Abstract
Algae, as a low-impact aquatic feedstock, is regarded as a promising biomass for producing valuable biofuel, syngas, and biochar. Algae, on the other hand, are mostly composed of lipids, proteins, and carbohydrates, as opposed to lignocellulosic biomass. Algal species have a faster growth rate and higher photosynthetic efficiency than terrestrial plants, making them an excellent alternative for a sustainable environment. Algal biomass has shown great promise as a raw material for biochar production in recent years. Algae biochar has a high potential for use as a material for contamination remediation and energy application. This review paper summarizes the applicability of algal biochar, algal biochar modification strategies, fabrication methods, and algal biochar properties. Carbon sequestration, sediment and water treatment, and energy applications are all thoroughly discussed. More emphasis should be placed on practical applications, and more research should be conducted to address existing problems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
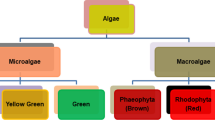
Algae, simply classified as macroalgae and microalgae, have been recognized as one of the most potential sustainable energy feedstocks for the future due to the ease with which they can be cultivated in great quantities in a variety of conditions [1]. More and more research is being done to determine the viability of these fast-growing plants as a source of renewable energy, nutritional or pharmaceutical supplements, and environmental remediation due to their high CO2 fixation efficiency and potential for the creation of valuable chemicals [2]. Consequently, algae biomass production from algae is offered as a cost-effective method for carbon sequestration and reutilization [3]. For algal biomass derived from green tide algae with a fast growth rate, biochar production is one potential solution for biomass conversion. Pyrolysis, a thermochemical method that might improve the conversion of biomass to biochar, is commendable in terms of energy and environmental remediation. Algal biomass is thermally decomposed into a variety of pyrolytic products including such bio-oil, syngas, and biochar through the process of algal pyrolysis. To improve the economic viability of biochar manufacturing methods with a faster rate, higher yield, and higher quality for applications, it is crucial to improve the thermochemical processes now in use.
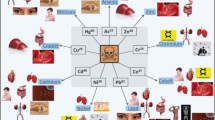
Biochar is the carbon-rich substance produced during pyrolysis process in low-oxygen conditions. Biomass and raw materials used to make biochar come from a wide range of sources. Eco-friendliness, affordability, and high efficacy for environmental remediation and energy application are just a few reasons why biochar has gained so much interest. As comparison to lignocellulose-based biochar, algal biochar is more likely to have a greater cation exchange capacity, pH value, and also higher nitrogen and trace element content that could be advantageous for increasing the chemical characteristics necessary for various applications. However, there have been just a few investigations on the physical and chemical properties of algal biochar as well as its applications. Therefore, this review provides an in-depth analysis of the relationship between production approaches, algal biochar properties, and potential applications. Importantly, this review intends to provide a comprehensive analysis of the prospective applications of algal biochar for carbon sequestration, water and sediment remediation, biofuel conversion, hydrogen production, and microbial fuel cell (Fig. 1). Detailed discussions were also held on the latest developments in developing techniques for efficiently turning waste algae into functionalized algal biochar for various environmental and energy applications. Additionally, the relevant chemical characteristics and proper implementation of algal biochar have been elucidated in order to expand its use.
Algal Biochar Production
Synthesis Method
Pyrolysis
Pyrolysis is the process of burning biomass at temperatures between 200 and 700 ℃ in the absence of air or oxygen to produce biochar [4]. Pyrolysis is one of the most powerful methods to synthesize biochar from biomass with various techniques such as conventional, fast, and flash pyrolysis [5]. Besides, pyrolysis is also improved by combining other techniques to increase the efficiency of the process, such as microwave-assisted pyrolysis [1]. The difference in operating conditions makes the difference between these techniques [4]. The properties of biochar products differ according to the pyrolysis techniques used.
Typically, there are two main factors affecting pyrolysis, including operating conditions and the characteristics of the feedstock [6]. The operating conditions that are often of interest are temperature, heating rate, and residence time. Previous studies have shown that biochar production increases with decreasing temperature, low heating rate, and increasing residence time [5, 7]. Besides, the feedstock characteristics, such as particle size and moisture content, directly affect biochar production [8•].
Algae was used as biomass feedstock to synthesize biochar in previous studies. For instance, in the study of Jung et al. [9], brown Laminaria japonica macroalgae was used to synthesize biochar with the high carbon content (78.34%) at 200 ℃ through slow pyrolysis. Similarly, Chang et al.[10] also showed that the carbon content of biochar achieved 65.0% at 700 ℃ using the slow pyrolysis process with Chlorella-based algal residue as feedstock. In the study of Yanik et al. [11], Laminaria digitata, Fucus serratus, and mixed macroalgae species from the Black Sea were used to synthesize biochar by fluidized-bed pyrolysis. The process was carried out at a temperature of 500 ℃ and achieved 29–36% of biochar yield.
Torrefaction
Torrefaction is a thermochemical technique used for biomass pre-treatment at temperatures 200–300 ℃ [12]. This process is operated in the absence of air at the atmospheric pressure under an inert condition [13]. Torrefaction was known as a useful technology in removing VOCs from biomass and creating biochar with high carbon content [14]. Therefore, torrefaction decreases the disadvantages of biomass and improves the quality of biochar. The biochar yield depends on the temperature of the process, residence time, and biomass characteristics. Wu et al. [15] presented that the biochar yield is dropped when the temperature is enhanced. At the torrefaction temperature of 300 °C, the biochar yield also decreases when residence time increases. Similarly, the results of the study by Uemura [16] also confirmed that biochar yield decreases with increasing temperature of torrefaction process on macroalga Laminaria japonica. Mwangi et al. [17] reported that the optimal temperature of microalgae torrefaction is 250 °C or below.
Torrefaction can be divided into two types, including wet and dry torrefaction [18]. The dry torrefaction is operated under inert nitrogen gas media with temperature of 200–300 °C, atmospheric pressure, and 80 min of residence time. Meanwhile, the wet torrefaction is performed under hot compressed water media with temperature 180–260 °C, at 200–700 psi pressure, and 5 min of residence time. Due to the high heat transfer rate in aqueous media, the wet torrefaction process has the main advantage of producing a dense product in a short residence time [12]. Biomass treated under high pressure hot water will produce biochar with lower ash content, better hydrophobicity, and higher calorific value [19]. These are essential properties that determine the effectiveness of biochar in its application to remove pollutants from soil and water. Microalgal was used for the wet torrefaction process in the study of Bach et al. [20]. This study result shows that 61.5% of microalgal biomass energy is protected, the calorific value is increased 21%, and the ash content is decreased.
Hydrothermal Carbonization
Recently, among thermochemical technology, hydrothermal carbonization (HTC) is known as a cost-effective and environment-friendly technology [21]. HTC transfers biomass into the solid product with high carbon content in water at temperatures between 180 and 250 °C and elevated pressure of 2–10 MPa [22]. The product of this process can be called hydrochar. Char from HTC process is usually uniform in shape and size [1]. During the conversion process, the temperature is the most influential factor on the efficiency of the HTC process, followed by residence time and the properties of the material feedstock [22].
HTC has many advantages over other conversion methods. Firstly, HTC synthesizes hydrochar under low-temperature conditions [23]. Lower temperature tends to generate a higher hydrochar yield. Secondly, HTC is a low-cost method to synthesize biochar [22]. Hydrochar is synthesized in the aqueous phase. Water is considered a solvent in the HTC process, leading to the wide application of this technology to remove pollutants in wastewater. Water present in the biomass or provided to the process is an effective solvent and reaction media during HTC. In recent studies, various feedstocks were selected for HTC, ranging from model substances to actual feedstock including cellulose, glucose, agricultural residue, animal manure, food waste, and agal residues [24]. The HTC process is clearly not limited to biomass, and feedstocks can be more complex.
There are many previous studies using algal as feedstock in the HTC process. Compared to terrestrial biomass, marine biomass, including less demand on agricultural land and higher photosynthetic activity. In the study of Levine et al. [25], Nannochloropsis oculata was used as feedstock in the HTC process at a temperature of 180–215 °C in the retention time of 15–45 min to generate the solid mass yield of 51%. Lipid extracted Spirulina was used in the HTC process with a temperature of 175 °C and a residence time of 30 min to produce the solid mass yield of 44.6% [26]. With the feedstock being Dunaliella salina, the solid mass yield can obtain 45.7% at the process temperature of 190–210 °C and the retention time of 30–120 min [27]. The chemical composition of the algae has a considerable impact on the hydrochar properties. However, one of the most difficult variables to investigate is algae composition. The positive link between feedstock carbon concentration and feedstock aromaticity and hydrophobicity has been attributed to the rise in hydrochar yield with feedstock carbon content. Feedstock solubility was shown to have a considerable impact on mass yield, which was explained by its role in the potential-rate limiting hydrolysis reaction of the HTC process.
Properties of Algal Biochar
Physical Properties
Several physical properties of algal biochar may be of interest, including structure, surface area, higher heating value, and biomass yield [1]. Algal biochar usually has a highly porous structure [28••]. Since most of the VOCs in the biomass has been removed during synthesis, algal biochar is easy to decompose [1]. The surface area of biochar is usually inversely proportional to the ash content of the feedstock [7]. Since algae have a high ash content, the surface area of algae biochar is often low [28••]. However, some studies show that increasing the temperature of the conversion process can achieve a higher surface area of solid products due to the escape of volatile substance and thus format channel structures during pyrolysis [28••]. These channel structures facilitate to improve the specific surface area and pore structure of biochar. A decrease in pore size formats internal pore structure and increases in porosity as a result of volatile release during carbonization. However, the properties of algal biochar vary slightly within its genus level and largely with the biochar from lignocellulosic biomass. For example, using HTC method, the biochar with more functional groups, high surface area, and spherical structure with a limited porosity can be prepared. Chemical activation improves the structural properties of biochar.
