Abstract
Wood decay activity of Omphalotus guepiniformis, one of the most frequently occurring fruiting bodies on beech coarse woody debris in cool temperate forests in Japan, was estimated in situ by chronosequence with a five decay class system. The decay columns of O. guepiniformis increased from decay class 1 to decay class 2, where they occupied 20.2% of the total area of cross sections, and was estimated to be a dominant basidiomycete. The decay columns of O. guepiniformis decreased after decay class 2 and were not detected in decay class 5. The relative density of the decay columns of O. guepiniformis decreased to 0.33 g cm−3 in decay class 2 (58.9% of fresh beech wood) but did not decrease thereafter. The lignocellulose index (LCI) of the decay columns of O. guepiniformis slightly decreased during the decay process while remaining in the range of white-rot. In contrast, the decay columns of microfungi increased in the later stages of decomposition and LCI of these decay columns decreased significantly alongside the decay process. These results suggest that O. guepiniformis has an important role in simultaneous decomposition of acid-unhydrolyzable residue (AUR, Klason lignin) and holocellulose in the early stages of beech log decomposition, while holocellulose selective decomposition by microfungi may occur in the late stages of decomposition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungi, and especially basidiomycetes, are the primary agents of coarse woody debris (CWD) decomposition in terrestrial forest ecosystems (Harmon et al. 1986; Boddy 1991). Differences in the fungal community structure and the timing of colonization could affect carbon and nutrient cycling and soil formation in forests via difference in CWD decomposition rates and decay types of wood (e.g., white-, brown-, and soft-rot) (Means et al. 1992; Progar et al. 2000; Barker 2008). However, few studies have demonstrated the extent to which fungi actually decay wood within CWD under field conditions or have evaluated the wood decay activity of certain fungi throughout the entire decay process (Temnuhin 1996).
Wood-inhabiting fungi, especially basidiomycetes and xylariaceous ascomycetes, form exclusive colonies called “decay columns” within CWD (Rayner and Boddy 1988). The decay column is covered with melanized hyphal mats called pseudosclerotial plates (PSPs) or pigmented hyphae growing at the confrontation regions of different mycelia that were observed in cross sections of the wood as zone lines (Lopez-Real and Swift 1975). Thus, the occurrence of wood decay fungi within decaying CWD can be quantified by measuring the decay columns (Coates and Rayner 1985a, 1985b, 1985c; Fukasawa et al. 2002), and wood decay activities of certain fungi in CWD can be quantified by measuring the decomposition that occurs in each decay column (Rayner and Boddy 1988; Boddy et al. 1989).
Omphalotus guepiniformis (Berk.) Neda (“Tsukiyotake” in Japanese) is a luminescent and poisonous pleurotoid basidiomycete growing on beech wood (Neda 2004). Our previous studies in a cool temperate natural forest revealed that (1) O. guepiniformis is the most frequently occurring fruiting body on beech CWD (Fukasawa et al. 2009a); (2) mycelia of O. guepiniformis (denoted as Lampteromyces japonicus) colonize the largest volume of beech CWD compared to the other fungal species in the intermediate stage of decomposition (Fukasawa et al. 2005b); (3) O. guepiniformis (denoted as L. japonicus) decays lignin and holocellulose of beech wood simultaneously under sterile laboratory culture conditions (Fukasawa et al. 2005a). Thus, it is estimated that O. guepiniformis has a central role in lignocellulose decomposition of beech CWD in cool temperate natural forests in Japan. O. guepiniformis is classified as “Vulnerable” in the Red Data Book because of the disappearance of beech forests (Environment Agency of Japan 2000). Quantitative research is required to evaluate the functional importance of O. guepiniformis in the decomposition process of beech CWD.
The aims of the present study are to demonstrate the dynamics of decay columns of wood-inhabiting fungi within decaying CWD of Japanese beech (Fagus crenata Blume) and the wood decay activities of those fungi especially focused on O. guepiniformis in a natural condition. We investigated beech logs at various stages of decomposition and estimated their decay processes by chronosequence method in which logs decaying on the forest floor are classified into decay classes based on combinations of visual and physical properties (Berg and McClaugherty 2003). Differences in CWD properties observed between decay classes are regarded as changes during the decay process. These chronosequence methods have been used to describe CWD decomposition on the forest floor (Lambert et al. 1980; Means et al. 1992; Bütler et al. 2007; Fukasawa et al. 2009a).
