Abstract
Effects of environmental variables on the distribution of benthic macroinvertebrates inhabiting sediments were studied at 25 sites along the shoreline of Lake Takkobu in the Kushiro wetland of northern Japan in summer 2003. During the last decade, the lake’s status has undergone a drastic shift from clear water dominated by submerged macrophytes to turbid water dominated by phytoplankton. The canonical correspondence analysis showed that four environmental variables explained the significant variation in the macroinvertebrate species composition: submerged plant biomass, bottom sediment organic matter content (OMC), distance from the mouth of the Takkobu River, and bottom-layer pH. Five species of Chironomidae [Chironomus sp. (except plumosus group), Psectrocladius sp., Corynoneura sp., Parachironomus sp. arcuatus group, and Zavreliella sp.] occurred in sites with relatively lower pH and a high submerged plant biomass, whereas three species of Tubificidae (Tubifex tubifex, Aulodrilus limnobius and Aulodrilus sp.) and two of Chironomidae (Nanocladius sp. and Monodiamesa sp.) occurred in sites with high pH and little vegetation. The three Tubificidae species also preferred organic-rich sediments. Irrespective of aquatic vegetation, Sphaerium sp. (Bivalvia) and Monodiamesa sp. (Chironomidae) occurred in low-OMC sites, whereas Tanypus sp. (Chironomidae) preferred high-OMC sites. The number of macroinvertebrate taxa showed the highest correlation with the number of submerged plants, suggesting that macroinvertebrate species richness was related mostly to submerged plant species diversity in this lake. The quantity and species richness of submerged plants and OMC are thus important determinants of the community structure of macroinvertebrates inhabiting sediments in Lake Takkobu.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The distribution and species composition of benthic macroinvertebrate communities inhabiting lakes are determined by independent or cumulative biotic and abiotic factors at different spatial scales. Recently, Johnson et al. (2004) revealed that in lakes the among-site variance in macroinvertebrate communities is best explained by habitat-scale characteristics. In littoral areas, differences of substrate (e.g., stone, gravel, cobbles, sand, silt, aquatic plants, or woody debris) are considered most important in determining the density, size structure, species composition, and species richness of the macroinvertebrate community (Hanson 1990; Oertli 1995; Dvořák 1996; van den Berg et al. 1997; Gardner et al. 2001; Tolonen et al. 2001; Weatherhead and James 2001).
An aquatic vegetation substrate has been shown to develop a unique macroinvertebrate fauna in littoral areas compared with other substrates such as stone, sand, or silt (Tolonen et al. 2001). Not only the presence or absence of vegetation but also the life form, species, and biomass of aquatic macrophytes can greatly influence the abundance, taxonomic composition, and size structure of the macroinvertebrate community (Hanson 1990; Rasmussen 1993; Strayer et al. 2003). In addition, submerged, coarse, woody debris in the littoral area (Bowen et al. 1998), wind exposure, presence of filamentous algae, the shore slope (Brodersen 1995), dissolved oxygen (DO) content, high nutrient levels due to the inflow of organic pollutants (Petridis 1993), and water depth (Petridis 1993; Kato et al. 1999) have been shown to affect the habitat-scale community structure of benthic macroinvertebrates.
Fish predation (e.g., Wellborn and Robinson 1991) also clearly affects the community structure of benthic macroinvertebrates. Fish are also known to influence the community structure of benthic macroinvertebrates indirectly to some extent, through their excretion (Matsuzaki et al. 2007), or direct or indirect habitat disturbance or modification by serving as “ecosystem engineers” (Moore 2006). Field manipulations have revealed that the presence of macrophyte vegetation decreases the efficiency of fish predation and provides refuge for benthic macroinvertebrates (Crowder and Cooper 1982; Diehl 1992, 1993, 1995; Hanson and Butler 1994).
