Abstract
Heterologous expression is an efficient strategy for target protein production. Dlt operon plays the important role in the d-alanylation of lipoteichoic acid, which might affect the net negative charge of cell wall for protein secretion. In this study, dlt operon was deleted to improve the target protein production, and nattokinase, α-amylase and β-mannanase with different isoelectric points (PIs) were served as the target proteins. Firstly, our results implied that deletions of dltA, dltB, dltC and dltD improved the net negative charge of cell wall for extracellular protein secretion respectively, and among which, the dltB deficient strain DW2ΔdltB showed the best performance, its nattokinase (PI: 8.60) activity was increased by 27.50% compared with that of DW2/pP43SacCNK. Then, the dltABCD mutant strain was constructed, and the net negative charge and nattokinase activity were increased by 55.57% and 37.13% respectively, due to the deficiency of dltABCD. Moreover, it was confirmed that the activities of α-amylase (PI: 6.26) and β-mannanase (PI: 5.75) were enhanced by 44.53% and 53.06% in the dltABCD deficient strains, respectively. Collectively, this study provided a strategy that deletion of dlt operon improves the protein secretion in B. licheniformis, and which strategy was more conducive to the target protein with lower PI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heterologous expression is an efficient strategy for protein production, and many expression systems (Escherichia coli, Bacillus, Saccharomyces cerevisiae, insect cell, mammalian cell etc.) have been developed in recent years. Among them, Bacillus species are considered as one of the most attractive workhouse for heterologous protein production, as their advantages of non-toxicity, convenience for gene modification and high yield of target protein (Harwood and Cranenburgh 2008; Kang et al. 2014; van Dijl and Hecker 2013).
Previously, many strategies have been conducted to improve the target protein production. Screening of promoters and signal peptides was confirmed as the efficient approaches for enhanced production of target protein. Yang et al. have screened 114 endogenous phase-dependent promoters in Bacillus subtilis, and PabrB, PspoVG and PlytR achieved the highest activities of esterase, keratinase and alkaline polygalacturonate lyase, respectively (Yang et al. 2017). The signal peptides have been screened for nattokinase production in our previous researches (Cai et al. 2016; Wei et al. 2015). Genetic engineering of host strain was regarded as the effective tactic, due to its universality and efficiency (Cai et al. 2017). A series of Bacillus host strains have been constructed via deleting the extracellular protease genes (Wei et al. 2015), and improving the expression levels of translocation element, chaperone PrsA, signal peptidase and signal peptide peptidase could also enhance the target protein production (Cai et al. 2017, 2016; Chen et al. 2015; Song et al. 2017). Currently, cell surface engineering of host strain has become an attractive and promising strategy for product production (Saeui et al. 2015; Ueda 2016; Wu et al. 2017). The yields of β-galactosidase, CGTase, xylanase etc. were all enhanced by over-expression of phospholipase C in E. coli (Su et al. 2017), also, the α-amylase activities were enhanced in the CDP-diacylglycerol-serine O-phosphatidyltransferase gene pssA and cardiolipin synthase gene clsA mutant strains, respectively (Cao et al. 2017).
Lipoteichoic acid (LTA) is a component of gram-positive bacteria cell wall, and it can absorb Mg2+ to support the activities of several synthetase on the cell membrane, also, it has the function of storage element and regulation of intracellular autolysinase activity (Wecke et al. 1996). d-alanylation of LTA mediated by dlt operon is a major process for LTA modification, which incorporates d-alanine into LTA, and resulting in the reduction of net negative charge on the cell surface (Kiriukhin and Neuhaus 2001; Spatafora et al. 1999). In Bacillus, LTA modification is controlled by dlt operon, which consists of dltA–E. Among this operon, the gene dltA [encoding for d-alanine–poly(phosphoribitol) ligase DCI] was responsible for the adenylation of d-alanine, dltB (encoding for d-alanyl–LTA synthase) owns the function of adenylated d-Ala secretion, dltC encodes for d-alanyl carrier protein DCP, and dltD owns the function for combination of DCP and DCI, however, dltE has no role in the d-alanylation of LTA. Previous researches implied that deletions of the genes dltA–D disrupted the modification of LTA, which further alter the micro-environment of cell surface and enhance the autolytic activity of cell (Hyyrylainen et al. 2000; Kiriukhin and Neuhaus 2001; Kovacs et al. 2006). Moreover, deficiency of dlt operon improved the net negative charge of cell wall, and the yields of cyclodextrin glycosyltransferase (Craynest et al. 2003), α-amylase (Cao et al. 2017) and rPA (Thwaite et al. 2002) were all enhanced in the dlt mutant strains.
