Abstract
Macrozoobenthos and submerged macrophytes interact closely. However, studies in China have focused on the middle and lower reaches of the Yangtze River, where shallow lakes are concentrated, rather than on temperate lakes. To clarify the responses of taxonomic and functional groups of macrozoobenthos in temperate lakes to changes in submerged macrophyte biomass (BMac) on a large scale, 19 temperate lakes within Baiyangdian Lake were investigated in this study. The BMac differed greatly across the 19 lakes, and Potamogeton crispus was the dominant species. According to the BMac, the 19 lakes were divided into 4 groups. One-way analysis of variance and Pearson correlation analysis showed that the water environmental parameters were different among the 4 groups, and the BMac was significant correlated with all the physical and chemical parameters of water bodies (except for water depth). Forty-one taxa of macrozoobenthos were identified in the 19 lakes, with oligochaetes, Hirudinea, gastropods, crustaceans, chironomid larvae, and aquatic insects (excluding chironomid larvae) represented by 9, 1, 4, 2, 19, and 6 species, respectively. Chironomid larvae and oligochaetes dominated by density, and gastropods and chironomid larvae dominated by biomass. Canonical correspondence analysis showed that the BMac was the most important factor affecting the macrozoobenthos community structure in group 1 to group 4. Macrozoobenthos with low pollution tolerance values were mainly found in areas with high BMac, while species with high pollution tolerance values were mainly distributed in areas with low BMac and high nutrient contents. Different taxonomic and functional groups of macrozoobenthos responded differently to changes in BMac. As BMac increased, density and biomass of oligochaetes and chironomid larvae tended to decrease, while those of gastropods and aquatic insects tended to first decrease and then increase. Collectors had more species than any other functional group in group 1 to group 4. As BMac increased, density and biomass of collectors gradually decreased, while density of predators, shredders, and scrapers tended to first decrease and then increase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shallow lakes have two stable states: a clear water state dominated by submerged macrophytes and a turbid water state dominated by algae (Scheffer and Jeppesen 2007; Hilt et al. 2017). In the context of human disturbances such as aquaculture, water eutrophication, and dam construction, an increasing number of shallow lakes around the world have experienced severe disruption of ecological functions and the gradual disappearance of submerged vegetation, so they have been gradually transformed from macrophytic lakes into algal lakes (Chambers et al. 2008; Zhang et al. 2017; Zhang et al. 2021). As an important component of lake ecosystems, macrozoobenthos interact closely with submerged vegetation (Newman 1991; Underwood et al. 1992; Jones and Sayer 2003; Wang et al. 2006; Tan et al. 2017). Therefore, the extinction of submerged vegetation will inevitably change the community structure and species diversity of macrozoobenthos (Liang and Wang 1999; Tan et al. 2017). However, there are few studies on the responses of taxonomic and functional groups of macrozoobenthos to changes in the biomass of submerged vegetation, and such studies have mainly focused on the subtropical regions where shallow lakes are concentrated (for example, the middle and lower reaches of the Yangtze River). Thus, the results of these studies do not provide a strong theoretical basis for the ecological management and restoration of temperate lakes.
Some studies on subtropical lakes have shown that submerged macrophytes play an important role in shaping the macrozoobenthos community structure (Wang et al. 2006; Pan et al. 2011, 2012; Chen et al. 2015). A large number of epiphytic algae attached to submerged plants provide an important food source for small gastropods. Therefore, with an increase in submerged macrophyte biomass (BMac), the standing crop of gastropods often increases (Liang and Wang 1999; Gong et al. 2000; Wang et al. 2006), while the density or biomass of chironomid larvae and oligochaetes that take up nutrients from sediments often decreases (Liang and Wang 1999; Jiang et al. 2006; Wang 2007). However, these studies have also drawn some inconsistent conclusions. For example, Gong et al. (2000) found that submerged vegetation not only increases the heterogeneity of habitats and the availability of food but also reduces the predation pressure of fish on macrozoobenthos, so the macrozoobenthos species number, density, and biomass are higher in macrophytic lakes than in algal lakes. In addition, scrapers and collectors had the highest abundances among the functional feeding groups in macrophytic and algal lakes, respectively. Although Pan et al. (2012) reported results similar to those of Gong et al. (2000), they found that the density of macrozoobenthos was significantly higher in algal lakes than in macrophytic lakes. In contrast, Liang et al. (1995) found that although there were fewer species of macrozoobenthos in algal lakes than in macrophytic lakes, the density and biomass of macrozoobenthos showed no obvious trend. Liang et al. (1995) also found that among different functional groups, the abundance of collectors was the highest in both macrophytic and algal lakes. On the one hand, the above differences may be mainly attributed to differences in research scale, and the results of studies on two or fewer lakes are often not representative. On the other hand, the above differences may be closely related to the physicochemical characteristics of water, species composition of submerged macrophytes, and the community structure of fish in different lakes. Some studies have shown that different gastropods differ notably in their preference for submerged macrophytes (Wang et al. 2006; Li et al. 2009; Hansen et al. 2011), and fish feeding on macrozoobenthos is also an important factor influencing their preference (Jones and Sayer 2003). Because temperate lakes are significantly different from subtropical lakes in their climatic conditions and dominant species of submerged macrophytes, it can be speculated that the response pattern of macrozoobenthos in temperate lakes to changes in BMac may be different from that of macrozoobenthos in subtropical lakes.