Lake Takkobu (surface area, 1.33 km2; mean depth, 1.0 m) is a lagoon in the east Kushiro wetland, and its catchment area (23.8 km2) is mostly covered with natural vegetation. However, a fragmentary summer survey by the Hokkaido Institute of Environmental Sciences and the National Institute for Environmental Studies revealed that the chlorophyll a (Chl a) concentrations rapidly increased between 1996 and 2000, and water blooms of cyanobacteria were observed in summer 2000 (Takamura et al. 2003). Concurrently, the number of aquatic plant species in the lake clearly decreased (Kadono 2007). According to the Geographic Survey Institute of Japan, most of the lake was occupied by submerged plants in 1992, but submerged vegetation remained only in the southern part of this lake in 2004 (Takamura et al. 2007). Therefore, in the last two decades, the state of Lake Takkobu has shifted from clear water dominated by submerged macrophytes to turbid water dominated by phytoplankton (Scheffer et al. 1993). Presumably, the benthic macroinvertebrate community structure in this lake also changed along with this drastic regime shift. An analysis of subfossil remains in a sediment core in Lake Søbygaad, Denmark, for example, showed distinct changes in chironomid communities reflecting eutrophication and a macrophyte succession through Chara, Ceratophyllum, and Potamogeton dominance to the present state, characterized by a complete loss of submerged vegetation and dominance of phytoplankton (Brodersen et al. 2001).
We aimed to elucidate the environmental factors that regulate the macroinvertebrate community inhabiting sediments in Lake Takkobu. Although this lake has undergone a rapid loss of submerged vegetation, a clear environmental gradient of water quality from south to north is present (Takamura et al. 2007). This gradient should influence the present macroinvertebrate community structure in the lake. Among environmental factors, we focused on water depth, distance from the main inflowing river, macrophyte biomass and the number of macrophytes species, organic matter content of sediments, bottom-layer DO, bottom-layer pH, Chl a content in the water, and abundance of benthivorous fish.
Methods
Study site
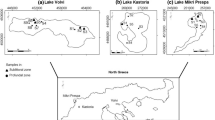
All surveys except for fish sampling were conducted during 23–25 July 2003. Fish were collected between 26 July and 5 August 2003. We selected 25 sampling sites along the lakeshore on the basis of the locations of river mouths and the main type of vegetation present (Fig. 1). The geographical location of each sampling site was recorded by differential global positioning system (DGPS) (Trimble Pro XR, Sunnyvale, CA, USA).
The main stream inflowing into this lake is the Takkobu River, and its catchment area accounts for 64.7% of the total catchment area of the lake. All other inflowing streams are small. In addition, the Kushiro River, which drains the lake, sometimes flows backward into the lake when the river’s water level is high. According to Takamura et al. (2007), the lake shows a clear environmental gradient during the summer, from the mouth of the Takkobu River in the south to the Takkobu River outlet in the north. Little vegetation remains in the northern part of the lake, where only small patches of Trapa vegetation close to the lakeshore are found, whereas the southern area is mostly covered with Trapa, along with a narrow belt of submerged macrophytes and sparse patches of Nuphar pumilum. The submerged plants are dominated by Potamogeton maackianus. In addition, Ceratophyllum demersum, Hydrilla verticillata, Myriophyllum verticillatum, and Potamogeton compressus were observed. Water blooms occur widely in the north, where the water quality tends to be uniform. In the south, however, the water quality is heterogeneous, influenced not only by water inflowing from the Takkobu River but also by water from springs or sediments having a high redox potential.
Measurements of environmental variables
We measured water depth, water temperature (WT), pH (HM-12P; DKK-TOA, Tokyo, Japan), and DO (YSI model 58; YSI, YellowSpring, Ohio) at the bottom of the water column at each sampling site. Water samples for measurement of the Chl a content were collected at 0.5-m depth at each site and were kept cool (4°C) in the field. The water samples were immediately passed through a filter (Whatman GF/F), and then the filter was frozen at −20°C for several days. Then, Chl a content was measured with a spectrophotometer (model 220A; Hitachi, Tokyo, Japan) after the filter was soaked in 99.9% methanol for 24 h (Marker et al. 1980).
The geographical location of each river mouth was determined on a 1:25,000 topographic map (Geographical Survey Institute, Tokyo, Japan) by using geographical information system (GIS) data (ArcGIS 9.1, Redlands, CA, USA). The distance between each river mouth and each sampling site was calculated from GIS data.
Sediment was collected with an Ekman grab sampler. The organic matter content in 5 g of the surface sediment was determined by combustion at 600°C to a constant weight. All plant bodies within a 1-m2 randomly established quadrat were collected by scuba divers. The plants were washed to remove attached organisms, separated according to species, and then allowed to dry at 90°C to a constant weight.