The higher heating value (HHV) is the amount of heat recovered when combustion takes place. The higher heating value recorded when microalgal and macroalgal were used as the feedstocks is around 7.6–23 MJ/kg and 5.2–5.21 MJ/kg, respectively [1]. Biochar derived from algal biomass generally has a lower HHV than those derived from lignocellulosic biomass, likely due to a lower carbon content but higher ash content [1].
The yield of algal biochar produced is a parameter of interest. Biochar is produced by algae in relatively high quantities per unit of biomass, as previously reported. However, compared to some other feedstocks, such as straw and green waste, the biochar yield generated using algal biomass is lower [8•]. Furthermore, by increasing the temperature and residence time of the conversion process, algal biochar yield tends to decrease [29].
Chemical Properties
Inorganic Ingredients
By pyrolyzing algae biomass, inorganic components (Cl, S, P, K, and Na) are volatilized and melted and can form molten sodium sulfate. While non-volatile and molten components are retained in the biochar. Some previous studies have shown that the inorganic content of algal biochar is higher than that of terrestrial biochar [30, 31]. With a high inorganic content, algal biochar offers potential agricultural applications as fertilizers to improve soil and provide nutrients to plants [6]. The mineral content of algal biochar varies depending on the type of algae biomass and their habitat [32]. For instance, some algae growing up in heavy metal pollution areas often show high levels of heavy metals in biochar upon pyrolysis. The inductively coupled plasma atomic emission spectrometry (ICP-AES) is commonly used to determine the mineral content of algal biochar.
Cation Exchange Capacity (CEC)
One of the important chemical properties of algal biochar is its cation exchange capacity (CEC). The cation exchange capacity of algal biochar plays a critical role in determining its ability to capture cationic nutrients [1]. The abundance of cationic nutrients in algal biochar opens up their application in nutrient retention and soil improvement. Previous studies have shown that the cation exchange capacity of algal biochar is higher than that of terrestrial biochar, especially for Ca, Na, Mg, and K cations [8, 31].
Functional Groups
Various functional groups have been detected in algal biochar, such as COOH, -OH, C = C, C = O, CH2, and C-O [33, 34]. A high abundance of these functional groups in algal biochar has been reported. This property is significant in water treatment by forming surface complexes between functional groups and pollutants in water [7]. The ability of microalgae to absorb organic and inorganic pollutants can be attributed to their high number of functional groups that enable them to perform biosorption [35, 36].
pH, Proximate, and Ultimate Analysis
The pH value of algae biochar is alkaline, ranging from 7.6 to 13.7 [1]. Pyrolysis temperature affects the pH value of the biochar. As pyrolysis temperature rises, acidic functional groups decompose and alkaline minerals are conversely enriched in biochar, resulting in an increase in pH [37]. When the pyrolysis temperature is increased from 250 to 600 °C, the pH of macroalgae-derived biochar rises from 8.7 to 13.7 [1, 8•].
Proximate analysis of algal biochar includes the measurement of volatile matter, moisture content, fixed carbon, and ash content. The volatile content in algal biochar is usually low due to the pyrolysis process [1]. After pyrolysis, the ash content and fixed carbon are increased. The removal of volatiles through pyrolysis and the accumulation of inorganic matter in algal biomass resulted in an increase in ash content [6].
For the ultimate analysis, the content of C, H, N, O, and S in algal biochar is of interest. Besides, the ratios H/C, O/C, and C/N are also assessed. The content of these elements in algal biochar is determined by the properties of the feedstock and the temperature of the pyrolysis process. The C content is observed to have an increase and the H, O, and S content decreased significantly in algal biochar [1]. Meanwhile, the N content is almost unchanged during the pyrolysis process [1]. Specifically, the atomic ratios of O/C to H/C have been used to describe the pyrolysis carbonization process, and more specially to describe several key biochar environmental longevity factors as a function of these ratios. Biochar with H/C ratios < 0.7 have greater fused aromatic ring structures and have been thermochemically altered as compared to biochar with H/C ratios > 0.7 [38]. Meanwhile, biochars with O/C ratios < 0.2 are highly stable (half-life > 1000 years), between 0.2 and 0.6 are moderately stable (half-life between 100 and 1000 years), and > 0.6 are relatively unstable (half-life less than 100 years) [38].
Factors Affecting the Properties of Algal Biochar
The properties of biochar reported by previous studies as a preliminary set for the biochar evaluation include pH, bulk density, volatile chemical, ash content, water-holding capacity, specific surface area, and porosity [39]. There are two major categories of biochar properties: physical properties and chemical properties, for example. Surface area, surface charge, total pore volume, porosity, pore size, and water holding capacity are listed as physical properties [40]. The chemical properties are investigated through proximate analysis (e.g., carbon content, ash, moisture, and volatile matter), ultimate analysis (e.g., organic and inorganic elemental analysis), pH, electrical conductivity, cation exchange capacity (CEC), and functional groups [1, 40].
The properties of biochar are varied by the kind of biomasses and production process [37, 40]. Each algal species contains unique components (lipids, cellulose, and protein, for example) and sizes (macroalgae and microalgae), which contribute to the unique properties of the biochar generated from the algae biomass [41•]. Biochar is made from biomass via a variety of thermochemical conversion processes, including hydrothermal carbonization, hydrothermal liquefaction, torrefaction, pyrolysis, and gasification [1, 41•, 42]. Each synthesis method has its own set of temperature parameters that influence the yield, and physicochemical properties of biochar.
The algal biomass component is the main factor that decides algal biochar composition. The proportion of main components (C, H, N, O, S, and P, as well as the ash content) in biochar derived from various algae species varies greatly [40]. Feedstocks are the main factor that determines the properties of biochar [39]. The type of feedstock affects the biochar properties in both physical and chemical properties directly such as surface area, ash content, fixed carbon content, pH, H/C ratio, cation exchange capacity [3, 43]. Biochar yield is highly dependent on the type of algal biomass. In terms of physical parameters, microalgal biochar has a smaller surface area than biochar generated from macroalgae. The yield percentages of biochar generated from microalgae and macroalgae are 20–63% and 8.1–62.4%, respectively [40]. For chemical properties, the previous studies dedicated that microalgal biochar is poor in cation exchange capacity, and C content yet rich in N, and harvestable inorganic nutrients [44]. Macroalgae have fixed carbon content and higher ash content than microalgae. The ash content of macroalgae is double greater than that of microalgae [3]. The fixed carbon content of macroalgae and microalgae ranges are 4.9–29.1% and 1.7–27.0% respectively (Karthik et al., 2021). Additionally, the component of biochar-based algae is also affected by abiotic and biotic factors such as algae species and their environment habitat [1]. The biochar made from seaweed showed low cation exchange capacity, and carbon content but high N, H, ash content, and electrical conductivity [42]. Roberts et al. [45] reported that the concentrations of C and H in biochar derived from red seaweeds are lower than those derived from brown seaweeds, although the concentrations of S and K are higher.
In addition to feedstock, biochar production methods and pyrolysis temperature are two significant parameters that determine the properties of algal biochar [1, 46]. The pyrolysis temperature affects algal biochar properties such as surface area, porosity, pH, zeta potential, graphitization degree, and electrical conductivity. Previous research demonstrated that when pyrolysis temperature increased, the surface area of algal biochar increased [2]. On the contrary, the biochar yield will reduce when the pyrolysis temperature rises. Yang et al. [44] revealed that as the pyrolysis temperature increased to 350 °C, the biochar yield reduced dramatically. Increasing the temperature from 500 to 900 °C enhanced the surface area and porosity by 25.4 to 67.6 m2 g−1 and 0.056 to 0.099 cm3 g−1, respectively, When the temperature was raised from 500 to 900 °C, the porosity and the surface area of biochar increased from 0.056 to 0.099 cm3 g−1 and from 25.4 to 67.6 m2 g−1, respectively [37]. As the pyrolysis temperature increases from 400 to 1000 °C, the zeta potential of biochar increases [37]. When the carbonization temperature of algal biomass exceeds 700 °C, the additional properties of algae biochar, such as crystallinity, electric conduction, and specific surface area, increase dramatically [37]. Besides the higher pyrolysis temperature, the decreased dissolved organic matter content and ash also could contribute to the increased surface area of algal biochar. [43].
Additionally, Yu et al. [1] dedicated that biochar-produced properties are affected by the operating conditions (e.g., heating rate, reaction vessel, chemical activation, residence time, and highest treatment temperature (HTT)). Especially, the highest treatment temperature showed the greatest effect [1]. In recent years, biochar promoted its characteristic for production purposes by activating one or some properties of biochar. The biochar activation method can improve its surface area. Zhou et al. [47] reported that KOH-treated Kelp biochar had a high specific surface area of 507.177 m2 g−1. According to Yang et al. [48], water washing enhanced the surface area of biochar derived from Ulva prolifera from 13.46 to 257.41 m2 g−1. Steam activation [49] and H3PO4 acid washing [50] also can increase the surface area of biochar, but further research is needed on algal biomass.
In general, many factors are affecting the properties of algae biochar. Every factor may affect one or many properties. At the same time, every characteristic of biochar is affected by one or some factors. Depending on algal biochar applications such as soil amendment, biofuel conversion, wastewater treatment, its properties will be chosen and promoted for suitable applications.
Activation Strategies of Biochar
The raw biochar is not possibly employed for various application due to the low specific surface area and porosimetry. Hence, biochar must be functionalized through activation processes. The morphology and chemical composition of the biochar are altered by activation processes such as physical, chemical, and biological method. The surface area and porosity of biochar are crucial parameters that must be thoroughly explored, either that or suitable properties should be created on biochar by the use of appropriate activation methods. This is significant in relation to the value of surface area, pore size, pore volume, and pore distribution in the biochar. Without adequate activation, the resulting biochar has (i) a large number of intermolecular gaps caused by the breakdown of bonds between the organic matters; (ii) obstructed pores, which result in the formation of tar; (iii) scarcity of pore channel in relation to narrow distribution of surface area; and (iv) impurities such as condensates and ashes, which cause the pore size and volume to decrease [42]. Numerous activation approaches are being investigated in order to develop an efficient biochar catalyst for environmental and energy applications. In general, there are two types of activation strategies for algal biochar modification, namely physical and chemical activation.