Materials and methods
Sampling site
The study was carried out in a cool temperate deciduous forest dominated by Fagus crenata and Quercus crispula in Ashiu Experimental Forest of Kyoto University (35°18′N, 135°43′E) about 40 km north of Kyoto, Japan. The mean annual temperature is about 10°C and the mean monthly temperature ranges from 0.4 in January to 25.5°C in August. The mean annual precipitation over a 56-year period has been 2,495 mm. The study area was located in the Sea of Japan coastal regions where heavy snow covered the ground (over 2 m depth in max) in the winter period from December to April.
Fungal isolation
A total of 50 logs of beech were marked along a 3-km walkway including the ridge and bottom of several slopes. All logs had fallen naturally in the sampling site. The time after their fall is unknown. The diameter of the logs ranged from 13 to 53 cm. Logs of beech were identified by bark texture and xylem ray distribution of wood tissues using the keys reported by Shimaji et al. (1980). We assigned each log of beech to 1 of the 5 decay classes (10 logs per decay class) using visual and physical criteria after Fukasawa et al. (2009a) as follows: (1) wood hard, knife penetrates only a few millimeters into the wood, bark and twigs (diam <1 cm) intact; (2) wood rather hard, a knife penetrates less than 1 cm into the wood, bark and twigs starting to lost, branches (diam 1–4 cm) intact; (3) wood distinctly softened, knife penetrates ca. 1–4 cm into the wood, bark and branches partly lost, original log circumference intact; (4) wood strongly decayed, knife penetrates ca. 5–10 cm into the wood, bark lost in most places, original log circumference disintegrating; (5) wood very strongly decayed, either to a very soft crumbly substance, or is flaky and fragile, knife penetrates in most places more than 10 cm into the wood, original log circumference not or hardly recognizable.
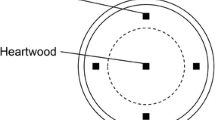
From each log in decay classes 1–4, a disk, approximately 10 cm in thickness, was cut from an intermediate part of the log using a chainsaw. The disks were bagged, stored at 5°C, and processed within 48 h. One cross section of each disk was observed and the fungal decay columns were distinguished by zone lines and wood discoloration. From each disk, small wood chips (approximately 2 mm3) were cut from the cross sections at nine fixed positions corresponding to the sapwood, outer, and inner heartwood (Fig. 1). Additional wood chips were taken from cross sections of all areas in which there was discoloration due to fungal colonies (Fig. 1). From each log in decay class 5, wood cubes (approximately 8 cm3) were collected from the cross sections at nine fixed positions described above (Fig. 1) in the field, and then a wood chip (approximately 2 mm3) was collected from each sample in the laboratory. The disks and wood cubes were collected between July and December 2006.
All wood chips were aseptically cut out from the disks or cubes using flamed chisel and transferred to Petri plates containing both of the two different media: malt extract agar (MA; 2% malt extract, 1.5% agar, w v−1; Nacalai Tesque, Kyoto, Japan), and malt extract agar containing benomyl (2 mg l−1; Sumitomokagaku, Tokyo, Japan). The first medium was intended to isolate all the fungi, and the second medium was intended to detect slow-growing fungi such as basidiomycetes by preventing rapid growth of ascomycetes by benomyl.
The plates were incubated at 20°C for 2 months. Once fungal colonies formed in the agar plates, each colony was transferred to a new agar plate to grow as a pure culture. Identification of these fungi was based on colony features of the cultures and their morphologies, which were determined microscopically. Mycelia of O. guepiniformis were identified by their thick, bright-orange-colored colonies with slightly purplish centers on MA medium and by their blue aerial hyphae with brown knots under the microscope (Fukasawa 2003). Other fungi were categorized into “other basidiomycetes”, “microfungi” and “sterile fungi”. Mycelia of basidiomycetes were distinguished by clamp connections or characteristic rhizomorphs. In this study, we call anamorphic ascomycetes and zygomycetes as “microfungi”. Further information about species composition and succession of microfungi obtained from this series of studies were described elsewhere (Fukasawa et al. 2009b).
