Abstract
Corynebacterium glutamicum is generally regarded as a safe microorganism, and widely used in the large-scale production of various amino acids and organic acids, such as l-glutamate, l-lysine and succinic acid. During the process of industrial fermentation, C. glutamicum is usually exposed to varying environmental stresses, such as variations in pH, salinity, temperature, and osmolality. Among them, pH fluctuations are regarded as one of the most frequent environmental stresses in microbial fermentation. In this review, we summarize the current knowledge of pH homeostasis mechanisms adopted by C. glutamicum for coping with low acidic pH and high alkaline pH stresses. Facing with low pH environments, C. glutamicum develops a variety of strategies to maintain intracellular pH homeostasis, such as lowering intracellular reactive oxygen species, the improvement of potassium transport, the regulation of mycothiol-related pathways, as well as the repression of sulfur assimilation. While during alkaline pH stresses, the Mrp-type Na+/H+ antiporters are shown to play a dominant role in conferring C. glutamicum cells resistance to alkaline pH. Furthermore, we also discuss the general strategies and prospects on metabolic engineering of C. glutamicum to improve alkaline or acid resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteria usually encounter diverse environmental stresses, such as variations in pH, salinity, temperature, and osmolality (Aertsen and Michiels 2004; Beales 2004). The external pH fluctuations, also known as pH challenges, might affect the intracellular pH levels, further resulting in deleterious effects on enzyme activity, protein stability, structure and function of biological macromolecules such as nucleic acids, proteins, lipids and carbohydrates (Krulwich et al. 2011). However, most microorganisms can maintain their internal pH ranges narrower than the external pH for growth, termed pH homeostasis (Baker-Austin and Dopson 2007; Krulwich et al. 2011; Padan et al. 2005; Slonczewski et al. 2009). In general, the neutralophilic bacteria can grow at external pH values of 5.5 to 9.0 but maintain their intracellular pH levels between 7.2 and 7.8 (Slonczewski et al. 2009). For example, the Gram-positive bacterium Corynebacterium glutamicum can maintain a cytoplasmic pH at 7.5 ± 0.5 units over the external pH values between 6.0 and 9.0 (Follmann et al. 2009b). However, at an external pH value below 6.0 or above 9.0, growth rates of C. glutamicum cells decrease drastically, and a sudden collapse in cytoplasmic pH homeostasis is observed when the external pH value is below 5.5 (Follmann et al. 2009b; Jakob et al. 2007). In order to survive in low pH environment, bacteria have evolved a large number of adaptive strategies for acidic pH homeostasis during the natural evolution (Cotter and Hill 2003; Kanjee and Houry 2013; Krulwich et al. 2011; Lund et al. 2014), mainly including direct active extrusion of cytoplasmic protons, proton consumption by amino acid-dependent decarboxylase systems, neutralization by the production of alkaline substances, the modification of cell membrane lipid compositions, the repair and damage prevention of biological macromolecules, and the reconstruction of specific metabolic pathways. Meanwhile, bacteria develop diverse mechanisms of intracellular pH homeostasis for coping with alkaline pH stress (Krulwich et al. 2011; Padan et al. 2005), such as proton retention by the activation of monovalent cation/H+ antiporters and F1F0-ATP synthase, metabolic alternations for acid substances production through sugar fermentation and amino acid deaminases, and changes of bacterial secondary cell wall polymer.
Corynebacterium glutamicum is well-known as a generally-recognized-as-safe (GRAS) organism in fermentation industry for more than 50 years, and has been widely engineered as an important platform for the production of various amino acids, organic acids, diamines, and alcohols (Becker et al. 2018; Lee et al. 2016). As an industrial workhorse, C. glutamicum is typically subjected to many abiotic factors such as temperature, pH, and osmotic stress, and pH fluctuations are considered as one of the grand environmental challenges in fermentation process (Michel et al. 2015). In recent years, some studies have focused on the investigation of adaptive mechanisms for intracellular pH homeostasis in C. glutamicum (Follmann et al. 2009b; Xu et al. 2019). In this review, we will compare and contrast the mechanisms of intracellular pH homeostasis in most bacteria and C. glutamicum, and summarize recent achievements of pH homeostasis mechanisms adopted by C. glutamicum for coping with low pH and alkaline pH stresses. We also discuss future prospects for metabolic engineering of C. glutamicum to improve pH adaptation.
General strategies for pH challenges in bacteria
Intracellular pH homeostasis is important for bacterial physiology, and it can allow most bacteria to tolerate or grow at the fluctuating pH environments (Krulwich et al. 2011). As a result, almost all bacteria have diverse adaptive strategies for maintaining pH homeostasis at the external pH values that exceed intracellular pH range to support growth. The strategies employed by most bacteria to cope with low pH challenges are listed in the left panel of Fig. 1, while the strategies that enable bacteria to manage high pH stresses are shown in left panel of Fig. 2.