For physical activation, the flow of CO2, steam, or a mixture of these gas agents was directed through the pores of the biochar at high temperature (> 700 °C). The most reactive carbon atoms were removed by oxidation during this procedure, resulting in the development of new pores, the enlargement of existing holes, and the formation of a large number of oxygenated functional groups (OFGs) on biochar [51]. The surface of biochar experiences the following chemical reactions during the pyrolysis process: (i) the oxygen in water molecules adsorbed the surface of the free binding sites of biochar, forming surface hydrogen complexes and CO; (ii) the water–gas shift process occurs, where CO combines with water to generate CO2 and H2 gas; and (iii) the resultant gases activate the biochar. With exception of unblocking pores, steam activation increases the aromaticity and decreases the polarity of the biochar. Shim et al. [52] reported that the surface area of algal biochar (Miscanthus) nearly doubled while the polarity was decreased after the steam activation process (activation temperature of 800 °C). Notably, the steam activation requires less energy than CO2 activation as reported by Ranguin et al. [53]. In contrast to CO2, which forms and widens micropores, steam produces both micropore and mesopore, resulting in a wider range of pore size distribution [54••].
Chemical activation is the process of impregnating a biomass with a chemical activating agent in an inert atmosphere, mainly pyrolyzed between 450 and 900 °C. Carbonization and activation of biomass occur simultaneously during the one-step modification process. Chemical activation is deemed more economically feasible than physical activation because it needs less processing time, decreases the activation temperature, produces high-quality porous biochar, and requires only one step. Activating agents that have the potential to act as a strong dehydrator during activation process are classified as follows: (i) acids, i.e., H2SO4 and H3PO4, (ii) alkalis, i.e., KOH and K2CO3, (iii) transition metal salts, i.e., AlCl3 and ZnCl2. Sevilla et al. [55] used KOH as activating agent for the producing biochar from microalgae. The resulting biochar had a large surface area of up to 2200 m2 g−1, which was exclusively attributed to micropores. Nguyen et al. reported algal biochar with a high surface area (1326 m2 g−1) and total pore volume (0.93 cm3 g−1) produced from ZnCl2-assisted pyrolysis of Ascophyllum nodosum. However, because ZnCl2 has negative environmental consequences, it is only used in a limited number of chemical activation procedures. In general, chemical activation significantly increases the surface area and porosity of activated carbon as compared to physical activation [56].
Environmental Remediation
Carbon Sequestration
The global warming induced by the increased greenhouse gas emissions has been increasingly noticed for 20 years. Therefore, proposing strategies to reduce carbon emission is essential. Different approaches such as chemical, mechanical method, and filtration have been explored for mitigating the CO2 emission [57]. Utilizing biochar for carbon capture and sequestration has been potentially studied for minimizing the climate change effects [58]. A past study highlighted that biochar possesses a long residence time in soil (> 1000 years), which favors for carbon sequestration [59]. In addition, due to its high specific surface area (SSA), porous structure, and suitable quantity of functional groups on surface, biochar generated from algae can be used as a CO2 adsorbent. It was found that biochar could sequester about 12% of greenhouse gases [60]. As demonstrated, microalgae were a powerful candidate to sequester carbon generated from the thermal power plant [61]. In detail, a previous work indicated that algae can efficiently capture the CO2 concentration of 5–15% form the flue gases [61]. Such outcomes attained a significant higher CO2 capture compared to the terrestrial plants. Ghorbani et al. [62] reported green algae species, i.e., CO2 fixation rates of 6.24 and 1.45 g L−1. day have been achieved by Chlorella Vulgaris and Anabaena species, respectively. Another species (Spirulina sp.) reached a maximum biofixation efficiency of 37.9% at 6% CO2 (v/v). Algae potential for mitigating CO2 emission was reinforced by a past work, which showed that 1 kg of cultivated microalgae can fix 1.83 kg of CO2 in the atmosphere [63]. Moreira and Pires [64] concluded that algae are capable of amassing a high lipid content, which favors storing twice as much energy per carbon. Yu et al. [65] reported the growth of the microalga C. vulgaris FSP-E and the pyrolysis-based generation of its microalgal biochar. At a feeding CO2 concentration of 2.5%, the maximum biomass productivity of C. vulgaris FSP-E was 0.87 g L−1 day−1. The yield of biochar from the pyrolysis of microalgal biomass was 26.9% of the total amount. The biochar made from C. vulgaris FSP-E has an alkali pH value and favorable O/C (< 0.4) and H/C (< 0.6) ratios for sequestering carbon and soil treatment. In considerations of the agricultural environment, the introduction of algal biochar to soil increased the retention period of key critical nutrients, hence improving soil fertility and crop production efficiency. The increased surface area of biochar boosts soil population of iron-reducing microorganisms, lowering CH4 production by competing with methanogens. As a result of using biochar in agricultural soil, carbon sequestration was increased, resulting in fewer emissions of CH4 gas into the environment [61]. Thus, employing algae to fix CO2 and converting algal biomass to biochar may be a viable strategy for carbon capture and the development of renewable energy sources.
Adsorbent
Numerous recent studies have focused on the application of tailored algal biochar as biosorbent for remediation of pollutant in aquatic environment. Algal biochar, a green biomass, was modified not only to satisfy critical standards (renewable, sustainable, and low-cost production) but also to improve remediation for inorganic (phosphate and heavy metal) and organic contaminants (micro-pollutants).
Algal biochar is an excellent adsorbent due to the presence of a significant number of functional groups. Inorganic removal by algal biochar is governed by the main mechanisms such as electrostatic attraction, physical adsorption, ion exchange, and inner sphere complexation. Recent years have seen the development of composites of algal biochar by combining biochar with various substances to boost its maximal adsorption capability. As shown in Table 1, several macroalgae species (e.g., Laminaria japonica and Undaria pinnatifida) were used as feedstocks for biochar production via modification techniques such as (1) magnesium/aluminum layered double hydroxide, (2) metal oxide/hydroxide (Fe, Mg) impregnation, and (3) electrochemical modification. The maximum phosphate adsorption capacity attained as high as 887 mg g−1 for macroalgae (Laminaria japonica) modified using magnesium/aluminum layered double hydroxide, which was consistent with Freundlich and Langmuir isotherm model. It has been observed that the Mg/Al doping in the biochar can generate a significant amount of colloidal or nanosized particles on the surface of biochar, leading to the formation of polynuclear complexes with P in aqueous solution via the surface interaction mechanism [66]. Biochar often has a negative zeta potential at neutral pH, indicating that it can readily absorb positively charged ions such as metal ions. In complexation, ligands and functional groups (-OH and -COOH) on the surface of biochar form complexes with different metals to absorb heavy metals. Additionally, given the high mineral content of marine algae, cation exchange and precipitation may be additional mechanism for heavy metal adsorption (Na, K, Mg, and Ca). Liu et al. [67] prepared biochar from blue algae (Microcystic) for the effective adsorption of Cd in different water matrixes (135.6 mg g−1). Precipitation with minerals was the primary mechanism, with a performance of 68.7–89.5%, according to the results of the characterization methods. Moreover, iron oxide-doped biochars derived from waste marine algae (kelp and hijikia) were able to effectively remove heavy metals (Cd2+, Cu2+, and Zn2+) from water, and the biochar was easily recovered from the aqueous solution using external magnetic forces [68•]. Wang et al. [69] reported the utilization of δ-MnO2-modified biochar derived from Rhizoclonium (MnO2/CB) for uranium (U(VI)) removal from aqueous solution. The complexation between U(VI) and surface OFGs mostly governed the U(VI) adsorption on MnO2/CB.
The adsorption of organic pollutants by biochar is found to be dependent on the properties of the pollutants and the surface chemistry of biochar. The major adsorption routes for organic contaminants are the electrostatic interaction, hydrogen bonding, pore-filling route, and π-π electron donor–acceptor (π-EDA) [37]. Zheng et al. [70] found that the more polarizable the OFGs in algal biochar, the stronger the attractive force that may be created during the organic adsorption process. Through electrostatic contact, the OFGs on the surface of algal charcoal can act as binding sites for ionized forms of organic contaminants. OFGs groups such as carbonyl (-COOH) and hydroxyl (-OH) serve as proton donors, attracting various contaminants (proton acceptors) to the active sorption sites via hydrogen bonding; nonetheless, this type of bonding is classified as form of weak ionic interaction [71]. For π-EDA process, the graphitic structure of algal biochar with high abundance of aromatic ring exhibits an attraction toward the aromatic ring on the pollutant such as dye and antibiotics. The algal biochar was rich in crystalline minerals and heterogeneous OFGs and NFGs than the terrestrial plant-derived biochar. High thermal pyrolysis temperature for algal biochar preparation facilitates excellent sorption capacities for sulfamethoxazole (SMX), which were dominated by cation bridging, then followed by π-EDA interaction and pore filling. [72]. To enhance the adsorption toward antibiotics, biochar derived from brown algae Ascophyllum nodosum was modified through hydrothermal method coupling with chemical activation (ZnCl2), which showed a good result of maximum adsorption capacity (150 − 400 mg g−−1) in different conditions.
