss from peat bogs. Proc Natl Acad Sci USA 103:19386–19389
Bremner JM (1996) Nitrogen-total. In: Sparks DL (ed) Methods of soil analysis. Part 3. Chemical methods. SSSA-ASA, Madison, pp 1085–1121
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365
Daniel RM, Curran MP (1981) A method for the determination of nitrate reductase. J Biochem Biophys Methods 4:131–132
DeForest JL, Zak DR, Pregitzer KS, Burton AJ (2004) Atmospheric nitrate deposition, microbial community composition, and enzyme activity in northern hardwood forests. Soil Sci Soc Am J 68:132–138
Dick WA, Cheng L, Wang P (2000) Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol Biochem 32:1915–1919
Gallo M, Amonette R, Lauber C, Sinsabaugh RL, Zak DR (2004) Microbial community structure and oxidative enzyme activity in nitrogen-amended north temperate forest soils. Microb Ecol 8:218–229
Galloway J, Cowling E (2002) Reactive nitrogen and the world: 200 years of change. Ambio 31:64–71
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Guan SY (1986) Soil enzyme and its research methods (in Chinese). Agricultural Press, Beijing
Haynes RJ, Mokolobate MS (2001) Amelioration of Al toxicity and P deficiency in acid soil by additions of organic residues: a critical review of the phenomenon and the mechanisms involved. Nutr Cycl Agroecosys 59:47–63
Hobbie SE (1996) Temperature and plant species control over litter decomposition in Alaskan Tundra. Ecol Monogr 66:503–522
Hobbie SE (2000) Interactions between litter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian montane forest. Ecosystems 3:484–494
Hobbie SH, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–1877
Hoyt PB, Turner RC (1975) Effects of organic materials added to very acid soils on pH, aluminum, exchangeable NH4, and crop yields. Soil Sci 119:227–237
Jenkinson DS, Adams DE, Wild A (1991) Model estimates of CO2 emissions from soil in response to global warming. Nature 351:304–306
Johnson DW, Cheng W, Ball JT (2000) Effects of CO2 and N fertilization on decomposition and N immobilization in ponderosa pine litter. Plant Soil 224:115–122
Kandeler E, Tscherko D, Spiegel H (1999) Long-term monitoring of microbial biomass, N mineralisation and enzyme activities of a Chernozem under different tillage management. Biol Fert Soils 28:343–351
Keller JK, Bridgham SD, Chapin CT, Iversen CM (2005) Limited effects of six years of fertilization on carbon mineralization dynamics in a Minnesota fen. Soil Biol Biochem 37:1197–1204
Kuperman RG (1999) Litter decomposition and nutrient dynamics in oak-hickory forests along a historic gradient of nitrogen and sulfur deposition. Soil Biol Biochem 31:237–244
Lovett GM (1994) Atmospheric deposition of nutrient and pollutants in North America: an ecological perspective. Ecol Appl 4:629–650
Magill AH, Aber JD (1998) Long-term effects of experimental nitrogen additions on foliar litter decay and humus formation in forest ecosystems. Plant Soil 203:301–311
Micks P, Downs MR, Magill AH, Nadelhoffer KJ, Aber JD (2004) Decomposition litter as a sink for 15 N-enriched additions to an oak forest and a red pine plantation. For Ecol Manage 196:71–87
Moorhead DL, Sinsabaugh RL (2000) Simulated patterns of litter decay predict patterns of extracellular enzyme activities. Appl Soil Ecol 14:71–79
Moran KK, Six J, Horwath WR, van Kessel C (2005) Role of mineral-nitrogen in residue decomposition and stable soil organic matter formation. Soil Sci Soc Am J 69:1730–1736
Naeem S, Hahn DR, Schuurman G (2000) Producer decomposer co-dependency influences biodiversity effects. Nature 403:762–764
Nannipieri P, Ceccanti B, Cervelli S, Matarese E (1980) Extraction of phosphatase, urease, proteases, organic-carbon, and nitrogen from soil. Soil Sci Soc Am J 44:1011–1016
Ohshima T, Tamura T, Sato M (2007) Influence of pulsed electric field on various enzyme activities. J Electrostat 65:156–161
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Osono T, Takeda H (2002) Comparison of litter decomposing ability among diverse fungi in a cool temperate deciduous forest in Japan. Mycologia 94:421–427
Perucci P, Casucci C, Dumontet S (2000) An improved method to evaluate the o-diphenol oxidase activity of soil. Soil Biol Biochem 32:1927–1933
Romani AM, Fischer H, Mille-Lindblom C, Tranvik LJ (2006) Interactions of bacteria and fungi on decomposing litter: differential extracellular enzyme activities. Ecology 87:2559–2569
Rudd JWM, Kelly CA, Schindler DW, Turner MA (1988) Disruption of the nitrogen cycle in acidified lakes. Science 240:1515–1517
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Sinsabaugh RL, Carreiro MM, Repert DA (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60:1–24
Sinsabaugh RL, Gallo ME, Lauber C, Waldrop M, Zak DR (2005) Extracellular enzyme activities and soil carbon dynamics for northern hardwood forests receiving simulated nitrogen deposition. Biogeochemistry 75:201–215
Tietema A (1998) Microbial carbon and nitrogen dynamics in coniferous forest floor material collected along a European nitrogen deposition gradient. For Ecol Manage 101:29–36
Trenberth KE (1999) Conceptual framework for changes of extremes of the hydrological cycle with climate change. Clim Change 42:327–339
van Antwerpen R, Meyer JH (1999) Soil degradation II. Effect of trash and inorganic fertilizer application on soil strength. Proc S Afr Sug Technol Assoc 73:14–20
Waldrop MP, Zak DR, Sinsabaugh RL (2004) Microbial community response to nitrogen deposition in northern forest ecosystems. Soil Biol Biochem 36:1443–1451
Wang TJ, Jiang F, Li S, Liu Q (2007) Trends in air pollution during 1996–2003 and cross-border transport in city clusters over the Yangtze River Delta region of China. Terr Atmos Ocean Sci 18:995–1009
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30870419, 40971151) and Project “948” of State Forestry Administration (2006-4-13). We are grateful to two anonymous referees for their helpful comments to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wang, C., Feng, X., Guo, P. et al. Response of degradative enzymes to N fertilization during litter decomposition in a subtropical forest through a microcosm experiment. Ecol Res 25, 1121–1128 (2010). https://doi.org/10.1007/s11284-010-0737-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-010-0737-8