Baiyangdian Lake is the largest shallow lake in the North China Plain and plays an important role in maintaining biodiversity and regional ecological security. In recent decades, due to the dual effects of human activities and climate change, the surface area of Baiyangdian Lake has drastically shrunk, and it is divided by thousands of ditches into more than 100 relatively independent sublakes of different sizes (Xu et al. 1998). Due to their different utilization models, the ecological environment of these lakes varies greatly. Some lakes are severely eutrophic due to degradation or extinction of submerged macrophytes, while some lakes are at high risk of turning into marshes due to the overgrowth of submerged macrophytes (Diao et al. 2013; Zhang et al. 2021). However, this provides good conditions for this study analysing the relationship between macrozoobenthos and submerged macrophytes in temperate lakes. In this study, the macrozoobenthos, submerged macrophytes, and physicochemical factors in 19 temperate lakes within Baiyangdian Lake were systematically investigated to answer the following two questions: (1) are submerged macrophytes the most important environmental factor that determines the community structure of macrozoobenthos in temperate lakes in China? (2) How do the taxonomic and functional groups of macrozoobenthos in temperate lakes respond to changes in BMac? Considering the submerged macrophytes cannot only directly affect the macrozoobenthos community, but also indirectly by changing the physicochemical properties of water, we hypothesize that it should be the key factor. This study is the first to investigate the relationship between macrozoobenthos and submerged macrophytes in temperate lakes in China on a large scale. Its results will be valuable for the ecological management and restoration of temperate lakes.
Materials and methods
Overview of the study area
Baiyangdian Lake is located in Baoding City, Hebei Province. It has a warm temperate continental monsoon climate with a mean annual temperature of 12.1°C and a mean annual rainfall of 524.9 mm. It is mainly recharged by surface runoff and rainfall. The historical water level of Baiyangdian Lake during the flooding season can reach 11.73 m, with a corresponding lake area of 366 km2, mean depth of 2.84 m, and water storage of 10.38 × 108 m3 (Wang and Dou, 1998). Since the 1960s, the serious decline in the surface area of Baiyangdian Lake has necessitated ecological water supplementation to maintain its water level. Notably, the surface area of Baiyangdian Lake was 177 km2 in 1995, 53 km2 in 2005 and 78.5 km2 in 2015 (Wang et al. 2020). At present, many mudflats in the lake area have been reclaimed as farmland and residential areas, and the entire lake is divided by thousands of ditches into more than 100 relatively independent sublakes of different sizes (Fig. 1). Among them, macrophytic lakes comprise less than 20% of the surface area of Baiyangdian Lake, and macrophytic algal transitional lakes comprise approximately 50% (Diao et al. 2013). In terms of the composition of aquatic plants, the main dominant species of emergent plants is Phragmites australis, and the main dominant species of submerged macrophytes include Potamogeton crispus, Stuckenia pectinatus, and Myriophyllum spicatum (Yang et al. 2020; Zhang et al. 2021).
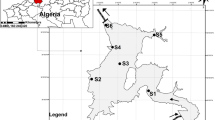
Distribution of the 19 temperate lakes within Baiyangdian Lake. (Three sampling sites were randomly set up at each lake. The codes of the 19 lakes are as follows: 1, Shaoche Lake; 2, Hele Lake; 3, Siwang Lake; 4, Guang Lake; 5, Longwang Lake; 6, Laowang Lake; 7, Xiaomai Lake; 8, Damai Lake; 9, Pingyang Lake; 10, Chiyu Lake; 11, Shihou Lake; 12, Houtang Lake; 13, Qiantang Lake; 14, Fanyu Lake; 15, Wanlou Lake; 16, Julong Lake; 17, Sanjiao Lake; 18, Baiyang Lake; 19, Yangjiao Lake)
Field survey
In May 2021, we conducted a field survey on the submerged macrophytes, macrozoobenthos, and physical and chemical parameters of the water in the 19 lakes within Baiyangdian Lake. Since all the 19 lakes are part of the Baiyangdian Lake, and they are interconnected with each other, the hydrological regime is basically the same. Three sampling sites were set for each lake, totalling 57 sampling sites (Fig. 1). Macrozoobenthos were collected from each sampling site using a Petersen grab (1/16 m2). The collected mud samples were sieved through a 100-mesh sieve and placed in white dissecting trays for sorting. The macrozoobenthos samples were fixed with 10% formaldehyde and then identified, counted, and weighed in the laboratory. The measurement results were converted to density and biomass per unit area. The submerged macrophytes were collected using submerged macrophyte-harvesting clamps with an opening area of 0.25 m2. Submerged macrophytes were randomly collected from each sampling site three times. The collected submerged macrophytes were drained and weighed (wet weight), and the weights were converted to biomass per unit area. At the same time, mixed water samples were taken from each sampling site and brought back to the laboratory to determine their contents of total nitrogen (TN) and total phosphorus (TP). The conductivity (Cond), dissolved oxygen (DO), water temperature (Temp), and pH of the water samples were measured with a portable water quality analyser (YSI ProPlus, YSI Inc., Ohio, USA). The Secchi depth (ZSD) was measured with a Secchi disk to gauge the transparency of the water samples. The mean water depth (ZM) at macrophytic and macrophyte-free sites was measured with a sounding lead and a portable sounder (SM-5, SpeedTech Instruments, Great Falls, VA, USA), respectively.