The area of decay columns of O. guepiniformis and fungi in the other categories were measured on a photocopy of the cross section of each disk in decay classes 1–4 using the computer program ImageJ version 1.41 (National Institutes of Health 2008) and expressed as percentage of the area within all the ten cross sections in each decay class. Although well-established decay columns of basidiomycetes are usually occupied exclusively by a single species (Rayner and Boddy 1988), in some cases, such as replacement or mycoparasite, there exist more than two species in single decay column (Boddy et al. 1989). In this study, the decay columns from which only O. guepiniformis was isolated were designated as decay columns of O. guepiniformis in order to evaluate sole decay functions of this fungus without effects of other organisms. If a decay column included more than two isolation points and from which fungi in the different categories were isolated, a boundary between the colonies of them was determined tentatively as a vertical line across the midpoint of a line between the two isolation points.
Physicochemical properties
Wood samples were taken from each isolation point (Fig. 1) of each disk using an electric drill (drill bit, length: 24 cm, diameter: 9 mm). The drill bit was drilled into a disk from one cross section to penetrate the disk, and all the wood particles drilled out were sampled. The depth of each drill hole was measured. In the logs of decay class 5, residues of wood cubes after fungal isolation were used for physicochemical analyses.
Wood samples were weighed fresh and then dried to a constant weight at 40°C. The water content of each wood sample was expressed as a percentage of a dry weight of the sample. The relative density (g cm−3) of the sample (also known as bulk density or apparent density) was calculated as dry weight of sample divided by the volume of drill hole. Relative density is an adequate measure of the degree of decomposition in dead wood as long as original shape and volume of the log was maintained (Yoneda 1975; Christensen 1984). Relative density was not calculated for wood of decay class 5 because the original wood shape and volume was not maintained.
The dried wood samples were ground in a laboratory mill to pass a 0.5-mm screen and used for chemical analyses. Analyses were conducted from February to May 2007. The amount of acid-unhydrolyzable residue (AUR, also known as the acid-insoluble residue or “Klason lignin” fraction) in the sample was estimated gravimetrically using hot sulphuric acid digestion (King and Heath 1967). Samples were extracted with alcohol–benzene at room temperature and the residues treated with 72% sulphuric acid (v v−1) for 2 h at room temperature with occasional stirring. The mixture was then diluted with distilled water to make a 2.5% sulphuric acid solution and autoclaved at 120°C for 60 min. After cooling, the residue was filtered and washed with water through a porous crucible (G4), dried at 105°C, and then weighed. The filtrate (autoclaved sulphuric acid solution) was used for total carbohydrate analysis by the phenol–sulphuric acid method (Dubois et al. 1956). Five percent phenol (v v−1) and 98% sulphuric acid (v v−1) were added to the solution. The optical density of the solution was then measured by a spectrophotometer at 490 nm using the known concentrations of d-glucose as standards. Since wood contains very little carbohydrate other than holocellulose, measured amounts of the total carbohydrate were regarded as the amount of holocellulose in this study.
The final residue left after acid hydrolysis during chemical analysis contains a mixture of organic materials that is not a single chemical compound such as lignin. Preston et al. (1997) demonstrated through NMR analysis that the organic residue prepared from acid hydrolysis of a variety of litter types contains a mixture of organic compounds including not only lignin but also condensed tannins and waxes. In the present study we use the term AUR to refer the organic residue left after chemical analysis.
Lignocellulose index (LCI) is a useful index of litter chemical quality (Osono and Takeda 2001) and is calculated according to the following equation:
Statistical analysis
A two-way ANOVA was employed to compare wood physicochemical properties (relative density, LCI, and water content) among the decay columns of O. guepiniformis, other basidiomycetes and microfungi. Decay classes 1–4 and the categories of the fungi were set as factors. Data of decay class 5 were not used in the analysis because no decay columns of O. guepiniformis were present in decay class 5. A one-way ANOVA was used to compare wood physicochemical properties among the decay classes, followed by Tukey’s HSD test (p < 0.05) for decay columns of each fungal category separately. All statistical tests were performed using JMP version 5.1.1 software (SAS Institute 2004).