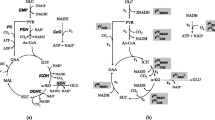
Strategies for acidic pH homeostasis in bacteria and C. glutamicum. General acid adaptive mechanisms in most bacteria are shown on the left, and possible molecular strategies adopted by C. glutamicum for coping with low pH are shown on the right. The detailed examples of adaptive mechanisms are described in the main text. Note: Glu, glutamate; GABA, gamma-aminobutyrate; Arg, arginine; Agm, agmatine; Lys, lysine; Cad, cadaverine; Orn, ornithine; Putr, putrescine; Nuo, NADH-ubiquinone oxidoreductase; Cyo, cytochrome bo; Gln, glutamine; Cit, citrulline; UFAs, unsaturated fatty acids; SFAs, saturated fatty acids; CFAs, cyclopropane fatty acids; ROS, reactive oxygen species; MSH, mycothiol; MSSM, mycothiol disulfide; Gad A/B, glutamate decarboxylase; GadC, glutamate-GABA antiporter; AdiA, arginine decarboxylase; AdiC, arginine-agmatine antiporter; CadA, lysine decarboxylase; CadB, lysine-cadaverine antiporter. SpeF, ornithine decarboxylase; PotE, putrescine-ornithine antiporter; Hyd, hydrogenase; YbaS, glutaminase; ArcA, arginine deiminase; ArcB, ornithine-carbamoyltransferase; ArcC, carbamate kinase; ArcD, arginine-ornithine antiporter; CglK, potassium channel protein; KatA, catalase; Dps, DNA protection during starvation protein; Mtr, mycothiol disulfide reductase; McbR, sulfur metabolism regulator; SucE, succinate exporter; Sqo, succinate:menaquinone oxidoreductase; Cg1261, putative lysine decarboxylase. σB, sigma B factor, “?” symbol indicates strategies that still need to be characterized functionally
Strategies for alkaline pH homeostasis in bacteria and C. glutamicum. The adaptive mechanisms responsible for alkaline pH tolerance in most bacteria are shown on the left, and possible strategies adopted by C. glutamicum are shown on the right. The detailed examples of adaptive mechanisms are described in the main text. Note: NhaA/B/P, Na+/H+ antiporter A/B/P; ChaA, Na+(Ca2+)/H+ antiporter; MdfA, multidrug efflux protein; NavBP, voltage-gated Na+ channel; TnaA, tryptophan deaminase; SdaA, serine deaminase, Mrp, multiple resistance and pH adaptation antiporter; TUP, teichuronopeptide; SCWP, secondary cell wall polymers; Cg1447, cobalt-zinc-cadmium efflux protein. “?” symbol indicates strategies that still need to be characterized functionally
Intracellular pH homeostasis mechanisms in low pH
Direct active proton extrusion
In some bacteria, the enzymatic complex F1F0-ATPase plays an important role in acid tolerance, which can expel protons using the energy released by ATP hydrolysis. The function of F1F0-ATPase in pH homeostasis was first discovered in Gram-positive bacterium Streptococcus spp. (Kobayashi et al. 1986). Subsequently, several Gram-negative bacteria such as Escherichia coli and Salmonella typhimurium, as well as Gram-positive bacteria Lactococcus lactis and Listeria monocytogenes have been demonstrated to exist such a mechanism (Lund et al. 2014). In addition, the respiratory chain complexes in many bacteria are also involved in proton export. For example, the expression of proton-pumping respiratory chain components has been shown to be upregulated under acidic pH stress in E. coli, supporting their roles in pH homeostasis (Krulwich et al. 2011; Slonczewski et al. 2009). It was also reported that some bacteria like E. coli harbor specific hydrogenases that catalyze the formation of hydrogen from cytoplasmic protons, thus contributing to acid survival (Noguchi et al. 2010).
Decarboxylase-mediated proton consumption
The mechanism of decarboxylase-mediated proton consumption resides in both Gram-positive and Gram-negative bacteria and involves four amino acid-dependent acid resistance systems (Foster 2004; Lund et al. 2014; Slonczewski et al. 2009), including glutamic acid-dependent acid resistance (GADR), arginine-dependent acid resistance (ADAR), lysine-dependent acid resistance (LDAR) and ornithine-dependent acid resistance (ODAR) systems. Each of these systems is composed of an amino acid decarboxylase that consumes protons in the process of catalyzing amino acid decarboxylation, and an antiporter that imports substrates coupling with the export of products. Generally, the GADR system plays a major role in conferring resistance to acid challenge in many bacteria, for example E. coli, L. monocytogenes and L. lactis (Lund et al. 2014).
Alkaline substances production
This mechanism mainly includes three enzyme systems: deiminase, deaminase and urease (Lund et al. 2014). These systems are capable of generating ammonia (NH3) which combines with intracellular protons to produce NH4+. For instance, arginine deiminase (ADI) system consisting of arginine deiminase, ornithine transcarbamoylase and carbamate kinase is commonly found in Gram-positive bacteria, which catalyzes the complete conversion from arginine to ornithine, NH3, CO2 and ATP, while ATP can be utilized by F1F0-ATPase to further expel protons (Ryan et al. 2009). Glutaminase is also found to offer acid resistance by the catalysis of l-glutamine to release ammonia in E. coli (Lu et al. 2013). Besides, urease catalyzes the urea decomposition to yield NH3 and carbamate, which further disintegrates into NH3 and CO2 (Scott et al. 2002).
Cell membrane modification
The alterations in membrane lipid composition are generally regarded as effective strategies to cope with various external stresses by regulating membrane permeability and fluidity (Qi et al. 2019; Siliakus et al. 2017; Sohlenkamp 2017). Cyclopropane fatty acids (CFAs) are observed in the phospholipids of many species of bacteria, which are formed by the addition of a methylene across the carbon–carbon double bond of unsaturated fatty acids (UFAs) (Grogan and Cronan 1997). The increased CFAs content has been proposed to be involved in the resistance to acid shock by the modification of membrane fluidity and proton permeability in E. coli (Brown et al. 1997; Shabala and Ross 2008) and several other bacteria, such as the acidophile (referred to bacteria that grow at external pH values below 3.0) Acidithiobacillus ferrooxidans (Mykytczuk et al. 2010), Salmonella typhimurium (Kim et al. 2005) and Oenococcus oeni (To et al. 2015). In response to acid stress, the shift from short-chained saturated fatty acids to long-chained monounsaturated fatty acids is observed in Streptococcus mutants (Fozo and Quivey 2004), whereas another opposite effect is also found in E. coli (Brown et al. 1997), A. ferrooxidans (Mykytczuk et al. 2010) and Lactobacillus casei (Broadbent et al. 2010), and those strains show an increase in the proportion of saturated fatty acids. Moreover, some bacteria such as Listeria monocytogenes display an increased anteiso-fatty acid content under acid stress conditions (Giotis et al. 2007). Thus, different bacteria may employ diverse strategies to alter membrane lipid composition for acid resistance, and more work is needed to further explore the potential molecular basis by which the modification of membrane lipids affects proton permeability in bacteria.