Data analysis
To improve the reliability and universality of the research results, this study analysed the relationship between macrozoobenthos and submerged macrophytes in different groups of lakes rather than at different sites. First, the 19 lakes were divided into 4 groups (group 1 to group 4) based on the range of mean BMac, namely, < 500, 500–2000, 2000–4000, and >4000 g/m2, which correspond to BMac levels of extremely low, low, moderate, and high, respectively. The differences in the physical and chemical parameters of the four lake groups were tested using one-way analysis of variance (ANOVA). To ensure the normality and homogeneity of variance of the data, the data were log transformed before analysis. If there was a significant difference in the variables that met the homogeneity of variance, Tukey’s honestly significant difference test was used to find differences between the groups; otherwise, Tamhane’s T2 method was used (Zhang et al. 2021). Pearson correlation was used to analyse the correlations between the BMac and the physicochemical factors of the water of the 19 lakes.
According to the species composition, the macrozoobenthos in this study were mainly divided into six taxonomic groups, namely, oligochaetes, Hirudinea, gastropods, crustaceans, chironomid larvae, and aquatic insects (chironomid larvae were not included in this taxonomic group). According to their feeding habits, macrozoobenthos are mainly divided into four functional groups, namely, collectors (including collector filters and collector gatherers), predators, scrapers, and shredders (Liang and Wang 1999; Mandaville 2002). According to the sensitivity of the different species to water pollution, their tolerance values were assigned as 1–10 (Mandaville 2002; Ministry of Ecology and Environment of China 2020). The dominance (Y) of macrozoobenthos was mainly calculated from the frequency of occurrence of different species (fi) and the ratio of the number of individuals of one species (Ni) to the total number of individuals (N). The calculation formula is Y = (Ni/N) * fi (Liu et al. 2016). When Y is ≥ 1%, the species is a dominant species. The diversity and equitability of macrozoobenthos at different sites was evaluated with the Shannon diversity index (H') and Pielou’s equitability index (E), respectively, which was calculated as H' = -Σ(Pi)(log Pi) and E = H' / H' max, where Pi is the proportion of an individual species i among the whole community. The differences in the mean species number, H', E, density, and biomass of macrozoobenthos among group 1 to group 4 were analysed with the nonparametric Kruskal–Wallis test. One-way ANOVA was used to examine the differences of density and biomass of different taxonomic and functional groups among the 4 groups. Before analysis, the data were log (x + 1) transformed to meet the assumptions of normality and homogeneity. If a significant difference was detected, post hoc pairwise comparisons were done using Student–Newman–Keuls method. All the above analyses were performed in SPSS 13.0 software.
The relationship between the macrozoobenthos community structure and environmental factors was analysed in Canoco 4.5 software. First, the density data from the 57 sampling sites were used to conduct detrended correspondence analysis (DCA), based on the length of the first axis of DCA ordination (redundancy analysis is used if the length is less than 3, canonical correspondence analysis (CCA) is used if the length is more than 4, and both redundancy analysis and CCA can be used if the length is 3–4). CCA was used to analyse the relationships between species and the environmental factors. The following environmental factors were included in the analysis: BMac, ZM, ZSD, ZSD/ZM, DO, pH, Cond, TN, TP, and Temp. Before data analysis, species with a frequency of occurrence less than 5% were excluded (Zhang et al. 2018). The Monte Carlo permutation test (P < 0.01) and forward selection were used to determine the main environmental factors.
Results
Characteristics of submerged macrophytes and water bodies
A total of six submerged macrophytes were found in the 19 lakes: Potamogeton crispus, Myriophyllum spicatum, Chara sp., Stuckenia pectinatus, Ceratophyllum demersum, and Utricularia vulgaris. P. crispus was the most dominant species, comprising 61.94% of the total BMac, followed by M. spicatum, Chara sp., and S. pectinatus, comprising 21.07%, 8.61%, and 7.38% of the total BMac, respectively. The other two submerged macrophytes each comprised less than 1% of the total BMac. The mean BMac in the 19 lakes ranged from 0 to 9653 g/m2 (Fig. 2). Based on the difference in the mean BMac, the 19 lakes were divided into group 1 to group 4, which included five, six, four, and four lakes, respectively (Fig. 2). The mean biomasses of group 1 to group 4 were 152, 1362, 2517, and 8165 g/m2, respectively.
As the BMac increased, the ZSD, ZSD/ZM, and DO of group 1 to group 4 gradually increased, while Cond, TN, and TP gradually decreased (Fig. 3). ZM was similar across group 1 to group 4, and there was no obvious pattern of changes in Temp. Group 4 had a significantly higher pH than the other three groups (Fig. 3).ANOVA indicated that group 1 to group 4 did not show significant differences in ZM but did show significant differences in the other eight indicators (Figure 3). Pearson correlation analysis showed that BMac was positively correlated with ZSD, ZSD/ZM, pH, and DO; negatively correlated with ZM, Cond, TN, and TP; and weakly correlated with Temp (Table 1).