Catalyst
Algal biochar–based catalysts have been widely applied in different systems for the degradation of contaminants in wastewater, including, Fenton like reactions, and photocatalytic systems [73, 74]. Ho et al. [75] fabricated N-doped graphitic biochars (SDBC) that were synthesized from Spirulina residue. SDBC was used as catalysts for peroxydisulfate (PDS) activation and sulfamethoxazole (SMX) oxidation. SMX was chosen as the target pollutant to assess the catalytic performance of SDBC in PDS activation. The SDBC/PDS system could degrade over 90% of SMX. Chen et al. [76] prepared a biochar catalyst using Enteromorpha, a kind of green algae that is rich in nitrogenous compounds, to activate peroxymonosulfate (PMS) for paracetamol degradation. It was found that the highest degradation efficiency of paracetamol was obtained at following conditions: pH = 10.5, [Fe–N@C] = 0.1 g L−1, [PMS] = 1 mM, and reaction time = 30 min. The authors demonstrated that the paracetamol degradation mechanisms were due to both the radical pathways of O2− and non-radical 1O2 generated in the Fe–N@C/PMS system. In another study, Qi et al. [77] fabricated three-dimensional graphene-like biochar derived from Enteromorpha (EGB) to activated persulfate (PS) for sulfamethoxazole (SMX) degradation. The results have shown that complete removal of SMX was achieved at EGB concentration of 0.05 g L−1 within 90 min and the kobs value was 0.0655 min−1. Wang et al. [78] investigated the catalytic ability of Taihu blue algae–derived biochar. The generated Fe (III)-ABC composites were synthesized by means of pyrolysis combining with KOH activation and Fe (III) loading. The biochar composites have excellent catalytic performance, degradation efficiency of nickel of about 98.87% at conditions of pH at 6, Fe (III)-ABC of 0.5 g, and H2O2 dosage of 20 mM, reaction time at 60 min. Kelp is a representative algae plant, a type of substances rich in nitrogen. Huang et al. [79] used kelp biomass to prepare N-doped kelp biochar (KB) material. Their material was applied to activate peroxymonosulfate (PMS) for ofloxacin degradation. The result showed that the KB degraded 40 mg L−1 Ofloxacin close to 100% within 60 min, applied with PMS. Algal biochar–based heterogeneous catalysts have also proven to be successful in photo-Fenton reactions. Photocatalyst has been studied to degrade textile dyes such as methylene blue (MB) and malachite green (MG) from aqueous solutions [80,81,82]. Zhou et al. [80] evaluated the catalytic potential of an Activated Kelp Biochar (AKB), modified by KOH impregnation, and Bi2MoO6–AKB composite (BKBC) was fabricated by a combination of pyrolysis and a solvothermal technique to remove the methylene blue in aqueous solution. It was found that adsorption and photodegradation progress of methylene blue (80 mg L−1) by AKB, BKBC was 94.12% and 61.39%. The photocatalytic degradation was initiated after sorption for 4 h. A type of algae was obtained from the Barog Kotla revolute in the village of Bhajol near Shoolini University in Solan and used as a precursor for the manufacture of algal biochar (AlBc) [81]. The catalyst, algal biochar@La/Cu/Zn trimetallic nanocomposite (AlBc@La/Cu/Zn/TNC), was fabricated to remove malachite green. The result has shown that 63% removal of the malachite green by AlBc@La/Cu/Zr TNC under dark conditions and photo-degradation of dye by AlBc@La/Cu/ZrTNC in solar light discovered that of 87% of malachite green was remediated in 5 h. The result revealed that the \(^{\bullet }\mathrm{OH}\) radical generated by composite was the main responsible for the photodegradation of malachite green. Fazal et al. [82] made macroalgae biochar-TiO2 composite (BCT). Photocatalytic performance of the prepared samples is evaluated by photocatalytic degradation of methylene blue under light illumination. The irradiation of BCT generated \(^{\bullet }\mathrm{OH}\), \(^{\bullet }{{\mathrm{O}}_{2}}^{-}\) radicals which degraded the methylene blue through the following reaction [82]:
The algal biochar-photocatalyst composite shows good photocatalytic behavior because of biochar provides adequate support for various photocatalyst, increase the attachment sites, increase the interaction of pollutant and photocatalyst, rapid transfer of the electron inhibiting the electrons/holes recombination, and reduce the band gap of the photocatalyst [74].
Sediment Remediation
Sediment pollution has emerged as a worldwide environmental issue since the 1980s and has received great attention [83, 84]. Pollutants in the sediment exist in both organic and inorganic forms [84, 85]. Thus, sediment remediation is urgently needed. In recent years, biochar produced from the algae has been employed to eliminate the contaminants in sediment such as 4-nonylphenol (4-NP), Di-(2-ethylhexyl) phthalate (DEHP), and polycyclic aromatic hydrocarbons (PAHs) [86,87,88,89]. Hung et al. [86] made the red algae raw material (RAB) for 4-nonylphenol (4-NP) degradation in the presence of sodium percarbonate (SPC). The calcium in RAB efficiently activated sodium percarbonate (SPC) to generate reactive radicals for the catalytic degradation of 4-NP at pH 9.0. In another study performed by Hung et al. [88], prepared red seaweed (Agardhiella subulata)-derived biochar (RSB) was used to activate sodium percarbonate (SPC) for the degradation of Di-(2-ethylhexyl) phthalate (DEHP) in contaminated surface sediments. The results have shown that the main species causing DEHP degradation was OH which was generated during the RSB activation of SPC. RSB was the best-performing SPC activator under the optimal initial pH of 9. The total DEHP degradation was 63% in 12 h. Hung et al. [87] prepared nitrogen-enrich biochar carbocatalyst (BAB) that was derived from brown algal (Sargassum duplicatum). BAB and melamine pyrolysis product (N-BAB) effectively activated peroxymonosulfate (PMS) for the degradation of polycyclic aromatic hydrocarbons (PAHs) in marine sediments. The results have shown that the catalyst N-BAB could degrade 86% of PAHs in 12 h under optimal condition: [PMS] = 1 × 10−4 M, [N-BAB] = 3.3 g L−1, and pH = 3.0. Hung et al. [89] prepared boron-doped biochar (B-BAB) from pristine brown algae (Sargassum duplicatum) and boric acid using a facile pyrolysis method. The B-BAB was then used as catalyst for the activation of peroxymonosulfate (PMS) for aromatic hydrocarbons (PAHs) degradation in marine sediments. Results revealed that B-BAB has superior catalytic capacity for PMS activation, degradation efficiency of PAH of about 93% at conditions of pH at 3, B-BAB of 1.0 g L−1 and PMS dosage of 1 × 10−4 M, reaction time at 12 h. The authors reported that the boron-doped biochar enhanced PAH degradation in the B-BAB/PMS system due to \({\mathrm{SO}_4}^{\bullet -}\), \(^{\bullet} \mathrm{OH}\), and 1O2. Though the algal biochar has the good properties to deal with recalcitrant organic contaminants in sediment, studies are at a lab-scale, which requires substantial efforts to scale up the treatment procedure for a large volume of the sediment.
Energy Application
Biofuel Conversion
Because of rapid growth rate and ability to be cultivated in waste water or waste land, microalgae are believed to be a good source to generate renewable energy in several ways. Carbohydrates, proteins, and lipid/natural oils are the three primary components of algal biomass. Carbohydrates are mainly formed in algal biomass in the form of polysaccharides and sugar monomers such as starch, cellulose, and glucose, similar to terrestrial plants [3]. As for the bio-oil and syngas production aspect, glucose and starch are desirable feedstock for bio-ethanol and H2 production [90]. Depending on the algal species, growing conditions, seasons, and location considerations, the lipid content of algae normally ranges from 0.9 to 71.5%wt. Microalgae like Chlorella pyrenoidose with lipid content of up to 71.5 wt% is the ideal feedstocks for lipid extraction and transesterification into biofuels [3]. Algal bio-oil also has low sulfide content, which results in negligible SOx emission after combustion. Therefore, microalgae biofuels appear to be among the most effective alternatives to petroleum. In addition to their role in mitigating greenhouse gas emissions, microalgae biomass is considered one of the most efficient renewable energy sources for biofuel production due to its high energy yield and low cost. According to reports, 1 kg of algal biomass can sequester 1.83 kg of carbon dioxide [91].
A wide range of technologies can be used to convert the organic molecules into a useable form of fuel based on the structural variability of the various types of biomass available. Algal biomass can be treated by pyrolysis to generate crude bio-oil or converted into methane by anaerobic digestion [92]. Lipid extraction from dry/wet microalgae followed by upgrading and hydrothermal liquefaction are two techniques which may process wet microalgae directly [44]. However, the conversion of biomass into the bio-oil using pyrolysis technologies is one of the most studied technologies over the past 50 years [93]. Fast pyrolysis is typically used for optimal bio-oil production because the short residence time of volatiles in the reactor does not allow extensive secondary reactions of volatile matters that would reduce the yield of bio-oil and increase the yield of biochar [3]. Moreover, the biochar produced form algae can be employed for energy application and environmental remediation.
Despite all these developments, algae bio-oil is not suitable for direct usage in engines or commercial production of valuable chemicals without further treatment. This is mainly due to the high acidity, high oxygen content, high viscosity, and low calorific value. One of the common problems for bio-oil from all biomass types comes from the fatty acid and aldehyde contents, which cause corrosiveness and low chemical stability of produced bio-oil [94]. Due to the aqueous growth environment, most of the algal biomass has high moisture content with over 95 wt%, leading to a high moisture content of the pyrolytic bio-oil, thereby affecting the quality of the bio-oil [7]. In any case, drying of algae is energy-intensive, which requires 3–5 MJ of energy to reduce the moisture content by 10–15 wt% [95]. The high oxygen content (10–30 wt%) of algal bio-oil compared to heavy petroleum fuel oil (about 1.0 wt%) can lead to low higher heating value (HHV) of bio-oil, which is a huge barrier for it to become the fossil fuel alternative [94]. Furthermore, algal bio-oil with the high protein content is potentially causing NOx emission [96]. Therefore, a considerable denitrification treatment of algal bio-oil is necessary after fast pyrolysis.
To overcome high moisture or oxygen content limitation, co-pyrolysis is a potential strategy for improving bio-oil yield and quality by blending algae with another feedstock. In the co-pyrosis of microalgae and low-density polyethylene (LDPE), the presence of LDPE promoted the formation of formic/acetic ester and long-chain alcohol, whereas it reduced O content from 45.5 to 10.2 wt% in Enteromorpha prolifera bio-oil and N content from 19.5 to 6.6 wt% in Spirrulina platensis bio-oil [97]. The bio-oil yield was significantly increased because the volatile content of LDPE can crack into liquid products. The co-pyrolysis of Enteromorpha prolifera with waste plastics (HDPE) showed the sharp decrease in the nitrogen-containing compounds, oxygenates, and acids in algal bio-oil while light hydrocarbons and aromatics contents were increased [98].
