Abstract
We examined how performance of Operophtera brumata (Lepidoptera) larvae was affected by nitrogen (N) fertilization of boreal forest understorey vegetation. We monitored larval densities on Vaccinium myrtillus plants for a period of 7 years in a field experiment. Preliminary results indicated that the N effect on larval densities was weak. To examine if this was due to indirect interactions with a plant pathogen, Valdensia heterodoxa, that share the same host plant, or due to top-down effects of predation, we performed both a laboratory feeding experiment (individual level) and a bird exclusion experiment (population level) in the field. At the individual level, altered food plant quality (changes in plant concentration of carbon, N, phenolics, or condensed tannins) due to repeated infection by the pathogen had no effect on larval performance, but both survival to the adult stage and adult weight were positively affected by N fertilization. Exclusion of insectivorous birds increased the frequency of larval damage on V. myrtillus shoots, indicating higher larval densities. This effect was stronger in fertilized than in unfertilized plots, indicating higher bird predation in fertilized plots. Predation may thus explain the lack of fertilization effect on larval densities in the field experiment. Our results suggest that top-down effects are more important for larval densities than bottom-up effects, and that bird predation may play an important role in population regulation of O. brumata in boreal forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growth rate, fecundity, and survival of insect herbivores may be limited by the quality of the food plants. In general, high food quality is equal to high nitrogen (N) concentration and low concentrations of carbon-based secondary metabolites (CBSMs) (Mattson 1980; White 1993; Scriber and Slansky 1981; Kause et al. 1999). Nitrogen fertilization of host plants may profoundly affect these components and the effect of fertilization is expected to be strongest in inherently N-poor environments (Chapin 1980; Tamm 1991). In such environments, fertilization of host plants may result in increased growth rate, higher potential fecundity, and shorter development time of insects (Mattson 1980; Scriber and Slansky 1981; White 1993), and ultimately in increased abundance of insects (Ritchie and Tilman 1993). However, the positive effects of N addition on individual insects may not necessarily result in increased densities or larger population sizes (Kytö et al. 1996; Sipura 1999; Ritchie 2000) as such effects may be overridden by either other abiotic factors (Hunter and Price 1998; Ritchie 2000) or by biotic factors, such as predation (Fowler and Lawton 1985; Kytö et al. 1996).
If the density of herbivores is influenced by strong top-down regulation from predation, the effect of the predator may also indirectly influence the plant community through what is known as trophical cascades (Paine 1980). Such trophical cascades may, at least theoretically, explain why herbivore densities may remain unchanged although the quality of their food plants has increased, e.g., due to fertilization (Oksanen et al. 1981; Oksanen and Oksanen 2000). Trophical cascades may influence the total biomass production of an entire trophical level (community-wide cascade) or only influence one or a few species at a trophical level (species-level cascade) (Polis 1999). The relative importance of trophical cascades in terrestrial systems has been intensively debated in ecological literature (e.g., Power 1992; Strong 1992; Oksanen and Oksanen 2000; Schmitz et al. 2000), and although species-level cascades seem to be fairly common in terrestrial systems (Schmitz et al. 2000), evidence for community-wide cascades is so far sparse or ambiguous (Persson 1999; Polis et al. 2000).
Another type of biotic interaction that may influence insect performance, and thereby insect densities, is indirect interactions with plant pathogens. Indirect interactions between plant pathogens and insect herbivores that temporally and spatially share the same host plant species or individuals are likely to be common, but have frequently been overlooked in ecological studies (see Hatcher 1995; Moran 1998 for overview). Several studies have shown that severity of fungal disease may increase following N fertilization of the host plants (Solomon and Oliver 2001 and references therein; Strengbom et al. 2002). Nitrogen fertilization often results in decrease of defensive carbon-based secondary metabolites (CBSMs), such as phenolics, which may increase the plant quality to pathogens, as well as to herbivores (see Witzell and Shevtsova 2004 and references therein). However, disease by plant pathogens may also induce increased production or accumulation of these compounds in plants (Hammond and Hardy 1988; Moran 1998), which could reduce the insect performance (Hatcher et al. 1995; Lappalainen et al. 1995). Hence, it may be hypothesized that plant pathogens can influence insects negatively by changed chemical composition of the shared host plant (i.e., bottom-up effect), and thereby offsetting any positive effects on the insects from N fertilization. However, such plant-mediated interactions may be complex and are not always readily explained by predictable bottom-up effects. For instance, Nordin et al. (1998) found that N addition to a boreal forest both increased disease incidence by the parasitic fungus Valdensia heterodoxa Peyronel and increased damage by Lepidoptera larvae on the common dwarf shrub, Vaccinium myrtillus L. This may seem to contradict the hypothesis that plant pathogens should negatively influence insect performance by plant-mediated changes. However, as the results reported by Nordin et al. (1998) represent short-term responses (1 year), the stress effect on the plants from increased disease may have been too small to cause any major effect on larval performance.