B. licheniformis DW2 is a bacitracin industrial production strain (Wang et al. 2017a, b), also, it could act as the host strain for protein expression (Cai et al. 2016). In this study, the dlt operon was deleted to improve the net negative charge for protein secretion, and the target proteins (nattokinase, α-amylase and β-mannanase) with different isoelectric points (PIs) were applied to evaluate the effects of dlt operon deficiency on protein production. This study confirmed that deletion of dlt operon could improve the target protein secretion efficiency significantly, and also provided an efficient host strain of B. licheniformis for protein production.
Materials and methods
Bacterial, plasmids and cultivation condition
The bacterial strains and plasmids used in this study were listed in Table 1. B. licheniformis DW2 was employed as the original strain for recombinant strain construction. The plasmid T2(2)-Ori was applied for gene knockout in B. licheniformis, and the expression vector was constructed based on pHY300PLK. All primers used for strain construction in this study were provided in Table S1 (Seeing in the Supplementary Materials). Lysogeny-broth (LB) medium was served as the basic medium for bacterial growth, and the corresponding antibiotic (50 µg/mL ampicillin, 20 µg/mL tetracycline or 20 µg/mL kanamycin) was added into the medium when necessary. The ME medium for cell growth contains 20 g/L glucose, 20 g/L sodium glutamate, 10 g/L sodium citrate, 7 g/L NH4Cl, 0.5 g/L K2HPO4·3H2O, 0.5 g/L MgSO4·7H2O, 0.04 g/L FeCl3·6H2O, 0.104 g/L MnSO4·H2O, 0.15 g/L CaCl2·2H2O, pH 7.2. The medium for nattokinase and α-amylase production were provided in our previous research (Cai et al. 2017). The medium for β-mannanase production containing: 25 g/L konjac flour, 50 g/L soybean meal, 5 g/L corn starch, 0.2 g/L MgSO4·3H2O, 1 g/L KH2PO4, pH 7.2. The inoculum (3%) was added into 30 mL of fermentation media in a 250 mL flask, and was incubated at 220 rpm and 37 °C for 48 h. All the fermentation experiments were repeated at least three times.
Gene knockout in B. licheniformis
The method for gene knockout in B. licheniformis was referred to our previously reported method (He et al. 2017), and the procedure for constructing dltA deficient strain was served as an example. Briefly, the upstream and downstream homology arms of dltA were amplified by the corresponding primers (dltA-KF1/R1 and dltA-KF2/R2), and fused by splicing-overlapping-extension PCR (SOE-PCR). Then, the fused fragments were digested by restriction enzymes XbaI and SacI, and inserted into the gene knockout vector T2(2)-Ori. Diagnostic PCR and DNA sequence confirmed that the vector, named as T2-dltA, was constructed successfully.
Then, the vector T2-dltA was transformed into B. licheniformis DW2, and verified by diagnostic PCR and plasmid extraction. The positive transformants were cultivated in the LB liquid medium with 20 µg/mL kanamycin at 45 °C, sub-cultured for three generations and transferred into kanamycin-free LB medium at 37 °C for another six generations. The cells were then plated on LB agar medium with or without kanamycin and incubated for 20 h, respectively, and the dltA deletion strain was verified by diagnostic PCR and DNA sequence, named as DW2∆dltA. Similarly, the genes dltB, dltC, dltD and dltABCD deletion strains were attained by the same method.
Construction of the protein expression vector
The nattokinase and α-amylase expression vectors used in this study were obtained in our previous research (Cai et al. 2017), and the β-mannanase expression vector was constructed as follows. Briefly, the alsSD promoter, β-mannanase gene gmuG (containing its own signal peptide) and amyL terminator TamyL of B. licheniformis DW2 were amplified by the corresponding primers, and fused by SOE-PCR to from the β-mannanase expression element. Then, the fused fragment was digested by XbaI and EcoRI and inserted into pHY300PLK, diagnostic PCR and DNA sequence confirmed that the β-mannanase expression vector was constructed successfully, named pHY-GumG.