We constructed a cubic frame (a 2-m2 quadrat, 2.1 m high) to estimate fish density at each sampling site and covered the sides of the cube with a 4-mm mesh net, weighting its bottom so that it would sink. The cube was thrown into each sampling site, and all fish in the quadrat were collected with scoop and seine nets. Seventeen species of fish were collected in Lake Takkobu in 2003 (Hariu et al. 2007). The total number of fish of four benthivorous species (Lefua nikkonis, Misgurnus anguillicaudatus, Rhinogobius sp. OR, and Gymnogobius castaneus) was used as the variable for predator of benthic macroinvertebrates.
Collection of benthic macroinvertebrates
Sediment samples for benthic macroinvertebrates were collected with a stainless core sampler (200 mm in diameter, 300 mm high) by scuba divers and washed through a 0.2-mm mesh sieve within 24 h. The collected organic materials, including plant debris, were preserved in 4% formaldehyde solution. In the laboratory, the samples were sorted, washed through a 0.2-mm mesh net to remove the formaldehyde, and separated, using the unaided eye, into taxonomic groups (T. Ito). Chironomidae were identified to the species level by R. Ueno following Webb et al. (1985) and Wiederholm (1983), and Oligochaeta were identified by A. Ohtaka after Brinkhurst and Jamieson (1971). Gastropoda and Bivalvia were identified by Y. Kuwahara after both Masuda and Uchiyama (2004) and Tsalolikhin (2004), Hirudinoidea by T. Itoh following Yang (1996), Mysidae by S. Hiruta following Murano (1997), Ceratopogonidae by S. Kitaoka following Borkent and Wirth (1997), and Trichoptera and others by T. Ito following Wiggins (1996).
Data analysis
A canonical correspondence analysis (CCA) was carried out with CANOCO 4.5 software (ter Braak and Šmilauer 2002) to elucidate the relationships between the species composition and their explanatory environmental variables. CCA can be appropriate to find a particular set of observed environmental variables to which the present macroinvertebrate taxa respond (McCune 1997). We identified 43 macroinvertebrate taxa, but seven taxa that occurred at only one site as well as eight taxa that included two or more species (Table 1) were excluded from the analysis, because casual and uncertain taxa might distort the species ordination. Then, we considered 28 taxa and 13 environmental variables (Table 2).
Before performimg CCA, we conducted a detrended correspondence analysis (DCA) with our data set, identified environmental variables (10 variables) that significantly correlated with the DCA site scores of the first or second axis by Kendall’s rank correlation (P < 0.05), and then calculated Pearson correlation coefficients among the 10 environmental variables. On the basis of this information, several CCA ordinations were performed to identify the significant environmental variables explaining the variance of species data in the CCA. We avoided using a combination of environmental variables that were correlated with one another (Table 3; |r| ≥ 0.5). The DCA was also performed with CANOCO 4.5 software. Statistical significance of eigenvalues and species-environment correlations for the first two axes generated by the CCA were tested by the Monte Carlo method based on 999 permutations. The density values of 28 macroinvertebrate taxa were ln(χ + 1)-transformed to obtain homogeneity of variance. Organic matter content (%) values of the sediments were arcsine-transformed, and biomass values of aquatic plants and abundance of fish were ln(χ + 1)-transformed. Kendall’s rank correlation and Pearson correlation were calculated with R software (Dalgaard 2002).
Results
Macroinvertebrates and environmental variables
Forty-three macroinvertebrate taxa were identified, including 1 Nematoda, 1 Gastropoda, 2 Bivalvia, 6 Oligochaeta, 2 Hirudinea, 1 Mysidae, 2 Hydroptilidae, 1 Ceratopogonidae, and 27 Chironomidae (Table 1). Limnodrilus hoffmeisteri (Tubificidae), Chironomus sp. plumosus group, Procladius sp., and Glyptotendipes spp. (Chironomidae) were the most common taxa. Each of these taxon was recorded at more than 17 of the 25 sampling sites. The most abundant taxa were Glyptotendipes spp., Chironomus sp. plumosus group, and L. hoffmeisteri, accounting for 28, 15, and 13%, respectively, of the total number of macroinvertebrate individuals. Mean density (no. m−2) of each taxon ranged from 32 to 3,141 when counted only at sites where the taxon was present (Table 1).
We hypothesized that the 13 selected environmental variables regulated the benthic macroinvertebrate community structure in Lake Takkobu (Table 2). The values of these variables show that there was considerable heterogeneity among sites. Among them, water depth, abundance of benthivorous fish, and Chl a content were excluded because they showed no significant correlations (P > 0.05) with the DCA site scores of the first or second axis by Kendall’s rank correlation. The Pearson correlation matrix among the 10 included environmental variables is shown in Table 3. The variables relating to aquatic plants, except between the biomasses of Trapa and Potamogenton, were significantly correlated with one another.