Results
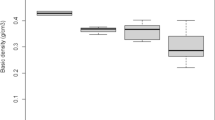
From 674 wood chips (average 13.5 chips per log), 1,490 isolations were made. Of these isolations, 1,394 yielded fungal isolates. Figure 2 shows the percentage area of the decay columns of the detected fungal categories in cross sections. O. guepiniformis increased from 5.8% in decay class 1 to 20.2% in decay class 2 and then decreased to 3.0% in decay class 4. O. guepiniformis was not detected in decay class 5. The percentage area of the decay columns of other basidiomycetes, as with O. guepiniformis, increased from decay class 1 to decay class 2 and then decreased to decay class 4. On the other hand, the decay columns of microfungi decreased from decay class 1 to decay class 2 due to the increase in basidiomycetes, then increased thereafter to 65.5% in decay class 4. The decay columns of sterile fungi were largest in decay class 1 and decreased during the decay process.
Figure 3 shows the distribution of the decay columns of O. guepiniformis observed in cross sections of beech logs. In decay class 1, the decay columns of O. guepiniformis were detected in three of ten logs. They were mainly distributed in the sapwood, from a very small area near the upper surface of the log (#649) to a larger area (#725 and #722). In decay class 2, the decay columns were detected in three of ten logs. They were not only largely distributed in the sapwood (#667 and #639) but also in the heartwood (#666). In decay class 3, the decay columns were detected in two of ten logs. They were largely distributed in the heartwood and to a lesser extent in the sapwood. In decay class 4, the decay columns were detected in two of ten logs. Their distributions were restricted to small parts of the cross sections.
Distributions of all decay columns of Omphalotus guepiniformis detected in cross sections of beech logs (grey area). The vertical direction of each cross section reflects the real direction in the field. Solid lines are boundaries between fungal decay columns determined by zone lines or wood discoloration. Dotted lines near the center of each cross section are the boundaries between the heartwood and sapwood. The identification number of each log is shown below each cross section. a Decay class 1; b decay class 2; c decay class 3; d decay class 4. Bar 10 cm
Two-way ANOVA showed both fungal categories and decay classes as well as their interaction, which significantly influenced physicochemical properties of wood (relative density, LCI, and water content) (Table 1). Mean values of the relative density of the decay columns of O. guepiniformis decreased from decay class 1 to decay class 2, reaching 0.33 g cm−3 in decay class 2 and increased again from decay class 2 to decay class 4 (Table 2). On the other hand, the relative densities of the decay columns of other basidiomycetes and microfungi decreased throughout the decay process and reached 0.28 and 0.17 g cm−3, respectively, in decay class 4. LCI of the decay columns of O. guepiniformis slightly decreased from decay class 1 to decay class 2 and then remained constant thereafter (Table 2). On the other hand, LCI of the decay columns of microfungi decreased throughout the decay process and reached 0.576 in decay class 5, suggesting selective decomposition of holocellulose. Water content of the decay columns of O. guepiniformis fluctuated between 45 and 93%, while the range was substantially smaller compared to the water contents of the decay columns of other basidiomycetes and microfungi that increased throughout the decay process and reached 631 and 574%, respectively, in decay class 5.
Discussion
Where does wood decomposition by O. guepiniformis occur in the decaying beech logs and when does it occur in the decay process? This study clearly shows that O. guepiniformis colonizes the sapwood of recently fallen beech logs and extends its decay columns from the sapwood to the heartwood throughout the decay process. The decay columns were mainly distributed in the sapwood near the upper surface of the logs in decay class 1, suggesting that O. guepiniformis probably colonizes the log via airborne spores. The percentage area of the decay columns of O. guepiniformis reached a maximum (20.2%) in decay class 2. We recorded 18 species of fruiting bodies of basidiomycetes (Fukasawa et al. 2009a) and 22 species of microfungi (Fukasawa et al. 2009b) from beech logs in decay class 2. Furthermore, many non-cultivable fungi that could not be isolated in this study may exist within the logs (Vainio and Hantula 2000). Therefore, O. guepiniformis, singly occupying 20.2% of the total area of the cross sections, is estimated to be a dominant fungus in beech logs for decay class 2. Nevertheless, even for decay class 2, the decay columns of O. guepiniformis were detected in only three of ten logs, suggesting that there is a significant variation among the logs as to whether O. guepiniformis dominates. We have previously found the same trend that the percentage of volume colonized by O. guepiniformis varied from 3.1 to 92.7% among five beech logs for decay class 3 (diameter 10.7–20.5 cm) (Fukasawa et al. 2005b). Biotic factors such as local spore source (Vasiliauskas et al. 2005) and abiotic factors such as moisture and temperature conditions (Rayner and Boddy 1988; Yang 2005) may affect fungal community structure within CWD.