In the present study, we examined the effect of N fertilization on the performance of Operophtera spp. (Lepidoptera) larvae on V. myrtillus, in a boreal forest of northern Sweden. We monitored larval densities for 7 years (from 1996 to 2002). Intriguingly, preliminary data indicated that the response of larvae densities to increased N input was either weak or absent. This may seem to contradict the general conception of increased performance following N fertilization as well as the short-term responses (1 year) reported by Nordin et al. (1998). However, at the site of the present experiment, fertilization has, compared with the study by Nordin et al. (1998), caused a stronger effect on the pathogen with repeated disease occurrence over several years (Strengbom et al. 2002). This raised the question if the lack of N effect on larval densities could be derived from chemical changes in the plant induced by repeated severe disease by the parasitic fungus. If indirect interactions with the parasitic fungus are important, negative effects induced by the pathogen may override any positive effects of fertilization on larval performance at the individual level and explain the weak response to fertilization under field conditions. To study this, we performed a feeding experiment, in which we provided larvae with V. myrtillus that had been repeatedly diseased and/or fertilized for seven consecutive years.
A lack of N effect on larval densities can also be derived from top-down effects. For instance, if fertilization results in higher densities of predators or more intense foraging by predators in fertilized areas (behavioral response), any positive effects of fertilization at the individual larval level may be overridden by increased predation pressure, resulting in no or only a weak response on insect densities at the population level. To elucidate this aspect, we performed a bird exclusion experiment to examine if bird predation had the potential to explain the lack of N effect on larval densities under field conditions.
Materials and methods
Study site
We conducted the field part of the study within the Svartberget Experimental Forest in northern Sweden (64°14′N, 19°46′E), 70 km NW of Umeå. The area is located in the middle boreal zone (Ahti et al. 1968). The experimental site is a late successional Norway spruce (Picea abies) forest, where the ericaceous dwarf shrub Vaccinium myrtillus dominates the understorey vegetation (for more detailed description of the field site see Strengbom et al. 2002). In 1995, we established a total of eighteen 1,000 m2 plots at this site. The shortest distance between two plots was 25 m. We randomly assigned the plots to three different N treatments: 0 (control), 12.5 (N1) or 50 (N2) kg N ha−1 year−1 (n=6 for each treatment). During the period 1996–2002, we fertilized the plots once a year using granulated NH4NO3, which was evenly distributed over the plots at the onset of each growth period, i.e., end of May or beginning of June.
Studied species
Operophtera brumata L. (Lepidoptera: Geometridae) is a polyphagous moth with one generation per year. In northern Sweden, eggs hatch in late May or early June and larvae feed until mid-July, when they drop to the ground and form a pupa. The larvae go through five instars before they pupate. Adult moths start emerging in late September to mid-October, when males locate the wingless females and mating takes place. After mating and egg laying, both females and males die and the species survives the winter as eggs. During its larval stage, O. brumata defoliates a wide range of plant species, including V. myrtillus (Kerslake et al. 1996; Tikkanen et al. 1999). In our study area, V. myrtillus is the main host plant of O. brumata, as well as for the closely related O. fagata Scharfenberg, which in the study area has the same biology as O. brumata.
The parasitic fungus Valdensia heterodoxa is a generalist pathogen commonly found on Vaccinium myrtillus. Its sexual stage is referred to as Valdesinia heterodoxa (Ascomycota, Sclerotiniaceae) (Eriksson 1974). The fungus overwinters as sclerotia in the veins of Vaccinium myrtillus leaves that have been infected and shed during the previous summer (Norvell and Redhead 1994). In early summer, ascospores of the fungus infect new V. myrtillus leaves. Conidia are produced on leaves during summer and this phase of infection is seen as a brown spot disease. If severe, it may result in premature leaf loss and the appearance of visible defoliated patches in the V. myrtillus cover (Strengbom et al. 2002). In the autumn of 1996, we marked all patches of V. myrtillus showing severe disease symptoms, as well as patches showing no or only a low level of disease symptoms (refereed to as nondiseased V. myrtillus) at the study site. The prevalence of disease on these patches remained more or less stable during the whole experimental period.
Larval density
In mid-June of each year, 1996–2002, we scored the density of Operopthera larvae within five permanently marked, circular, 0.1 m2 subplots in each of the 1,000 m2 plots. Data from the five subplots were used as an estimate of the larval density for each plot. Because the larval density was low during several of the years investigated, we pooled the data for the two closely related Operophtera species (O. brumata and O. fagata). Since the ecology of these two species is very similar at our experimental site, we do not expect this to introduce any marked variance to the data. Although the plot size used in this study is limited, we consider that data from the 1,000 m2 plots provide a reasonable estimate of the larval responses at the population level.
Feeding experiment
During the summer of 2002 we performed a feeding experiment with O. brumata, the more abundant of the two Operophtera species. In early June, we collected 120 larvae (4th instar) by sweep netting at a small, nonfertilized forest site with the same characteristics as the experimental site. After collection we placed the larvae one by one in plastic boxes (67×100×100 mm). A damped piece of paper was also added to the boxes in order to increase the humidity. To prevent larvae from escaping, we covered the boxes with a mesh net. To mimic the summer light conditions at high latitudes, the boxes were then kept in a room (18°C, 78% relative humidity) with constant light.
