Abstract
The decomposition rate of CWD is a key missing link for a quantitative understanding of forest ecosystem functioning. We examined factors influencing decomposition rates of bark, roots and branches from aspen (Populus tremula), birch (Betula pendula, B. pubescens), fir (Abies sibirica), spruce (Picea abies, P. obovata), Scots pine (Pinus sylvestris) and Siberian pine (Pinus sibirica) CWD in three primeval European boreal forests. The chronosequence approach with estimates of single exponential decomposition rate (k) based on calculation of mass loss was used. The k of non-stem parts increased in the order: branches (0.006 year−1 for P. sibirica and 0.020 year−1 for other species), roots in poorly drained sites (0.025 year−1), roots in well-drained sites (0.034 year−1) and bark (0.110 and 0.138 year−1 and 0.147 and 0.255 year−1 under poorly and well-drained conditions and from 1 to 3 m and >3 m above the root collar, respectively). Our results predict that the rate of decomposition of whole CWD pieces in European boreal forests is a function of vegetation zone, site conditions, tree species and size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coarse woody debris (CWD) represented by standing dead trees, downed woody debris and stumps (Harmon et al. 1986) has been acknowledged as an important component of forest biodiversity, carbon and nutrient cycling (Laiho and Prescott 2004; Palviainen et al. 2011; Stokland et al. 2012; Dittrich et al. 2014). It has also been used as an indicator of forest ecosystem services (Helfenstein and Kienast 2014). The aboveground volume of CWD stem parts in European boreal forests can reach over one thousand m3 ha−1 (Shorohova and Kapitsa 2015) with the residence time varying from tens to more than 500 years (Shorohova and Kapitsa 2014b). Non-stem components can double the aboveground CWD pools of organic matter and nutrients. For example, the volume of bark, branches and roots can vary from 8 to 17 %, from 4 to 10 % and from 18 to 30 %, respectively, of the stem volume of the dominant boreal tree species (Tetioukhin et al. 2004).
Decomposition of woody debris is the result of microbial decay, physical degradation (i.e., fragmentation and weathering), leaching and biological transformation. Of these, microbial decay is considered the main process (Chambers et al. 2001; Bond-Lamberty and Gower 2008). There have been many studies in European boreal forests that estimate regional decomposition rates of stem wood (Krankina and Harmon 1995; Harmon et al. 2000; Yatskov et al. 2003; Mäkinen et al. 2006), whereas less is known about decomposition rates of non-stem components (Yin 1999; Palviainen and Finér 2015).
The estimates of microbial respiration and fragmentation rates of aboveground stem parts are used for calculating carbon budgets on the country level (Mackensen and Bauhus 1999; Kudeyarov et al. 2007; Zamolodchicov et al. 2011) and in vegetation and climate models (Cramer et al. 2001). Less frequently, belowground roots and branches on the forest floor are included in the models (Beets et al. 2011; Garrett et al. 2012). The lack of information on coarse root decomposition rates and C pool changes has been identified as one of the biggest source of errors in forest C-stock reporting (Tobin et al. 2007). Considering all CWD components separately, taking into account ecosystem attributes, would improve those models significantly. In general, uncertainties in predicting the rate of CWD decomposition can lead to uncertainties in our understanding of forests and their role in the sequestration and emission of CO2. Those uncertainties, in turn, result in an inability to develop appropriate strategies for achieving deadwood-related objectives, including climate change mitigation, biodiversity protection and procurement of forest bioenergy feedstocks (Russell et al. 2015).
In boreal zones, over a narrow range of conditions, the climatic effects on the decomposition rate of stem wood have been shown to be less important or nonsignificant compared to site and substrate characteristics (Yatskov et al. 2003; Mäkinen et al. 2006; Shorohova and Kapitsa 2014a, b). On an ecosystem scale, higher decomposition rates were observed for stem wood on sites with moderate moisture compared to those on dry and moist sites (Shorohova and Kapitsa 2014a, b) and can be hypothesized for non-stem CWD components as well.
Data on the decomposition rate of belowground coarse roots are scarce and show the same, faster and slower root decomposition rates compared to aboveground material (Fahey et al. 1988; Janisch et al. 2005; Olajuyigbe et al. 2011; Shorohova et al. 2012; Palviainen and Finér 2015). These contradictions can be explained by the interplay between differences in moisture content and oxygen concentration below and above ground. The influence of soil drainage may be a significant driver of decomposition rates, particularly for roots, where decomposition can be retarded under the saturated conditions of very poorly drained soils or facilitated under well-drained conditions, where the availability of moisture and nutrients for decomposing fungi can be improved (Erickson et al. 1985). The decomposition of roots above ground, as part of uprooted trees, has not been studied yet. The decomposition rates of roots below ground and above ground for different tree species, different regions and different tree mortality modes (i.e., snags and snapped trees as well as of other aboveground CWD parts) need to be compared under well and poorly drained site conditions in different parts of boreal zone.