What proportion of the wood components was decayed by O. guepiniformis? This study demonstrates that the mean values of the wood relative density of the decay columns of O. guepiniformis decreased to 0.33 g cm−3, which represents 58.9% of fresh beech wood (0.56 g cm−3, Fukasawa et al. 2009a). During this period, LCI values of the decay columns slightly decreased but were in the ranges of ‘normal-like’ white-rotted wood (0.65–0.84) determined by Kawase (1962), i.e., not different from LCI of fresh wood. These results suggest that O. guepiniformis decays AUR and holocellulose of beech wood simultaneously in their decay columns under field conditions. We previously reported that O. guepiniformis decayed AUR and holocellulose simultaneously under sterile laboratory culture conditions (Fukasawa et al. 2005a). Although the decay columns of O. guepiniformis extended to the heartwood in decay classes 3 and 4, progressive wood decomposition was not detected in the decay columns of this fungus during this period. This may be partly due to the replacement of O. guepiniformis by microfungi in well-decayed areas, as shown in Fig. 2, and thus, the decay columns of O. guepiniformis may be restricted to relatively undecayed areas such as the heartwood. Wood decomposition by O. guepiniformis may be reduced in the heartwood because the heartwood of beech is called ‘false heartwood’ or ‘red heart’ and includes polyphenolics that have antifungal activity (Hillis 1987).
On the other hand, the relative density of the decay columns of other basidiomycetes and microfungi decreased even after decay class 2. We previously reported that fruiting bodies of white-rot basidiomycetes, such as Steccherinum rhois and Trichaptum biforme, frequently occurred on the beech logs in decay classes 1 and 2 (Fukasawa et al. 2009a). Since both of these species were revealed as capable of decaying AUR and holocellulose simultaneously (Fukasawa et al. 2005a), the role of other basidiomycetes, especially in the early stages of beech log decomposition, may be similar to that of O. guepiniformis. In the later stages of decomposition, fruiting bodies of basidiomycetes such as Mycena haematopus and Pluteus spp. have frequently been recorded, although their role in beech log decomposition is still obscure (Fukasawa et al. 2009a).
Because the percentage area of the decay columns of O. guepiniformis and other basidiomycetes decreased and that of microfungi increased after decay class 2, it is estimated that a part of the decay columns of microfungi in decay classes 3–5 had been colonized by O. guepiniformis or other basidiomycetes during the earlier decay classes. Our previous report showed that several species of microfungi known as soft-rot fungi, such as Trichoderma spp., were frequently isolated from later stages of beech log decomposition (Fukasawa et al. 2009b). Holocellulose decay abilities of soft-rot microfungi would be stimulated by the prior delignification by white-rot fungi (Tanaka et al. 1988). The high water content observed in the decay columns of microfungi is suitable for soft-rot fungal activity (Eriksson et al. 1990; Worrall and Wang 1991) instead of the decay columns of O. guepiniformis, where the range of the water content is within that level suitable for basidiomycetes (Schmidt 2006).
