Abstract
Non-aerated compost teas (NCTs) are water extracts of composted organic materials and are used to suppress soil borne and foliar disease in many pathosystems. Greenhouse trials were used to test the effectiveness of NCTs to suppress potato bacterial wilt caused by Ralstonia solanacearum on plants grown in soils inoculated with a virulent isolate of the pathogen (biovar II). NCTs prepared from matured compost sources: agricultural waste (AWCT), vermicompost (VCT) and solid municipal waste (SMWCT) were evaluated at three initial application times (7 days before inoculation, at time of inoculation and 7 days after inoculation) prior to weekly applications, in a randomized complete-block design. AWCT applied initially at the time of inoculation resulted in the greatest disease suppression, with the disease severity index 2.5-fold less than the non-treated plants and the “area under the disease progress curve” (AUDPC) 3.2-fold less. VCT and SMWCT were less suppressive than AWCT regardless of initial application time. Next generation sequencing of the v4 region of 16S rRNA gene and the internal transcribed spacer region (ITS1) revealed that diversity and composition of the bacterial and fungal communities across the NCTs varied significantly. Dominant bacterial phyla such as Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, Verrucomicrobia, Chloroflexi, Planctomycetes, Acidobacteria, and a fungal phylum Ascomycota were detected in all NCTs. AWCT had optimum physico-chemical measurements with higher bacterial Shannon diversity indices (H) and fungal richness (S) than the other treatments. We conclude that bacterial wilt of potatoes grown in controlled conditions can be suppressed by a non-aerated compost tea with a high microbial diversity when applied at planting and weekly thereafter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bacterial wilt, caused by Ralstonia solanacearum (Yabuuchi et al. 1995), is regarded as one of the most severe diseases of potato, causing great economic loss to production worldwide (Ding et al. 2013). Mostly found in tropical and subtropical regions, the bacterium is known to affect more than 200 plant species distributed among 50 botanical families (Hayward 1991). Various strains of R. solanacearum have been associated with the loss in yield and quality of important crops such as tomato, eggplant, pepper, tobacco, banana, chili, and peanut (Álvarez et al. 2010; Kempe and Sequeira 1983). Control strategies for a range of hosts have been developed; these include synthetic chemical pesticides (Lee et al. 2012), cultural practices such as field sanitation, clean seed production and crop rotation (Kassa and Chindi 2013), induced resistance by natural products and elemental nutrients such as silicon and calcium treatments (Dannon and Wydra 2004; Gado 2013), and non-pesticide chemicals such as Acibenzolar-S-methyl (ASM) (Pradhanang et al. 2005). The use of pesticides as fumigants or disinfectants is not only associated with environmental contamination and human health risks (Acero et al. 2008), but also with depletion of beneficial soil microbes associated with the suppression of the pathogen population (Gamliel et al. 2000). Similarly, resistance inducing non-pesticide chemicals options are less practical because they are more expensive than other options (Yuliar and Toyota 2015).
Biologically based treatments particular to specific hosts have shown promising results as crop protectants in different experimental settings. Amendment of soil organic content by incorporating composted animal and crop residues (Li and Dong 2013), and a range of isolated rhizosphere and endophytic beneficial microbes (Tan et al. 2013) have been reported as effective and environmentally friendly crop protectants. Populations of R. solanacearum are genetically variable (Álvarez et al. 2010) and capable of adapting to a range of environmental conditions (van Elsas et al. 2000) thus making biological control less effective. Bacterial wilt control is generally an ongoing challenge for farmers and a universally accepted management option for many plant hosts is lacking.
Integrated management of bacterial wilt, by incorporation in the soil of a range of solid organic matters such as compost, slurries of animal wastes, bio-organic fertilizers, as well as their extracts, offer a promising tool that primarily enhance microbial antagonism against R. solanacearum. Bioorganic fertilizer derived from a mixture of animal and plant-based organic products mixed in water without subsequent fermentation was found to suppress bacterial wilt development in certain hosts, such as tobacco (Wu et al. 2014) and potato (Ding et al. 2013).
The suitability of water extracts of composted organic materials (“compost teas”) in suppressing various diseases of a wide range of horticultural crops has been studied extensively (Evans and Percy 2014). Effectiveness of compost tea against a range of soil borne fungal pathogen such as Verticillium dahliae, Fusarium oxysporum f. sp. Lycopersici and Rhizoctonia solani has been reported in various horticultural crops (Pane et al. 2014). There are few reports that have indicated the suitability of compost tea for suppression of bacterial disease. For example, compost teas of different sources have significantly reduced the severity of tomato foliar spot caused by Xanthomonas vesicatoria (Al-Dahmani et al. 2003). Similarly, Islam et al. (2014) reported that a soil drench of compost tea suppressed the severity of bacterial wilt in brinjal, caused by R. solanacearum. There do not appear to be any reports of bacterial and fungal diversities from NCTs used for suppressing bacterial wilt of potato caused by R. solanacearum.