Several studies have reported a significant yield of biodiesel with the use of algal biochar as a catalyst during the trans-esterification process [99••]. Large pore size, more active sites, and the presence of hydrophilic sulfonic acid group (-SO3H) make the biochar catalyst an easily accessible one for the reactants. Fu et al. [100] developed a novel biocatalyst (MBC) for biodiesel production via an in situ hydrothermal partially carbonization using microalgae residue as the raw material. The sulfonated MBC catalyst exhibited excellent catalytic activity when used to esterify oleic acid and trans-esterify triolein with methanol. The high density of -SO3H and OFGs in the carbon catalyst made a good affinity between the hydrophilic parts of the reactants and active sites of biocatalyst, favoring the dispersion of the catalyst in methanol and subsequently resulting in good catalytic conversion [101]. With appropriate treatment, algal biochar has proven to be a good heterogeneous biocatalyst for biodiesel production via esterification and trans-esterification.
Hydrogen Production
Biochar is considered an emerging catalyst/support for hydrogen production from biomass gasification. Hydrogen production is facilitated by biochar catalysts that have been discussed [2, 99••]. Because of its low cost, environmental friendliness, wide surface area, and ability to be reused, biochar can be used as heterogeneous catalysts in the generation of biohydrogen. Furthermore, biohydrogen, with a calorific value of 140 kJ g−1, is considered the cleanest and purest type of fuel, as its byproduct during combustion process is solely water vapor, posing no significant environmental danger. In a membrane reactor, microalgae Galdieria sulphuraria–derived biochar was converted to hydrogen using hydrothermal liquefaction (HTL), one of the most effective thermochemical conversion methods [102]. In the same conversion technique, 23.7% hydrogen gas was produced by Chorella vulgaris [103] and Scenedesmus obliquus microalgae produced 11.2% hydrogen [104]. Algal biochar from Sargassum was discovered to produce just 3 mmol of hydrogen per gram of Sargassum [105]. Cladophora glomerata algae impregnated with iron had a greater hydrogen yield of 7.99 mmol/g [106]. In another study, Cladophora glomerata was converted into magnetic biochar that produced 22% hydrogen via a slow pyrolysis process promoted with iron [107•].
Microbial Fuel Cell
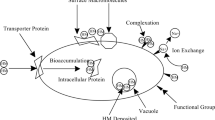
To deal with energy crisis worldwide, an alternative to fossil fuels is to use renewable energy sources including solar, hydro, wind, and biomass. But their utilizations are not effective to make them fail to satisfy the growing demand for energy due to the increase of population and industrialization. The developments of the microbial fuel cell (MFC) have contributed a great concept for bioelectricity generation as promising, cost-effective, environmentally friendly, and sustainable energy production approaches [108, 109]. To develop a low-cost MFC system, biochar could be incorporated in three possible options, including electrodes (anode and cathode), electrocatalyst, and developing polymer electrolyte membrane with it [110]. As major components of an MFC, electrodes are required to facilitate exoelectrogenic biofilm growth and electrochemical reactions and improve the electrochemical performance of MFC. Therefore, developing cost-effective and environmentally friendly electrode material for MFC such as biochar could be a potential opportunity for this MFC moving forward to be an advanced technology for our future (Fig. 2). Although there are numerous types of raw materials employed for biochar production, algal biomass is a viable source for the construction of biochar electrodes, and several applications of biochar in MFC have been reviewed [51, 111].
Furthermore, the activated biochar has been proven that it is applicable to be an economic electrode material in MFC, and microalgae-derived biochar has been demonstrated as catalyst that required less synthesis cost than that of Pt-C cathode catalyst, which was 112% extremely higher than that of microalgae-based activated biochar [112]. In a MFC, limiting factors such as slow reaction kinetics of oxidation reduction reaction (ORR) could be solved by applying catalyst resulting in high performance and the utilization of non-toxic source was useful to commercialize the MFC for practical application [112]. Another way to dealt with this issue is the use of air–cathode catalyst as a factor to enhance ORR. Doping heteroatoms containing nitrogen and phosphorous in carbon-based materials could be utilized to improve ORR. For example, the power density of 2068 mW m−2 was achieved using Chlorella pyrenoidosa as the precursor in a green and inexpensive N, P doped carbon catalysts, which was 13% higher than the result obtained using Pt as catalyst [112]. On the other hand, the use of microalgae was also helpful since a harmful algal bloom was harvested and then treated by pyrolysis to fabricate biochar anode to enhance electron uptake produced by Shewanella oneidensis MR-1 in MFC [113]. As a result, there was a 4.1-fold difference between the anodes made of normal graphite (2.2 µA cm−2) and those made of algal biochar (9.1 µA cm−2) in terms of current density. The decreased charge transfer resistance of the algal biochar anode resulted in faster charge transfer as a result of more bacteria adhering to the biochar anode, and the intimate contact between of MR-1 cells and algal biochar electrode may boost the performance. According to electrochemical impedance spectroscopy (EIS) measurement, biochar-derived electrode could, directly and indirectly, create electron pathways for generating current [113]. Various methods have been proposed to enhance efficiencies of electron transfer in bioelectrochemical system such as materials containing functional groups. Among cobalt and chitosan immobilized on Chlorella pyrenoidosa, microalgae-derived biochar was used as mediator for electron transfer and produced power density of 3.1 mW cm−2 [114•].
Future Research Needs
Algal biochar has the potential to influence global carbon sequestration and mitigation of climate change, from energy production to environmental remediation. However, the cost of algal biochar production and applications is determined by a variety of factors such as feedstock availability, raw material preparation, pyrolysis condition, and biochar reusability. More attention should be placed on practical applications, and more research should be performed to address existing problems: (1) Current research on the stability of algal biochar-based composites and their biological toxicity to aquatic and soil microorganisms is lacking; (2) different parameters or conditions used in the synthesis procedures need to be optimized for modifying the engineered algal biochar efficiently; (3) consideration should be given to the economic efficiencies beyond the lab scale stage as well as possible tradeoffs in practical applications as part of a life cycle assessment of algal biochar.
Conclusion
The biochar produced from algae is a carbon-rich porous solid that already has applications in environmental remediation and energy application. In terms of the algal biochar produced, it is dependent on the method and process parameters employed to make it. Pyrolysis condition and algae type are the main factors influencing the applicability of biochar. In addition to its direct value as a tool for sediment and water treatment, as well as energy applications, algal biochar could generate substantial revenue as a carbon sequestrant. Future environmental sustainability is anticipated to be enhanced by the development of algal biochar technologies.
Data Availability
All data generated or analysed during this study are included in this published article.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Yu KL, Lau BF, Show PL, Ong HC, Ling TC, Chen WH, et al. Recent developments on algal biochar production and characterization. Bioresour Technol. 2017;246:2–11. https://doi.org/10.1016/j.biortech.2017.08.009.
Bird MI, Wurster CM, Silva PHD, Bass AM, de Nys R. Algal biochar - production and properties. Bioresour Technol. 2011;102(2):1886–91. https://doi.org/10.1016/j.biortech.2010.07.106.
Lee XJ, Ong HC, Gan YY, Chen WH, Mahlia TMI. State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers Manag. 2020;210:34. https://doi.org/10.1016/j.enconman.2020.112707.
Demirbas A, Arin G. An overview of biomass pyrolysis. Energy Sources. 2002;24(5):471–82. https://doi.org/10.1080/00908310252889979.
Chen W-H, Lin B-J, Huang M-Y, Chang J-S. Thermochemical conversion of microalgal biomass into biofuels: a review. Bioresour Technol. 2015;184:314–27. https://doi.org/10.1016/j.biortech.2014.11.050.
Bird MI, Wurster CM, de Paula Silva PH, Bass AM, De Nys R. Algal biochar–production and properties. Bioresour Technol. 2011;102(2):1886–91. https://doi.org/10.1016/j.biortech.2010.07.106.
Sun J, Norouzi O, Mašek O. A state-of-the-art review on algae pyrolysis for bioenergy and biochar production. Bioresour. Technol. 2021:126258. https://doi.org/10.1016/j.biortech.2021.126258.
• Tag AT, Duman G, Ucar S, Yanik JJJ. Pyrolysis a. Effects of feedstock type and pyrolysis temperature on potential applications of biochar. J J Anal Appl Pyrolysis. 2016;120:200–6. https://doi.org/10.1016/j.jaap.2016.05.006. This article provides basic information about pyrolysis condition for biochar production.
Jung K-W, Jeong T-U, Kang H-J, Ahn K-H. Characteristics of biochar derived from marine macroalgae and fabrication of granular biochar by entrapment in calcium-alginate beads for phosphate removal from aqueous solution. Bioresour Technol. 2016;211:108–16. https://doi.org/10.1016/j.biortech.2016.03.066.
Chang Y-M, Tsai W-T, Li M-H. Chemical characterization of char derived from slow pyrolysis of microalgal residue. J Anal Appl Pyrolysis. 2015;111:88–93. https://doi.org/10.1016/j.jaap.2014.12.004.
Yanik J, Stahl R, Troeger N, Sinag A. Pyrolysis of algal biomass. J Anal Appl Pyrolysis. 2013;103:134–41. https://doi.org/10.1016/j.jaap.2012.08.016.
Bergman PC, Kiel JH. Torrefaction for biomass upgrading. Proc 14th European Biomass Conference, Paris, France. 2005:17–21.
Chen W-H, Huang M-Y, Chang J-S, Chen C-Y. Thermal decomposition dynamics and severity of microalgae residues in torrefaction. Bioresour Technol. 2014;169:258–64. https://doi.org/10.1016/j.biortech.2014.06.086.
Nhuchhen DR, Basu P, Acharya B. A comprehensive review on biomass torrefaction. Inter J Renew Energy Biofuels. 2014;2014:1–56. https://doi.org/10.5171/2014.506376.
Wu K-T, Tsai C-J, Chen C-S, Chen H-W. The characteristics of torrefied microalgae. Appl Energy. 2012;100:52–7. https://doi.org/10.1016/j.apenergy.2012.03.002.