To summarize, this study clearly shows that O. guepiniformis decayed beech logs at the sampling site as follows: (1) during the period of decay classes 1–2; (2) mainly in the sapwood; (3) 20.2% of the total area of cross sections of the logs were colonized by O. guepiniformis; (4) wood mass was decomposed to 58.9% of the original mass in the decay columns of O. guepiniformis at decay class 2; (5) wood organic components (AUR and holocellulose) were decayed simultaneously (simultaneous white-rot). These results suggest that O. guepiniformis has an important role in beech log decomposition, causing simultaneous white-rot in the early stages of decomposition. Further research such as laboratory decay tests is needed to reveal the effects of prior decomposition by O. guepiniformis on successive wood decomposition by other fungi; moreover, actual identification of wood-inhabiting mycelia of basidiomycetes by molecular techniques are required to reveal the mechanisms of resource partitioning among wood-inhabiting fungi for a better understanding of the fungal decay processes in beech CWD.
References
Barker JS (2008) Decomposition of Douglas-fir coarse woody debris in response to differing moisture content and initial heterotrophic colonization. For Ecol Manage 255:598–604
Berg B, McClaugherty C (2003) Plant litter. Decomposition, humus formation, carbon sequestration. Springer, Berlin
Boddy L (1991) Importance of wood decay fungi in forest ecosystems. In: Arora DK, Rai B, Mukerji KG, Knudsen GR (eds) Handbook of applied mycology, vol. 1: soil and plants. Marcel Dekker, New York, pp 507–539
Boddy L, Owens EM, Chapela IH (1989) Small scale variation in decay rate within logs one year after felling: effect of fungal community structure and moisture content. FEMS Microbiol Ecol 62:173–184
Bütler R, Patty L, Bayon R-CL, Guenat C, Schlaepfer R (2007) Log decay of Picea abies in the Swiss Jura Mountains of central Europe. For Ecol Manage 242:791–799
Christensen O (1984) The states of decay of woody litter determined by relative density. OIKOS 42:211–219
Coates D, Rayner ADM (1985a) Fungal population and community development in cut beech logs. I. Establishment via the aerial cut surface. New Phytol 101:153–171
Coates D, Rayner ADM (1985b) Fungal population and community development in cut beech logs. II. Establishment via the buried cut surface. New Phytol 101:173–181
Coates D, Rayner ADM (1985c) Fungal population and community development in cut beech logs. III. Spatial dynamics, interactions and strategies. New Phytol 101:183–198
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Environment Agency of Japan (2000) Threatened wildlife of Japan—Red data book 2nd ed.—vol. 9: bryophytes, algae, lichens, fungi (in Japanese with English abstract). Japan Wildlife Research Center, Tokyo
Eriksson K-EL, Blanchette RA, Ander P (1990) Microbial and enzymatic degradation of wood and wood components. Springer, Berlin
Fukasawa Y (2003) Heterogeneity of wood chemical property within decomposing beech logs reflecting fungal community structure and fungal decay ability. Master Thesis, Kyoto University, Kyoto
Fukasawa Y, Osono T, Takeda H (2002) Fungal community structure and physical and chemical properties of decaying beech logs in a cool temperate natural forest (in Japanese with English abstract). Appl For Sci 11:7–14
Fukasawa Y, Osono T, Takeda H (2005a) Decomposition of Japanese beech wood by diverse fungi isolated from a cool temperate deciduous forest. Mycoscience 46:97–101
Fukasawa Y, Osono T, Takeda H (2005b) Small-scale variation in chemical property within logs of Japanese beech in relation to community structure and functional diversity of fungi. Mycoscience 46:209–214
Fukasawa Y, Osono T, Takeda H (2009a) Dynamics of physicochemical properties and occurrence of fungal fruit bodies during decomposition of coarse woody debris of Fagus crenata. J For Res 14:20–29
Fukasawa Y, Osono T, Takeda H (2009b) Microfungus communities of Japanese beech logs at different stages of decay in a cool temperate deciduous forest. Can J For Res 39:1606–1614