Repair and damage prevention of macromolecules
Excessive protons can potentially lead to the damage for intracellular biological macromolecules, including proteins and nucleic acids. It has been suggested that cytoplasmic (DnaK and GroEL), periplasmic (HdeA and HdeB) chaperones and Dps (DNA-binding protein in starved cells) are crucial for bacteria to survive in acid stress (Lund et al. 2014). Besides, the CIp protease complex is also observed to eliminate irreversibly damaged proteins caused by acid stress in many bacteria (Frees et al. 2007; Lund et al. 2014).
Reconstruction of metabolic pathways
Bacteria can change their metabolic pathways for the sake of survive in external stresses. In L. casei, physiological and metabolomic analyses reveal that acid stress results in higher intracellular aspartate and arginine levels, supporting that aspartate biosynthesis may be involved in acid tolerance (Wu et al. 2013). Similarly, the presence of glutamate, arginine and lysine improves acid tolerance in Bacillus cereus (Senouci-Rezkallah et al. 2011). Malolactic fermentation involving in the decarboxylation of l-malate to yield l-lactic acid is also found to be important for low acid tolerance in Streptococcus mutants and certain other oral lactic acid bacteria (Lemme et al. 2010). In addition, the uptake and biosynthesis of compatible solutes such as trehalose are often regarded as basic strategies against multiple stresses, and should be supposed to contribute to acid tolerance (Iordachescu and Imai 2008).
Intracellular pH homeostasis mechanisms in high pH
Proton capture or retention
A transformation to alkaline settings is also stressful for bacteria. So bacteria have evolved several strategies to manage alkaline stresses (Krulwich et al. 2011; Padan et al. 2005). Among them, monovalent cation/H+ antiporters are most crucial in the maintenance of alkaline pH homeostasis (Ito et al. 2017; Krulwich et al. 2009). As members of monovalent cation/H+ antiporters, Na+/H+ antiporters are ubiquitous in plenty of microorganisms. For instance, the best-characterized NhaA antiporter from E. coli is essential for the growth at alkaline pH in the presence of sodium, while the ChaA or MdfA antiporter could support alkaline pH homeostasis in the absence of sodium (Padan 2008; Tirosh et al. 2012). The Mrp (Multiple resistance and pH) system is regarded as the critical antiporter for cytoplasmic pH homeostasis in the alkaliphilic (referred to bacteria that grow at external pH values above 10.0) and neutralophilic (referred to bacteria that can grow at external pH values of 5.5–9.0) Bacillus species (Ito et al. 2017). The voltage-gated Na+ channel NaVBP in alkaliphilic Bacillus pseudofirmus OF4, is also found to support pH homeostasis under alkaline stress conditions (Ito et al. 2004). Besides, ATP synthesis capacity also represents an important factor for intracellular pH homeostasis at alkaline pH, and the elevated levels of F1F0-ATP synthase are shown to promote the entry of proton into the cell for ATP generation (Hicks et al. 2010).
Acid substances production
At high pH, some bacteria tend to upregulate catabolic pathways or amino acid deaminases to produce organic acids (Padan et al. 2005; Slonczewski et al. 2009). The favored pathways of sugar fermentation at alkaline pH are those related to glucose, which can produce more acid substances such as acetate (Slonczewski et al. 2009). Several kinds of amino acid deaminases that remove ammonia and direct carbon into the TCA cycle are also increased at alkaline pH, including tryptophan deaminase TnaA and serine deaminase SdaA (Stancik et al. 2002).
Variations in the cell surface layers
Secondary cell wall polymers (SCWP) that associated with the peptidoglycan layer is majorly found in the alkaliphile, and it is found to be a contributing factor in response to alkaline pH stress. Teichuronopeptide, a major structural component of the cell wall, is shown to play a role in high pH tolerance of Bacillus halodurans C-125 (Aono et al. 1999), and SlpA, an acidic Slayer protein, also has an important role in alkaline pH homeostasis of B. pseudofirmus OF4 (Padan et al. 2005). These above acidic polymers are predicted to enhance proton uptake by increasing proton concentrations near the membrane surface at high pH in alkaliphiles.