The suppressiveness of compost tea is mostly ascribed to its biotic component (Koné et al. 2010). Previous studies suggested that total numbers of culturable microorganisms (microbial abundance within the range studied) was not associated with the degree of disease suppression (Pane et al. 2012), whereas microbial community structure appeared to play a role in the suppression of gray mold on geranium after foliar application of compost tea (Scheuerell and Mahaffee 2006). Palmer et al. (2010) also reported an association between the diversity of culturable bacterial and fungal microbes and disease suppressive ACTs. Therefore, understanding the microbial community structure in compost tea is likely to be important in optimising production protocols. Microbial community composition and diversity in compost tea have been mostly studied by culture based techniques (Koné et al. 2010), or cultivation independent approaches using fingerprinting techniques (Shrestha et al. 2011), that provide limited taxonomic resolutions. Application of next generation sequencing technologies provides a powerful tool to source information about microbial community structure by extracting community DNA and sequencing the phylogenetic gene targets (16S rRNA, bacteria) and ITS regions for fungi (Caporaso et al. 2012; Lindahl et al. 2013) from environmental samples. Therefore, this study was designed to evaluate the potential of non-aerated compost teas produced from variable compost sources in suppressing bacterial wilt of potato. Microbial communities of the NCTs were studied to determine if variation diversity, abundance, and richness of both bacterial and fungal microbes at different taxonomic levels were related to efficacies of compost teas applications.
Materials and methods
Composting conditions and feedstock composition
Agricultural waste compost (AWC) and vermicompost (VC) were prepared in an experimental field of the Ambo Plant Protection Research Centre, located at 8°57′N, 37°52′E, Ethiopia between April to June, 2013.
Briefly, various types of agricultural waste, composed of plant and animal based materials (maize and wheat straw, chopped grasses, wood ash, cow dung, and forest soils), were piled in equal proportion. The composting process was initiated with wetting of the piles by agricultural well water and then the piles were allowed to decompose for 65 days in a pit composting system (size approx. 1 cubic m), which is a typical composting technique in the area. The compost was left for further curing and maturation for 5 months until it was used for the trials. In vermicomposting, earthworms (Eisenia fetida) were used to decompose the starting organic waste substrates. The bedding, consisting of animal waste (donkey manure), and plant based waste (wheat straw and corn stalks) were mixed in equal amounts in plastic composting boxes, with holes drilled in the bottom for aeration and drainage, according to the local practice. Solid municipal waste compost (SMWC) was made by the Addis Ababa Environmental Protection Authority. Compostable and sorted household solids consisting of vegetable and fruit peelings and other food wastes were collected from residential houses near the site and composted in an open windrow method for a period of 3 months and further cured for 3 months.
Preparation of compost tea
Matured samples of AWC, VC, and SMWC were used for preparation of non-aerated compost teas (NCTs) according to the procedure outlined by Koné et al. (2010). Plastic buckets (30 l capacity) were used to prepare the different batches of NCTs in 1:5 ratios (w/v) using agricultural well water for a period of 14 days at room temperature. Layered muslin cloth was used to filter the respective compost ferments. The compost teas were stored in a cool room (5–8 °C) until used for the trials.
Isolation and culture of Ralstonia solanacearum
Ralstonia solanacearum used in this trial was isolated from a local farmer’s tomato (Solanum lycopersicum L.) field near Ambo, Ethiopia. Tomato plants showing typical symptoms of bacterial wilt were selected and brought to the laboratory for isolation, identification and maintenance of the pathogen. Samples from stems and roots of the plants were surface sterilized with 1% sodium hypochlorite for 3 min, cut into pieces and separately transferred to a selective 2, 3, 5-triphenyltetrazolium chloride agar (TZC) medium (Kelman 1954). After 48 h, 11 mucoid, reddish and irregularly shaped colonies having a central white colour typical of wild/virulent types of R. solanacearum were purified by selecting individual colonies and subculturing onto the same media (Kelman 1954). A hypersensitivity test was conducted; single colonies of the isolates from the 48 h old culture were transferred to 250 ml of liquid nutrient broth and grown for 48 h on a rotary shaker at 150 rpm at room temperature. A 3 ml volume from the stock bacterial solution (adjusted to 109 cfu) of each isolate was intravenously injected into the leaves of tobacco (Nicotiana tabacum) and the leaf reaction observed for 24–72 h (Kempe and Sequeira 1983). Based on cultural characteristics and hypersensitivity response, three isolates were selected and maintained in sterile distilled water at room temperature for further biochemical tests and soil inoculation in the greenhouse experiments.