Relationship of species composition to environmental gradients
In the CCA, distance from the mouth of the Takkobu River (DISTANCE), biomass of submerged plants (BIO-SUB), organic matter content of the bottom sediments (OMC), and bottom-layer pH (BpH) explained the significant variation in the macroinvertebrate species composition (Fig. 2). The first and second canonical axes explained 10.3% (eigenvalue 0.232) and 9.5% (eigenvalue 0.214), respectively, of the variance in the species data, and 39.9 and 36.7%, respectively, of the variance in species-environment relationships (Table 4). Intraset correlations showed that the first axis was a gradient of both decreasing BIO-SUB and increasing BpH and that the second axis was a gradient of decreasing OMC (Table 4). The species-environment correlation of the first axis became significant in the Monte Carlo permutation test (P < 0.05) only when we selected BIO-SUB among the six variables relating to aquatic plants as the environmental variable.
Distribution of 28 macroinvertebrate taxa in relation to environmental variables: CCA ordination diagrams showing species (left, open triangles), sites (right, open circles), and environmental variables (left, arrows); the first axis is horizontal, the second vertical. The abbreviations of 28 species are defined in Table 1. The environmental variables are DISTANCE distance from the mouth of the Takkobu River, BIO-SUB biomass of submerged plants, OMC organic contents of the sediments, and BpH bottom-layer pH
Species responses to environmental gradients
Five species belonging to Chironomidae (Chironomus sp. except plumosus group, Psectrocladius sp., Corynoneura sp., Parachironomus sp. arcuatus group, and Zavreliella sp.) occurred at sites with relatively lower pH and a high biomass of submerged plants (Fig. 2). Three Tubificidae species (T. tubifex, A. limnobius and Aulodrilus sp.) and two Chironomidae species (Nanocladius sp. and Monodiamesa sp.) occurred at sites with high pH and little vegetation. In addition, the three Tubificidae species preferred organic-rich sediments (Fig. 2). Sphaerium sp. (Bivalvia) as well as Monodiamesa sp. (Chironomidae) occurred at sites with low OMC, whereas Tanypus sp. (Chironomidae) was found at sites with high OMC. These three species showed little relation to aquatic plants.
Relationships between number of taxa and environmental variables
The number of macroinvertebrate taxa was significantly and positively correlated with the number of species of submerged macrophytes (n = 25, r = 0.490, P = 0.012), the biomass of floating-leaved macrophytes (n = 25, r = 0.443, P = 0.027), and the biomass of Trapa (n = 25, r = 0.453, P = 0.023) among the 13 environmental variables.
Discussion
The variance in the distribution of the benthic macroinvertebrate community in this lake was well explained by environmental gradients related to BIO-SUB and OMC. The six variables relating to aquatic plants were closely related to one another (Table 3). Although Trapa is the most conspicuous vegetation in the lake, it was not the Trapa biomass but BIO-SUB that was selected as the best explanatory variable among them. The life form of aquatic plants seems to influence macroinvertebrate community structure. For example, the total biomass of macroinvertebrates was found to be higher, and the peak of their biomass size spectrum smaller, in Chara beds than in rooted-plant (mostly Isoetes sp.) weed beds (Hanson 1990; Rasmussen 1993). Chironomids, anisopterans, gastropods, and sphaerid clams dominated the macroinvertebrate community in Chara beds, whereas amphipods dominated the community in rooted-plant (mostly Isoetes sp.) weed beds (Hanson 1990). Benthic macroinvertebrates were found to dominate in Vallisneria americana beds, whereas epiphytic species not only dominated in Trapa natans vegetation but also increased the total density of macroinvertebrates, because of the high plant biomass of Trapa (Strayer et al. 2003). Although how Trapa or submerged vegetation differently influenced the macroinvertebrate community structure was not clear in our study, the presence of aquatic vegetation was shown to be the most important determinant of the macroinvertebrate community in this lake.
Generally, most studies seem to evaluate the macroinvertebrate community in aquatic vegetation by including both epiphytic and benthic communities. The present study, however, showed that the influence of aquatic vegetation was prominent even when the target was restricted to the benthic community inhabiting sediments. Presumably, vegetation is important to the benthic community because it provides refuge from predators, physically stable habitats, better food conditions, and DO. Among these, the roots of some macrophytes have been shown previously to provide benthic macroinvertebrates with DO (Ságová-Marecková and Kvet 2002).