Adaptive strategies adopted by C. glutamicum for coping with pH challenges
Corynebacterium glutamicum is typically regarded as a moderately alkali-tolerant organism with an optimal growth pH between 7.0 and 8.5 (Follmann et al. 2009b), and the minimal pH value for growth of C. glutamicum cells is found to be 5.5 (Jakob et al. 2007). In recent years, many studies have focused on elucidating physiological mechanisms of the acid-alkaline stress tolerance in C. glutamicum, and have found and characterized several molecular adaptation mechanisms underlying low pH response (right panel of Fig. 1) or high pH response (right panel of Fig. 2) that are specific to C. glutamicum. However, the current understanding of the molecular mechanisms adopted by C. glutamicum for controlling intracellular pH homeostasis is still limited.
pH homeostasis mechanisms for coping with low pH in C. glutamicum
Various general strategies to cope with acid stress have been described in most bacteria, such as proton-consuming reactions mediated by glutamate decarboxylase or lysine decarboxylase, as well as alkaline neutralization dependent on ADI and Urea systems. However, the situation in C. glutamicum is a little different (Fig. 1). The homologous proteins of glutamate or arginine decarboxylase that can consume protons during the decarboxylation of amino acids are missing in C. glutamicum (Follmann et al. 2009b). Although the protein encoded by cg1261 is annotated to be a lysine decarboxylase, its function still needs to be characterized functionally (Ikeda and Nakagawa 2003). Moreover, the homolog of the arcA gene that encodes an important component of ADI system is likewise absent in C. glutamicum (Ikeda and Nakagawa 2003). Although a putative urease complex is found in C. glutamicum, its involvement in acid tolerance needs further investigation (Beckers et al. 2004). In general, these common strategies known to be important for acidic pH homeostasis in most bacteria seem to be missing for C. glutamicum.
Several studies have revealed that multiple cellular processes are implicated in the resistance to acidic pH stress in C. glutamicum. For instance, Follmann et al. (2009b) offered a functional link between pH homeostasis, oxidative stress, iron homeostasis, and methionine biosynthesis. Xu et al (2019) also reported that several important cellular processes, such as the repression of oxidative stress and sulfur assimilation, play important roles in sustaining pH homeostasis. In addition, Jakob et al. (2017) provided the global gene expression profile of C. glutamicum subjected to long-term lactic acid adaptation, and confirmed that the absence of sigma factor SigB leads to an obviously increased sensitivity to low pH. These findings support the importance of some sigma factors in response to various environmental stresses (Patek and Nesvera 2011).
Given that acid challenge can lead to the accumulation of intracellular reactive oxygen species (ROS) (Lund et al. 2014; Slonczewski et al. 2009), the reduction of ROS levels represents a promising method to confer acid resistance. Several components implicated in intracellular ROS scavenging have been characterized in C. glutamicum (Fig. 1, right panel). For example, the regulation of ROS homeostasis by mycothiol-related proteins, such as mycothiol peroxidase MPx, mycothiol disulfide reductase Mtr or mycothiol glycosyltransferase MshA, can confer C. glutamicum cells with the ability to adapt to acid stress (Liu et al. 2016; Si et al. 2016; Wang et al. 2016). Xu et al (2019) found that the catalase KatA and DNA-protection protein Dps cooperatively mediate intracellular ROS scavenging, and are also required for resistance to low pH stress in C. glutamicum. In addition, the repression of sulfur metabolism by the McbR regulator shows beneficial effects on acid stress tolerance. Moreover, the CglK-mediated potassium accumulation is also found to be crucial for growth and intracellular pH homeostasis at acidic pH in C. glutamcium (Follmann et al. 2009a). Besides, Michel et al (2015) provided the opinions that other possible reactions are also assumed to be relevant for proton extrusion under acid stress conditions, including the export of lactate or succinate, and the fumarate reduction reaction mediated by the succinate:menaquinone oxidoreductase. However, those possibilities still need to be characterized functionally.
pH homeostasis mechanisms for coping with high pH in C. glutamicum
Corynebacterium glutamicum is regarded to be a moderately alkali-tolerant organism with optimal growth at pH 7.0 to 8.5, and shows a marked Na+ resistance at alkaline pH (Xu et al. 2016). Previous studies illustrated that the pH-regulated antiporters such as NhaA and MdfA play crucial roles in the maintenance of alkaline pH homeostasis in E. coli, but the homologs of these antiporters are missing in C. glutamicum (Fig. 2). Xu et al (2016) characterized the catalytic capacity of three putative Na+/H+ antiporters derived from C. glutamicum, and found that the Mrp1 antiporter has significant Na+ (Li+)/H+ antiport activities with an apparently low Km and alkaline pH optimum. Further physiological experiments confirm that the Mrp-type antiporters, especially Mrp1 antiporter, play a predominant role in conferring alkaline tolerance in C. glutamicum (Xu et al. 2018). In addition, the Cg1447 encoding putative transporter of the cation diffusion facilitator family also plays a role in alkaline pH tolerance in C. glutamicum (Takeno et al. 2008).
Metabolic engineering of C. glutamicum for improving acid or alkaline resistance
Corynebacterium glutamicum is an important workhorse for the production of various bio-based chemicals in biotechnology. Given that the growth and production of C. glutamicum are commonly hampered by the environmental stresses, especially pH challenges during the fermentation conditions, the improvement of physiological performance of industrial microbes will contribute to a more efficient biotechnological production of desired products (Jia et al. 2014; Liu et al. 2015; Zhu et al. 2012). The identification and characterization of pH-resistant components or pH-survival strategies will provide increasingly powerful modules for engineering physiological robustness against pH challenges.