Biochemical characteristics of Ralstonia solanacearum isolates
Selected biochemical characterization of three chosen isolates, including Gram staining, KOH solubility test, oxidase test, catalase test and starch solubility test were performed according to standard procedures (Goszczynska et al. 2000). For the biovar identification, carbon source utilization tests were conducted based on the ability of each isolate to utilize disaccharides (sucrose, lactose and maltose) and sugar alcohols (mannitol, sorbitol and dulcitol) according to the procedure described by Aley and Elphinstone (1995) using Hayward’s basal medium (Hayward 1964). The carbohydrates were prepared in 10% w/v water, sterilised and transferred to the previously autoclaved basal medium in test tubes. Loops full of 48 h old culture inoculum of each of the three isolates were prepared in 300 μl of sterile water to make individual bacterial suspensions. A volume of 30 μl of each isolate suspension was added to basal media amended with the carbohydrate sources, incubated for up to 3 weeks and then observed at 3, 7, and 14 days for the formation of top to downward yellow coloration due to the change in pH (Aley and Elphinstone 1995).
Characterisation of compost and NCTs
Physicochemical and microbial analysis of compost and NCTs
Physical and chemical parameters such as electrical conductivity (EC), pH (1: 2.5 H2O) and extractable chemical nutrients (Na, K, Ca, Mg, Fe, Mn, Cu, and Zn) were analysed according to standard procedures for both the compost and nonaerated compost teas at the Soil Testing Laboratory of Ethiopia. Quality parameters including organic carbon content (OM) and total nitrogen (TN) were measured for the parent composts at the start of the trial to quantify the C:N ratio of the matured compost used for production of the NCTs.
Microbial community analysis
In order to characterise the microbial communities, genomic DNA was extracted from the last batch of each NCTs used for the disease trial, using the Power Soil DNA kit (MO BIO Laboratories, Inc., USA) according to the manufacturer’s instruction. A 0.25 g pellet collected from centrifugation of 30 ml of NCTs samples at a speed of 2900 g for 30 min was added to bead-beating tubes and further purified through the subsequent steps of the extraction procedure. DNA was extracted in triplicate from each NCTs sample and purity was measured using the spectrophotometer at wavelengths of 260/280 nm (NanoDrop 8000 Spectrophotometer, Thermo Fisher Scientific Inc., Wilmington, DE, U.S.A.). To determine the diversity and composition of the bacterial and fungal communities in the non-aerated compost teas, high throughput amplicon sequencing was conducted according to the methods described by Caporaso et al. (2011).
NCTs microbial genes sequencing was performed at MR DNA (Shallowater, TX, USA) on the Illumina MiSeq platform following the manufacturer’s guidelines. From DNA samples of the NCTs, the V4 variable region of the 16S rRNA gene (for bacteria and archaea) was amplified with the PCR primers 515F/806R and the internal transcribed spacer region (ITS1) of the nuclear ribosomal RNA gene (for fungi) was amplified with primers ITS1-F/ITS2. Amplifications were performed in a 30 cycle PCR using the HotStarTaq Plus Master Mix Kit (Qiagen, USA). The PCR conditions were: 94 °C for 3 min, followed by 28 cycles of 94 °C for 30 s, 53 °C for 40 s and 72 °C for 1 min, after which a final elongation step at 72 °C for 5 min was performed. PCR products were checked by gel electrophoresis using a 2% agarose gel to determine the success of amplification and the relative intensity of bands. Multiple PCR product samples were pooled together in equal proportions based on their molecular weight and DNA concentrations. Pooled samples were purified using calibrated Ampure XP beads. Then the pooled and purified PCR products were used to prepare a DNA library by following the Illumina TruSeq DNA library preparation protocol. Sequence data were processed using the MR DNA analysis pipeline (MR DNA, Shallowater, TX, USA). Briefly, sequences were joined, depleted of barcodes, denoised, then ambiguous base calls, chimeras and sequences with length of <150 bp were removed. Operational taxonomic units (OTUs) were defined by clustering at 3% divergence (97% similarity). Final OTUs were taxonomically classified using BLASTn against a curated database derived from GreenGenes (DeSantis et al. 2006), RDPII and NCBI (http://www.ncbi.nlm.nih.gov, http://rdp.cme.msu.edu). Sequence data from the project have been deposited in the MG-RAST (metagenomics analysis server) under accession numbers for bacteria: MGP15434, and for fungi: MGP15436.