OMC was shown to be an important environmental variable, followed by BIO-SUB. Beaty et al. (2006) experimentally showed that higher OMC enhances the growth of Chironomus, but few studies have suggested that OMC determines the community structure of benthic macroinvertebrates in lake littoral areas. Irrespective of aquatic vegetation, in this lake, Tanypus sp. was shown to prefer organic-rich sediments, whereas Monodiamesa sp. and Sphaerium sp. preferred environments with the least OMC. Monodiamesa bathyphila has been reported to inhabit sandy sediments beneath oligotrophic waters (Sæther 1983), in accordance with our result.
Although bottom DO was not retained as a variable in the CCA, low concentrations of bottom DO might be one important factor (although associated with other factors) affecting species composition at lake littoral sites around the mouth of an organically polluted river (e.g., Petridis 1993). In our study, bottom DO (with a range of 5.6–11.1 mg l−1) did not decrease near the mouth of any river. DO often decreases to below the critical threshold for aquatic life below dense Trapa vegetation (Caraco and Cole 2002) or submerged macrophytes (Takamura et al. 2003). In this lake, however, concentrations of bottom DO did not correlate with the Trapa biomass, probably because some sites with dense vegetation also had high bottom DO owing to the presence of springs (Takamura et al. 2007).
Chironomidae is the most numerous taxonomic group inhabiting the sediments in this lake. Their densites were similar to or higher than those reported in Trapa and Vallisneria vegetation of the freshwater tidal Hudson River (Strayer et al. 2003). The CCA revealed that the occurrence of several chironomid species largely depended on biomass of submerged plants in this lake. Among them, five species, Chironomus sp. except plumosus group, Psectrocladius sp., Corynoneura sp., Parachironomus sp. arcuatus group, and Zavreliella sp., preferred more organic-rich sediments than Endochironomus tendens, Dicrotendipes sp. and Polypedilum sordens. According to Brodersen et al. (2001), E. tendens, Parachironomus arcuatus, and several species of Psectrocladius and Polypedilum show high levels of dependence on aquatic macrophytes. Many species of Dicrotendipes and Psectrocladius inhabit aquatic plants (Epler 1988; Kornijów 1989a, b). Chironomus sp. plumosus group and Procladius sp. occurred at 21 and 19 sites, respectively; therefore, these taxa are widely distributed in Lake Takkobu and as a result might not show specific demands in relation to the environmental gradient in this lake. Chironomus sp. plumosus group and species of Procladius and Tanypus have been shown to prefer pelagic or profundal waters to the littoral area (Serruya 1978; Ueno et al. 1993; Tolonen et al. 2001).
Among the six oligochaete species, the CCA (Fig. 2) revealed that the three species of T. tubifex, A. limnobius, and Aulodrilus sp. occurred in relatively organic-rich sediments with less vegetation, farther from the mouth of the Takkobu River, than L. hoffmeisteri, Limnodrilus udekemianus, and Bothrioneurum vejdovskyanum. Generally, all six oligochaete species are reported to be able to tolerate extremely oxygen-deficient environments (Brinkhurst 1974; Ohtaka and Kikuchi 1997). However, the present study showed that the former three species seem to have different habitat preferences from the latter three species. L. hoffmeisteri has been reported to occur abundantly in lake littorals with low bottom DO and high ammonium concentrations around the mouth of an organically polluted river (Petridis 1993). However, in our study, this species appeared at 22 sites regardless of bottom DO concentrations and was widely distributed in Lake Takkobu.
The species richness of benthic macroinvertebrates (Table 1) was correlated with three variables relating to aquatic vegetation: the species number of submerged macrophytes and the biomasses of Trapa and floating-leaved macrophytes. The abundance and richness of the macroinvertebrate community seem to increase as the density or biomass of freshwater macrophytes increases (e.g., Crowder and Cooper 1982; Lodge 1991). One reason why aquatic plants, particularly submerged macrophytes, support an abundant and diverse macroinvertebrate community is that they increase the available substrate area. More surface area provides the macroinvertebrate community with more habitat and more food (e.g., epiphytic algae) (Warfe and Barmuta 2006). Independently of habitat area, architectural complexity increased the abundance and richness of epiphytic invertebrates in an artificial plant experiment (Taniguchi et al. 2003). Complex macrophyte architecture might result in the trapping of more organic matter, which means more food for the macroinvertebrates. It might also provide more interstitial space, which functions as a refuge from predation (Crowder et al. 1998; Diehl and Kornijów 1998). In general, macrophyte vegetation decreases the efficiency of fish predation and provides a refuge for benthic macroinvertebrates (Crowder and Cooper 1982; Diehl 1992, 1993, 1995; Hanson and Butler 1994), although a complex macrophyte architecture per se does not necessarily help the macroinvertebrate community to escape from their predators (Warfe and Barmuta 2006).