Multiple strategies have been reported to enhance pH resistance in bacteria and C. glutamicum, such as the elimination of intracellular ROS induced by pH stress, the regulation of metabolic imbalance, the activation of specific ion transporter, and adaptive laboratory evolution method (Fig. 3). At present, several examples of enhancing pH tolerance are described in C. glutamicum. Xu et al (2019) reported that the over-expression of katA and dps obviously reduces intracellular ROS levels caused by acid stress, thereby contributing to conferring C. glutamicum cells resistance to low pH challenge. Likewise, the over-expression of mycothiol pathway genes, such as mpx or mshA, can protect C. glutamicum cells against acid stress by scavenging intracellular ROS (Liu et al. 2016; Wang et al. 2016). Moreover, the repression of sulfur assimilation pathway by the McbR regulator contributes to reducing l-cysteine accumulation, and shows beneficial effects for the growth under acidic pH conditions (Xu et al. 2019). Follmann et al (2009a) revealed that C. glutamicum exhibits a potassium-dependent growth limitation at low pH, and found that potassium uptake via CglK channel is crucial for C. glutamicum acid stress resistance. Xu et al (2018) reported that the over-expression of Mrp1 Na+/H+ antiporter slightly improves growth ability under mixed salt and alkaline stress conditions. Adaptive laboratory evolution (ALE) is a widely used method to quickly improve the robustness of strains against multiple stresses. By the ALE strategy, Xu et al (2019) obtained a C. glutamicum acid-adapted strain that exhibits clearly enhanced growth compared to wild-type cells at pH values ranging from 5.2 to 6.0.
Engineering strategies of C. glutamicum to improve pH tolerance. ROS engineering is used to reduce deleterious ROS levels induced by acid stress. Regulatory protein engineering is used for directed modification of metabolic pathways to adapt with pH challenges. Transporter engineering is performed to enhance the transport of potassium at low pH or the proton uptake at high pH. Membrane lipid engineering is expected to enhance membrane permeability and fluidity. Repair pathway engineering can confer improved stress resistance by the repair or damage prevention of DNA or protein. Adaptive laboratory evolution is an effective method to rapidly evolve organisms for desired stress tolerance. Note: KatA, catalase; Dps, DNA protection during starvation protein; Mpx, mycothiol peroxidase; MshA, mycothiol glycosyltransferase; McbR, sulfur metabolism regulator; DtxR, iron regulator; Fas, fatty acid synthase; DnaK, protein-repair chaperone; IrrE, exogenous global regulator that stimulate DNA repair gene transcription. “?” symbol indicates strategies that still need to be confirmed experimentally
In addition, some strategies have also been proven effective in engineering robustness of microbes against pH stress, such as the modification of cell membrane compositions and the enhancement of repair and damage prevention processes (Lund et al. 2014; Qi et al. 2019). The alteration of membrane lipid compositions such as saturation and chain length fatty acids is feasible to enhance the stress tolerance of microorganisms. For example, the increased cyclopropane fatty acid (CFAs) content is regarded as a major acid resistance strategy in E. coli (Foster 2004). The repair and damage prevention of biological macromolecules are also essential for maintaining the physiological function of DNA or protein under stress conditions (Lund et al. 2014). Heterogeneous expression of a chaperone protein DnaK or a DNA repair regulatory protein IrrE is shown to be an effective strategy to enhance multiple stresses tolerance of E. coli, including low pH challenge (Lemme et al. 2010). Although transcriptome data reveal that the expression patterns of the above two cellular processes are affected by pH levels, no example has been established on the engineering of C. glutamicum for improved pH resistance from the aspects of cell membrane and repair pathway. Nevertheless, we still believe that the regulation of cell membrane functions or repair pathways may offer a feasible and promising strategy for C. glutamicum pH resistance in future.
Conclusion and prospects
To survive and grow under external pH stresses, several general adaptations have been evolved to maintain intracellular pH homeostasis in most bacteria. Among them, the proton-consuming decarboxylation dependent on glutamate (GADR system) plays a crucial role in conferring acid resistance in bacteria such as E. coli, L. monocytogenes, as well as lactic acid bacteria. Moreover, the formation of alkaline substances is also a relatively common strategy to regulate intracellular pH level in many Gram-positive bacteria, depending on the arginine deiminase (ADI system) and urea breakdown. However, genes encoding homologous proteins of the above GADR or ADI system are missing in C. glutamicum, implying that C. glutamicum must have some novel undiscovered acid-resistant components, and its physiological adaptation mechanisms in an acidic environment still need further exploration. Alternatively, the dominant role of Na+/H+ antiporters in the maintenance of alkaline pH homeostasis has been functionally characterized in many bacteria. Xu et al (2018) has also reported that Mrp-type Na+/H+ antiporters play a crucial role in conferring alkaline resistance in C. glutamicum. However, the involvement of other strategies in response to alkaline pH stresses, such as the F1F0-ATP synthase and amino acid deaminases, has not been characterized functionally. In conclusion, more efforts are still required to unveil the pH tolerance-related genes and mechanisms adopted by C. glutamicum in further research.
The engineered microorganism with enhanced stress tolerance is essential for achieving higher yields and productivity in the fermentation (Jia et al. 2014). However, conventional metabolic engineering methods such as knockout, down-regulation or over-expression of pH tolerance-related genes often result in the imbalance and irreversible alternations of metabolic fluxes, thereby impeding cell growth (Choi et al. 2019). Improvements in synthetic biology, in particular genetic circuit design, have increased the potential for improving the robustness of industrial microbes against diverse stresses (Gao et al. 2019). As an example shown in Fig. 4, we may be able to achieve a specific spatiotemporal expression of the given pH-resistant component through dynamic regulation of pH tolerance-related genes via genetic circuit strategy. Certainly, many efforts still need to be made to design the circuit architecture and identify efficient pH tolerance-related modules for establishing a feasible dynamic control system. For instance, more elementary functional building blocks such as rigorous pH-responsive promoters are still required to be characterized in C. glutamicum. Although the current challenge in genetic circuit design is still present, a dynamic control of pH-resistant components will hold great potential in achieving smart microorganisms with desired robustness.