The decomposition of branches and the fungal communities associated with them have been studied mainly when they were attached to standing trees under a forest canopy (Boddy and Rayner 1983; Boddy et al. 1987) or on the forest floor (Miller 1983; Erickson et al. 1985; Mukhin 1993; Hyvönen et al. 2000; Garrett et al. 2012). When branches start to decompose when a tree is alive, complete decay of sapwood can occur in the canopy if it is supported by heartwood, but if not, branches and twigs will fall to the floor, where decay continues (Boddy and Heilmann-Clausen 2008). Knowledge of the decomposition of the side branches, or branches attached to logs, is very limited (Ganjegunte et al. 2004).
Decomposition rates of bark have been shown to differ from those of wood. However, the character of this difference depends on tree species and environmental conditions influencing microbial respiration (mineralization) rates as well as on fragmentation induced by biotic agents (Rypaček 1957; Parameswaran et al. 1976; Ganjegunte et al. 2004; Shorohova and Kapitsa 2012, 2014b) and requires further research.
In this study, we aimed at estimating the decomposition dynamics of non-stem CWD components and whole CWD pieces in primeval European boreal forests using a chronosequence approach. Our specific objectives were to: (1) estimate the initial conditions and mass loss of non-stem CWD components: roots, branches and bark; (2) calculate the decomposition rates of CWD components depending on vegetation zone, soil moisture, CWD tree species, tree mortality mode and log size; and (3) estimate the CWD piece scale decomposition rates of the dominant tree species in European boreal forests. We hypothesized that the substrate moisture during decomposition influenced by position of CWD component and soil conditions, together with initial density and mass, are the most important predictors of the decomposition rate. We hypothesized that the decomposition rate at the CWD piece scale would increase from north to south and would be higher under better than poor soil drainage. Comparing an assumed higher, equal and lower decomposition rate of bark, roots and branches, respectively, to stem wood and relative weight proportion of each component, we hypothesized that the decomposition rate of whole CWD pieces would be higher than that of stem wood alone.
Materials and methods
Study sites and sample plots
The studies were carried out from 1997 to 2009 in three primeval old-growth forests located in Russia (Fig. 1). Mean annual temperatures vary from −3.0 to +3.6 °C, and mean annual precipitation varies from 600 to 750 mm. The forested areas of Vepssky forest (VF1) and Central Biosphere Forest Reserve (CFR) landscapes are dominated by Norway spruce (Picea abies Karst., P. fennica) with an admixture of birch (Betula pubescens Ehrh. and Betula pendula Roth.), aspen (Populus tremula L.) and Scots pine (Pinus sylvestris L.). In the CFR, the broadleaved species Norway maple (Acer platanoides L.) and European white elm (Ulmus laevis Pall.) also occur. The forests of Yugyd-Va National Park sites (Komi) are mixed Siberian spruce (Picea obovata Ledeb.), Siberian fir (Abies sibirica Ledeb.), Siberian pine (Pinus sibirica Du Tour or (Loudon) Mayr) and birch. According to the information from the archives, the forests of all sampled sites have never been commercially harvested. Standing growing stock varied from 0 in the gap left by stand-replacing windthrow in CFR to 502 m3 ha−1 in an over-mature mixed even-aged stand in VF1. All forest stands were uneven-aged with the tree age ranging from 1 to 460 years. The period of time since last disturbance (determined by dating fire scars or uprooted trees) varied from 1 to 380 years. The CWD stocks varied greatly among the plots, ranging from 46 to 1267 m3 ha−1, depending on the natural disturbance regime. Maximum CWD values were found after stand-replacing windthrows in highly productive forests in CFR.
Location of the study sites. Komi—Yugyd-va National Park, VF1—Vepssky forest, Saint-Petersburg region, CFR—Central Forest State Natural Biosphere Reserve, Tver region. NB, MB, SB and HB—northern, middle, southern boreal and hemi-boreal zones, respectively, according to the forest vegetation zoning mapped for the natural vegetation of Europe (Bonh et al. 2000, 2000/2003)
The main forest types in the study sites were herb-rich, Oxalidosum, Equisetum, Hylocomioso-Myrtillosum, Myrtillosum, Ledosum, Polytrichoso-Myrtillosum and Sphagnoso-Myrtillosum according to the classification of forest ecosystems in the northwest of Russia (Fedorchuk et al. 2005). The forest types herb-rich, Oxalidosum and Myrtillosum are classified as well-drained; the forest types Equisetum, Ledosum and Sphagnoso-Myrtillosum are classified as poorly drained.