We investigated only the benthic macroinvertebrate community in the sediments. Ito et al. (2005) collected 46 additional macroinvertebrate taxa from macrophyte vegetation in Lake Takkobu, including several species highly dependent on macrophytes. Thus, aquatic vegetation is surely important for supporting a diverse macroinvertebrate community in Lake Takkobu. The present study not only showed that the rapid loss of submerged vegetation has altered the macroinvertebrate community structure but also that the decrease in species richness of submerged plants has decreased the number of macroinvertebrate species. Therefore, the restoration of the species diversity of the submerged macrophytes as well as the quantity of the submerged vegetation, the levels of both of which have decreased in the past decade (Kadono 2007), is desirable for maintaining a diverse and unique macroinvertebrate community in this lake.
References
Beaty SR, Fortino K, Hershey AE (2006) Distribution and growth of benthic macroinvertebrates among different patch types of the littoral zones of two arctic lakes. Freshw Biol 51:2347–2361
Borkent A, Wirth WW (1997) World species of biting midges (Diptera: Ceratopogonidae). Bull Am Mus Nat Hist 233:1–257
Bowen KL, Kaushik NK, Gordon AM (1998) Macroinvertebrate communities and biofilm chlorophyll on woody debris in two Canadian oligotrophic lakes. Arch Hydrobiol 141:257–281
Brinkhurst RO (1974) The benthos of lakes. Macmillan, London
Brinkhurst RO, Jamieson BGM (1971) Aquatic Oligochaeta of the world. Oliver and Boyd, Edinburgh
Brodersen KP (1995) The effect of wind exposure and filamentous algae on the distribution of surf zone macroinvertebrates in Lake Esrom, Denmark. Hydrobiologia 297:131–148
Brodersen KP, Odgaard BV, Vestergaard O, Anderson NJ (2001) Chironomid stratigraphy in the shallow and eutrophic Lake Sǿbygaard, Denmark: chironomids-macrophyte co-occurrence. Freshw Biol 46:253–267
Caraco NF, Cole JJ (2002) Contrasting impacts of a native and alien macrophyte on dissolved oxygen in a large river. Ecol Appl 12:1496–1509
Crowder LB, Cooper WE (1982) Habitat structural complexity and the interaction between bluegills and their prey. Ecology 63:1802–1813
Crowder LB, McCollum EW, Martin TH (1998) Changing perspectives on food web interaction in lake littoral zones. In: Jeppesen E, Søndergaard M, Søndergaard M, Christoffersen K (eds) The structuring role of submerged macrophytes in lakes. Springer-Verlag, New York, pp 240–249
Dalgaard P (2002) Introductory statistics with R. Springer, Tokyo
Diehl S (1992) Fish predation and benthic community structure: the role of omnivory and habitat complexity. Ecology 73:1646–1661
Diehl S (1993) Effects of habitat structure on resource availability, diet and growth of benthivorous perch, Perca fluviatilis. Oikos 67:403–414
Diehl S (1995) Direct and indirect effects of omnivory in a littoral lake community. Ecology 76:1727–1740
Diehl S, Kornijów R (1998) Influence of submerged macrophytes on trophic interactions among fish and macroinvertebrates. In: Jeppesen E, Søndergaard M, Søndergaard M, Christoffersen K (eds) The structuring role of submerged macrophytes in lakes. Springer-Verlag, New York, pp 24–46
Dvořák J (1996) An example of relationships between macrophytes, macroinvertebrates and their food resources in a shallow eutrophic lake. Hydrobiologia 339:27–36
Epler JH (1988) Biosystematics of the genus Dicrotendipes Kieffer, 1913 (Diptera: Chironomidae: Chironominae) of the world. Mem Am Entomol Soc 36:1–214
Gardner SC, Grue CE, Major WW III, Conquest LL (2001) Aquatic invertebrate communities associated with purple loosestrife (Lythrum salicaria), cattail (Typha latifolia) and bulrush (Scirpus acutus) in central Washington, USA. Wetlands 21:593–601