A possible example of dynamic regulation for pH-tolerant phenotypes by genetic circuits. In the presence of low pH stress signal, it induces the expression of low pH-sensing genetic promoter or riboswitch PSR1, and allows the transcription of both acid stress-resistant element-1 (sre1) and sspB genes. The adaptor protein SspB delivers SsrA-tagged proteins to the ClpXP proteases for degradation. In the absence of xylose, the expression of high pH-sensing genetic devices PSR2 is inactive owing to the impediment of XylR-xylO complex. In the presence of both xylose and high pH stress signal, the binding of xylose to XylR repressor relieves the repression and allows the sufficient expression of PSR2, thereby activates alkaline stress-resistant element-2 (sre2) and lacI expression. Moreover, the elevated LacI repressor will further inhibit the expression of PSR1 and abolish the expression of SspB adaptor
References
Aertsen A, Michiels CW (2004) Stress and how bacteria cope with death and survival. Crit Rev Microbiol 30:263–273. https://doi.org/10.1080/10408410490884757
Aono R, Ito M, Machida T (1999) Contribution of the cell wall component teichuronopeptide to pH homeostasis and alkaliphily in the alkaliphile Bacillus lentus C-125. J Bacteriol 181:6600–6606
Baker-Austin C, Dopson M (2007) Life in acid: pH homeostasis in acidophiles. Trends Microbiol 15:165–171. https://doi.org/10.1016/j.tim.2007.02.005
Beales N (2004) Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: a review. Compr Rev Food Sci Food Saf 3:1–20. https://doi.org/10.1111/j.1541-4337.2004.tb00057.x
Becker J, Rohles CM, Wittmann C (2018) Metabolically engineered Corynebacterium glutamicum for bio-based production of chemicals, fuels, materials, and healthcare products. Metab Eng 50:122–141. https://doi.org/10.1016/j.ymben.2018.07.008
Beckers G, Bendt AK, Kramer R, Burkovski A (2004) Molecular identification of the urea uptake system and transcriptional analysis of urea transporter- and urease-encoding genes in Corynebacterium glutamicum. J Bacteriol 186:7645–7652. https://doi.org/10.1128/Jb.186.22.7645-7652.2004
Broadbent JR, Larsen RL, Deibel V, Steele JL (2010) Physiological and transcriptional response of Lactobacillus casei ATCC 334 to acid stress. J Bacteriol 192:2445–2458. https://doi.org/10.1128/JB.01618-09
Brown JL, Ross T, McMeekin TA, Nichols PD (1997) Acid habituation of Escherichia coli and the potential role of cyclopropane fatty acids in low pH tolerance. Int J Food Microbiol 37:163–173. https://doi.org/10.1016/S0168-1605(97)00068-8
Choi KR, Jang WD, Yang D, Cho JS, Park D, Lee SY (2019) Systems metabolic engineering strategies: Integrating systems and synthetic biology with metabolic engineering. Trends Biotechnol 37:817–837. https://doi.org/10.1016/j.tibtech.2019.01.003
Cotter PD, Hill C (2003) Surviving the acid test: Responses of gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67:429–453. https://doi.org/10.1128/mmbr.67.3.429-453.2003
Follmann M, Becker M, Ochrombel I, Ott V, Kramer R, Marin K (2009a) Potassium transport in Corynebacterium glutamicum is facilitated by the putative channel protein CglK, which is essential for pH homeostasis and growth at acidic pH. J Bacteriol 191:2944–2952. https://doi.org/10.1128/Jb.00074-09
Follmann M et al (2009b) Functional genomics of pH homeostasis in Corynebacterium glutamicum revealed novel links between pH response, oxidative stress, iron homeostasis and methionine synthesis. BMC Genom 10:621. https://doi.org/10.1186/1471-2164-10-621
Foster JW (2004) Escherichia coli acid resistance: Tales of an amateur acidophile. Nat Rev Microbiol 2:898–907. https://doi.org/10.1038/nrmicro1021
Fozo EA, Quivey RG (2004) Shifts in the membrane fatty acid profile of Streptococcus mutans enhance survival in acidic environments. Appl Environ Microbiol 70:929–936. https://doi.org/10.1128/aem.70.2.929-936.2004
Frees D, Savijoki K, Varmanen P, Ingmer H (2007) Clp ATPases and ClpP proteolytic complexes regulate vital biological processes in low GC, Gram-positive bacteria. Mol Microbiol 63:1285–1295. https://doi.org/10.1111/j.1365-2958.2007.05598.x
Gao C, Xu P, Ye C, Chen X, Liu L (2019) Genetic circuit-assisted smart microbial engineering. Trends Microbiol. https://doi.org/10.1016/j.tim.2019.07.005
Giotis ES, McDowell DA, Blair IS, Wilkinson BJ (2007) Role of branched-chain fatty acids in pH stress tolerance in Listeria monocytogenes. Appl Environ Microbiol 73:997–1001. https://doi.org/10.1128/AEM.00865-06
Grogan DW, Cronan JE Jr (1997) Cyclopropane ring formation in membrane lipids of bacteria. Microbiol Mol Biol Rev 61:429–441
Hicks DB, Liu J, Fujisawa M, Krulwich TA (2010) F1F0-ATP synthases of alkaliphilic bacteria: Lessons from their adaptations. Biochim Biophys Acta Bioenerg 1797:1362–1377. https://doi.org/10.1016/j.bbabio.2010.02.028