Comparison of physical and chemical parameters of water bodies in group 1 to group 4. (Note: ZM, mean water depth; ZSD, Secchi depth; ZSD/ZM, ratio of Secchi depth to water depth; Temp, water temperature; Cond, conductivity; TN, total nitrogen; TP, total phosphorus. Different superscript letters in the figure indicate significant differences.)
Characteristics of macrozoobenthos communities
A total of 41 taxa of macrozoobenthos were identified in the 19 lakes, including 9 oligochaetes, 1 Hirudinea, 4 gastropods, 2 crustaceans, 19 chironomid larvae, and 6 other aquatic insects (Appendix Table 4). According to density, chironomid larvae and oligochaetes dominated, comprising 82.0% and 13.1% of the total density, respectively (Fig. 4). According to biomass, gastropods and chironomid larvae dominated, comprising 57.3% and 38.0%, respectively (Fig. 4). According to the tolerance values, most macrozoobenthic species were pollution-tolerant, including Limnodrilus hoffmeisteri, Limnodrilus claparedianus, and Chironomus sp.; only a few species were pollution-intolerant, including Eukiefferiella sp., Ecnomus sp., and Caenis sp. (Appendix Table 4).
The number of macrozoobenthos species at the 57 sampling sites varied from 1 to 9, with a mean of 4.2. With an increase in BMac, the Shannon diversity index and Pielou’s equitability index of macrozoobenthos in group 1 to group 4 increased, density gradually decreased, and biomass first decreased and then increased (Table 2). The chi-squared test showed no significant differences in the above five parameters (species number, Shannon diversity index, Pielou’s equitability index, density, and biomass of macrozoobenthos) between groups (Table 2). There were clear differences in the dominant species across group 1 to group 4. Chironomus sp. was the main dominant species in group 1 to group 3, with dominances of 35.61, 78.86, and 69.76, respectively. The dominance of Chironomus sp. in group 4 (8.44) was significantly weaker than its dominance in group 1 to group 3 (Table 3). Three of the four dominant species in group 1 were collectors, and all of them were pollution-tolerant. The proportion of collectors in group 3 and group 4 was significantly lower than that in group 1, and Parachironomus sp. (a predator) and Cricotopus sp. (a shredder) were common dominant species in group 3 and group 4 (Table 3).
Influencing factors
Results of the forward selection in the CCA and Monte Carlo permutation tests indicated that the major environmental factors influencing the macrozoobenthos community structure in group 1 to group 4 included BMac, TP, ZSD/ZM, and DO (Figure 5). The eigenvalues of the first and second axes of ordination were 0.513 and 0.378, respectively. The Monte Carlo permutation test showed that both ordination axes were significant at P < 0.01. The first and second axes explained 30.3% and 20.5% of the species–environment relationship, respectively. Among the four major environmental factors, BMac was the most important influencing factor, followed by TP. The ordination diagram of the sampling sites showed that the sampling sites of group 4 were mainly in areas with high BMac and low nutrient contents (Fig. 5A). From the species ordination diagram, it can be seen that species with low tolerance values, such as Eukiefferiella sp., Synorthocladius sp., Glyptotendipes sp., and Gammarus sp., were mainly found in the areas with high BMac, while pollution-tolerant species, such as L. hoffmeisteri, Limnodrilus sp., Chironomus sp., and Tanypus sp., were mainly found in the areas with low BMac and high nutrient contents (Fig. 5B).
CCA ordination diagrams based on sampling site–environment A and species–environment relationships B. (The value in parentheses following each species in B represents the tolerance value of the species. Abbreviations: Synort, Synorthocladius sp.; Eukief, Eukiefferiella sp.; Parata, Paratanytarsus sp.; Parafo, Parafossarulus striatulus; Glypto, Glyptotendipes sp.; Endoch, Endochironomus sp.; Polype, Polypedilum sp.; Gammar, Gammarus sp.; Cricot, Cricotopus sp.; Parach, Parachironomus sp.; Chaobo, Chaoboridae sp.; Chiron, Chironomus sp.; Radix, Radix sp.; Ilyodr, Ilyodrilus sp.; Procla, Procladius sp.; Microc, Microchironomus sp.; Limhof, Limnodrilus hoffmeisteri; Tanypus, Tanypus sp.; Limnod, Limnodrilus sp.; Brasow, Branchiura sowerbyi; Propsi, Propsilocerus sp.; Einfel, Einfeldia sp.; Cerato, Ceratopogonidae sp.)
Responses of macrozoobenthos to submerged macrophytes
Different taxonomic and functional groups responded differently to changes in BMac. As the BMac increased, the density and biomass of oligochaetes and chironomids all showed decreasing trends, and the density and biomass of gastropods and aquatic insects all showed a trend of first decreasing and then increasing (Fig. 6). However, ANOVA analyses indicated that only biomass of chironomids among the 4 groups had significant difference (F = 5.155, P = 0.003). There were more species of collectors than any other functional group in all 4 groups, comprising 50.0%, 54.5%, 63.6%, and 42.9% of the total species number, respectively. As the BMac increased, the density and biomass of collectors gradually decreased, while the density and biomass of predators, shredders, and scrapers all showed a trend of first decreasing and then increasing (Fig. 7). ANOVA analyses showed that both the density and biomass of collectors among the 4 groups were significantly different (density, F = 3.310, P = 0.027; biomass, F = 5.269, P = 0.003), while only the density of shredders among the 4 groups was significantly different (F = 4.194, P = 0.010).