We provided larvae with V. myrtillus shoots (current annual shoots, hereafter referred to as shoots) that were collected from the field experiment. We used plant material from repeatedly severely diseased and from nondiseased V. myrtillus from controls and plots receiving 50 kg N ha−1 year−1 from the fertilization experiment (see above). At this time of the year, early June, the plant material that we provided to the larvae showed no visual signs of disease. This implies that we tested the indirect effect on food quality, i.e., chemical changes in C and N pools induced by repeated disease, rather than the direct effect of visible leaf lesions. This also corresponds to the situation in the field. The first lesions of Valdensia usually appear in late June, when the first larvae start to pupate. Hence, the possibility for direct interactions between the fungus and larvae is small under natural conditions. Each larva was provided with one of four food types: unfertilized or fertilized V. myrtillus that either had been repeatedly and heavily diseased by V. heterodoxa or had experienced only low level or no disease at all (30 larvae per food type). Before the start of the experiment, we weighed all larvae (hereafter referred to as start weight). Each day, we replaced the plant material in the boxes with fresh V. myrtillus shoots. At the same time, we also checked the status of the larvae. We removed all larvae that showed signs of parasitoid infestations (in total 34% of the larvae). Plant material provided to the larvae was stored in a fully illuminated room at 15°C and the cut ends of the shoots were kept in water to prevent desiccation. Every third day we brought new plant material from the experimental site. We provided the larvae with new shoots until pupal formation was completed. Average time to pupation was 7 days and all larvae had formed pupae after 11 days.
After pupation, the pupae were left in the boxes under the same conditions for 88 additional days. Thereafter, we gradually reduced the temperature and illumination to 10°C and 10 h a day, respectively. The pupae were kept under these conditions and regularly checked until metamorphosis was completed. The first adult emerged 127 days after the first pupa was formed. After emergence, we collected the adults for determination of sex and body mass. The adult biomass was measured as dry weight (40°C for 24 h). We terminated the experiment when 7 days had passed without any new adults emerging (172 days after the start of the experiment). The pupae remaining at this time were considered as dead. Of the 120 larvae at the start of the experiment, 45% survived to the adult stage. When survival probability was calculated, individuals infested with parasitoids were excluded.
To estimate the effect of food type on fecundity, and thereby its potential effect on population dynamics, we used the known relationship between adult female body mass and egg production established for O. brumata by Holliday (1977). The equation y=0.954x−67.88 (g×10−4) describes the relationship between adult fresh weight and fecundity. As we only had adult dry mass, we could not use this relationship to calculate the exact fecundity, but the relationship can still be used to obtain an estimate of the relative effect size between the food types in our experiment. By using dry weights, we might introduce some unexpected variation, as the insects might have differed in fresh weight. However, this difference is likely to reflect differences in water content, which should have only a minor effect on fecundity compared to, for example, fat and protein content.
Chemical analysis
Plant material collected from the feeding experiment was analyzed for concentrations of total N and C, and for individual phenolic metabolites and condensed tannins. For the analyses, three current annual shoots per treatment combination (n=4) were dried (35°C for 72 h) and pulverized using a ball mill. Individual phenolics were analyzed using HPLC (modified from Witzell et al. 2003). Vanillin-HCl assay (Sun et al. 1998) was used to estimate the levels of condensed tannins. Carbon and N concentrations were analyzed by an elemental analyzer (2400 CHN Analyzer, Perkin Elmer, Norwalk, Conn.).
Bird exclusions
The most important insectivorous birds at the experimental site are probably chicks of Capercaillie (Tetrao urogallus) and Hazel grouse (Bonasa bonasia). In addition, passerine birds, such as Robin (Erithacus rubecula), Common redstart (Phoenicurus phoenicurus), Song thrush (Turdus philomelos), Redwing (Turdus iliacus), Pied flycatcher (Ficedula hypoleuca), and Chaffinch (Fringilla coelebs) are frequent foragers in the shrub layer (J. Strengbom, personal observations). In mid-June 1997, we built 24 bird exclosures with a basal size of 2×2 m and 1 m in height at the field site. The exclosures were constructed of mink net with a gap size of 11 mm. The construction allowed free access for voles and shrews, but prevented birdsaccess to the V. myrtillus dwarf shrubs growing inside. We used a classical split-plot design for this experiment. Half of the exclosures were placed in plots that received an annual dose of 50 kg N ha−1 year−1. Six of these fertilized plots were the same as those used for larval density estimates (see above). The other six exclosures were placed in plots sized 5,000 m2 that were fertilized with the same annual N dose and located at the same forest site. The remaining 12 exclosures were placed in nonfertilized control plots (not the same controls as those where we measured larval density). We scored the proportion of larvae-damaged shoots inside the exclosures and in permanently marked plots adjacent to the exclosures once a year during the 3 years from 1998 to 2000. The scoring was done by randomly choosing 100 current annual shoots of V. myrtillus and classifying them as either damaged or not damaged. All shoots that showed any signs of herbivory typical for Operophtera larvae (i.e., folivory or feeding damage on young shoots) were classified as damaged.