Uemura Y, Matsumoto R, Saadon S, Matsumura Y. A study on torrefaction of Laminaria japonica. Fuel Process Technol. 2015;138:133–8. https://doi.org/10.1016/j.fuproc.2015.05.016.
Mwangi JK, Lee W-J, Whang L-M, Wu TS, Chen W-H, Chang J-S, et al. Microalgae oil: Algae cultivation and harvest, algae residue torrefaction and diesel engine emissions tests. Aerosol Air Qual Res. 2015;15(1):81–98. https://doi.org/10.4209/aaqr.2014.10.0268.
Yan W, Acharjee TC, Coronella CJ, Vasquez VR. Thermal pretreatment of lignocellulosic biomass. Environ Prog Sustain Energy. 2009;28(3):435–40. https://doi.org/10.1002/ep.10385.
Bach Q-V, Chen W-H, Sheen H-K, Chang J-S. Gasification kinetics of raw and wet-torrefied microalgae Chlorella vulgaris ESP-31 in carbon dioxide. Bioresour Technol. 2017;244:1393–9. https://doi.org/10.1016/j.biortech.2017.03.153.
Bach Q-V, Chen W-H, Lin S-C, Sheen H-K, Chang J-SJEC, Management. Wet torrefaction of microalga Chlorella vulgaris ESP-31 with microwave-assisted heating. Energy Convers Manag. 2017;141:163–70. https://doi.org/10.1016/j.enconman.2016.07.035.
Erlach B, Harder B, Tsatsaronis G. Combined hydrothermal carbonization and gasification of biomass with carbon capture. Energy. 2012;45(1):329–38. https://doi.org/10.1016/j.energy.2012.01.057.
Titirici M-M, White RJ, Falco C, Sevilla M. Black perspectives for a green future: hydrothermal carbons for environment protection and energy storage. Energy Environ Sci. 2012;5(5):6796–822. https://doi.org/10.1039/C2EE21166A.
Tekin K, Karagöz S, Bektaş SJR, Reviews SE. A review of hydrothermal biomass processing. Renew. Sustain. Energy Rev. 2014;40:673–87. https://doi.org/10.1016/j.rser.2014.07.216.
Wang TF, Zhai YB, Zhu Y, Li CT, Zeng GM. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: process conditions, fundamentals, and physicochemical properties. Renew Sustain Energy Rev. 2018;90:223–47. https://doi.org/10.1016/j.rser.2018.03.071.
Levine RB, Sierra COS, Hockstad R, Obeid W, Hatcher PG, Savage PE. The use of hydrothermal carbonization to recycle nutrients in algal biofuel production. Environ Prog Sustain Energy. 2013;32(4):962–75. https://aiche.onlinelibrary.wiley.com/journal/19447450.
Broch A, Jena U, Hoekman SK, Langford J. Analysis of solid and aqueous phase products from hydrothermal carbonization of whole and lipid-extracted algae. Energies. 2014;7(1):62–79. https://doi.org/10.3390/en7010062.
Heilmann SM, Davis HT, Jader LR, Lefebvre PA, Sadowsky MJ, Schendel FJ, et al. Hydrothermal carbonization of microalgae. Biomass Bioenerg. 2010;34(6):875–82. https://doi.org/10.1016/j.biombioe.2010.01.032.
•• Leng L, Xiong Q, Yang L, Li H, Zhou Y, Zhang W, et al. An overview on engineering the surface area and porosity of biochar. Sci Total Environ. 2021;763: 144204. https://doi.org/10.1016/j.scitotenv.2020.144204. This review paper highlights the relationships between the surface area/porosity of biochar and its application in environmental remeidation.
Ronsse F, Van Hecke S, Dickinson D, Prins WJGB. Production and characterization of slow pyrolysis biochar: influence of feedstock type and pyrolysis conditions. GCB-Bioenergy. 2013;5(2):104–15. https://doi.org/10.1111/gcbb.12018.
Wang K, Brown RC, Homsy S, Martinez L, Sidhu SS. Fast pyrolysis of microalgae remnants in a fluidized bed reactor for bio-oil and biochar production. Bioresour Technol. 2013;127:494–9. https://doi.org/10.1016/j.biortech.2012.08.016.
Roberts DA, Paul NA, Dworjanyn SA, Bird MI, de Nys R. Biochar from commercially cultivated seaweed for soil amelioration. Sci Rep. 2015;5(1):1–6. https://doi.org/10.1038/srep09665.
Sun D, Lan Y, Xu EG, Meng J, Chen WJWM. Biochar as a novel niche for culturing microbial communities in composting. Waste Manage. 2016;54:93–100. https://doi.org/10.1016/j.wasman.2016.05.004
Jin H, Hanif MU, Capareda S, Chang Z, Huang H, Ai Y. Copper (II) removal potential from aqueous solution by pyrolysis biochar derived from anaerobically digested algae-dairy-manure and effect of KOH activation. J Environ Chem Eng. 2016;4(1):365–72. https://doi.org/10.1016/j.jece.2015.11.022.
Liu P, Rao D, Zou L, Teng Y, Yu H. Capacity and potential mechanisms of Cd (II) adsorption from aqueous solution by blue algae-derived biochars. Sci Total Environ. 2021;767: 145447. https://doi.org/10.1016/j.scitotenv.2021.145447.
Guo W-Q, Zheng H-S, Li S, Du J-S, Feng X-C, Yin R-L, et al. Removal of cephalosporin antibiotics 7-ACA from wastewater during the cultivation of lipid-accumulating microalgae. Bioresour Technol. 2016;221:284–90. https://doi.org/10.1016/j.biortech.2016.09.036.
Zeraatkar AK, Ahmadzadeh H, Talebi AF, Moheimani NR, McHenry MPJJ. Potential use of algae for heavy metal bioremediation, a critical review. J Environ Manage. 2016;181:817–31. https://doi.org/10.1016/j.jenvman.2016.06.059.
Chen YD, Liu FY, Ren NQ, Ho SH. Revolutions in algal biochar for different applications: state-of-the-art techniques and future scenarios. Chin Chem Lett. 2020;31(10):2591–602. https://doi.org/10.1016/j.cclet.2020.08.019.
Ippolito JA, Cui LQ, Kammann C, Wrage-Monnig N, Estavillo JM, Fuertes-Mendizabal T, et al. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: a comprehensive meta-data analysis review. Biochar. 2020;2(4):421–38. https://doi.org/10.1007/s42773-020-00067-x.
Sohi SP, Krull E, Lopez-Capel E, Bol R. A review of biochar and its use and function in soil. In: Sparks DL, editor. Advances in Agronomy, Vol 105. Advances in Agronomy. San Diego: Elsevier Academic Press Inc; 2010. p. 47–82.
Karthik V, Kumar PS, Vo DVN, Sindhu J, Sneka D, Subhashini B, et al. Hydrothermal production of algal biochar for environmental and fertilizer applications: a review. Environ Chem Lett. 2021;19(2):1025–42. https://doi.org/10.1007/s10311-020-01139-x.
• Kumar G, Shobana S, Chen WH, Bach QV, Kim SH, Atabani AE, et al. A review of thermochemical conversion of microalgal biomass for biofuels: chemistry and processes. Green Chem. 2017;19(1):44–67. https://doi.org/10.1039/c6gc01937d. This article summarizes the production of renewable biofuels from algaes via thermal conversion processes.
Anto S, Sudhakar MP, Ahamed TS, Samuel MS, Mathimani T, Brindhadevi K, et al. Activation strategies for biochar to use as an efficient catalyst in various applications. Fuel. 2021;285:8. https://doi.org/10.1016/j.fuel.2020.119205.
Sun YN, Gao B, Yao Y, Fang JN, Zhang M, Zhou YM, et al. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem Eng J. 2014;240:574–8. https://doi.org/10.1016/j.cej.2013.10.081.
Yang CY, Li R, Zhang B, Qiu Q, Wang BW, Yang H, et al. Pyrolysis of microalgae: a critical review. Fuel Process Technol. 2019;186:53–72. https://doi.org/10.1016/j.fuproc.2018.12.012.
Roberts DA, Cole AJ, Paul NA, de Nys R. Algal biochar enhances the re-vegetation of stockpiled mine soils with native grass. J Environ Manage. 2015;161:173–80. https://doi.org/10.1016/j.jenvman.2015.07.002.
Mukome FND, Zhang XM, Silva LCR, Six J, Parikh SJ. Use of Chemical and physical characteristics to investigate trends in biochar feedstocks. J Agric Food Chem. 2013;61(9):2196–204. https://doi.org/10.1021/jf3049142.
Zhou YR, Zhang HL, Cai L, Guo J, Wang YN, Ji LL, et al. Preparation and characterization of macroalgae biochar nanomaterials with highly efficient adsorption and photodegradation ability. Materials. 2018;11(9):14. https://doi.org/10.3390/ma11091709.
Yang CH, Miao SC, Li TJ. Influence of water washing treatment on Ulva prolifera-derived biochar properties and sorption characteristics of ofloxacin. Sci Rep. 2021;11(1):12. https://doi.org/10.1038/s41598-021-81314-4.
Rajapaksha AU, Vithanage M, Lee SS, Seo DC, Tsang DCW, Ok YS. Steam activation of biochars facilitates kinetics and pH-resilience of sulfamethazine sorption. J Soils Sediments. 2016;16(3):889–95. https://doi.org/10.1007/s11368-015-1325-x.
Zhao L, Zheng W, Masek O, Chen X, Gu BW, Sharma BK, et al. Roles of phosphoric acid in biochar formation: synchronously improving carbon retention and sorption capacity. J Environ Qual. 2017;46(2):393–401. https://doi.org/10.2134/jeq2016.09.0344.
Singh A, Sharma R, Pant D, Malaviya P. Engineered algal biochar for contaminant remediation and electrochemical applications. Sci Total Environ. 2021;774:25. https://doi.org/10.1016/j.scitotenv.2021.145676.