Discussion
Submerged macrophytes and water physicochemical parameters
As shown by the changes in the species composition and BMac in the 19 studied temperate lakes, the water environments of these lakes differed greatly. Among the six submerged macrophytes surveyed in 19 lakes, pollution-tolerant species, pollution-intolerant species, and pioneer species were found. Among them, highly pollution-tolerant P. crispus, M. spicatum, S. pectinatus, and C. demersum are widespread, while U. vulgaris is only distributed in water bodies with low nutrient contents (Koller-Peroutka et al. 2015). In this study, U. vulgaris was observed only in Shaoche Lake, which had a high BMac and clear water. This finding indirectly indicates that the water environment of Shaoche Lake was better than that of the other lakes. Because Chara sp. can grow under low-light conditions, it is generally a pioneer species in the restoration of submerged macrophytes in temperate lakes (Blindow 1992; Van den Berg et al. 1999). The biomass of Chara sp. was high in some lakes in Baiyangdian Lake, indicating that the submerged macrophytes in these lakes may be in the early stage of recovery. In terms of biomass, during the survey in spring, P. crispus had the highest biomass, which may be mainly related to its unique life history. P. crispus generally germinates in autumn, grows rapidly in spring, and dies at high temperatures in summer (Wang et al. 2016), while other submerged macrophytes generally germinate in spring and reach their maximum biomass in summer (Ni 1999). In addition, the lower water temperatures in temperate regions than in subtropical regions may also be more conducive to the growth and development of P. crispus and may prolong its dominant period. Therefore, P. crispus generally became the main dominant species of submerged macrophyte in these temperate lakes in spring. In some subtropical lakes in the middle and lower reaches of the Yangtze River, perennial submerged macrophytes, such as Potamogeton maackianus, Vallisneria natans, and M. spicatum, often become the dominant species in spring (Li and Cheng 1999; Gong et al. 2000; Zhang et al. 2019), and the dominant position of P. crispus is not as obvious in subtropical lakes as in temperate lakes. It seems that climatic conditions are an important factor causing significant differences in the community structure of submerged macrophytes in the two regions.
Pearson correlation analysis showed that the BMac was significantly correlated with all the environmental factors except for ZM (Table 1). In addition, as the BMac increased, ZSD, ZSD/ZM, and DO gradually increased, while Cond, TN, and TP gradually decreased. This may be closely related to the structuring roles of submerged macrophytes. As the main primary producers of lake ecosystems, submerged macrophytes cannot only release oxygen through photosynthesis but also absorb nutrients in water bodies, reduce sediment resuspension, and raise water transparency (Jeppesen et al. 1998). Similar results in other lakes have also been reported (Wang et al. 2005; Yang et al. 2020). These results also explain why BMac was the most important environmental factor affecting the macrozoobenthos community structure in the 19 lakes in the CCA results (Fig. 5). Submerged macrophytes cannot only directly affect the food source of macrozoobenthos but also indirectly change the habitat conditions of macrozoobenthos by affecting the physicochemical properties of water bodies. Therefore, macrozoobenthos with low tolerance values (e.g. Eukiefferiella sp., Synorthocladius sp., and Glyptotendipes sp.) are mostly distributed in lakes with high BMac, while species with high tolerance values (e.g. L. hoffmeisteri, Chironomus sp., and Tanypus sp.) are mostly distributed in lakes with high nutrient contents.
Response of macrozoobenthos communities to changes in BMac
In this study, a total of 41 taxa of macrozoobenthos were identified in the 19 lakes. Compared with recent historical data, the number of species identified in this study was significantly higher. For example, the survey results of Baiyangdian Lake in 2006–2007, 2009, and 2018 showed that it had only 23, 21, and 24 taxa, respectively (Xie et al. 2010; Qin et al. 2021). However, from the perspective of macrozoobenthic species composition, most of the species were pollution-tolerant, with a tolerance value generally above 6.0; only a few species were pollution-intolerant, including Eukiefferiella sp., Ecnomus sp., and Caenis sp. The above results indicate that despite the recently improved ecological environment and increased biodiversity in Baiyangdian Lake, water pollution is still one of the major problems in this lake.