Statistical analysis
PRIMER 6 (version 6.13) and PERMANOVA+ (version 1.0.3) software packages (PRIMER-E, Plymouth, Ivybridge, United Kingdom) were used for the assessment of microbial community composition of the samples. Bray Curtis similarity coefficients were calculated between pairs of samples based on untransformed percentages of the OTUs and created as a lower triangular resemblance matrix. Permutational multivariate analysis of variance (PERMANOVA) was used to assess the effect of nonaerated compost tea type on observed bacterial and fungal composition. Principal coordinate analysis by canonical analysis of principal coordinates (CAP) was used to make the ordination diagram showing the structural difference between communities (Anderson and Willis 2003). The relative proportions of bacterial and fungal taxa, at phylum, class and genus levels, were calculated and used to construct a table illustrating the variation in relative abundance of both bacterial and fungal microbes in the replicates (n = 3) of the compost teas samples. Alpha diversities (species richness S and Shannon diversity index H) of both fungal and bacterial communities were determined based on the number of OTUs observed.
Greenhouse experiment
Plant material, inoculum and inoculation method
Sprouted tubers of potato (Solanum tuberosum L.) of the variety Jalene were obtained from the seed multiplication section of Holetta agricultural research laboratories. Single tubers were planted in pots (20 cm diameter) which were filled with a mixture of soil prepared from field soil, compost and sand in 2:1:1 ratio, respectively. The soil mixture was autoclaved at 121 °C for 2 h and cooled before tubers were planted. Each planted tuber gave rise to three to five main stems.
For the soil inoculation, a pathogenic isolate of R. solanacearum was grown on TZC medium for 48 h. It was then transferred for mass production in a nutrient broth for an additional 48 h at a room temperature. Pots allocated for inoculation with R. solanacearum were drenched with 200 ml bacterial suspension of 109 cfu/ml. Inoculations occurred 7 days before or after sprouted tubers were transplanted to the pots according to the treatment, as outlined below. Control pots were drenched with 200 ml of distilled water.
The temperature of the greenhouse was maintained in the range 25–32 °C and relative humidity was approximately 70% for the experimental period of 80 days.
Treatments
NCTs extracted from the three compost sources above were evaluated for their ability to suppress bacterial wilt development using the potted potato plants. A randomised complete block design with three blocks (replicates) was used. Within each block, there were 10 treatment combinations comprising compost tea type and application timing (Table 1). Each compost tea was either applied as a 500 ml drench 7 days before soil inoculation with R. solanacearum (AE, SE, VE) at the same time as the inoculum (AO, SO, VO) or 7 days after pathogen inoculation (AI, SI, VI). Each treated plant then received 500 ml of the designated compost tea on a weekly basis throughout the remaining experimental period. The control treatment plants were inoculated and non-treated; rather, distilled water was applied on each of the days a compost tea treatment was applied (Table 1).
Disease assessment
Yellowing and stunting of the aboveground plant parts, typical of the symptoms of bacterial wilt, were first observed 1 month after soil inoculation. Disease severity was assessed for each potted plant at 32, 42, 52, 62 and 72 days after planting using a 0–4 scale (Kempe and Sequeira 1983), where 0 = no symptoms, 1 = up to 25% of the foliage wilted, 2 = 25–50% of the foliage wilted, 3 = 50–75% of the foliage wilted and 4 = 75–100% of the foliage wilted.
Data analyses
The mean disease rating was calculated from the severity scores for each of the three replicates per treatment. This mean was then expressed as a percentage of the maximum possible score (4) to express disease severity on a scale of 0–100 (Winstead and Kelman 1952). Disease severity index (DSI) (%) assessed at the different times after planting was used to calculate the area under the disease progress curve (AUDPC) by the method of Campbell and Madden (1990).The presence or absence of treatment effects was tested using one way analysis of variance (ANOVA) with the GLM procedure for the final DSI and AUDPC response variables using the SAS software program (SAS Institute 2011). When effects of treatments were significant (p < 0.05), means were compared using Fisher’s protected least significant difference (LSD) test.