Sampling, calculations and data analysis
The non-stem components of logs larger than 8 cm in DBH of the following tree species were sampled: Norway spruce and Siberian spruce, hereinafter referred to as spruce; Scots pine, birch, aspen and Siberian fir, hereinafter referred to as fir; and Siberian pine. The CWD formed after the death of mature trees ranged from 60 to a few hundred years old. We used a chronosequence approach to estimate the dynamics of CWD decomposition. The dating of CWD pieces was determined from permanent plot records (VF1, 14–34 year period, CFR, 22–37 year period), year of windfall (CFR, 7–64 year period) and dendrochronological methods of cross-dating, growth release patterns and mechanical scars of neighboring trees (Komi, period covered up to 168 years). Undecayed wood of roots and branches and log bark were sampled from trees that died in the current year or 1 year before sampling to serve as controls. In total, 1768 samples were taken (Table 1). When the year of windthrow was known, the determination of time since death (i.e., time elapsed from the date of tree death to the date of sampling) was precise. Permanent plot inventories had been conducted every 3–5 years, so the accuracy of time since death estimates averaged 2 years.
Three to five “representative” roots and branches with diameters ranging from 4 to 15 cm were selected by CWD piece. The roots were sampled aboveground from (1) trees after windthrow when a tree was uprooted (49 trees), (2) trees that died standing and then fell down so that the root system turned up totally or partly aboveground (15 trees) and (3) trees that were damaged by wind or snow so that part of their root system remained belowground and part of it emerged aboveground (67 trees). Belowground roots were not excavated; thus, only the decomposition of roots that decomposed totally or partly aboveground was studied. Side branches were sampled at a minimum distance of 20 cm from the place of attachment to a log. We did not study the branches that fell from live trees or any CWD that decomposed on the forest floor.
Cross sections from side branches and roots were extracted by a chain saw and axe or knife and debarked. The samples from branch and root wood were taken at ca. 0.5 m from the stem. At this distance, bulk density of branch and root wood reaches their mean values (Polubojarinov 1976). The proportional area in different degrees of decomposition was visually estimated on a scale of 1–4, from intact to completely decomposed wood, respectively, with white and brown rot considered separately. An adjustment for volume losses during decomposition was made; the initial shape of fragmented and highly decomposed fractions was reconstructed. Holes due to fragmentation or complete decomposition were counted as well. Small samples of regular shape up to 100 cm3 in size were taken from each cross section according to degree and type of rot. Their length, width and thickness of the samples were measured. The area percentage was assumed to correspond to the proportional volume for each sample. Samples were oven-dried at 103 °C for 48 h and weighed. Bulk density (ρ, g m−3) was calculated by dividing the dry mass by the fresh volume of the sample. A weighted average for bulk density (as weighted by the proportion of different degrees of decomposition represented in the sampled cross sections) was calculated for each root or branch piece.
We studied only the decomposition of bark attached to logs. Bark on the ground or in litter was not considered. The decomposition of bark was analyzed based on mass loss per surface area. The length of all logs (L, m) was measured, and diameters were recorded at the stem base, at 1.3 m and the top of all logs. The lateral surface area of logs (S, m2) was calculated by the formula:
where R and r are, respectively, the maximum and minimum radii at opposite ends of a broken log segment in m, and L is a slant height of a log in m.
To account for the loss of bark fragments by mechanical damage or insects, the percent cover of bark remaining on a log (f) was visually estimated. Two to three regularly shaped bark samples of a few cm2 were taken from the stem base and the middle of each log and measured in two dimensions. Then, the area-specific mass of bark (mass unit per surface area) was calculated as:
where M b is the area-specific mass of bark (g m−2), m is the dry mass of a bark sample (Mg), and s is the area of sample (m2).
In order to determine the total area of remaining bark (S f ), the lateral surface area of log (S) was multiplied by the percent of remaining bark (f):
The total mass of log bark (M sb) was calculated as:
In order to separate the factors controlling the rate of decomposition from the factors controlling initial bulk density and mass (as measured in the control samples), we first analyzed the bulk density of roots and braches as well as the initial mass per surface area and total mass from the bark of trees that died in the year of observation or the year before. An analysis of variance (ANOVA) type III sum of square for unbalanced design, when the numbers of observations for different groups were unequal (Shaw and Mitchell-Olds 1993), and Duncan post hoc multiple comparison tests (Statistica 6.0) were implemented to estimate the effect of: (a) vegetation zone (northern boreal, southern/middle boreal, hemi-boreal); (b) soil moisture (well-drained vs. poorly drained); (c) tree species; (d) component (root vs. branch); (e) log part (0–3 m above root collar vs. >3 m above root collar for bark); (f) log at breast height, or 1.3 m (DBH) (expressed as three categories: 0–20, 21–40 and >41 cm); and (g) the interrelationship of some of the above factors on the initial bulk density and mass variation.
We calculated the percent dry mass remaining (M, %) based on the loss of the bulk density (ρ, g m−3), area-specific (M b) and total (M sb) mass of bark related to the initial density (ρ 0), and specific (M b0) and total (M sb0) mass, respectively. The initial parameters were calculated based on the results of ANOVA, i.e., the values were grouped when the differences were not statistically significant. The remaining mass was adjusted to 100 % when ρ exceeded ρ 0, M b exceeded M b0, and M sb exceeded M sb0. The annual decomposition rates (k, year−1) were calculated based on a single exponential model (Olson 1963).