With an increase in BMac, the Shannon diversity index of the macrozoobenthos in group1 to group 4 showed an increasing trend, and the mean number of species in group 3 and group 4 was also obviously higher than that in group 1 and group 2 (Table 2), which was largely consistent with the results from some lakes in the middle and lower reaches of the Yangtze River (Liang et al. 1995; Gong et al. 2000; Pan et al. 2012). However, the gradually decreasing trend of macrozoobenthos density and the first increasing and then decreasing trend of biomass in this study (Table 2) differ from the findings of Gong et al. (2000). The main reason for this difference is related to the changes in the density and biomass of dominant taxonomic groups. Because many species of chironomid larvae and oligochaetes are pollution-tolerant, their density or biomass often decreases with increasing BMac (Liang and Wang 1999; Jiang et al. 2006; Wang 2007). In this study, the densities of the two dominant taxonomic groups of chironomid larvae and oligochaetes (comprising 95.1% of the total density) both decreased with increases in BMac, which was consistent with the above trend and was the cause of the gradual decrease in macrozoobenthos density (Figure 6). The changes in the macrozoobenthos biomass in group 1 to group 4 were mainly due to the changes in the biomass of gastropods. In this study, gastropods accounted for the largest proportion (53.7%) of the total biomass. More benthic gastropods appeared in group 1 and group 2 with lower BMac, and more epiphytic gastropods appeared in group 4 with higher BMac. Therefore, the biomass of gastropods showed a trend of first decreasing and then increasing. The main cause of this phenomenon may be related to the life habits of different gastropods. Because adults of benthic gastropods (such as Bellamya sp.) live at the bottom of water bodies and feed on algae that live on the lake bottom; they are not closely related to submerged macrophytes, whereas epiphytic gastropods (such as Alocinma longicornis) are more affected by submerged macrophytes (Liang and Wang 1999; Wang et al. 2006).
Collectors had the most species number among all functional groups in the 4 groups, which is consistent with the results of Liang et al. (1995). However, it does not support the conclusion that the macrophytic and algal lakes had the highest abundances of scrapers and collectors, respectively (Gong et al. 2000; Pan et al. 2012). Among the 41 taxa of macrozoobenthos identified in this study, there were 19 species of chironomid larvae and nine species of oligochaetes but only four species of gastropods (scrapers). Compared with that in subtropical lakes (Pan et al. 2011, 2012), the proportion of scraper species in this study was significantly lower. This phenomenon may be mainly the result of the dual effects of overfishing and submerged macrophyte species composition. Because of the high edible value of gastropods, local residents often catch them for food or selling. Therefore, some large individual gastropods that have been widely seen in historical surveys (such as Cipangopaludina chinensis and Cipangopaludina cathayensis) (Xie et al. 2010) were not observed in this survey. The complexity and life history of submerged macrophytes also affect the diversity of gastropods (Wang et al. 2006; Hansen et al. 2011). P. crispus is characterized by a simple morphological structure and death in summer, which causes many gastropods to lose their habitats soon after they settle down. Therefore, many epiphytic gastropods have a low preference for P. crispus. As the BMac increased, the density and biomass of collectors gradually decreased, supporting the conclusion of Pan et al. (2012). In this study, the density and biomass of both shredders and predators were extremely low, and this phenomenon is also common in some subtropical lakes (Liang and Wang 1999). The density and biomass of the above two functional groups showed a trend of first decreasing and then increasing, which may be mainly caused by the changes in the dominant species.
Suggestions for lake management
Macrozoobenthos and submerged macrophytes are the main components of lake ecosystems, and the study of the relationship between the two is of great significance for the ecological management and restoration of shallow lakes (Tan et al. 2017). In this study, the responses of the taxonomic and functional groups of macrozoobenthos in 19 temperate lakes to changes in BMac were preliminarily analysed. Based on the current status of submerged macrophytes and macrozoobenthos, the following two suggestions are proposed:
-
1)
Due to its unique life history, P. crispus has become the main dominant species in many temperate lakes in spring. However, in summer, when P. crispus may die, nutrients return to the water body, which is not conducive to the maintenance of clear water in the lake. In addition, many epiphytic gastropods have a significantly lower preference for P. crispus (Wang et al. 2006), resulting in a significantly lower diversity of gastropods in lakes. Therefore, how to appropriately reduce the dominance of P. crispus in temperate lakes (especially in some macrophytic lakes with P. crispus as the dominant species) in spring while increasing the dominance of other perennial submerged macrophytes is the top issue to consider. Because the underwater light level is the main factor that restricts the germination and growth of submerged macrophytes (Scheffer 1998; Ni 1999), appropriately increasing the lake level and reducing the underwater light level during the germination period of P. crispus can inhibit the germination and growth of P. crispus. In addition, the lake level should be appropriately lowered during the germination period of other submerged macrophytes in spring to promote early establishment of a dominant position for submerged macrophytes with complex structures that gastropods prefer, such as M. spicatum and S. pectinatus.
-
2)
The optimal coverage of submerged macrophytes in temperate lakes is approximately 50% (Zhang et al. 2021). Therefore, keeping the BMac in macrophytic lakes and algal lakes at an appropriate level is also important. Another study on temperate lakes showed that the feeding strength of macrozoobenthos on epiphytic algae on the leaf surface of submerged macrophytes is one of the main driving factors for the shift between macrophytic lakes and algal lakes (Jones and Sayer 2003). Because there is a significant trophic cascade effect between aquatic organisms of different trophic levels in lake ecosystems, the recovery of submerged vegetation in lakes with severe degradation of submerged macrophytes should be promoted by removing fishes that feed on gastropods and releasing small epiphytic gastropods, in addition to reducing the nutrient load of the water body and improving the water transparency. In contrast, the control of submerged vegetation in lakes with overgrowth of submerged macrophytes can be promoted by appropriately increasing the number of fishes that feed on gastropods, in addition to removing submerged macrophytes and increasing the number of herbivorous fishes, to reduce the number of epiphytic gastropods and control the growth of submerged macrophytes.