Results
Characteristics of Ralstonia solanacearum
Eleven isolates were screened for pathogenicity and hypersensitivity tests, and of these three isolates of R. solanacearum from symptomatic tomato plants showed cultural characteristics typical of virulent strains. These isolates formed highly opaque and smooth colonies on the nutrient agar medium (NA) and produced fluid, brown/tan pigment with whitish-pink centres on TZC medium after 48 h of incubation at 28 °C, which is similar to the documented morphology of R. solanacearum (Kelman 1954). They were all gram negative, rod-shaped and non-spore forming isolates. They induced chlorotic symptoms on injected tobacco leaves within 3 days, however were not pathogenic to tobacco. The isolates showed positive results when tested for oxidization of maltose and lactose typical of biovar II, unlike the strains of the biovar I which do not produce acid because the carbohydrates supplied are not utilized. The three isolates considered were all positive to oxidase, catalase and KOH tests and were pathogenic to potato and hence we randomly chose one of them for the subsequent activities. Earlier reports indicated strains isolated from solanaceous vegetables (mainly tomato and potato) in Ethiopia were mainly classified as biovar II, with recent records of biovar I strains from the same host plants (Lemessa and Zeller 2007; Yaynu 1989).
Physicochemical characteristics of parent compost and compost teas
The parent compost sources had pH values ranging from 7.1 to 8.5 and the C:N ratio of the AWC (19.8) was higher than that of the SMC and VC (3.1 and 3.7, respectively). The EC and concentration of extractable cations in the AWC were lower than the values recorded for the SMWC and VC, and this difference was also reflected in the EC for the respective compost teas.
The pH of the parent composts and compost teas was neutral with the SMC being slightly alkaline (pH 8.5) (Table 2). Values for the concentration of available cations and anions were variable among parent composts and compost teas. AWCT appeared to have a lower concentration of the extractable ions K, Ca, Fe, Cu and Zn (Table 2).
Effect of NCT on bacterial wilt disease
Individual treatments showed variability in disease suppression on each assessment date, as reflected by the values for the final mean DSI and AUDPC (Table 3). All NCT treatments, except treatments SI and VI, had lower mean DSIs and AUPDC values than the non-treated control.
AWCT applied concurrently with the pathogen (AO) resulted in a mean DSI of 33%, which was significantly lower than 7 days before pathogen inoculation (AE, 45%), 7 days after pathogen inoculation (AI, 50%) and the non-treated control (CI, 83.3%) (Table 3). All treatments resulted in lower AUDPC than the control treatment; the AO treatment resulted in the lowest AUDPC of all treatments, followed by the same type of compost tea applied as a protective treatment (AE).
Effects of the weekly application of the treatments on symptom progression were also evaluated at four assessment points (day 32, 42, 52 and 62 after planting) prior to the final disease assessment (Fig. 1a–d). No disease was detected in any of the plants treated with SMWCT until 42 days after planting. Approximately 50 days after planting, there appeared to be a steep increase in the severity of wilting of plants treated with SMWCT, although the mean DSI and AUDPC remained significantly lower than the control and VCT treatments 72 days after planting (Table 3). AWCT appeared to restrict symptom development consistently across all disease scoring dates compared to the other tea types. Plants treated with vermicompost teas (VI, VO, and VE) showed progressive wilting with time and disease severity indices were often as high as the control treatment at each assessment date. By 52 days, the severity of wilting for plants receiving SMWCTs in all the three application timings (SO, SE, and SI) was similar to the severity observed in the non-treated plants.
a–d Mean bacterial wilt severity (%) at different times after planting (a–d) of potato variety Jalene. The soil of potted plants, except non-inoculated controls, was drenched with 200 ml of 1 × 109 cfu/ml pathogenic Ralstonia solanacearum. A 500 ml volume of each non-aerated compost tea of types A, S or V (Table 1) was applied initially at I, O or E (Table 1) and weekly thereafter. Mean (n = 3) bars sharing the same letter within each sub-figure (a–d) are not significantly different according to Fisher’s LSD and α = 0.05
Microbial community structure and diversity
A total of 477,335 and 201,374 16S rRNA and fungal ITS rRNA gene sequences were obtained by amplicon sequencing after they had been filtered to remove poor quality reads. Among replicates of the NCT samples (n = 3), the number of bacterial gene sequences varied from 27, 625 to 70,712 (median = 46,606), whereas the number of the fungal ITS gene sequences varied from 6628 to 36,956 (median = 24,070).