Factorial ANOVA (type III sum of square test for unbalanced design) and Duncan tests were implemented to estimate the effects of vegetation zone (northern boreal, southern/middle boreal, hemi-boreal), soil moisture (well-drained vs. poorly drained), tree species, tree mortality mode (uprooted, died standing, broken), component (roots, branches, bark), log part (0–3 m above root collar vs. >3 m above root collar), log DBH (expressed as three categories: 0–20, 21–40 and >41 cm) and the interrelation of some of the above factors on the variation in k. The effect of log DBH on k variation was also analyzed using linear regression analysis. All graphs were created in R (R Core Team 2013).
The decomposition rates for whole CWD pieces were estimated as follows. First, mean DBH and height for sampled CWD pieces were calculated by tree species, vegetation zones and site conditions. The volumes of those mean stems with bark were calculated using taper functions (Tetioukhin et al. 2004). The volumes of stems without bark were acquired by subtracting the proportion of bark estimated depending on tree species and DBH (Tetioukhin et al. 2004). Second, the initial mass of CWD components was estimated. The stem mass was calculated by multiplying the volumes of mean trees by mean bulk density of control samples (Shorohova and Kapitsa 2014b). The initial bark mass from this study was used. The tree species and age-specific biomass expansion factors depending on vegetation zone (Zamolodchicov et al. 1998) were applied to calculate the initial mass of roots and branches related to the volume of a mean tree. Finally, mean decomposition rate of CWD pieces was calculated as a sum of the decomposition rate for each component multiplied by its mass proportion in the total mass of a CWD piece.
Results
Roots
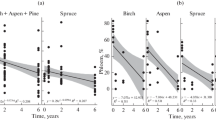
Initial density of root wood did not depend on tree species. The decomposition of roots depended on soil moisture regime and tree species independently of tree mortality mode and log size (Table 2; Fig. 2). Aspen roots decomposed at the fastest rate and fir roots decomposed at the slowest rate (Table 3).
Decomposition of roots over time. The dots represent remaining mass of roots; lines are models based on calculated mean decomposition rates for the following cases: good_A—roots of aspen under well-drained conditions; good_B, F, P, S—roots of birch, fir, Scots pine and spruce under well-drained conditions; poor_B, S, SP—roots of birch, spruce and Siberian pine under poorly drained conditions; and poor_F—roots of fir under poorly drained conditions
Branches
Before decomposition, the wood of branches was denser than that of roots independent of vegetation zone and site conditions. Initial wood bulk density of branches depended on tree species, with the highest values for fir and spruce. The decomposition rate of branches depended only on the tree species. Branches of Siberian pine decomposed at the slowest rate (Table 2; Fig. 3).
Bark
The area-specific mass and total mass of bark before decomposition were higher at the stem base than at the top and increased with log diameter. The regional climate and site conditions did not influence the initial bark mass. The rate of area-specific mass loss of bark increased with log diameter (Table 3; Fig. 4). The percent of bark left on the sampled stems decreased with time since tree death and averaged 94, 76, 68 and 41 % for aspen and birch, fir and spruce, Siberian pine and Scots pine, respectively. The rate of bark decomposition estimated as a total mass loss, i.e., including fragmentation as sloughing from logs and mineralization, depended on the log part and site moisture regime. It increased with the distance from the root collar. Higher fragmentation and consequently decomposition rates were recorded on well-drained sites compared to poorly drained ones (Table 3; Fig. 5).
Decomposition of bark over time (including fragmentation). The dots represent remaining total mass of bark; lines are models based on calculated mean decomposition rates for the following cases: Base_good_—bark from 0 to 3 m from the stem base under well-drained conditions; Base_poor—bark from 0 to 3 m from the stem base under poorly drained conditions; Top_good—bark from >3 m from the stem base under well-drained conditions; and Top_poor—bark from >3 m from the stem base under poorly drained conditions
The CWD piece scale decomposition rates varied depending on vegetation zone, moisture regime and tree species. The slowest rates were found for Siberian pine in northern boreal forest under poorly drained conditions. The fastest decomposition rates were found for spruce on well-drained southern boreal sites. In general, the decomposition of whole CWD piece was faster than decomposition of stem wood (Table 4; Fig. 6).
Discussion
Range of variability in the decomposition rates of CWD components and decomposition factors
Our results concur with the results from our previous studies and with information found in the available literature (Polubojarinov 1976). The initial density of woody CWD components increases in the order: roots, stems and branches. With some exceptions, the wood density of a component determines its decomposition rate. This result supports an earlier study on the negative interrelationship between initial density and decomposition rate (Harmon et al. 1995).