Hanson JM (1990) Macroinvertebrate size-distributions of two contrasting freshwater macrophyte communities. Freshw Biol 24:481–491
Hanson MA, Butler MG (1994) Responses to food web manipulation in a shallow waterfowl lake. Hydrobiologia 279/280:457–466
Hariu T, Nakajima H, Takamura N (2007) Distribution and present status of fishes in Lake Takkobu and adjacent rivers (in Japanese with English abstract). Jpn J Limnol 68:157–167
Ito T, Ohtaka A, Ueno R, Kuwahara Y, Ubukata H, Hori S, Itoh T, Hiruta S, Tomikawa K, Matsumoto N, Kitaoka S, Togashi S, Wakana I, Ohkawa A (2005) Aquatic macroinvertebrate fauna in Lake Takkobu, Kushiro Marsh, northern Japan (in Japanese with English abstract). Jpn J Limnol 66:117–128
Johnson RK, Goedkoop W, Sandin L (2004) Spatial scale and ecological relationships between the macroinvertebrate communities of stony habitats of streams and lakes. Freshw Biol 49:1179–1194
Kadono Y (2007) Changes in macrophytic flora of Lake Takkobu, Kushiro, Japan, in past 30 years (in Japanese with English abstract). Jpn J Limnol 68:105–108
Kato H, Takamura N, Mikami H (1999) Distribution of macroinvertebrates in deep and oligotrophic Lake Towada, Japan. Acta Hydrobiol Sinica 23:96–105
Kornijów R (1989a) Macrofauna of elodeids of two lakes of different trophy. I. Relationships between plants and structure of fauna colonizing them. Ekol Pol 37:31–48
Kornijów R (1989b) Macrofauna of elodeids of two lakes of different trophy. II. Distribution of fauna living on plants in the littoral of lakes. Ekol Pol 37:49–57
Lodge DM (1991) Herbivory on freshwater macrophytes. Aquat Bot 41:195–224
Marker AFH, Nusch EA, Rai H, Riemann B (1980) The measurement of photosynthetic pigments in freshwaters and standardization of methods: conclusions and recommendations. Arch Hydrobiol Beih Erg Limnol 14:91–106
Masuda O, Uchiyama R (2004) Freshwater mollusks of Japan, including brackish water species. Pisces, Tokyo
Matsuzaki SS, Usio N, Takamura N, Washitani I (2007) Effects of common carp on nutrient dynamics and littoral community composition: roles of excretion and bioturbation. Fundam Appl Limnol 168:27–38
McCune B (1997) Influence of noisy environmental data on canonical correspondence analysis. Ecology 78:2617–2623
Moore JW (2006) Animal ecosystem engineers in streams. Bioscience 56:237–246
Murano M (1997) Order Mysidacea. In: Chihara M, Murano M (eds) An illustrated guide to marine plankton in Japan (in Japanese). Tokai University Press, Tokyo, pp 1010–1084
Oertli B (1995) Spatial and temporal distribution of the zoobenthos community in a woodland pond (Switzerland). Hydrobiologia 300/301:195–204
Ohtaka A, Kikuchi H (1997) Composition and abundance of zoobenthos in the profundal region of Lake Kitaura, central Japan during 1980–1985, with special reference to Oligochaetes. Pub Itako Hydrobiol Stn 9:1–14
Petridis D (1993) Macroinvertebrate distribution along an organic pollution gradient in Lake Lysimachia (Western Greece). Arch Hydrobiol 128:367–384
Rasmussen JB (1993) Patterns in the size structure of littoral zone macroinvertebrate communities. Can J Fish Aquat Sci 50:2192–2207
Sæther OA (1983) The larvae of Prodiamesinae (Diptera: Chironomidae) of the Holarctic region—keys and diagnoses. Entomol Scand Suppl 9:141–147
Ságová-Marecková M, Kvet J (2002) Impact of oxygen released by the roots of aquatic macrophytes on composition and distribution of benthic macroinvertebrates in a mesocosm experiment. Arch Hydrobiol 155:567–584
Scheffer M, Hosper SH, Meijer ML, Moss B, Jeppesen E (1993) Alternative equilibria in shallow lakes. Trends Ecol Evol 8:275–279
Serruya C (1978) Vertical distribution of benthic fauna. In: Serruya C (ed) Lake Kinneret. Junk, The Hague, pp 391–394