Shim T, Yoo J, Ryu C, Park Y, Jung J. Effect of steam activation of biochar produced from a giant Miscanthus on copper sorption and toxicity. Bioresour Technol. 2015;197:85–90. https://doi.org/10.1016/j.biortech.2015.08.055.
Ranguin R, Delannoy M, Yacou C, Jean-Marius C, Feidt C, Rychen G, et al. Biochar and activated carbons preparation from invasive algae Sargassum spp. for Chlordecone availability reduction in contaminated soils. J Environ Chem Eng. 2021;9(4):9. https://doi.org/10.1016/j.jece.2021.105280.
•• Sajjadi B, Chen WY, Egiebor NO. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev Chem Eng. 2019;35(6):735–76. https://doi.org/10.1515/revce-2017-0113. This communication presents a comprehensive review of physical activation/modification strategies and their effects on biochar properties and related environmental application fields.
Sevilla M, Gu W, Falco C, Titirici MM, Fuertes AB, Yushin G. Hydrothermal synthesis of microalgae-derived microporous carbons for electrochemical capacitors. J Power Sources. 2014;267:26–32. https://doi.org/10.1016/j.jpowsour.2014.05.046.
Patra BR, Mukherjee A, Nanda S, Dalai AK. Biochar production, activation and adsorptive applications: a review. Environ Chemi Lett. 2021;19(3):2237–59. https://doi.org/10.1007/s10311-020-01165-9.
Rizwan M, Mujtaba G, Memon SA, Lee K, Rashid N. Exploring the potential of microalgae for new biotechnology applications and beyond: a review. Renew Sustain Energy Rev. 2018;92:394–404. https://doi.org/10.1016/j.rser.2018.04.034.
Molina M, Zaelke D, Sarma KM, Andersen SO, Ramanathan V, Kaniaru D. Reducing abrupt climate change risk using the Montreal Protocol and other regulatory actions to complement cuts in CO2 emissions. Proc Natl Acad Sci USA. 2009;106(49):20616–21. https://doi.org/10.1073/pnas.0902568106.
Zhang SP, Wang L, Wei W, Hu JJ, Mei SH, Zhao QY, et al. Enhanced roles of biochar and organic fertilizer in microalgae for soil carbon sink. Biodegradation. 2018;29(4):313–21. https://doi.org/10.1007/s10532-017-9790-0.
Ennis CJ, Evans AG, Islam M, Ralebitso-Senior TK, Senior E. Biochar: carbon sequestration, land remediation, and impacts on soil microbiology. Crit Rev Environ Sci Technol. 2012;42(22):2311–64. https://doi.org/10.1080/10643389.2011.574115.
Mona S, Malyan SK, Saini N, Deepak B, Pugazhendhi A, Kumar SS. Towards sustainable agriculture with carbon sequestration, and greenhouse gas mitigation using algal biochar. Chemosphere. 2021;275:17. https://doi.org/10.1016/j.chemosphere.2021.129856.
Ghorbani A, Rahimpour HR, Ghasemi Y, Zoughi S, Rahimpour MR. A review of carbon capture and sequestration in iran: microalgal biofixation potential in iran. renew. Sustain Energy Rev. 2014;35:73–100. https://doi.org/10.1016/j.rser.2014.03.013.
Cheah WY, Show PL, Chang JS, Ling TC, Juan JC. Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour Technol. 2015;184:190–201. https://doi.org/10.1016/j.biortech.2014.11.026.
Moreira D, Pires JCM. Atmospheric CO2 capture by algae: Negative carbon dioxide emission path. Bioresour Technol. 2016;215:371–9. https://doi.org/10.1016/j.biortech.2016.03.060.
Yu KL, Show PL, Ong HC, Ling TC, Chen WH, Salleh MAM. Biochar production from microalgae cultivation through pyrolysis as a sustainable carbon sequestration and biorefinery approach. Clean Technol Environ Policy. 2018;20(9):2047–55. https://doi.org/10.1007/s10098-018-1521-7.
Jung KW, Jeong TU, Choi JW, Ahn KH, Lee SH. Adsorption of phosphate from aqueous solution using electrochemically modified biochar calcium-alginate beads: batch and fixed-bed column performance. Bioresour Technol. 2017;244:23–32. https://doi.org/10.1016/j.biortech.2017.07.133.
Liu PY, Rao DA, Zou LY, Teng Y, Yu HY. Capacity and potential mechanisms of Cd(II) adsorption from aqueous solution by blue algae-derived biochars. Sci Total Environ. 2021;767. https://doi.org/10.1016/j.scitotenv.2021.145447.
• Son EB, Poo KM, Chang JS, Chae KJ. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci Total Environ. 2018;615:161–8. https://doi.org/10.1016/j.scitotenv.2017.09.171. This paper reported the application of magnetic biochar derived from marine in heavy metal removal.
Wang BL, Zheng JL, Li YY, Zaidi A, Hu YW, Hu BW. Fabrication of delta-MnO2-modified algal biochar for efficient removal of U(VI) from aqueous solutions. J Environ Chem Eng. 2021;9(4):10. https://doi.org/10.1016/j.jece.2021.105625.
Zheng HS, Guo WQ, Li S, Chen YD, Wu QL, Feng XC, et al. Adsorption of p-nitrophenols (PNP) on microalgal biochar: analysis of high adsorption capacity and mechanism. Bioresour Technol. 2017;244:1456–64. https://doi.org/10.1016/j.biortech.2017.05.025.
Nguyen VT, Nguyen TB, Chen CW, Hung CM, Vo TDH, Chang JH, et al. Influence of pyrolysis temperature on polycyclic aromatic hydrocarbons production and tetracycline adsorption behavior of biochar derived from spent coffee ground. Bioresour Technol. 2019;284:197–203. https://doi.org/10.1016/j.biortech.2019.03.096.
Zhao M, Ma XH, Liao XR, Cheng SY, Liu Q, Wang HF, et al. Characteristics of algae-derived biochars and their sorption and remediation performance for sulfamethoxazole in marine environment. Chem Eng J. 2022;430:13. https://doi.org/10.1016/j.cej.2021.133092.
Enaime G, Baçaoui A, Yaacoubi A, Lübken M. Biochar for wastewater treatment—conversion technologies and applications. Appl Sci. 2020;10(10):3492. https://doi.org/10.3390/app10103492.
Sutar S, Otari S, Jadhav J. Biochar based photocatalyst for degradation of organic aqueous waste: a review. Chemosphere. 2022;287: 132200. https://doi.org/10.1016/j.chemosphere.2021.132200.
Ho S-H, Li R, Zhang C, Ge Y, Cao G, Ma M, et al. N-doped graphitic biochars from C-phycocyanin extracted Spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation. Water Res. 2019;159:77–86. https://doi.org/10.1016/j.watres.2019.05.008.
Chen C, Ma T, Shang Y, Gao B, Jin B, Dan H, et al. In-situ pyrolysis of Enteromorpha as carbocatalyst for catalytic removal of organic contaminants: considering the intrinsic N/Fe in Enteromorpha and non-radical reaction. Appl Catal B Environ. 2019;250:382–95. https://doi.org/10.1016/j.apcatb.2019.03.048.
Qi Y, Ge B, Zhang Y, Jiang B, Wang C, Akram M, et al. Three-dimensional porous graphene-like biochar derived from Enteromorpha as a persulfate activator for sulfamethoxazole degradation: role of graphitic N and radicals transformation. J Hazard Mater. 2020;399: 123039. https://doi.org/10.1016/j.jhazmat.2020.123039.
Wang H, Wang H, Zhao H, Yan Q. Adsorption and Fenton-like removal of chelated nickel from Zn-Ni alloy electroplating wastewater using activated biochar composite derived from Taihu blue algae. Chem Eng J. 2020;379: 122372. https://doi.org/10.1016/j.cej.2019.122372.
Huang Y-m, Li G, Li M, Yin J, Meng N, Zhang D, et al. Kelp-derived N-doped biochar activated peroxymonosulfate for ofloxacin degradation. Sci. Total Environ. 2021;754:141999. https://doi.org/10.1016/j.scitotenv.2020.141999.
Zhou Y, Zhang H, Cai L, Guo J, Wang Y, Ji L, et al. Preparation and characterization of macroalgae biochar nanomaterials with highly efficient adsorption and photodegradation ability. Materials. 2018;11(9):1709. https://doi.org/10.3390/ma11091709.
Sharma G, Bhogal S, Gupta VK, Agarwal S, Kumar A, Pathania D, et al. Algal biochar reinforced trimetallic nanocomposite as adsorptional/photocatalyst for remediation of malachite green from aqueous medium. J Mol Liq. 2019;275:499–509. https://doi.org/10.1016/j.molliq.2018.11.070.
Fazal T, Razzaq A, Javed F, Hafeez A, Rashid N, Amjad US, et al. Integrating adsorption and photocatalysis: a cost effective strategy for textile wastewater treatment using hybrid biochar-TiO2 composite. J Hazard Mater. 2020;390: 121623. https://doi.org/10.1016/j.jhazmat.2019.121623.
Burton GA. Metal bioavailability and toxicity in sediments. Criti Rev Environ Sci Technol. 2010;40(9–10):852–907. https://doi.org/10.1080/10643380802501567.
Perelo LW. In situ and bioremediation of organic pollutants in aquatic sediments. J Hazard Mater. 2010;177(1–3):81–9. https://doi.org/10.1016/j.jhazmat.2009.12.090.
Vandenbossche M, Jimenez M, Casetta M, Traisnel M. Remediation of heavy metals by biomolecules: a review. Crit Rev Environ Sci Technol. 2015;45(15):1644–704. https://doi.org/10.1080/10643389.2014.966425.
Hung C-M, Huang C, Hsieh S-L, Tsai M-L, Chen C-W, Dong C-D. Biochar derived from red algae for efficient remediation of 4-nonylphenol from marine sediments. Chemosphere. 2020;254: 126916. https://doi.org/10.1016/j.chemosphere.2020.126916.