The decomposition rates of CWD components differed greatly. Local ecosystem characteristics and substrate attributes were more important predictors of decomposition rates than vegetation zone. This confirms the conclusion that the common assumption that climate is a predominant control on decomposition is supported only when local-scale variation is aggregated into mean values (Mackensen and Bauhus 1999; Bradford et al. 2014).
Roots
Our estimates of root decomposition rates in boreal forests were of the same order of magnitude as reported in the literature (Tarasov and Birdsey 2001; Melin et al. 2009; Palviainen and Finér 2015). The decomposition rates of roots under well-drained soil conditions were comparable with the regional averages for decomposition rates of log wood of the same tree species (Krankina and Harmon 1995; Harmon et al. 2000; Tarasov and Birdsey 2001; Yatskov et al. 2003; Mäkinen et al. 2006; Shorohova and Kapitsa 2014b). The decomposition rates of roots under poorly drained conditions in this study were much lower than regional averages of the same tree species. As we hypothesized, soil moisture regime had a strong effect on the decomposition rates of root wood. Under poorly drained conditions, the lack of oxygen necessary for decomposers (Rayner and Boddy 1988) can explain the slow decomposition rates observed in our study. The influence of tree species on decomposition rates was only marginal and related to soil moisture. Only the decomposition rate of aspen and fir root wood was different from that of other tree species. Globally, there were clear differences found for decomposition rates of stem wood of gymnosperms compared to angiosperms (Weedon et al. 2009). Case studies from North America suggest that decomposition rate of woody roots varied between coniferous tree species either strongly (Janisch et al. 2005) or marginally (Chen et al. 2001). There was no variation between 80 native and non-native species in deciduous forest species (Jo et al. 2016). The effect of species traits and log diameter on the decomposition rates of woody roots is probably dominated by other effects such as moisture and aeration. Tree mortality mode did not influence the decomposition rate of root wood. This means no difference in the root decomposition rate was observed when decomposition started belowground when a tree died standing and then was blown down, or if a tree was first broken and then blown down, or when decomposition started aboveground in case of uprooting. Probably, the high initial moisture content of roots partly covered by soil or in many cases by mosses predetermines quick colonization of the substrate by wood-decomposing organisms which start the decay process.
Branches
The decomposition rates of attached and elevated side branches in this study were much lower than regional averages of stem wood for the same tree species (Krankina and Harmon 1995; Harmon et al. 2000; Tarasov and Birdsey 2001; Yatskov et al. 2003; Mäkinen et al. 2006; Shorohova and Kapitsa 2014b) and lower than the decomposition of branches of the same tree species on the forest floor (Mukhin 1993). As in the case with roots, decomposition rate of branches only slightly depended on tree species and did not depend on log diameter. Side branches decomposed at a common rate except Siberian pine, which showed the lowest decomposition rate. No significant differences were found between decomposition rates of deciduous tree branches in a mixed deciduous woodland (Swift et al. 1976). This evidence suggests that neither species traits nor log size is an important predictor of decomposition rates of attached branches.
Bark
The decomposition rate of bark attached to logs determined in this study is within the range reported for the decomposition rates of stumps (Shorohova et al. 2012) and log bark (Shorohova and Kapitsa 2014a) in European boreal forests. The much higher decomposition rates of bark compared to wood may be one of the reasons that our estimates of decomposition rates for whole CWD pieces were in the upper limit of the regional ranges for CWD decomposition rates. Moisture regime has both direct and indirect influence on CWD decomposition rates. When losses due to fragmentation are considered, the decomposition rate of bark becomes significantly higher on well-drained sites compared to poorly drained ones. This result can lead to an assumption of higher activity of bark-boring insects under more favorable site conditions compared to poor conditions. In wet forests in Komi sites, bark cover of coniferous trees slowly decreases during decomposition (Shorohova and Kapitsa 2014a). In this study, when Komi sites are combined with more well-drained CF and partly VF1 sites, many trees have already been affected by bark beetles before death. The bark of such trees sloughs from logs relatively quickly. Stem diameter negatively influenced the mineralization and fragmentation rates of the bark of all tree species showing the importance of the initial substrate quality for decomposition. The area-specific mass loss of bark caused by mineralization and volume loss was faster compared to decomposition of wood. Likewise, when considering the whole CWD piece and taking into account fragmentation, bark decomposed faster than other components.
With a few exceptions for some tree species, the decomposition rate increased in the following order, from slowest to fastest: branches, roots in poorly drained sites, leaning logs, fallen logs and roots in well-drained sites and bark. The slowest rate of decomposition of side branches among other CWD components was recorded also for Pinus radiata CWD due to unfavorable microclimate conditions (most of the side branches were not in contact with soil even after a few years of decomposition). Polyphenol and greater lignin concentrations may also have been a factor (Ganjegunte et al. 2004). The same study showed the faster decomposition of Pinus radiata log wood compared to bark; however, bark fragmentation was not taken into account.