Statistical analysis
To test for differences in Operophtera larvae density between fertilization treatments in the field experiment, we used repeated-measure ANOVA. In order to obtain an estimate of larval density for each plot, we pooled data from the five subplots within each treatment plot. Analysis was performed on log (x+1) transformed data (Zar 1996). In order to test if larval herbivory was affected by fertilization and exclusion of birds, we used a repeated-measure ANOVA, where proportion of damaged V. myrtillus shoots (arcsin transformed) was used as response variable, and fertilization, exclosure and year as factors. Fertilization was treated as a between-plot effect, and exclosure and exclosure × fertilization were treated as within-plot effects. To test if there was a relationship between larval damage and larval density, we performed a linear regression between larval density [log (x+1) transformed] and the proportion of damaged shoots outside the exclosures (arcsin transformed). We performed this test only for the high N treatment (N2), as no data was available for the other treatments (see above). Differences between treatments in time to pupa formation and adult weight were tested with ANCOVA, where fertilization, disease by V. heterodoxa and sex of the larvae were used as factors. Since there was no significant difference in starting weight between the groups (one-way ANOVA, P=0.49), we used starting weight as a covariate. To test if the survival (proportion of adults that emerged from the pupae) differed between treatments, we used chi-square tests. The frequency of emerging adults within each treatment group was compared with the expected frequency. Differences in C/N ratio, concentration of phenolic acids and condensed tannins in the V. myrtillus shoots between treatments were tested with a two-way ANOVA, where fertilization and disease by the fungus were used as factors. Since a large number of tests were performed (one test per individual compound), we adjusted the P value with a sequential Bonferroni correction (Rice 1989). All statistical tests were performed with the SPSS for Windows software package (release 11.0.1 15 November 2001).
Results
Larval density in the field (population level)
Over the experimental period, the effect of N fertilization on Operophtera larval densities was weak or absent (Fig. 1). During the first years of the experiment the year-to-year variation in larval density was small, but after the 4th year there was a steep increase in larval density in all treatments. Although the density of larvae was higher on average in the N2 treatment than in the controls in all of the 7 years studied, we detected no significant effect of fertilization on larval density in the field (ANOVA: Year×N: F12, 90=0.917, P=0.553; N: F2,15=2.084, P=0.159), indicating that N fertilization had no or only a weak effect on larval densities.
Feeding experiments (individual insect level)
In the feeding experiment, fertilization was found to have a positive effect on larval performance, while repeated disease from the parasitic fungus V. heterodoxa on the shared host plant V. myrtillus had no effect. Larval survival was significantly influenced by food type (χ2=25.83, df=3, P<0.001). Fertilization (χ2=24.51, df=1, P<0.001), but not repeated disease by the parasitic fungus (χ2=0.96, df=1, P=0.33) had a significant effect on survival to the adult stage. The probability of reaching the adult stage was lowest (10/19=0.53) for the larvae-provided unfertilized V. myrtillus plants, and highest (17/19=0.89) for larvae-provided fertilized plants. For the larvae feeding on unfertilized diseased plants the survival probability was 0.65 (17/26), and 0.71 (10/14) for those feeding on diseased and fertilized plants.
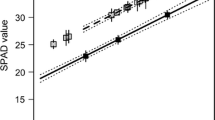
We found no main effect of either fertilization (ANCOVA: F1, 45=0.394, P=0.553) or disease (F1, 45=0.399, P=0.531) on the time to pupation (Fig. 2). However, females had shorter development time when reared on fertilized V. myrtillus, while the pattern was the opposite for males, i.e., there was an interaction between fertilization and sex (F1, 45=6.204, P=0.017) for time to pupation. Larvae reared on fertilized plant material had a larger adult body mass than those reared on shoots of unfertilized plants (F1, 45=7.855, P=0.007), while disease had no effect on the adult body mass (F1, 45=0.585, P=0.448). This shows that fertilization had a positive effect on larval performance, but repeated disease by the parasitic fungus did not influence the performance (Fig. 2). Females were on average larger than males, but there was no interaction between sex and food type (Sex × N: F1, 45=0.635, P=0.430; Sex × disease: F1, 45=0.002, P=0.967), which shows that the dietary response did not differ between females and males.
Performance of Operophtera brumata larvae reared on Vaccinium myrtillus. Larvae were provided with fertilized or unfertilized V. myrtillus shoots that either had been heavily diseased for several years or had a history of no or only moderate disease (nondiseased) by the parasitic fungus Valdensia heterodoxa. a and b show response on time to pupation for males and females, respectively. c and d show response on adult hatch weight for males and females, respectively. Error bars represent SE
To estimate the effect of fertilization on insect fecundity, and thereby its potential effect on population density, we used the relationship between adult female body mass and egg production established for O. brumata by Holliday (1977) (see Materials and methods). The difference in adult body mass between females reared on fertilized and unfertilized V. myrtillus (Fig. 2) would, according to this relationship, correspond to on average 6.9 times higher egg production for females reared on fertilized plants. If the survival probabilities for fertilized and unfertilized food plants are taken into account (survival probability × calculated fecundity), females reared on fertilized V. myrtillus would on average have 11.7 times higher fecundity than females reared on unfertilized V. myrtillus. These estimates suggest that fertilization may have a positive influence on larval performance at the individual insect level.