References
Blindow I (1992) Long- and short-term dynamics of submerged macrophytes in two shallow eutrophic lakes. Freshw Biol 28:15–27. https://doi.org/10.1111/j.1365-2427.1992.tb00558.x
Chambers PA, Lacoul P, Murphy KJ, Thomaz SM (2008) Global diversity of aquatic macrophytes in freshwater. Hydrobiologia 595:9–26. https://doi.org/10.1007/s10750-007-9154-6
Chen L, Zhang Y, Liu Q, Hu Z, Chen L (2015) Spatial variations of macrozoobenthos and sediment nutrients in Lake Yangcheng: emphasis on effect of pen culture of Chinese mitten crab. J Environ Sci 37:118–129. https://doi.org/10.1016/j.jes.2015.06.008
Diao XJ, Huang CH, He LS, Meng R, Yuan DH (2013) Difference in community structure of submerged macrophytes and related influence factors between macrophytic and algal regions of Baiyangdian lake. Wetl Sci 11:366–371
Gong Z, Xie P, Wang S (2000) Macrozoobenthos in 2 shallow, mesotrophic Chinese Lakes with contrasting sources of primary production. J N Am Benthol Soc 19:709–724. https://doi.org/10.2307/1468128
Hansen JP, Wikström SA, Axemar H, Kautsky L (2011) Distribution differences and active habitat choices of invertebrates between macrophytes of different morphological complexity. Aquat Ecol 45:11–22. https://doi.org/10.1007/s10452-010-9319-7
Hilt S, Brothers S, Jeppesen E, Veraart AJ, Kosten S (2017) Translating regime shifts in shallow lakes into changes in ecosystem functions and services. BioScience 67:928–936. https://doi.org/10.1093/biosci/bix106
Jeppesen E, Sondergaard M, Sondergaard M, Christoffersen KE (1998) The structuring role of submerged macrophytes in lakes. Springer – Verlag, New York
Jiang PH, Liang XM, Chen F, Zhou YY, Wang HZ (2006) Indication of macrophyte-restorable area by spatial pattern of macrobenthos in Yuehu Lake. Resour Environ Yangtze Basin 15:502–505
Jones JI, Sayer CD (2003) Does the fish–invertebrate–periphyton cascade precipitate plant loss in shallow lakes? Ecology 84:2155–2167. https://doi.org/10.1890/02-0422
Koller-Peroutka M, Lendl T, Watzka M, Adlassnig W (2015) Capture of algae promotes growth and propagation in aquatic Utricularia. Ann Bot 115:227–236. https://doi.org/10.1093/aob/mcu236
Li W, Cheng Y (1999) Quantitative analysis on the main submerged communities in Honghu Lake. 1. Potamogeton maackianus community. Acta Hydrobiol Sin 23:53–58
Li KY, Liu ZW, Hu YH, Yang HW (2009) Snail herbivory on submerged macrophytes and nutrient release: implications for macrophyte management. Ecol Eng 35:1664–1667. https://doi.org/10.1016/j.ecoleng.2008.05.009
Liang YL, Wang HZ (1999) Zoobenhos. In: Liu JK (ed) Advanced Hydrobiology. Science Press, Beijing, pp 241–259
Liang Y, Wu T, Xie Z (1995) On the current conditions of zoobenthos in Baoan Lake with an assessment of its potential fishery production capacity. In: Liang YL, Liu HQ (eds) Resources, Environment and Fishery Ecological Management of Macrophytic Lakes. Science Press, Beijing
Liu X, Chen K, Chen Q, Wang M, Wang L (2016) The community structure of macroinvertebrate and its relationship to the environmental factors in summer and autumn within typical reaches of Huai River Basin. Acta Sci Circumst 36:1928–1938. https://doi.org/10.13671/j.hjkxxb.2015.0575
Mandaville SM (2002) Benthic macroinvertebrates in freshwaters-taxa tolerance values, metrics, and protocols. Soil and Water Conservation Society of Metro Halifax, Nova Scotia
Ministry of Ecology and Environment of China (2020) Technical guidelines for monitoring and evaluating aquatic ecological of the lakes and the reservoirs (draft for comments). Ministry of Ecology and Environment of China, Beijing
Newman RM (1991) Herbivory and detritivory on freshwater macrophytes by invertebrates: a review. J N Am Benthol Soc 10:89–114. https://doi.org/10.2307/1467571
Ni LY (1999) Aquatic macrophytes. In: Liu JK (ed) Advanced Hydrobiology. Science Press, Beijing, pp 224–240
Pan B, Wang H, Wang H, Wang Z (2011) Macrozoobenthic assemblages in relation to environments of the Yangtze-isolated Lakes. Front Environ Sci Eng 6:246–254. https://doi.org/10.1007/s11783-011-0381-8
Pan BZ, Wang HJ, Liang XM, Wang HZ (2012) Macrozoobenthos in Yangtze floodplain lakes: patterns of density, biomass, and production in relation to river connectivity. J N Am Benthol Soc 30:589–602. https://doi.org/10.1899/10-025.1
Qin S, Cui JS, Ju ZJ, Shen LN, Zhang LL, Fu Y (2021) Changes of benthic invertebrate community in the Baiyangdian Lake and analysis of main environmental factors under the condition of human disturbance. Acta Sci Circumst 41:1123–1133