Hung C-M, Chen C-W, Huang C-P, Dong C-D. Activation of peroxymonosulfate by nitrogen-doped carbocatalysts derived from brown algal (Sargassum duplicatum) for the degradation of polycyclic aromatic hydrocarbons in marine sediments. J Environ Chemi Eng. 2021;9(6): 106420. https://doi.org/10.1016/j.jece.2021.106420.
Hung C-M, Huang C-P, Chen C-W, Dong C-D. The degradation of di-(2-ethylhexyl) phthalate, DEHP, in sediments using percarbonate activated by seaweed biochars and its effects on the benthic microbial community. J Clean Prod. 2021;292: 126108. https://doi.org/10.1016/j.jclepro.2021.126108.
Hung C-M, Chen C-W, Huang C-P, Cheng J-W, Dong C-D. Algae-derived metal-free boron-doped biochar as an efficient bioremediation pretreatment for persistent organic pollutants in marine sediments. J Clean Prod. 2022:130448. https://doi.org/10.1016/j.jclepro.2022.130448
Chew KW, Yap JY, Show PL, Suan NH, Juan JC, Ling TC, et al. Microalgae biorefinery: high value products perspectives. Bioresour Technol. 2017;229:53–62. https://doi.org/10.1016/j.biortech.2017.01.006.
Sarwer A, Hamed SM, Osman AI, Jamil F, Al-Muhtaseb AH, Alhajeri NS, et al. Algal biomass valorization for biofuel production and carbon sequestration: a review. Environ Chem Lett. 2022;20(5):2797–851. https://doi.org/10.1007/s10311-022-01458-1.
Zhong WZ, Chi LN, Luo YJ, Zhang ZZ, Zhang ZJ, Wu WM. Enhanced methane production from Taihu Lake blue algae by anaerobic co-digestion with corn straw in continuous feed digesters. Bioresour Technol. 2013;134:264–70. https://doi.org/10.1016/j.biortech.2013.02.060.
Wu Y, Wu SL, Zhang HY, Xiao R. Cellulose-lignin interactions during catalytic pyrolysis with different zeolite catalysts. Fuel Process Technol. 2018;179:436–42. https://doi.org/10.1016/j.fuproc.2018.07.027.
Saber M, Nakhshiniev B, Yoshikawa K. A review of production and upgrading of algal bio-oil. Renew Sustain Energy Rev. 2016;58:918–30. https://doi.org/10.1016/j.rser.2015.12.342.
Gan YY, Ong HC, Show PL, Ling TC, Chen WH, Yu KL, et al. Torrefaction of microalgal biochar as potential coal fuel and application as bio-adsorbent. Energy Convers Manag. 2018;165:152–62. https://doi.org/10.1016/j.enconman.2018.03.046.
Kim SW, Koo BS, Lee DH. A comparative study of bio-oils from pyrolysis of microalgae and oil seed waste in a fluidized bed. Bioresour Technol. 2014;162:96–102. https://doi.org/10.1016/j.biortech.2014.03.136.
Tang ZY, Chen W, Hu JH, Li SQ, Chen YQ, Yang HP, et al. Co-pyrolysis of microalgae with low-density polyethylene (LDPE) for deoxygenation and denitrification. Bioresour Technol. 2020;311:7. https://doi.org/10.1016/j.biortech.2020.123502.
Xu SN, Cao B, Uzoejinwa BB, Odey EA, Wang S, Shang H, et al. Synergistic effects of catalytic co-pyrolysis of macroalgae with waste plastics. Process Saf Environ Prot. 2020;137:34–48. https://doi.org/10.1016/j.psep.2020.02.001.
•• Chi NTL, Anto S, Ahamed TS, Kumar SS, Shanmugam S, Samuel MS, et al. A review on biochar production techniques and biochar based catalyst for biofuel production from algae. Fuel. 2021;287:9. https://doi.org/10.1016/j.fuel.2020.119411. This review highilights why biochar catalyst is important for fuel production and its advantages.
Fu XB, Li DH, Chen J, Zhang YM, Huang WY, Zhu Y, et al. A microalgae residue based carbon solid acid catalyst for biodiesel production. Bioresour Technol. 2013;146:767–70. https://doi.org/10.1016/j.biortech.2013.07.117.
Qian KZ, Kumar A, Zhang HL, Bellmer D, Huhnke R. Recent advances in utilization of biochar. Renew Sustai Energy Rev. 2015;42:1055–64. https://doi.org/10.1016/j.rser.2014.10.074.
Ibrahim AFM, Dandamudi KPR, Deng SG, Lin JYS. Pyrolysis of hydrothermal liquefaction algal biochar for hydrogen production in a membrane reactor. Fuel. 2020;265:8. https://doi.org/10.1016/j.fuel.2019.116935.
Arun J, Varshini P, Prithvinath PK, Priyadarshini V, Gopinath KP. Enrichment of bio-oil after hydrothermal liquefaction (HTL) of microalgae C. vulgaris grown in wastewater: bio-char and post HTL wastewater utilization studies. Bioresour. Technol. 2018;261:182–7. https://doi.org/10.1016/j.biortech.2018.04.029.
Mahima J, Sundaresh RK, Gopinath KP, Rajan PSS, Arun J, Kim SH, et al. Effect of algae (Scenedesmus obliquus) biomass pre-treatment on bio-oil production in hydrothermal liquefaction (HTL): biochar and aqueous phase utilization studies. Sci Total Environ. 2021;778:9. https://doi.org/10.1016/j.scitotenv.2021.146262.
Taghavi S, Norouzi O, Tavasoli A, Di Maria F, Signoretto M, Menegazzo F, et al. Catalytic conversion of Venice lagoon brown marine algae for producing hydrogen-rich gas and valuable biochemical using algal biochar and Ni/SBA-15 catalyst. Int J Hydrog Energy. 2018;43(43):19918–29. https://doi.org/10.1016/j.ijhydene.2018.09.028.
Norouzi O, Di Maria F. Catalytic effect of functional and Fe composite biochars on biofuel and biochemical derived from the pyrolysis of green marine biomass. Fermentation-Basel. 2018;4(4):9. https://doi.org/10.3390/fermentation4040096.
• Salimi P, Norouzi O, Pourhoseini SEM, Bartocci P, Tavasoli A, Di Maria F, et al. Magnetic biochar obtained through catalytic pyrolysis of macroalgae: a promising anode material for Li-ion batteries. Renew Energy. 2019;140:704–14. https://doi.org/10.1016/j.renene.2019.03.077. This study reported the utilization of algal biochar as an electrode in Li-ion battery in energy production and storage systems.
Logan BE, Hamelers B, Rozendal RA, Schrorder U, Keller J, Freguia S, et al. Microbial fuel cells: methodology and technology. Environmen Sci Technol. 2006;40(17):5181–92. https://doi.org/10.1021/es0605016.
Lefebvre O, Uzabiaga A, Chang IS, Kim BH, Ng HY. Microbial fuel cells for energy self-sufficient domestic wastewater treatment-a review and discussion from energetic consideration. Appl Microbiol Biotechnol. 2011;89(2):259–70. https://doi.org/10.1007/s00253-010-2881-z.
Chakraborty I, Sathe SM, Dubey BK, Ghangrekar MM. Waste-derived biochar: applications and future perspective in microbial fuel cells. Bioresour Technol. 2020;312:12. https://doi.org/10.1016/j.biortech.2020.123587.
Bhatia SK, Palai AK, Kumar A, Bhatia RK, Patel AK, Thakur VK, et al. Trends in renewable energy production employing biomass-based biochar. Bioresour Technol. 2021;340:12. https://doi.org/10.1016/j.biortech.2021.125644.
Chakraborty I, Bhowmick GD, Ghosh D, Dubey BK, Pradhan D, Ghangrekar MM. Novel low-cost activated algal biochar as a cathode catalyst for improving performance of microbial fuel cell. Sustain Energy Technol Assess. 2020;42:10. https://doi.org/10.1016/j.seta.2020.100808.
Wang YS, Li DB, Zhang F, Tong ZH, Yu HQ. Algal biomass derived biochar anode for efficient extracellular electron uptake from Shewanella oneidensis MR-1. Front Environ Sci Eng. 2018;12(4):9. https://doi.org/10.1007/s11783-018-1072-5.
• Lee JH, Kim DS, Yang JH, Chun Y, Yoo HY, Han SO, et al. Enhanced electron transfer mediator based on biochar from microalgal sludge for application to bioelectrochemical systems. Bioresour Technol. 2018;264:387–90. https://doi.org/10.1016/j.biortech.2018.06.097. This study is focused on the utilization of microalgal sludge in biochar conversion and its application as an electrode material in enzymatic fuel cell system.
Jung KW, Ahn KH. Fabrication of porosity-enhanced MgO/biochar for removal of phosphate from aqueous solution: application of a novel combined electrochemical modification method. Bioresour Technol. 2016;200:1029–32. https://doi.org/10.1016/j.biortech.2015.10.008.
Jung KW, Jeong TU, Kang HJ, Chang JS, Ahn KH. Preparation of modified-biochar from Laminaria japonica: simultaneous optimization of aluminum electrode-based electro-modification and pyrolysis processes and its application for phosphate removal. Bioresour Technol. 2016;214:548–57. https://doi.org/10.1016/j.biortech.2016.05.005.
Jung KW, Jeong TU, Hwang MJ, Kim K, Ahn KH. Phosphate adsorption ability of biochar/Mg-Al assembled nanocomposites prepared by aluminum-electrode based electro-assisted modification method with MgCl2 as electrolyte. Bioresour Technol. 2015;198:603–10. https://doi.org/10.1016/j.biortech.2015.09.068.
Acknowledgements
We acknowledge the support from the Ho Chi Minh City University of Technology (HCMUT), VNU-HCM, and other collaborating universities for this study.
Funding
This research is partially funded by the Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number NCM2021-20–01.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Water and Sediment Pollution
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, TB., Nguyen, VT., Hoang, HG. et al. Recent Development of Algal Biochar for Contaminant Remediation and Energy Application: A State-of-the Art Review. Curr Pollution Rep 9, 73–89 (2023). https://doi.org/10.1007/s40726-022-00243-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-022-00243-6