Methodological considerations and uncertainties
Most uncertainties in our estimates of decomposition rates are related to the chronosequence approach, as discussed earlier for ecological studies in general (Johnson and Miyanishi 2008) and for decomposition studies in particular (Shorohova and Kapitsa 2014a, b; Palviainen and Finér 2015).
Initial substrate condition is unknown, and decomposition rates are overestimated in cases where decomposition starts and/or bark sloughs before tree death. In this study, we tried to select CWD pieces without traces of rot caused by biotrophic fungi. Logs without bark were not sampled either.
The trees that decomposed most slowly are measured at the extreme end of time since death, hence underestimating the decomposition rate. The processes of bark and wood colonization by decomposing organisms and leaching are not separated from fragmentation and mineralization. In general, uncertain predictions for CWD decomposition rates, and their global C-cycle implications, will persist until interactions of CWD microbial communities are better understood (Liu et al. 2013). In chronosequence studies, including this one, CWD pieces which were decomposed by different fungal species are combined in the same dataset.
When estimating the whole CWD piece decomposition rates, we used the biomass expansion factors acquired from different case studies. The length of time when branches are attached to logs remains uncertain. This leads to uncertainties in estimating the whole CWD piece decomposition rate. In addition, our estimates of CWD decomposition rates cannot be scaled up for buried wood (Moroni et al. 2015).
Conclusions
Assessments of CWD decomposition need to take into account fragmentation effects and differences among components. The different decomposition rates of wood and bark suggest that if wood and bark together are considered as one substrate, the rate of decomposition will likely be underestimated. Attached branches decomposed at the slowest rate compared to other components and cannot be associated with branches on the forest floor. The whole CWD piece decomposition rate can be predicted as a function of vegetation zone, site conditions, tree species and size in European boreal forests.
CWD decomposition rates and turnover time affect the quality and persistence of this substrate in forest ecosystems and, consequently, its role for carbon and nutrient storage and as a substrate for deadwood-dependent organisms (Stokland et al. 2012). Thus, our results can be applied in modeling carbon dynamics and for predicting the quality of CWD in biodiversity studies in European boreal forests.
References
Beets PN, Kimberley MO, Paul TSH, Garrett LG (2011) Planted forest carbon monitoring system—forest carbon model validation study for Pinus radiata. New Zeal J For Sci 41:177–189
Boddy L, Heilmann-Clausen J (2008) Basidiomycete community development in temperate angiosperm wood, Chapter 12. In: Boddy L, Frankland JS, West P (eds) Ecology of saprotrophic basidiomycetes. Academic press/Elsevier, London, pp 155–179
Boddy L, Rayner ADM (1983) Ecological roles of basidiomycetes forming decay communities in attached oak branches. New Phytol 93:77–88
Boddy L, Bardsley DW, Gibbon OM (1987) Fungal communities in attached ash branches. New Phytol 107:143–154
Bond-Lamberty B, Gower ST (2008) Decomposition and fragmentation of coarse woody debris: re-visiting a boreal black spruce chronosequence. Ecosystems 11:831–840
Bonh U, Gollub G, Hettwer C [Bearb.] (2000) Karte der natürlichen vegetation Europas/Map of the natural vegetation of Europe. Maßstab/Scale 1:2.500.000. Teil 2/Part 2: Legende/Legend, 153 S.; Teil 3/Part 3: Karten/Maps (9 Blätter/Sheets, Legendenblatt/LegendSheet, Übersichtskarte 1:10 Mio./General Map 1:10 million)—Münster (Landwirtschaftsverlag)
Bonh U, Neuhäusl R, unter Mitarbeit von Gollub G, Hettwer C, Neuhäuslová Z, Shlüter H, Weber H (2000/2003) Karte der natürlichen Vegetation Europas/Map of the Natural Vegetation of Europe. Maßstab/Scale 1:2.500.000. Teil 1/Part 1: Erläuterungsband/Explanatory Text, 655 S.; Teil 2/Part 2: Legende/Legend, 153 S.; Teil 3/Part 3: Karten/Maps (9 Blätter/Sheets, Legendenblatt/Legend Sheet, Übersichtskarte 1:10 Mio./General Map 1:10 million)—Münster (Landwirtschaftsverlag)
Bradford MA, Warren Ii RJ, Baldrian P, Crowther TW, Maynard DS, Oldfield EE, Wieder WR, Wood SA, King JR (2014) Climate fails to predict wood decomposition at regional scales. Nat Clim Change 4:625–630