Harmon ME, Franklin JF, Swanson FJ, Sollins P, Gregory SV, Lattin JD, Anderson NH, Cline SP, Aumen NG, Sedell JR, Lienkaemper GW, Cromack K, Cummins KW (1986) Ecology of coarse woody debris in temperate ecosystems. Adv Ecol Res 15:133–302
Hillis WE (1987) Heartwood and tree exudates. Springer, Berlin
Kawase K (1962) Chemical components of wood decayed under natural condition and their properties. J Fac Agric Hokkaido Univ 52:186–345
King HGC, Heath GW (1967) The chemical analysis of small samples of leaf material and the relationship between the disappearance and composition of leaves. Pedobiologia 7:192–197
Lambert RL, Lang GE, Reiners WA (1980) Loss of mass and chemical change in decaying boles of a subalpine balsam fir forest. Ecology 61:1460–1473
Lopez-Real JM, Swift MJ (1975) The formation of pseudosclerotia (‘zone lines’) in wood decayed by Armillaria mellea and Stereum hirsutum. II. Formation in relation to the moisture content of the wood. Trans Br Mycol Soc 64:473–481
Means JE, MacMillan PC, Cromack K (1992) Biomass and nutrient content of Douglas-fir logs and other detrital pools in an old-growth forest, Oregon, U.S.A. Can J For Res 22:1536–1546
National Institute of Health (2008) ImageJ: image processing and analysis in Java. Version 1.41 [online]. http://rsb.info.nih.gov/ij/. Accessed 24 May 2009
Neda H (2004) Type studies of Pleurotus reported from Japan. Mycoscience 45:181–187
Osono T, Takeda H (2001) Organic chemical and nutrient dynamics in decomposing beech leaf litter in relation to fungal ingrowth and succession during 3-year decomposition processes in a cool temperate deciduous forest in Japan. Ecol Res 16:649–670
Preston CM, Trofymow JA, Sayer BG, Niu J (1997) 13CPMAS NMR investigation of the proximate analysis of fractions used to assess litter quality in decomposition studies. Can J Bot 75:1601–1613
Progar RA, Schowalter TD, Freitag CM, Morrell JJ (2000) Respiration from coarse woody debris as affected by moisture and saprotroph functional diversity in Western Oregon. Oecologia 124:426–431
Rayner ADM, Boddy L (1988) Fungal decomposition of wood: its biology and ecology. Wiley, Chichester
SAS Institute (2004) JMP statistical discovery software (ver. 5.1.1). SAS Institute Inc., Cary
Schmidt O (2006) Wood and tree fungi: biology, damage, protection, and use. Springer, Berlin
Shimaji K, Sudou S, Harada H (1980) Mokuzai no soshiki (in Japanese). Morikita Publishing, Tokyo
Tanaka H, Enoki A, Fuse G, Nishimoto K (1988) Interactions in successive exposure of wood to varying wood-inhabiting fungi. Holzforschung 42:29–35
Temnuhin VB (1996) Preliminary quantitative estimation of wood decomposition by fungi in a Russian temperate pine forest. For Ecol Manage 81:249–257
Vainio EJ, Hantula J (2000) Direct analysis of wood-inhabiting fungi using denaturing gradient gel electrophoresis of amplified ribosomal DNA. Mycol Res 104:927–936
Vasiliauskas R, Lygis V, Larsson K-H, Stenlid J (2005) Airborne fungal colonisation of coarse woody debris in North Temperate Picea abies forest: impacts of season and local spatial scale. Mycol Res 109:487–496
Worrall JJ, Wang CJK (1991) Importance and mobilization of nutrients in soft rot of wood. Can J Microbiol 37:864–868
Yang D-Q (2005) Isolation of wood-inhabiting fungi from Canadian hardwood logs. Can J Microbiol 51:1–6
Yoneda T (1975) Studies on the rate of decay of wood litter on the forest floor. I. Some physical properties of decaying wood. Jpn J Ecol 25:40–46
Acknowledgments
We thank Nobu Endo, Osamu Tateno, Hitomi Mizuta, Akihiro Shimizu, Yusuke Hagiwara, and members of Laboratory of Forest Ecology, Kyoto University, for their help in field and laboratory works. Thanks are extended to the staff of Kamigamo Experimental Forest of Kyoto University for technical support. Financial support for this study was provided by the Research Fellowship Division, Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Fukasawa, Y., Osono, T. & Takeda, H. Beech log decomposition by wood-inhabiting fungi in a cool temperate forest floor: a quantitative analysis focused on the decay activity of a dominant basidiomycete Omphalotus guepiniformis . Ecol Res 25, 959–966 (2010). https://doi.org/10.1007/s11284-010-0720-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-010-0720-4