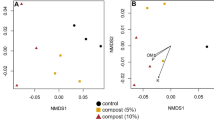
The community structure varied significantly with compost tea source. Permutational multivariate analysis of variance showed bacterial and archaeal communities harboured in the NCTs were significantly different (p = 0.004 and p = 0.003, respectively). Fungal communities among NCTs were also significantly different (p = 0.003). Similarly, canonical analysis of principal coordinates (CAP) also showed that both the bacterial and fungal communities in the different NCT types were significantly different (p = 0.003 for both analyses) (Fig. 2a, b).
a, b Canonical analysis of principal coordinates (CAP) for a bacterial, and b fungal communities in three types of non-aerated compost tea: circles represent vermicompost tea (VCT), triangles represent solid municipal waste compost teas (SMWCT) and squares indicate agricultural waste compost teas (AWCT)
The three NCTs were found to contain very diverse bacterial communities at all levels of taxonomic classification. The main bacterial phyla included Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, Verrucomicrobia, Chloroflexi, Planctomycetes, and Acidobacteria (Table 4). Proteobacteria, mainly Betaproteobacteria, was the most common phylum in the AWCT whereas the Bacteroidetes was the most common phylum in VCT and SMWCT. The class Bacteroidia dominated in SMWCT whereas Flavobacteriia dominated the VCT community.
Ascomycota was the dominant fungal phylum in all NCT types (Table 5). Eurotiomycetes, Leotiomycetes and Sordariomycetes were the dominant fungal classes of the phylum Ascomycota in all NCTs. SMWCT harboured species of the phylum Basidiomycota in higher abundance compared to the other two compost teas (Table 5). AWCT contained a significant proportion of Ascomycota (30.65% relative abundance) for which the class could not be determined, while the presence of unidentified classes was low in the other two compost teas (relative abundance of <0.5%) (Table 5). Alpha diversity measures varied among NCTs for both the bacterial and fungal communities (Table 6). The bacterial communities in the VCT had the highest richness but lowest Shannon diversity. The fungal communities in the AWCT had the highest richness but Shannon’s diversity did not vary greatly between the three compost teas.
The percentage contribution of the most common (top 10%) genera is shown in Fig. 3. Genera including Thiobacillus, Malikia, Hydrogenophaga, Desulfomicrobium and Prolixibacter were only observed in AWCT whereas genera including Oligosphaera, Paracoccus, Synergistes, and Anoxybacillus were only observed in SMWCT. VCT appeared to be dominated by the genus Flavobacterium constituting about 32% of the top 10% of taxa. In contrast, the most even distribution of bacterial genera was observed in AWCT, an observation that is consistent with the alpha diversity statistics (Fig. 3).
Discussion
Application of each type of NCTs to the soil of potted potato plants reduced the severity of bacterial wilt disease compared to the non-treated plants (Fig. 1; Table 3). These compost teas varied in their physico-chemical and biological characteristics as influenced by the composition of the compost used to make each tea. The application of high-throughput sequencing in this study revealed that the bacterial and fungal communities across the three NCTs varied markedly. The variable level of bacterial wilt suppression observed in this experiment was also associated with the initial timing of NCT application in relation to the time of inoculation with the target pathogen.
AWCT suppressed disease symptom development to a greater degree than SMWCT or VCT for all initial application times evaluated. The choice of composting substrates is known to have a significant impact in producing disease suppressive compost teas for certain pathosystems (Al-Dahmani et al. 2003; Scheuerell and Mahaffee 2006). Generally, composts (and presumably their watery extracts) must have attributes within a consistent range to be used successfully in biological control of horticultural crops (Hoitink et al. 1997). Biotic and physico-chemical properties of the parent compost and their NCTs also play a major role in disease suppression. For example, the C:N ratio of parent composts should not be too high to prevent nitrogen immobilization, which results in competition between the microbes and plants for nutrients in the soil, or too low to prevent the release of phytotoxic compounds such as ammonia to the soil (Gao et al. 2010). In this trial, the AWCT made from both animal and plant-based substrates in equal proportion, had a relatively low EC, neutral pH, and C:N ratio (19:1) of the parent compost, which appeared to be in the optimum C:N range (<25:1) (Kuo et al. 2004). Presumably these characteristics favour microbial activity in the soil (Moral et al. 2009) that in turn suppresses the severity of soil borne disease (Hoitink et al. 1997). In contrast, SMWCT and VCT had a higher concentration of extractable cations and EC, and very low C:N ratio in the respective parent compost sources. The higher wilt severity which occurred on plants treated by these two NCTs might be due to less favourable conditions for growth and proliferation of antagonistic microorganisms, due to high salt content and low C:N ratio as previously reported for different pathosystems (Aryantha et al. 2000). Hoitink et al. (1993) showed that application of SMWC with a high salt concentration at planting time reduced soybean yield due to promotion of root rot caused by Phytophthora. Similarly, Md Islam and Toyota (2004) reported that tomato plants treated with bark and coffee compost with high Ca and Mg concentration, had a higher incidence of bacterial wilt than plants treated with farm yard manure (FYM) containing significantly lower concentration of extractable cations. Therefore, it is evident that the quality of parent composts influences the degree to which NCTs suppress bacterial wilt.