Vaccinium myrtillus chemistry
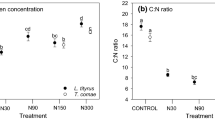
A lower C/N ratio, mainly due to a higher N concentration (Table 1), was found both in fertilized (ANOVA: F1, 12=13.58, P=0.003) and annually diseased plants (F1, 12=15.27, P=0.002). We analyzed several individual phenolic acids and flavonoids, as well as condensed tannins, but found that V. myrtillus secondary metabolic response to fertilization or disease was generally weak (for full ANOVA table see Electronic Supplementary Material S1). Fertilization (F1, 12=3.93, P=0.071, n.s. after Bonferroni correction) and disease (F1, 12=5.48, P=0.037, n.s. after Bonferroni correction), individually, tended to increase concentration of condensed tannins, while the combined effect (N × disease: F1, 12=11.84, P=0.005) resulted in lower concentration (Table 1). The opposite pattern was found for an individual flavonoid compound (tentatively identified as kaempferol-3-glucoside). The concentration of this compound tended to decrease in plants that were either fertilized or diseased, but increased when the two were combined (N × disease: F1, 12=15.45, P=0.002; Table 1). In fertilized or diseased plants the sum of HPLC phenolics as well as the levels of most of the individual phenolics tended to be lower than in control plants (Table 1). However, after Bonferroni correction none of these effects were significant (S1).
Bird exclusion
In the bird exclusion experiment, we found a higher frequency of larval-damaged V. myrtillus shoots inside the exclosures than outside (ANOVA: F1, 22=128.33, P< 0.001). The proportion of damaged shoots showed some temporal variation during the 3-year monitoring period, but the damage was always higher inside than outside the exclosures (Fig. 3). On average, damage was 41% higher when birds were excluded compared to when birds had access to the dwarf shrubs. The effect of bird exclusion on fertilized plots was larger than on unfertilized plots, i.e., there was an interaction between exclosure and fertilization (F1, 22=4.04, P=0.057). On average over the 3 years, the damage levels inside bird exclosures were 22% higher on fertilized plots than on unfertilized ones (Fig. 3). The effect of exclosure did not change over time, i.e., there was no interaction between year and exclosure (F2, 44=2.72, P=0.077). This indicates that the effect of reduced predation was not transferred from 1 year to the next. We also found a positive relationship between larval density [log (x+1) transformed] and larval damage on V. myrtillus (arcsin transformed) (y=4.44x−0.76, R2=0.61, P<0.001), indicating that larval damage can be used as an estimate of larval density. Due to the experimental setup this relationship was derived only from N2 plots, which may introduce some error to the estimated larval densities.
Discussion
Our study shows that N fertilization had a positive effect on the performance of O. brumata larvae at the individual level: the feeding experiment showed that larvae reared on fertilized V. myrtillus had both higher survival probability and larger adult body mass than larvae reared on unfertilized V. myrtillus. Since fertilization resulted in an increased N concentration of V. myrtillus, our results are in accordance with the general concept of improved insect performance when plant N concentration increases (Mattson 1980; White 1993). Increased weight or body size of insects may contribute to increased population size, as larger individuals (especially females) tend to have a higher potential fecundity (Scriber and Slansky 1981; Leather 1988). Based on the observed differences in survival probability and adult body mass among females reared on fertilized and unfertilized V. myrtillus, we estimated that females feeding on fertilized V. myrtillus should have more than 11 times higher potential fecundity compared to females feeding on unfertilized V. myrtillus. Despite this, we could not detect higher larvae densities at the population level in the field experiment. Over the 7-year period that we monitored larval densities in the forest fertilization experiment, the response on larval densities from increased N input was weak or absent. This weak response to fertilization at the population level supports the view that translation of effects from the individual level to the population level may be complicated (Leather 1988; Larsson 1989; Kytö et al. 1996), and that positive effects on the individual level may be overridden by other abiotic or biotic factors.
In accordance with other studies (Holmes et al. 1979; Atlegrim 1989; Marquis and Whelan 1994; Sipura 1999), we found larval damage to increase following exclusion of insectivorous birds. On average, bird predation reduced larval damage by more than 40%. Although extrapolation of the damage frequencies into larval densities by using the relationship that we established may introduce some uncertainties (R2=0.61), our results suggest that the top-down effects of bird predation may be an important factor for regulating larval densities in boreal forests, and further that this effect may be large enough to explain the lack of N response in the field experiment. The interaction between fertilization and bird exclusion suggests that the importance of predation increases with increasing fertility, and is in accordance with theoretical models suggesting that the top-down effect should increase with increasing productivity (Oksanen et al. 1981; Oksanen and Oksanen 2000) as well as with some empirical data (Stiling and Rossi 1997; Fraser and Grime 1998).
For several reasons we may have underestimated the top-down effects in our system. The reduced predation rates from birds did not carry over from 1 year to the next, probably a consequence of the small size of the exclosures (4 m2), which implies that we may have underestimated the effect size on larval damage. Moreover, we may also have underestimated the top-down effect on the insects as we only examined the potential role of one predator. Considering that ants (Baylis and Pierce 1991; Kytö et al. 1996) or parasitoids (Häggström and Larsson 1995; Parry et al. 1998) are known to be important predators on larvae, and assuming that their effect on larvae is similar to what was observed for birds, the overall top-down effect on larvae may be substantially larger than indicated by this study.