Strayer DL, Lutz C, Malcom HM, Munger K (2003) Invertebrate communities associated with a native (Vallisneria americana) and an alien (Trapa natans) macrophyte in a large river. Freshw Biol 48:1938–1949
Takamura N, Kadono Y, Fukushima M, Nakagawa M, Kim BH (2003) Effects of aquatic macrophytes on water quality and phytoplankton community in shallow lakes. Ecol Res 18:381–395
Takamura N, Nakagawa M, Wakana I, Igarashi S, Tsuji N (2007) Water quality and factors influencing its distribution in Lake Takkobu (in Japanese with English abstract). Jpn J Limnol 68:81–95
Taniguchi H, Nakano S, Tokeshi M (2003) Influences of habitat complexity on the diversity and abundance of epiphytic invertebrates on plants. Freshw Biol 48:718–728
Ter Braak CJF, Šmilauer P (2002) CANOCO reference manual and Cano Draw for Windows user’s guide: software for Canonical Community Ordination (v. 4.5). Microcomputer Power, Ithaca
Tolonen KT, Hämäläinen H, Holopainen IJ, Karjalainen J (2001) Influence of habitat type and environmental variables on littoral macroinvertebrate communities in a large lake system. Arch Hydrobiol 152:39–67
Tsalolikhin SJ (ed) (2004) Key to freshwater invertebrates of Russia and adjacent lands. Molluscs, polychaetes, nemerteans, vol 6. Nauka, St. Petersburg
Ueno R, Iwakuma T, Nohara S (1993) Chironomid fauna in the emergent plant zone of Lake Kasumigaura, Japan. Jpn J Limnol 54:293–303
van den Berg MS, Coops H, Noordhuis R, van Schie J, Simons J (1997) Macroinvertebrate communities in relation to submerged vegetation in two Chara-dominated lakes. Hydrobiologia 342/343:143–150
Warfe DM, Barmuta LA (2006) Habitat structural complexity mediates food web dynamics in a freshwater macrophyte community. Oecologia 150:141–154
Weatherhead MA, James MR (2001) Distribution of macroinvertebrates in relation to physical and biological variables in the littoral zone of nine New Zealand lakes. Hydrobiologia 462:115–129
Webb CJ, Scholl A, Ryser HM (1985) Comparative morphology of the larval ventromental plates of European species of Chironomus Meigen (Diptera: Chironomidae). Syst Entomol 10:373–385
Wellborn GA, Robinson JV (1991) The influence fish predation in an experienced prey community. Can J Zool 69:2515–2522
Wiederholm T (ed) (1983) Chironomidae of the Holarctic region. Keys and diagnoses. Part 1. Larvae. Entomol Scand Suppl 19:1–457
Wiggins GB (1996) Larvae of the North American caddisfly genera (Trichoptera), 2nd edn. University of Toronto Press, Toronto
Yang T (ed) (1996) Annelida, Hirudinea (in Chinese with English abstract). Fauna Sinica. Science Press, Beijing
Acknowledgements
We are grateful to Dr. Tetsuya Itoh of the School of Science, Hokkaido University, and Dr. Shigeo Kitaoka of Kashiwazaki City, Dr. Shin-ichi Hiruta of Hokkaido University of Education at Kushiro, and Dr. Yasuhiro Kuwahara of Hokkaido Abashiri Fisheries Experimental Station for the identification of Hirudinea, Ceratopogonidae, Mysidae, and Gastropoda and Bivalvia, respectively. Our thanks are also extended to Dr. Naotoshi Kuhara of the Chitose Board of Education, Dr. Hidenori Ubukata of Hokkaido University of Education at Kushiro, Dr. Tsutomu Hariu of Kushiro City Museum, Dr. Masaki Kyono of Sapporo Technical College, and Dr. Munemitsu Akasaka of the National Institute for Environmental Studies for their help throughout this study. This study was supported by the Kushiro nature restoration project of the Ministry of the Environment, Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Takamura, N., Ito, T., Ueno, R. et al. Environmental gradients determining the distribution of benthic macroinvertebrates in Lake Takkobu, Kushiro wetland, northern Japan. Ecol Res 24, 371–381 (2009). https://doi.org/10.1007/s11284-008-0514-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-008-0514-0