Chambers JQ, Schimel JP, Nobre AD (2001) Respiration from coarse wood litter in central Amazon forests. Biogeochemistry 52:115–131
Chen H, Harmon M, Griffiths RP (2001) Decomposition and nitrogen release from decomposing woody roots in coniferous forests of the Pacific Northwest: a chronosequence approach. Can J For Res 31:246–260
Cramer W, Bondeau A, Woodward FI, Prentice IC, Betts RA, Brovkin V et al (2001) Global response of terrestrial ecosystem structure and function to CO2 and climate change: results from six dynamic global vegetation models. Glob Change Biol 7:357–373
Dittrich S, Jacob M, Bade C, Leuschner C, Hauck M (2014) The significance of deadwood for total bryophyte, lichen, and vascular plant diversity in an old-growth spruce forest. Plant Ecol 215(10):1123–1137
Erickson HE, Edmonds RL, Peterson CE (1985) Decomposition of logging residues in Douglas-fir, western hemlock, Pacific silver fir, and ponderosa pine ecosystems. Can J For Res 15:914–921
Fahey TJ, Hughes JW, Pu M, Arthur MA (1988) Root decomposition and nutrient flux following whole-tree harvest of northern hardwood forest. For Sci 34(3):744–768
Fedorchuk VN, Neshataev VYu, Kuznetsova ML (2005) Forest ecosystems of the north-western regions of Russia: typology, dynamics, management features. Forestry Scientific Research Institute, St. Petersburg, p 382 (in Russian)
Ganjegunte GK, Condron LM, Clinton PW, Davis MR, Mahieu N (2004) Decomposition and nutrient release from radiata pine (Pinus radiata) coarse woody debris. For Ecol Manag 187:197–211
Garrett LG, Kimberley MO, Oliver GR, Pearce SH, Beets PN (2012) Decomposition of coarse woody roots and branches in managed Pinus radiata plantations in New Zealand—a time series approach. For Ecol Manag 269:116–123
Harmon ME, Franklin JF, Swanson FJ, Sollins P, Gregory SV, Lattin JD, Anderson NH, Cline SP, Aumen NG, Sedell JR, Liencamper GW, Cromack K.Jr, Cummins KW (1986) Ecology of coarse woody debris in temperate ecosystems. Adv Ecol Res 15:133–202
Harmon ME, Whigham DF, Sexton J, Olmsted I (1995) Decomposition and mass of woody detritus in the dry tropical forests of the northeastern Yucatan Peninsula, Mexico. Biotropica 27(3):305–316
Harmon ME, Krankina ON, Sexton J (2000) Decomposition vectors: a new approach to estimating woody detritus decomposition dynamics. Can J For Res 30:76–84
Helfenstein J, Kienast F (2014) Ecosystem service state and trends at the regional to national level: a rapid assessment. Ecol Ind 36:11–18
Hyvönen R, Olsson BA, Lundkvist H, Staaf H (2000) Decomposition and nutrient release from Picea abies (L.) Karst. and Pinus sylvestris L. logging residues. For Ecol Manag 126:97–112
Janisch JE, Harmon ME, Chen H, Fasth B, Sexton J (2005) Decomposition of coarse woody debris originating by clearcutting of an old-growth conifer forest. EcoScience 12(2):151–160
Jo I, Fridley JD, Frank DA (2016) More of the same? In situ leaf and root decomposition rates do not vary between 80 native and nonnative deciduous forest species. New Phytol 209(1):115–122
Johnson EA, Miyanishi K (2008) Testing the assumptions of chronosequences in successions. Ecol Lett 11:419–431
Krankina ON, Harmon ME (1995) Dynamics of the dead wood carbon pool in northern-western Russian boreal forests. Water Air Soli Pollut 82:227–238
Kudeyarov VN, Zavarzin GA, Blagodatskaya SA (2007) Carbon pools and fluxes in Russia’s terrestrial ecosystems. Science Publ., Moscow (in Russian)
Laiho R, Prescott CE (2004) Decay and nutrient dynamics of coarse woody debris in northern coniferous forests: a synthesis. Can J For Res 34:763–777
Liu W, Schaefer D, Qiao L, Liu X (2013) What controls the variability of wood-decay rates? For Ecol Manag 310:623–631
Mackensen J, Bauhus J (1999) The decay of coarse woody debris (National Carbon Accounting System technical report no. 6) Commonwealth of Australia
Mäkinen H, Hynynen J, Siitonen J, Sievänen R (2006) Predicting the decomposition of Scots pine, Norway spruce and birch stems in Finland. Ecol Appl 16:1865–1879
Melin Y, Petersson H, Nordfjell T (2009) Decomposition of stump and root systems of Norway spruce in Sweden: a modeling approach. For Ecol Manag 257:1445–1451
Miller WE (1983) Decomposition rates of aspen bole and branch litter. For Sci 29:351–356
Moroni MT, Morris DM, Shaw C, Stokland JN, Harmon ME, Fenton NJ, Merganičová K, Merganič J, Okabe K, Hagemann U (2015) Buried wood: a common yet poorly documented form of deadwood. Ecosystems 18(4):605–628
Mukhin VA (1993) Biota of xylotrophic basidiomycetes of the West-Siberian Plain. Nauka Publ., Moscow (in Russian)