It is difficult to interpret if the magnitude of larval damage observed in the present study is large enough to have any major impact on plant community biomass, i.e., cause a sensu Polis (1999) community-wide trophical cascade. In a review of terrestrial trophical cascades, Schmitz et al. (2000) concluded that species-level cascades are likely to be common in terrestrial systems, but caution should be taken before changes in damage frequencies are interpreted as a community-wide effect on plants. However, reduced damage from leaf-chewing Lepidoptera larvae has previously been found to increase tree growth (Marquis and Whelan 1994) and as pointed out, e.g., by Holt (2000), even small effects that may seem insignificant from the short-term perspective may have a marked impact on plant community structure from a longer-term perspective, which may be especially important for long-lived clonal plants, such as V. myrtillus. Since V. myrtillus is by far the most common plant species in the understorey plant community of mesic boreal forests, any changes that influence the abundance or biomass of this species may also have a substantial effect on the plant community as a whole. However, to determine the long-term impact on the vegetation, long-term studies of the vegetation following reduced damage are necessary.
Community-wide trophical cascades seem to be more common in aquatic or marine ecosystems than in terrestrial systems (Strong 1992; Persson 1999). Consequently, the most unambiguous examples of strong top-down regulation (planktivorous fish) that prevent increased densities of herbivores (zooplankton) following nutrient input have been found in aquatic systems (e.g., Carpenter et al. 2001 and references therein). Some studies on terrestrial systems have, in accordance with our findings, found that bird predation may limit herbivore densities and reduce damage in plants (Atlegrim 1989; Marquis and Whelan 1994; Sipura 1999); however, others have found that, although top-down effects may be present, the effect may attenuate at lower trophical levels, resulting in no or only weak effects on plants (Forkner and Hunter 2000; Gruner 2004). Lower heterogeneity and higher uniformity have been suggested as an explanation as to why trophical cascades should be more common in aquatic than in terrestrial systems (e.g., Persson 1999; Polis et al. 2000), and the level of species heterogeneity within and between trophical levels may explain the differences between terrestrial studies as well. Terrestrial systems where top-down effects cause trophical cascades have been found tend to come from “simple” systems, with few or uniform plant and herbivore species, while studies that have found weak effects tend to come from more complex systems (Polis et al. 2000). Thus, strong top-down effects and trophical cascades may be more likely in terrestrial systems that in these aspects resemble aquatic systems. Our study site may represent a relatively simple and uniform system with a few dominant species, which may explain why top-down effects appeared to be strong in the present study.
The incidence of disease by the parasitic fungus Valdensia heterodoxa has more than doubled on the plots receiving the high N dose at our experimental site. This in turn has resulted in severe premature leaf shedding in Vaccinium myrtillus during consecutive years, apparently causing marked stress on the plants, which could be expected to induce alterations in plant metabolism, e.g., in phenolic pool (Dixon and Paiva 1995). Because insect performance may be affected by changed plant quality, indirect interactions between V. heterodoxa and Operophtera larvae seemed possible. Our results, however, suggest that this parasitic fungus has no indirect effect on larval performance. Although V. heterodoxa may induce local increase in concentrations of some phenolics in V. myrtillus leaves (Witzell and Shevtsova 2004), we found no support for the hypothesis that V. heterodoxa would cause any systemic increase in the concentrations of phenolic compounds that would be strong enough to negatively influence larvae performance.
In general, the chemical status of V. myrtillus changed in response to fertilization and disease somewhat unexpectedly. In addition to the expected increase of N concentration in fertilized plants, N concentration was also elevated in repeatedly diseased plants. The origin of the increased N in diseased plants is not yet known. Similar results have, however, been reported by Solomon and Oliver (2001), who found that concentrations of plant (apoplastic) amino acids increased after infection by an intercellular pathogen Cladosporium fulvum. Moreover, as expected, condensed tannins tended to increase following repeated disease, which could represent a defensive response (cf. Dixon and Paiva 1995). However, the interaction between fertilization and disease seemed to produce stronger effects on phenolics. After Bonferroni correction the only significant effects on phenolics were due to their interaction. Vaccinium myrtillus that had been exposed to the combination of fertilization and repeated disease showed, contrary to the individual effects of the treatments, a drastically decreased concentration of tannins, whereas the concentration of an individual flavonoid increased. These findings suggest that in V. myrtillus, mainly higher molecular weight phenolics responded to treatments. The exact mechanisms behind these unexpected alterations are so far unknown and need further examination.
We also found that the larvae responded to changes in V. myrtillus chemistry in a slightly unexpected manner. The fact that larval performance did not correspond to the observed significant changes in tannins indicates that these compounds were of minor importance for larval performance, contradicting other studies that have found a decrease in performance of O. brumata larvae feeding on oak leaves (Quercus robur) with a high concentration of tannins (Feeny 1970; Tikkanen and Julkunen-Tiitto 2003). However, as we used larvae of the 4th instar, we cannot exclude the possibility that the observed differences in tannin concentrations may influence the performance of earlier instars, as they may be more sensitive to low food quality (Wint 1983; Tikkanen et al. 1999). Nonetheless, our data suggest that if there are plant-mediated interactions between Operophtera larvae and V. heterodoxa, they are not linked to the phenolic compounds of V. myrtillus in a simple manner.