Ikeda M, Nakagawa S (2003) The Corynebacterium glutamicum genome: features and impacts on biotechnological processes. Appl Microbiol Biotechnol 62:99–109. https://doi.org/10.1007/s00253-003-1328-1
Iordachescu M, Imai R (2008) Trehalose biosynthesis in response to abiotic stresses. J Integr Plant Biol 50:1223–1229. https://doi.org/10.1111/j.1744-7909.2008.00736.x
Ito M, Morino M, Krulwich TA (2017) Mrp antiporters have important roles in diverse bacteria and archaea. Front Microbiol 8:2325. https://doi.org/10.3389/Fmicb.2017.02325
Ito M, Xu HX, Guffanti AA, Wei Y, Zvi L, Clapham DE, Krulwich TA (2004) The voltage-gated Na+ channel NavBP has a role in motility, chemotaxis, and pH homeostasis of an alkaliphilic Bacillus. Proc Natl Acad Sci USA 101:10566–10571. https://doi.org/10.1073/pnas.0402692101
Jakob K, Satorhelyi P, Lange C, Wendisch VF, Silakowski B, Scherer S, Neuhaus K (2007) Gene expression analysis of Corynebacterium glutamicum subjected to long-term lactic acid adaptation. J Bacteriol 189:5582–5590. https://doi.org/10.1128/JB.00082-07
Jia H, Fan Y, Feng X, Li C (2014) Enhancing stress-resistance for efficient microbial biotransformations by synthetic biology. Front Bioeng Biotechnol 2:44. https://doi.org/10.3389/fbioe.2014.00044
Kanjee U, Houry WA (2013) Mechanisms of acid resistance in Escherichia coli. Annu Rev Microbiol 67:65–81. https://doi.org/10.1146/annurev-micro-092412-155708
Kim BH, Kim S, Kim HG, Lee J, Lee IS, Park YK (2005) The formation of cyclopropane fatty acids in Salmonella enterica serovar Typhimurium. Microbiology 151:209–218. https://doi.org/10.1099/mic.0.27265-0
Kobayashi H, Suzuki T, Unemoto T (1986) Streptococcal cytoplasmic pH is regulated by changes in amount and activity of a proton-translocating Atpase. J Biol Chem 261:627–630
Krulwich TA, Hicks DB, Ito M (2009) Cation/proton antiporter complements of bacteria: why so large and diverse? Mol Microbiol 74:257–260. https://doi.org/10.1111/j.1365-2958.2009.06842.x
Krulwich TA, Sachs G, Padan E (2011) Molecular aspects of bacterial pH sensing and homeostasis. Nat Rev Microbiol 9:330–343. https://doi.org/10.1038/nrmicro2549
Lee JY, Na YA, Kim ES, Lee HS, Kim P (2016) The actinobacterium Corynebacterium glutamicum, an industrial workhorse. J Microbiol Biotechnol 26:1341–1341. https://doi.org/10.4014/jmb.2016.2607.1341
Lemme A, Sztajer H, Wagner-Dobler I (2010) Characterization of mleR, a positive regulator of malolactic fermentation and part of the acid tolerance response in Streptococcus mutants. BMC Microbiol 10:58. https://doi.org/10.1186/1471-2180-10-58
Liu YB et al (2016) Mycothiol protects Corynebacterium glutamicum against acid stress via maintaining intracellular pH homeostasis, scavenging ROS, and S-mycothiolating MetE. J Gen Appl Microbiol 62:144–153. https://doi.org/10.2323/jgam.2016.02.001
Liu YP, Tang HZ, Lin ZL, Xu P (2015) Mechanisms of acid tolerance in bacteria and prospects in biotechnology and bioremediation. Biotechnol Adv 33:1484–1492. https://doi.org/10.1016/j.biotechadv.2015.06.001
Lu PL, Ma D, Chen YL, Guo YY, Chen GQ, Deng HT, Shi YG (2013) L-glutamine provides acid resistance for Escherichia coli through enzymatic release of ammonia. Cell Res 23:635–644. https://doi.org/10.1038/cr.2013.13
Lund P, Tramonti A, De Biase D (2014) Coping with low pH: molecular strategies in neutralophilic bacteria. FEMS Microbiol Rev 38:1091–1125. https://doi.org/10.1111/1574-6976.12076
Michel A, Koch-Koerfges A, Krumbach K, Brocker M, Bott M (2015) Anaerobic growth of Corynebacterium glutamicum via mixed-acid fermentation. Appl Environ Microbiol 81:7496–7508. https://doi.org/10.1128/Aem.02413-15
Mykytczuk NC, Trevors JT, Ferroni GD, Leduc LG (2010) Cytoplasmic membrane fluidity and fatty acid composition of Acidithiobacillus ferrooxidans in response to pH stress. Extremophiles 14:427–441. https://doi.org/10.1007/s00792-010-0319-2
Noguchi K, Riggins DP, Eldahan KC, Kitko RD, Slonczewski JL (2010) Hydrogenase-3 contributes to anaerobic acid resistance of Escherichia coli. PLoS ONE 5:e10132. https://doi.org/10.1371/journal.pone.0010132
Padan E (2008) The enlightening encounter between structure and function in the NhaA Na+-H+ antiporter. Trends Biochem Sci 33:435–443. https://doi.org/10.1016/j.tibs.2008.06.007
Padan E, Bibi E, Ito M, Krulwich TA (2005) Alkaline pH homeostasis in bacteria: New insights. Biochim Biophys Acta Biomembr 1717:67–88. https://doi.org/10.1016/j.bbamem.2005.09.010
Patek M, Nesvera J (2011) Sigma factors and promoters in Corynebacterium glutamicum. J Biotechnol 154:101–113. https://doi.org/10.1016/j.jbiotec.2011.01.017