Analytical methods
The cell biomass was measured by determination of the optical density at 600 nm. The volume of 2 mL fermentation broth was centrifuged at 10,000×g for 5 min, and re-suspended by suitable volume of normal saline, and OD600 was measured using a spectrophotometer (Bio-Rad, USA). The extracellular activities of nattokinase and α-amylase were measured by the method described in the previous researches (Cai et al. 2017). β-mannanase activity was determined by the following method (Song et al. 2017). The volume of 0.9 mL 5 g/L bean gum was insulated at 50 °C for 5 min, added with 0.1 mL fermentation supernatant and reacted at 50 °C for 5 min. Then, added 2 mL DNS and boiled for 5 min, cooled and volume to 25 mL. The absorbance was detected under 540 nm wavelength. One Unit (U) of β-mannanase activity was refined as the amount of enzyme that liberates 1 µmol reducing glucose (d-mannose) per minute under the given condition. The concentrations of total extracellular proteins were determined by the means of bovine serum albumin (BSA), which method was described in our previous research (Wei et al. 2015). Briefly, the volume of 100 µL fermentation supernatant was mixed with equal volume of 0.01% Coomassie brilliant blue, and detected the absorbance at 595 nm. Then, the concentration of extracellular total protein was calculated according to the strand standard curve made by different concentrations of BSA. The concentration of target proteins were determined by the method described in the previous research (Cai et al. 2017). The fermentation supernatant was applied for SDS-PAGE, and different concentrations of BSA were served as the standard. The concentrations of target proteins were calculated by the area and deepness of the bands with those of BSA on the SDS-PAGE, using the software of Quantity One by GS800 calibrated densitometer Bio-Rad.
Determination of the cation binding rate
The net negative charge of cell wall was measured by determining the cation binding rate of cell surface in ME medium, and the cation binding rate was analyzed by the cationic dyealcian blue 8GX binding assay (Cao et al. 2017). Briefly, the bacteria cells at mid-logarithmic phase were washed twice with 20 mM morpholinepropanesulfonic acid (MOPS) buffer, and re-suspended to a final OD600 of 0.5. Added with 65 mg/L alcian blue and rotated at the room temperature for 10 min at 3 rpm. Then, centrifuged at 10,000×g for 5 min, and the absorbance was detected under 530 nm wavelength. As a negative control, MOPS buffer with 65 mg/L alcian blue was incubated under the same conditions without bacteria. The cation binding rate was calculated as A1 (The absorbance of sample containing bacteria)/A2 (The absorbance of negative control).
Statistical analyses
All samples were analyzed in triplicate, and the data were presented as the mean ± the standard deviation for each sample point. All data were conducted to analyze the variance at P < 0.05 (*) and P < 0.01 (**), and an approximate Duncan’s multiple range test was applied to compare the mean values (Cai et al. 2017).
Results
Construction of the individual dlt deficient strains and their effects on the total extracellular protein production
In Bacillus, the dlt operon owns the function of d-alanylation of LTA, which might influence the net negative charge of cell wall for protein secretion (Kiriukhin and Neuhaus 2001). In order to analyze the effect of dlt operon deficiency on protein production, the individual gene of dlt operon was deleted respectively in B. licheniformis DW2. The construction procedure of dltA deletion strain was served as an example (Cai et al. 2018). Briefly, the upstream and downstream homology arms of dltA were amplified by the corresponding primers in Table S1 (seeing in the Supplementary Materials), and fused by SOE-PCR. Then, the fused fragment was inserted into the gene knockout vector T2(2)-Ori at the restriction enzyme rates XbaI and SacI, diagnostic PCR and DNA sequence confirmed that the gene knockout vector was constructed successfully, named as T2-dltA. Then, the vector T2-dltA was electro-transferred into B. licheniformis DW2, and the gene dltA was knocked out by homologous reorganization. Diagnostic PCR and DNA sequence confirmed that dltA was deleted successfully in DW2, named as DW2∆dltA. Similarly, the genes dltB, dltC and dltD were deleted successfully, and the gene deficient strains were named as DW2∆dltB, DW2∆dltC and DW2∆dltD, respectively.