Olajuyigbe SO, Tobin B, Gardiner P, Nieuwenhuis M (2011) Stocks and decay dynamics of above- and belowground coarse woody debris in managed Sitka spruce forests in Ireland. For Ecol Manag 262:1109–1118
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–330
Palviainen M, Finér L (2015) Decomposition and nutrient release from Norway spruce coarse roots and stumps—a 40-year chronosequence study. For Ecol Manag 358:1–11
Palviainen M, Finér L, Laiho R, Shorohova E, Kapitsa E, Vanha-Majamaa I (2011) Phosphorus and base cation accumulation and release patterns in decomposing Scots pine, Norway spruce and silver birch stumps. For Ecol Manag 260:1478–1489
Parameswaran N, Wilhelm GE, Liese W (1976) Ultrastructural aspects of beech bark degradation by fungi. Eur J For Pathol 6:274–286
Polubojarinov OI (1976) Density of wood, vol 159. Nauka Publ., Moskow (in Russian)
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rayner ADM, Boddy L (1988) Fungal decomposition of wood: its biology and ecology. Wiley, Chichester
Russell MB, Fraver S, Aakala T, Gove JH, Woodall CW, D’Amato AW, Ducey MJ (2015) Quantifying carbon stores and decomposition in dead wood: a review. For Ecol Manag 350:107–128
Rypaček V (1957) Biologie dřeva dřevokaznŷch hub. Praha (in Czech)
Shaw RG, Mitchell-Olds T (1993) Anova for unbalanced data: an overview. Ecology 74:1638–1645
Shorohova E, Kapitsa E (2014a) Mineralization and fragmentation rates of bark attached to logs in a northern boreal forest. For Ecol Manag 315:185–190
Shorohova E, Kapitsa E (2014b) Influence of the substrate and ecosystem attributes on the decomposition rates of coarse woody debris in European boreal forests. For Ecol Manag 356:273–284
Shorohova E, Kapitsa E (2015) Stand and landscape scale variability in the amount and diversity of coarse woody debris in primeval European boreal forests. For Ecol Manag 315:185–190
Shorohova E, Ignatyeva O, Kapitsa E, Kauhanen H, Kuznetsov A, Vanha-Majamaa I (2012) Stump decomposition rates after clear-felling with and without prescribed burning in southern and northern boreal forests in Finland. For Ecol Manag 263:74–84
Stokland JN, Siitonen J, Jonsson BG (2012) Biodiversity in dead wood. Cambrigde Univ. Press, Cambrigde
Swift MJ, Healey IN, Hibberd JK, Sykes JM, Bampoe V, Nesbitt ME (1976) The decomposition of branch-wood in the canopy and floor of a mixed deciduous woodland. Oecologia 26(2):139–149
Tarasov ME, Birdsey RA (2001) Decay rate and potential storage of course woody debris in the Leningrad region. Ecol Bull 49:137–149
Tetioukhin SV, Minayev VN, Bogomolova LP (2004) Forest Mensuration and inventory: reference book for the North-Western Russia. StP. FTA Publ, St. Petersburg (in Russian)
Tobin B, Black K, Mcgurdy M, Nieuwenhuis M (2007) Estimates of decay rates of components of coarse woody debris in thinned Sitka spruce forests. Forestry 80(4):455–469
Weedon JT, Cornwell WK, Cornelissen JHC, Zanne AE, Wirth C, Coomes DA (2009) Global meta-analysis of wood decomposition rates: a role for trait variation among tree species? Ecol Lett 12:45–56
Yatskov M, Harmon M, Krankina O (2003) A chronosequence of wood decomposition in the boreal forests of Russia. Can J For Res 33:1211–1226
Yin X (1999) The decay of forest woody debris: numerical modeling and implications based on some 300 data cases from North America. Oecologia 121:81–98
Zamolodchicov DG, Utlin AI, Korovin GN (1998) Determination of carbon stores by conversion-volumetric coefficients related to age of stands. Lesovedenie 3:84–93 (in Russian)
Zamolodchicov DG, Grabovsky VI, Kraev VN (2011) Dynamics of carbon budget of Russia’s forests during the last two decades. Lesovedenie 6:16–28 (in Russian)
Acknowledgments
The research was supported by the Russian Science Foundation (15-14-10023). The data were collected with financial support from the Russian Foundation of Basic Research (09-04-00209-a). The staff of Nature Park “Vepssky Forest,” Central Forest Biosphere State Natural Reserve and Yugyd-va National Park helped with practicalities during expeditions. We sincerely thank all people who helped us in the fieldwork. Olga Lisitsyna created the map of the study sites. Carla Burton revised the language.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shorohova, E., Kapitsa, E. The decomposition rate of non-stem components of coarse woody debris (CWD) in European boreal forests mainly depends on site moisture and tree species. Eur J Forest Res 135, 593–606 (2016). https://doi.org/10.1007/s10342-016-0957-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-016-0957-8