Scheffer M (1998) Ecology of shallow lakes. Kluwer Academic Publishers, London
Scheffer M, Jeppesen E (2007) Regime shifts in shallow lakes. Ecosystems 10:1–3. https://doi.org/10.1007/s10021-006-9002-y
Tan SY, Li Z, Cheng S (2017) Ecological interaction between submerged macrophytes and zoobenthos. SDRP J Earth Sci Environ Stud 2:1–10. https://doi.org/10.25177/jeses.2.2.2
Underwood GJC, Thomas JD, Baker JH (1992) An experimental investigation of interactions in snail-macrophyte-epiphyte systems. Oecologia 91:587–595. https://doi.org/10.1007/bf00650335
Van den Berg MS, Scheffer M, Van Nes E, Coops H (1999) Dynamics and stability of Chara sp. and Potamogeton pectinatus in a shallow lake changing in eutrophication level. Hydrobiologia 408:335–342. https://doi.org/10.1023/A:1017074211970
Wang HJ (2007) Predictive limnological studies on small to medium sized lakes along the mid-lower Yangtze River. [doctoral dissertation]. Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan
Wang SM, Dou HS (1998) Lakes of China. Science Press, Beijing
Wang HJ, Pan BZ, Liang XM, Wang HZ (2006) Gastropods on submersed macrophytes in Yangtze Lakes: community characteristics and empirical modelling. Int Rev Hydrobiol 91:521–538. https://doi.org/10.1002/iroh.200510846
Wang J, Song Y, Wang G (2016) Causes of large Potamogeton crispus L. population increase in Xuanwu Lake. Environ Sci Pollut Res 24:5144–5151. https://doi.org/10.1007/s11356-016-6514-7
Wang HZ, Wang HJ, Liang XM, Ni LY, Liu XQ, Cui YD (2005) Empirical modelling of submersed macrophytes in Yangtze Lakes. Ecol Model 188:483–491. https://doi.org/10.1016/j.ecolmodel.2005.02.006
Wang D, Men B, Zhang M (2020) Sediment water ecotone and sediment removal of shallow lake: a case study of Baiyangdian Lake in Xiong’an new area. Acta Sci Circumst 40:1550–1559
Xie S, Huang BS, Wang HW, Song CY, Shi BZ (2010) Assessment of water quality in Baiyangdian Lake by zoobenthos biodiversity. J Hydroecol 3:43–48
Xu M, Zhu J, Huang Y, Gao Y, Wang ZJ (1998) The ecological degradation and restoration of Baiyangdian Lake, China. J Freshw Ecol 13:433–446. https://doi.org/10.1080/02705060.1998.9663640
Yang W, Yan J, Wang Y, Zhang BT, Wang H (2020) Seasonal variation of aquatic macrophytes and its relationship with environmental factors in Baiyangdian Lake, China. Sci Total Environ 708:135112. https://doi.org/10.1016/j.scitotenv.2019.135112
Zhang Y, Jeppesen E, Liu X, Qin B, Shi K, Zhou Y, Thomaz SM, Deng J (2017) Global loss of aquatic vegetation in Lakes. Earth Sci Rev 173:259–265. https://doi.org/10.1016/j.earscirev.2017.08.013
Zhang Y, Cheng L, Tolonen KE, Yin H, Gao J, Zhang Z, Li K, Cai Y (2018) Substrate degradation and nutrient enrichment structuring macroinvertebrate assemblages in agriculturally dominated Lake Chaohu Basins, China. Sci Total Environ 627:57–66. https://doi.org/10.1016/j.scitotenv.2018.01.232
Zhang XK, Liu X, Yang ZD, Wang HZ (2019) Restoration of aquatic plants after extreme flooding and drought: a case study from Poyang Lake National nature reserve. Appl Ecol. Environ Res 17:15657–15668. https://doi.org/10.15666/aeer/1706_1565715668
Zhang X, Zhang J, Li Z, Wang G, Xie J (2021) Optimal submerged macrophyte coverage for improving water quality in a temperate lake in China. Ecol Eng 162:106177. https://doi.org/10.1016/j.ecoleng.2021.106177
Acknowledgements
We would like to thank Heyin Wang and Xiao Wang of Anqing Normal University for their help in the field survey.
Funding
This study was supported by the National Key R&D Program of China (2019YFD0900604), the Shanghai Sailing Program (No. 18YF1407500), and the Baiyangdian Aquatic Biological Resources Investigation and Water Ecological Restoration Demonstration Project (2018 LKY007).
Author information
Authors and Affiliations
Contributions
Hao Zhu and Xiaoke Zhang designed the study and performed the experiments. Hao Zhu and Xuan Che performed the experiments. Hao Zhu, Shuiping Cheng, and Xiaoke Zhang prepared the figures and tables. Xiaoke Zhang, Shuiping Cheng, and Xingguo Liu analysed the data, and Hao Zhu wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Thomas Hein
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Rights and permissions
About this article
Cite this article
Zhu, H., Cheng, S., Zhang, X. et al. Responses of macrozoobenthos communities to changes in submerged macrophyte biomass in 19 temperate lakes in China. Environ Sci Pollut Res 29, 59211–59223 (2022). https://doi.org/10.1007/s11356-022-20007-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20007-5