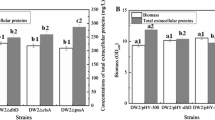
Then, these mutants, as well as the control strain DW2, were cultivated in the ME medium for 24 h, and the cell biomass and concentrations of total extracellular proteins were determined to evaluate the effects of individual gene deficiency on protein secretion. Based on our results of Fig. 1a, the cell growths were decreased in the dlt deficient strains, and the cell biomass were decreased by 9.07–12.31%, compared with that of DW2, respectively. Moreover, the concentrations of total extracellular proteins were increased obviously in the gene deletion strains (19.78%–53.47%), which were positively correlated with the SDS-PAGE results of extracellular proteins in Fig. 1b.
Effects of the individual dlt deletion on the extracellular protein production. a The concentrations of total extracellular proteins. Data are represented as the means of three replicates and bars represent the standard deviations, ∗P < 0.05; and ∗∗P < 0.01 indicate the significance levels between recombinant strains and control strain; b SDS-PAGE, M—protein marker (170, 130, 100, 70, 55, 40, 35 and 25 kDa); Lane 1—DW2; Lane 2—DW2∆dltA; Lane 3—DW2∆dltB; Lane 4—DW2∆dltC; Lane 5—DW2∆dltD
Effect of the individual dlt deletion on nattokinase production
Then, the nattokinase (PI: 8.60) expression vector pP43SacCNK were electro-transferred into the gene deletion strains, as well as the control strain DW2, and these recombinant strains were cultivated in the nattokinase fermentation medium to evaluate the effects of individual dlt gene deletion on nattokinase production. As shown in Fig. 2a, DW2/pP43SacCNK produced 27.71 FU/mL nattokinase, and deficiency of dltA, dltB, dltC and dltD improved nattokinase production. Among them, DW2∆dltB showed the highest nattokinase activity (35.33 FU/mL), which was 27.50% higher than that of DW2/pP43SacCNK. Furthermore, our results implied that the nattokinase yield produced by dlt mutant strains were 127.15 mg/L, 142.49 mg/L, 126.93 mg/L and 134.66 mg/L, increased by 9.23%, 23.53%, 10.04% and 16.74% compared with that of DW2/pP43SacCNK (115.35 mg/L). Also, the results of SDS-PAGE in Fig. 2b were consistent with those of nattokinase yields in Fig. 2a.
Nattokinase produced by B. licheniformis DW2 and dlt deletion strains. a Nattokinase activity. Data are represented as the means of three replicates and bars represent the standard deviations, ∗P < 0.05; and ∗∗P < 0.01 indicate the significance levels between recombinant strains and control strain; b SDS-PAGE, M—protein marker (170, 130, 100, 70, 55, 40, 35 and 25 kDa); Lane 1—DW2/pP43SacCNK; Lane 2—DW2∆dltA/pP43SacCNK; Lane 3—DW2∆dltB/pP43SacCNK; Lane 4—DW2∆dltC/pP43SacCNK; Lane 5—DW2∆dltD/pP43SacCNK
Effect of the individual dlt deletion on the net negative charge of cell wall
Since the dlt operon owns the function of d-alanylation of LTA, deficiency of dlt operon might affect the modification of LTA and cell surface microenvironment. Also, previous research has reported that the net negative charge of cell wall was improved in the dlt deletion strain of B. subtilis (Cao et al. 2017). In this research, the cell surface net negative charges of each strain were measured by determining the cation binding rate. Our results showed that the cation binding rate of DW2 was 35.18%, and deletion of individual gene of dltB, dltC and dltD could all improve the surface charge, which were 43.83%, 30.53% and 35.02% higher than that of DW2, respectively. Also, knocking out of dltA has no effect on the surface charge (Fig. 3). The stronger cation binding rate heralded the higher net negative charge of cell wall, thus, our results indicated that deletion of dlt operon improved the net negative charge of cell wall of B. licheniformis.
Construction of dltABCD deficient strain and its effects on the surface net negative charge and nattokinase production
Furthermore, the dltABCD operon was deleted to improve the net negative charge for target protein production. The dltABCD deletion vector T2-dltABCD was constructed according to the method described in the above section, and the gene operon dltABCD was knocked out successfully by homologous reorganization, resulting in the dltABCD deficient strain DW2∆dltABCD. Our results implied that deletion of dltABCD improved the cation binding rate, which was 55.57% higher than that of DW2 (Fig. 4a). Furthermore, after the transformation of nattokinase expression vector pP43SacCNK, the nattokinase activities produced by DW2 and DW2∆dltABCD were determined in the nattokinase production medium. Our results showed that 38.02 FU/mL nattokinase was produced by DW2∆dltABCD/pP43SacCNK, increased by 37.13% compared to DW2/pP43SacCNK (Fig. 4a). Moreover, the nattokinase yield produced by DW2∆dltABCD/pP43SacCNK was 154.86 mg/L, increased by 34.25% compared to DW2/pP43SacCNK (Fig. 4b).