Qi YL, Liu H, Chen XL, Liu LM (2019) Engineering microbial membranes to increase stress tolerance of industrial strains. Metab Eng 53:24–34. https://doi.org/10.1016/j.ymben.2018.12.010
Ryan S, Begley M, Gahan CGM, Hill C (2009) Molecular characterization of the arginine deiminase system in Listeria monocytogenes: regulation and role in acid tolerance. Environ Microbiol 11:432–445. https://doi.org/10.1111/j.1462-2920.2008.01782.x
Scott DR, Marcus EA, Weeks DL, Sachs G (2002) Mechanisms of acid resistance due to the urease system of Helicobacter pylori. Gastroenterology 123:187–195. https://doi.org/10.1053/gast.2002.34218
Senouci-Rezkallah K, Schmitt P, Jobin MP (2011) Amino acids improve acid tolerance and internal pH maintenance in Bacillus cereus ATCC14579 strain. Food Microbiol 28:364–372. https://doi.org/10.1016/j.fm.2010.09.003
Shabala L, Ross T (2008) Cyclopropane fatty acids improve Escherichia coli survival in acidified minimal media by reducing membrane permeability to H+ and enhanced ability to extrude H+. Res Microbiol 159:458–461. https://doi.org/10.1016/j.resmic.2008.04.011
Si MR et al (2016) Overexpression of mycothiol disulfide reductase enhances Corynebacterium glutamicum robustness by modulating cellular redox homeostasis and antioxidant proteins under oxidative stress. Sci Rep 6:29491. https://doi.org/10.1038/Srep29491
Siliakus MF, van der Oost J, Kengen SWM (2017) Adaptations of archaeal and bacterial membranes to variations in temperature, pH and pressure. Extremophiles 21:651–670. https://doi.org/10.1007/s00792-017-0939-x
Slonczewski JL, Fujisawa M, Dopson M, Krulwich TA (2009) Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv Microb Physiol 55:1–79. https://doi.org/10.1016/S0065-2911(09)05501-5
Sohlenkamp C (2017) Membrane homeostasis in bacteria upon pH challenge. In: Geiger O (ed) Biogenesis of fatty acids, lipids and membranes. Springer, Cham, pp 1–13. https://doi.org/10.1007/978-3-319-43676-0_57-1
Stancik LM, Stancik DM, Schmidt B, Barnhart DM, Yoncheva YN, Slonczewski JL (2002) pH-dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli. J Bacteriol 184:4246–4258. https://doi.org/10.1128/Jb.184.15.4246-4258.2002
Takeno S, Nakamura M, Fukai R, Ohnishi J, Ikeda M (2008) The Cgl1281-encoding putative transporter of the cation diffusion facilitator family is responsible for alkali-tolerance in Corynebacterium glutamicum. Arch Microbiol 190:531–538. https://doi.org/10.1007/s00203-008-0401-7
Tirosh O, Sigal N, Gelman A, Sahar N, Fluman N, Siemion S, Bibi E (2012) Manipulating the drug/proton antiport stoichiometry of the secondary multidrug transporter MdfA. Proc Natl Acad Sci U S A 109:12473–12478. https://doi.org/10.1073/pnas.1203632109
To TMH, Grandvalet C, Alexandre H, Tourdot-Marechal R (2015) Cyclopropane fatty acid synthase from Oenococcus oeni: expression in Lactococcus lactis subsp cremoris and biochemical characterization. Arch Microbiol 197:1063–1074. https://doi.org/10.1007/s00203-015-1143-y
Wang TT et al (2016) Mycothiol peroxidase MPx protects Corynebacterium glutamicum against acid stress by scavenging ROS. Biotechnol Lett 38:1221–1228. https://doi.org/10.1007/s10529-016-2099-y
Wu C, Zhang J, Du G, Chen J (2013) Aspartate protects Lactobacillus casei against acid stress. Appl Microbiol Biotechnol 97:4083–4093. https://doi.org/10.1007/s00253-012-4647-2
Xu N, Lv HF, Wei L, Liang Y, Ju JS, Liu J, Ma YH (2019) Impaired oxidative stress and sulfur assimilation contribute to acid tolerance of Corynebacterium glutamicum. Appl Microbiol Biotechnol 103:1877–1891. https://doi.org/10.1007/s00253-018-09585-y
Xu N, Wang L, Cheng HJ, Liu QD, Liu J, Ma YH (2016) In vitro functional characterization of the Na+/H+ antiporters in Corynebacterium glutamicum. FEMS Microbiol Lett 363:fnv237. https://doi.org/10.1093/femsle/fnv237
Xu N, Zheng YY, Wang XC, Krulwich TA, Ma YH, Liu J (2018) The lysine 299 residue endows the multisubunit Mrp1 antiporter with dominant roles in Na+ resistance and pH homeostasis in Corynebacterium glutamicum. Appl Environ Microb 84:e00110–e118. https://doi.org/10.1128/AEM.00110-18
Zhu LJ, Zhu Y, Zhang YP, Li Y (2012) Engineering the robustness of industrial microbes through synthetic biology. Trends Microbiol 20:94–101. https://doi.org/10.1016/j.tim.2011.12.003
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grant No. 2018YFA0901000), the National Natural Science Foundation of China (Grant Nos. 31972061, 31801526), and the Natural Science Foundation of Tianjin City (Grant Nos. 17JCYBJC24000, 17JCQNJC09600).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, J., Ma, Z., Gao, J. et al. Recent advances of pH homeostasis mechanisms in Corynebacterium glutamicum. World J Microbiol Biotechnol 35, 192 (2019). https://doi.org/10.1007/s11274-019-2770-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-019-2770-2