It is known that the biotic components of composts, originating from the organic waste composts, contribute to the suppression of wilt disease on potato and other hosts caused by the soil-borne R. solanacearum (Ding et al. 2013). Few studies, however, have investigated the diversity of microbial communities in compost teas in relation to their potential to suppress potato wilt and other diseases. The greater bacterial and fungal diversities of AWCT relative to the other NCTs might have contributed to the higher level of disease suppression observed. Md Islam and Toyota (2004) incorporated composted farm–yard manure into soil and observed enhanced microbial activities and a greater diversity of bacterial and fungal communities. This soil amendment resulted in poor survival in terms of the population of R. solanacearum. A diverse microbial community in the soil contains a diverse array of functional properties which places pressure on the population of soil-borne plant pathogens (van Elsas et al. 2012). One example is the work of Shiomi et al. (1999) who found that soil with a high microbial diversity was suppressive to R. solanacearum.
In recent years, genera not explored previously as biological control agents (BCAs) for bacterial wilt have been identified from diverse sources (Yuliar and Toyota 2015). Our analysis revealed genera that were identified in one type of NCT but not another and also dissimilarities in microbial composition that may have affected the biological activity and function of the communities. Even though common genera used as BCAs—Bacillus, Pseudomonas, and Burkholderia—were identified in all NCTs and are known to be antagonistic to R. solanacearum, their frequency was variable and they were present within a background microbial community that varied across NCTs effecting different levels of disease suppression. Moreover, the species and strains of the genera present were not studied, although these are known to influence the efficacy of biological control. It is apposite to note that biological based control measures of crop disease are mostly influenced by strain specificity, among other factors. For instance, Thomas and Upreti (2014) studied three isolates of the endophytic bacterium Bacillus pumilus isolated from grape and watermelon. Only one isolate from grape inhibited the in vitro growth of R. solanacearum while the two isolates from watermelon were not consistently antagonistic. Apart from chemical and biological attributes of NCTs, we suggest that strain specificity might have also played a role in the apparent variability in disease suppression of NCTs tested in the different times of application.
This study showed that the greatest suppression of bacterial wilt was achieved when the initial application of NCT was timed to coincide with tuber planting and inoculation. In a related study, Anith et al. (2004) reported that application of treatments including plant growth promoting rhizobacteria (PGPR) strains and mixtures of organic amendments at the time of seeding and a week before inoculation with R. solanacearum significantly reduced bacterial wilt in tomato. Plants first treated with NCTs 1 week after inoculation (curative) showed significantly greater disease than application on or before inoculation. R. solanacearum survives in the soil and replicates prior to entering the tuber buds/eyes or lateral roots via natural openings or wounded tissues (Álvarez et al. 2008). Although the fate of R. solanacearum after treatment application was not rigorously examined in this research, we hypothesise that the mechanism of disease suppression is competitive exclusion of the pathogen from the infection court and/or direct antagonism of the pathogen population by the microbes present in NCT. In some cases biological control agents work through the production of specific metabolites and it is possible that similar mechanisms were also present in our system. However, both this, and other studies (Palmer et al. 2010; Scheuerell and Mahaffee 2006) indicate that the presence of a diverse microbial community is essential for effective suppression of disease. It is also acknowledged that different results might have been observed if the experiment had been conducted with non-autoclaved field soil because its associated microbial community may have interacted with that present in the NCTs.
We conclude that compost tea produced as a non-aerated, water ferment of composted agricultural wastes comprising maize and wheat straw, chopped grasses, ash, and animal manure substrates has potential to be used as part of an integrated disease management strategy. The microbial diversity of NCT, especially bacterial diversity, appears to contribute to the level of disease suppression observed. Further studies are needed to enhance the efficacy of compost teas more generally by elucidating the mechanism/s of action.