Effects of dltABCD deficiency on the nattokinase production and cation binding rate. a Nattokinase activity and cation binding rate. Data are represented as the means of three replicates and bars represent the standard deviations, ∗P < 0.05; and ∗∗P < 0.01 indicate the significance levels between recombinant strains and control strain; b SDS-PAGE, M—protein marker (170, 130, 100, 70, 55, 40, 35 and 25 kDa); Lane 1—DW2/pP43SacCNK; Lane 2—DW2∆dltABCD/pP43SacCNK
Furthermore, the fermentation process curves of DW2/pP43SacCNK and DW2∆dltABCD/pP43SacCNK were measured, and the cell biomass and nattokinase activities were determined during the nattokinase production. Based on the results of Fig. 5, the nattokinase activities of DW2∆dltABCD/pP43SacCNK were higher than those of DW2/pP43SacCNK throughout the fermentation process, and the maximum activity was increased by 37.13% due to the deficiency of dltABCD. Additionally, the cell biomass was decreased by 15.72% compared with that of control strain, and the specific nattokinase activity produced by DW2∆dltABCD/pP43SacCNK was 2.92 FU/OD600, increased by 63.12% compared to DW2/pP43SacCNK (1.79 FU/OD600) (Fig. 5). Collectively, our results confirmed that deficiency of dlt operon improved the cell surface net negative charge, and the nattokinase yield was increased obviously in the dltABCD deficient strain.
Effects of dlt operon deficiency on the α-amylase and β-mannanase expression
In order to evaluate the effects of dlt operon deficiency on other proteins production. The α-amylase (PI: 6.26) and β-mannanase (PI: 5.75) expression vectors pHY-SAT and pHY-GumG were electro-transferred into DW2 and DW2∆dltABCD, respectively. The α-amylase expression vector pHY-SAT was obtained in our previous research (Cai et al. 2017), and β-mannanase expression vector pHY-GumG harboring alsSD promoter (BLi03848), gene gumG encoding for β-mannanase (containing its own signal peptide) (ACR84370) and amyL terminator TamyL (BALI_RS03660) was verified by diagnostic PCR and DNA sequence. Then, the extracellular activities of α-amylase and β-mannanase produced by recombinant strains were measured. As shown in Fig. 6, the activities of α-amylase and β-mannanase produced by the dltABCD deficient strains were 104.03 U/mL and 10.76 U/mL, increased by 44.53% and 53.06% compared with those of the control strains, respectively. Moreover, the specific activities of α-amylase and nattokinase of DW2∆dltABCD were 13.19 U/OD600 and 2.11 U/OD600, which were 73.2% and 96.82% higher than those of DW2, respectively. In addition, the α-amylase and β-mannanase yields produced by dltABCD deficient strains were 77.50 mg/L and 48.44 mg/L, which were 37.84% and 47.78% higher than those of DW2 (56.23 mg/L and 32.78 mg/L), respectively (Fig. S2). Collectively, based on these above results, our results confirmed that deficiency of dlt operon improved protein secretion, which was more conducive to the target protein with lower PI.
Discussion
Heterologous expression is an efficient approach for improvement production of target protein. Recently, many strategies have been conducted to improve the yields of target proteins, including screening of promoters and signal peptides, deleting the intracellular and extracellular proteases, over-expressing the translocation element, chaperone, signal peptidase and signal peptide peptidase, etc (Degering et al. 2010; Kang et al. 2014; Vitikainen et al. 2005; Zhang et al. 2013). Among these strategies, genetic engineering of host strain has been regarded as the effective tactics for enhancement of protein production, as its universality and efficiency. In this study, we have provided an efficient host strain DW2∆dltABCD for protein secretion via deleting the dlt operon in B. licheniformis DW2, and the yields of target proteins (nattokinase, α-amylase and β-mannanase) with different PIs were all increased obviously in DW2∆dltABCD.