In conclusion, larvae reared on fertilized V. myrtillus under laboratory conditions showed both a higher probability of survival and larger body mass than larvae reared on unfertilized V. myrtillus. However, N fertilization had no or only a weak positive effect on larval densities under field conditions. Thus, some mechanism(s) seemed to decouple the N effects at the individual level from those at the population level. We found no evidence suggesting that annually repeated pathogen disease of the host plant would change its quality as a food source for O. brumata larvae, thereby affecting larval performance and explaining the lack of N effect. However, we found that predation by birds may substantially reduce larvae densities. We suggest that the negative effect of an increased predation rate can explain the difference in response to N fertilization between the individual and the population level and thus also explain the lack of N response observed under field conditions. These findings also suggest that top-down effects may have an important role for population dynamics of Operophtera species in boreal forests.
References
Ahti T, Hämet-Ahti L, Jalas J (1968) Vegetation zones and their sections in Northwestern Europe. Ann Bot Fenn 5:169–211
Atlegrim O (1989) Exclusion of birds from bilberry stands: impact on insect larval and damage to the bilberry. Oecologia 79:136–139
Baylis M, Pierce NE (1991) The effect of host-plant quality on the survival of larvae and oviposition by adults of an ant-tended lyceaid butterfly, Jalmenus evagoras. Ecol Entomol 16:1–9
Carpenter SR, Cole J, Hodgson JR, Kitchell JF, Pace ML, Bade D, Cottingham KL, Essington TE, Houser JN, Schindler DE (2001) Trophic cascades, nutrients, and lake productivity: whole-lake experiments. Ecol Monogr 71:163–186
Chapin III SF (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Eriksson B (1974) On Deuteromycetes on Diapensales and Ericales in Fennoscandia. Sven Bot Tidskr 68:235–253
Feeny P (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding winter moth caterpillars. Ecology 51:565–581
Forkner RE, Hunter MD (2000) What goes up must come down? Nutrient additions and predation pressure on oak herbivores. Ecology 81:1588–1600
Fowler SV, Lawton JH (1985) Rapidly induced defenses and talking trees: the devil’s advocate position. Am Nat 126:181–195
Fraser LH, Grime JP (1998) Top-down control and its effect on the biomass and composition of three grasses ay high and low soil fertility in outdoor microcosmos. Oecologia 113:239–246
Gruner DS (2004) Attenuation of top-down and bottom-up forces in a complex terrestrial community. Ecology 85:3010–3022
Häggström H, Larsson S (1995) Slow larval growth on a suboptimal willow results in high predation mortality in the leaf beetle Galerucella lineola. Oecologia 104:308–315
Hammond AM, Hardy TN (1988) Quality of diseased plants as hosts for insects. In: Heinrichs EA (ed) Plant stress-insect interactions. Wiley, New York, pp 341–382
Hatcher PE (1995) Three-way interactions between plant pathogenic fungi, herbivorous insects and their host plants. Biol Rev 70:639–694
Hatcher PE, Paul ND, Ayers PG, Whittaker JB (1995) Interactions between Rumex spp., herbivores and a rust fungus: the effect of Uromyces rumicis infection on leaf nutritional quality. Funct Ecol 9:97–105
Holliday RT (1977) Population ecology of winter moth (Operophtera brumata) on apple in relation to larval dispersal and time of bud burst. J Appl Ecol 14:803–813
Holmes RT, Schultz JC, Nothnagel P (1979) Bird predation on forest insects: an exclusure experiment. Science 206:462–463
Holt RD (2000) Trophic cascades in terrestrial ecosystems. Reflections on Polis et al. Trends Evol Ecol 15:444–445
Hunter MD, Price PW (1998) Cycles in insect populations: delayed density dependence or exogenous driving variables? Ecol Entomol 23:216–222
Kause A, Ossipov V, Haukioja E, Lempa K, Hanhimäki S, Ossipova S (1999) Multiplicity of biochemical factors determining quality of growing birch leaves. Oecologia 120:102–112
Kerslake JE, Kruuk LEB, Hardey SE, Woodin SJ (1996) Winter moth (Operophtera brumata (Lepidoptera: Geometridae)) outbreaks on Scottis heather moorlands: effects of host plant and parasitoids on larval survival and development. Bull Entomol Res 86:155–164
Kytö M, Niemelä P, Larsson S (1996) Insects on trees: population and individual response to fertilization. Oikos 75:148–159
Lappalainen J, Helander ML, Palokangas P (1995) The performance of the autumnal moth is lower on trees infected by birch rust. Mycol Res 99:994–996
Larsson S (1989) Stressful times for plant stress-insect performance hypothesis. Oikos 56:277–283
Leather SR (1988) Size reproductive potential and fecundity in insects: things aren’t as simple as they seem. Oikos 51:386–389
Marquis RJ, Whelan CJ (1994) Insectivorous birds increase growth of white oak trough consumption of leaf-chewing insects. Ecology 75:2007–2014
Mattson WJ (1980) Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst 11:119–161
Moran PJ (1998) Plant-mediated interactions between insects and a fungal plant pathogen and the role of plant chemical response to infection. Oecologia 115:523–530