Previously, it was confirmed that the dlt operon was in charge of the d-alanylation of LTA, and the genes dltA, dltB, dltC and dltD all acted as the important roles during this process in B. subtilis (Kiriukhin and Neuhaus 2001; Kovacs et al. 2006). Thus, deletion of dltA, dltB, dltC and dltD might affect the d-alanylation of LTA, which further influences the net negative charge for protein production in B. licheniformis. In this study, the net negative charge of cell wall and protein secretion efficiencies were all enhanced in the dlt operon deficient strains, and the dltB deletion strain DW2∆dltB showed the best performance, which nattokinase activity was increased by 27.50% compared with that of the control strain. Furthermore, the dltABCD deficient strain DW2∆dltABCD was constructed, and the net negative charge was increased by 55.57% compared to DW2. Moreover, the yields of nattokinase, α-amylase and β-mannanase were all increased significantly in the dltABCD deletion strains, respectively. Thus, our results confirmed that deficiency of dlt operon improved the net negative charge and electrostatic interaction, which further promote the protein secretion in B. licheniformis (Cao et al. 2017; Hyyrylainen et al. 2007). Moreover, it was reported that deletion of dlt could improve the protein folding capability by up-regulating the expression level of chaperone PrsA in B. subtilis (Hyyrylainen et al. 2000), and this might be the reason that the increase rates of target protein activities were all higher than those of target protein yield. Additionally, since the deficiency of teichoic acid d-alanylation might cause the membrane damage, which would weaken the cell resistance to the changes in the environment (Lopez et al. 2006; Saar-Dover et al. 2012), thus, the cell growth were decreased in the dlt deficient strains. Therefore, strengthening of the microbial membrane has been applied in the production of biorenewable fuels and chemicals, which is toxic to microbial (Sherkhanov et al. 2014; Tan et al. 2017).
Previously, Cao et al. implied that the characteristics of cell membrane phospholipid bilayer and PI of heterologous protein were two important factors during protein secretion process. Based on their results, the α-amylase secretion efficiency was increased obviously in the pssA deficient strain, and α-amylase variant AmyBm with lower PI (5.70) showed the best performance compared with that of the α-amylase variants with PIs at 6.72, 6.41 and 5.88, which might due to that the electrostatic interaction between the cell surface and secreted proteins would promote the secretion of target proteins. Also, their results implied that the secretion efficiency of α-amylase variant with too low PI (4.77) was decreased obviously (Cao et al. 2017). Meanwhile, Stephenson and coworkers also implied that the positively charged protein could interact the cell wall in a manner, which might influence its secretion efficiency (Stephenson et al. 2000). In this study, the net negative charge was increased by 55.57% in the dlt operon deletion strain DW2∆dltABCD, and the activities of nattokinase, α-amylase and β-mannanase were increased by 37.13%, 44.53% and 53.06% in DW2∆dltABCD, respectively. Since the PIs of nattokinase, α-amylase and β-mannanase were 8.60, 6.26 and 5.75 respectively, our results indicated that deletion of dlt operon was beneficial for the target protein with lower PI, and the increase rate of β-mannanase was higher than those of nattokinase and α-amylase in this research.
Conclusion
In this study, deletion of dltA, dltB, dltC and dltD could all improve the net negative charge of cell wall for nattokinase production, and the dltB mutant strain DW2∆dltB showed the best performance among these strains, which nattokinase activity was increased by 27.50% compared with that of DW2. Furthermore, the dltABCD mutant strain was constructed, and our results implied that the net negative charge and nattokinase activity were increased by 55.57% and 37.13% in the dltABCD deletion strain, respectively. Moreover, the activities of α-amylase and β-mannanase were improved by 44.53% and 53.06% respectively, due to the deficiency of dltABCD. Collectively, this study provided a strategy that deletion of dlt operon improves target protein secretion in B. licheniformis, and which strategy was more conducive to the target protein with low PI.
References
Cai D, Wei X, Qiu Y, Chen Y, Chen J, Wen Z, Chen S (2016) High-level expression of nattokinase in Bacillus licheniformis by manipulating signal peptide and signal peptidase. J Appl Microbiol 121(3):704–712
Cai D, Wang H, He P, Zhu C, Wang Q, Wei X, Nomura CT, Chen S (2017) A novel strategy to improve protein secretion via overexpression of the SppA signal peptide peptidase in Bacillus licheniformis. Microb Cell Fact 16(1):70
Cai D, Hu S, Chen Y, Liu L, Yang S, Ma X, Chen S (2018) Enhanced production of poly-gamma-glutamic acid by overexpression of the global anaerobic regulator Fnr in Bacillus licheniformis WX-02. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-018-2693-7
Cao H, van Heel AJ, Ahmed H, Mols M, Kuipers OP (2017) Cell surface engineering of Bacillus subtilis improves production yields of heterologously expressed alpha-amylases. Microb Cell Fact 16(1):56
Chen J, Fu G, Gai Y, Zheng P, Zhang D, Wen J (2015) Combinatorial sec pathway analysis for improved heterologous protein secretion in Bacillus subtilis: identification of bottlenecks by systematic gene overexpression. Microb Cell Fact 14:92
Craynest M, Jorgensen S, Sarvas M, Kontinen VP (2003) Enhanced secretion of heterologous cyclodextrin glycosyltransferase by a mutant of Bacillus licheniformis defective in the D-alanylation of teichoic acids. Lett Appl Microbiol 37(1):75–80
Degering C, Eggert T, Puls M, Bongaerts J, Evers S, Maurer KH, Jaeger KE (2010) Optimization of protease secretion in Bacillus subtilis and Bacillus licheniformis by screening of homologous and heterologous signal peptides. Appl Environ Microbiol 76(19):6370–6376
Harwood CR, Cranenburgh R (2008) Bacillus protein secretion: an unfolding story. Trend Microbiol 16(2):73–79
He P, Zhang Z, Cai D, Chen Y, Wang H, Wei X, Li S, Chen S (2017) High-level production of alpha-amylase by manipulating the expression of alanine racamase in Bacillus licheniformis. Biotechnol Lett 39(9):1389–1394
Hyyrylainen HL, Vitikainen M, Thwaite J, Wu H, Sarvas M, Harwood CR, Kontinen VP, Stephenson K (2000) D-Alanine substitution of teichoic acids as a modulator of protein folding and stability at the cytoplasmic membrane/cell wall interface of Bacillus subtilis. J Biol Chem 275(35):26696–26703
Hyyrylainen HL, Pietiainen M, Lunden T, Ekman A, Gardemeister M, Murtomaki-Repo S, Antelmann H, Hecker M, Valmu L, Sarvas M, Kontinen VP (2007) The density of negative charge in the cell wall influences two-component signal transduction in Bacillus subtilis. Microbiology 153(Pt 7):2126–2136
Kang Z, Yang S, Du G, Chen J (2014) Molecular engineering of secretory machinery components for high-level secretion of proteins in Bacillus species. J Ind Microbiol Biotechnol 41(11):1599–1607
Kiriukhin MY, Neuhaus FC (2001) D-alanylation of lipoteichoic acid: role of the D-alanyl carrier protein in acylation. J Bacteriol 183(6):2051–2058
Kovacs M, Halfmann A, Fedtke I, Heintz M, Peschel A, Vollmer W, Hakenbeck R, Bruckner R (2006) A functional dlt operon, encoding proteins required for incorporation of d-alanine in teichoic acids in gram-positive bacteria, confers resistance to cationic antimicrobial peptides in Streptococcus pneumoniae. J Bacteriol 188(16):5797–5805
Lopez CS, Alice AF, Heras H, Rivas EA, Sanchez-Rivas C (2006) Role of anionic phospholipids in the adaptation of Bacillus subtilis to high salinity. Microbiology 152(Pt 3):605–616
Saar-Dover R, Bitler A, Nezer R, Shmuel-Galia L, Firon A, Shimoni E, Trieu-Cuot P, Shai Y (2012) D-Alanylation of lipoteichoic acids confers resistance to cationic peptides in group B Streptococcus by increasing the cell wall density. PLoS Pathog 8(9):e1002891
Saeui CT, Mathew MP, Liu L, Urias E, Yarema KJ (2015) Cell surface and membrane engineering: emerging technologies and applications. J Funct Biomater 6(2):454–485
Sherkhanov S, Korman TP, Bowie JU (2014) Improving the tolerance of Escherichia coli to medium-chain fatty acid production. Metab Eng 25:1–7
Song Y, Fu G, Dong H, Li J, Du Y, Zhang D (2017) High-Efficiency secretion of beta-mannanase in Bacillus subtilis through protein synthesis and secretion optimization. J Agric Food Chem 65(12):2540–2548