Nordin A, Näsholm T, Ericson L (1998) Effects of simulated N deposition on understorey vegetation of boreal coniferous forest. Funct Ecol 12:691–699
Norvell LL, Redhead SA (1994) Valdensinia heterodoxa (Sclerotiniaceae) in the United States. Can J For Res 24:1981–1983
Oksanen L, Oksanen T (2000) The logic and realism of the hypothesis of exploitation ecosystems. Am Nat 155:703–723
Oksanen L, Fretwell SD, Arrunda J, Niemela P (1981) Exploitation systems in gradients of primary productivity. Am Nat 118:240–262
Paine RT (1980) Food webs: linkage, interaction strength, and community infrastructure. J Anim Ecol 49:667–685
Parry D, Spence JR, Volney JA (1998) Budbreak phenology and natural enemies mediate survival of first-instar forest tent caterpillar (Lepidoptera:Lasiocampidae). Environ Entomol 27:1368–1374
Persson L (1999)Trophic cascades: abiding heterogeneity and the trophic level concept at the end of the road. Oikos 85:385–397
Polis GA (1999) Why are parts of the world green? Mulitipel factors control productivity and the distribution of biomass. Oikos 86:3–15
Polis GA, Sears ALW, Huxel GR, Strong DR, Maron J (2000) When is a trophical cascade a trophical cascade? Trends Ecol Evol 15:473–475
Power ME (1992) Top-down and bottom-up forces in food webs: do plants have primacy? Ecology 74:673–684
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Ritchie ME (2000) Nitrogen limitation and trophic vs. abiotic influences on insect herbivores in a temperate grassland. Ecology 8:1601–1612
Ritchie ME, Tilman D (1993) Predictions of species interactions from consumer-resource theory: experimental test with grasshoppers and plants. Oecologia 94:516–527
Schmitz OJ, Hambäck PA, Beckerman AP (2000) Trophic cascades in terrestrial systems: a review of the effects of carnivore removal on plants. Am Nat 155:141–153
Scriber JM, Slansky F Jr (1981) The nutritional ecology of immature insects. Annu Rev Entomol 26:183–211
Sipura M (1999) Tritrophic interactions: willows, herbivorous insects and insectivorous birds. Oecologia 121:537–545
Solomon PS, Oliver RP (2001) The nitrogen content of tomato leaf apoplast increases during infection by Cladosporium fulvum. Planta 213:241–249
Stiling P, Rossi AM (1997) Experimental manipulations of top-down and bottom-up factors in a tri-trophic system. Ecology 78:1602–1606
Strengbom J, Nordin A, Näsholm T, Ericson L (2002) Parasitic fungus mediates vegetation change in nitrogen exposed boreal forest. J Ecol 90:61–67
Strong DR (1992) Are trophic cascades all wet? The redundant differentiation in trophic architecture of high diversity ecosystems. Ecology 73:747–754
Sun B, Ricardo-da-Silva J, Spranger I (1998) Critical factors of vanillin assay for catechins and proanthocyanidins. J Agri Food Chem 46:4267–4274
Tamm CO (1991) Nitrogen in terrestrial ecosystems. Ecological studies no 81. Springer, Berlin Heidelberg New York
Tikkanen O-P, Julkunen-Tiitto R (2003) Phenological variation as protection against defoliating insects: the case of Quercus robur and Operophtera brumata. Oecologia 136:244–251
Tikkanen O-P, Carr TG, Roininen H (1999) Factors influencing the distribution of a generalist spring-feeding moth, Operophtera brumata (Lepidoptera: Geometridae) on host plants. Environ Entomol 3:461–469
White TCS (1993) The inadequate environment. Nitrogen and the abundance of animals. Springer, Berlin Heidelberg New York
Wint W (1983) The role of alternative host-plant species in the life of a polyphagous moth, Operophtera brumata (Lepidoptera: Geometridae). J Anim Ecol 52:439–450
Witzell J, Shevtsova A (2004) Nitrogen-induced changes in phenolics of Vaccinium myrtillus—implications for the interaction with a parasitic fungus. J Chem Ecol 30:1919–1938
Witzell J, Gref R, Näsholm T (2003) Plant-part specific and temporal variation in phenolic compounds of boreal bilberry (Vaccinium myrtillus L.) plants. Biochem Syst Ecol 31:115–127
Zar JH (1996) Biostatistical analysis. Prentice-Hall, New Jersey
Acknowledgements
We would like to thank The Svartberget Experimental Forest and its personnel for assistance with the annual fertilization of the experimental plots; Prof. Matt Ayres, Dr. Olli-Pekka Tikkanen, and two anonymous reviewer for valuable comments on an earlier version of this paper. This study was financially supported through grants from the ASTA program financially supported by MISTRA (Swedish Foundation for Strategic Environmental Research) (to LE and AN), by the Swedish Research Council (VR) (to LE), by FORMAS (Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning) (to AN and JW), and by the Gunnar & Ruth Björkman fod För Norrländsk Botanisk Forskning (to JS). The work presented in this paper conforms to the legal requirements of the country in which it was carried out, including those relating to conservation and welfare.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Strengbom, J., Witzell, J., Nordin, A. et al. Do multitrophic interactions override N fertilization effects on Operophtera larvae?. Oecologia 143, 241–250 (2005). https://doi.org/10.1007/s00442-004-1799-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1799-5