Spatafora GA, Sheets M, June R, Luyimbazi D, Howard K, Hulbert R, Barnard D, el Janne M, Hudson MC (1999) Regulated expression of the Streptococcus mutans dlt genes correlates with intracellular polysaccharide accumulation. J Bacteriol 181(8):2363–2372
Stephenson K, Jensen CL, Jorgensen ST, Lakey JH, Harwood CR (2000) The influence of secretory-protein charge on late stages of secretion from the Gram-positive bacterium Bacillus subtilis. Biochem J 350 (Pt 1):31–9
Su L, Jiang Q, Yu L, Wu J (2017) Enhanced extracellular production of recombinant proteins in Escherichia coli by co-expression with Bacillus cereus phospholipase C. Microb Cell Fact 16(1):24
Tan Z, Khakbaz P, Chen Y, Lombardo J, Yoon JM, Shanks JV, Klauda JB, Jarboe LR (2017) Engineering Escherichia coli membrane phospholipid head distribution improves tolerance and production of biorenewables. Metab Eng 44:1–12
Thwaite JE, Baillie LW, Carter NM, Stephenson K, Rees M, Harwood CR, Emmerson PT (2002) Optimization of the cell wall microenvironment allows increased production of recombinant Bacillus anthracis protective antigen from B. subtilis. Appl Environ Microbiol 68(1):227–234
Ueda M (2016) Establishment of cell surface engineering and its development. Biosci Biotechnol Biochem 80(7):1243–1253
van Dijl JM, Hecker M (2013) Bacillus subtilis: from soil bacterium to super-secreting cell factory. Microb Cell Fact 12:3
Vitikainen M, Hyyrylainen HL, Kivimaki A, Kontinen VP, Sarvas M (2005) Secretion of heterologous proteins in Bacillus subtilis can be improved by engineering cell components affecting post-translocational protein folding and degradation. J Appl Microbiol 99(2):363–375
Wang D, Wang Q, Qiu Y, Nomura CT, Li J, Chen S (2017a) Untangling the transcription regulatory network of the bacitracin synthase operon in Bacillus licheniformis DW2. Res Microbiol 168(6):515–523
Wang Q, Zheng H, Wan X, Huang H, Li J, Nomura CT, Wang C, Chen S (2017b) Optimization of inexpensive agricultural by-products as raw materials for bacitracin production in Bacillus licheniformis DW2. Appl Biochem Biotechnol 183(4):1146–1157
Wecke J, Perego M, Fischer W (1996) D-Alanine deprivation of Bacillus subtilis teichoic acids is without effect on cell growth and morphology but affects the autolytic activity. Microb Drug Resist 2(1):123–129
Wei X, Zhou Y, Chen J, Cai D, Wang D, Qi G, Chen S (2015) Efficient expression of nattokinase in Bacillus licheniformis: host strain construction and signal peptide optimization. J Ind Microbiol Biotechnol 42(2):287–295
Wu T, Ye L, Zhao D, Li S, Li Q, Zhang B, Bi C, Zhang X (2017) Membrane engineering—A novel strategy to enhance the production and accumulation of beta-carotene in Escherichia coli. Metab Eng 43(Pt A):85–91
Yang S, Du G, Chen J, Kang Z (2017) Characterization and application of endogenous phase-dependent promoters in Bacillus subtilis. Appl Microbiol Biotechnol 101(10):4151–4161
Zhang J, Kang Z, Ling Z, Cao W, Liu L, Wang M, Du G, Chen J (2013) High-level extracellular production of alkaline polygalacturonate lyase in Bacillus subtilis with optimized regulatory elements. Bioresour Technol 146:543–548
Acknowledgements
This work was supported by the National Program on Key Basic Research Project (973 Program, No. 2015CB150505), the Technical Innovation Special Fund of Hubei Province (2018ACA149), the Science and Technology Program of Wuhan (20160201010086).
Author information
Authors and Affiliations
Contributions
DC and SC designed the study. YC, DC and PH carried out the molecular biology studies and construction of engineering strains. YC, FM and QZ carried out the fermentation studies. YC, DC, XM and SC analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Y., Cai, D., He, P. et al. Enhanced production of heterologous proteins by Bacillus licheniformis with defective d-alanylation of lipoteichoic acid. World J Microbiol Biotechnol 34, 135 (2018). https://doi.org/10.1007/s11274-018-2520-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-018-2520-x