References
Acero JL, Benítez FJ, Real FJ, González M (2008) Chlorination of organophosphorus pesticides in natural waters. J Hazard Mater 153:320–328. doi:10.1016/j.jhazmat.2007.08.051
Al-Dahmani JH, Abbasi PA, Miller SA, Hoitink HA (2003) Suppression of bacterial spot of tomato with foliar sprays of compost extracts under greenhouse and field conditions. Plant Dis 87:913–919
Aley EFLGP, Elphinstone J (1995) Culture media for Ralstonia solanacearum isolation, identification and maintenance. Fitopatologia 30:126–130
Álvarez B, Vasse J, Le-Courtois V, Trigalet-Démery D, López M, Trigalet A (2008) Comparative behavior of Ralstonia solanacearum biovar 2 in diverse plant species. Phytopathology 98:59–68
Álvarez B, Biosca EG, López MM (2010) On the life of Ralstonia solanacearum, a destructive bacterial plant pathogen. Curr Res Technol Educ Topics Appl Microbiol Microbial Biotechnol 1:267–279
Anderson MJ, Willis TJ (2003) Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84:511–525
Anith KN, Momol MT, Kloepper JW, Marois JJ, Olson SM, Jones JB (2004) Efficacy of plant growth-promoting rhizobacteria, acibenzolar-S-methyl, and soil amendment for integrated management of bacterial wilt on tomato. Plant Dis 88:669–673. doi:10.1094/PDIS.2004.88.6.669
Aryantha IP, Cross R, Guest DI (2000) Suppression of Phytophthora cinnamomi in potting mixes amended with uncomposted and composted animal manures. Phytopathology 90:775–782. doi:10.1094/phyto.2000.90.7.775
Campbell CL, Madden LV (1990) Introduction to plant disease epidemiology. Wiley, New York
Caporaso JG et al (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci 108:4516–4522
Caporaso JG et al. (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624
Dannon EA, Wydra K (2004) Interaction between silicon amendment, bacterial wilt development and phenotype of Ralstonia solanacearum in tomato genotypes. Physiol Mol Plant Pathol 64:233–243
DeSantis TZ et al (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072
Ding C, Shen Q, Zhang R, Chen W (2013) Evaluation of rhizosphere bacteria and derived bio-organic fertilizers as potential biocontrol agents against bacterial wilt (Ralstonia solanacearum) of potato. Plant Soil 366:453–466
Evans KJ, Percy AK (2014) Integrating compost teas in the management of fruit and foliar diseases for sustainable crop yield and quality. In: Maheshwari DK (ed) Composting for sustainable agriculture. Springer, Cham, pp 173–198
Gado E (2013) Induction of resistance in potato plants against bacterial wilt disease under Egyptian condition. J Appl Sci Res 9:170–177
Gamliel A, Austerweil M, Kritzman G (2000) Non-chemical approach to soilborne pest management—organic amendments. Crop Prot 19:847–853 doi:10.1016/S0261-2194(00)00112-5
Gao M, Liang F, Yu A, Li B, Yang L (2010) Evaluation of stability and maturity during forced-aeration composting of chicken manure and sawdust at different C/N ratios. Chemosphere 78:614–619
Goszczynska T, Serfontein J, Serfontein S (2000) Introduction to practical phytobacteriology. SAFRINET The Southern African (SADC) Loop of BioNet-International
Hayward AC (1964) Characteristics of Pseudomonas solanacearum. J Appl Bacteriol 27:265–277. doi:10.1111/j.1365-2672.1964.tb04912.x
Hayward A (1991) Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol 29:65–87
Hoitink H, Boehm M, Hadar Y (1993) Mechanisms of suppression of soilborne plant pathogens in compost-amended substrates. In: Hoitink HAJ, Keener HM (eds) Science and engineering of composting: design, environmental, microbiological and utilization aspects. Renaissance Publications, Worthington, OH, pp 601–621
Hoitink H, Stone A, Han D (1997) Suppression of plant diseases by composts. HortScience 32:184–187
Islam M, Mondal C, Hossain I, Meah M (2014) Compost tea as soil drench: an alternative approach to control bacterial wilt in brinjal. Arch Phytopathol Plant Prot 47:1475–1488
Kassa B, Chindi A (2013) Seed tuber cycle and latent infection for the spread of potato bacterial wilt Ralstonia solanacearum (Smith) a threat for seed production in Ethiopia Asian. J Plant Pathol 7:74–83
Kelman A (1954) The relationship of pathogenicity of Pseudomonas solanacearum to colony appearance in a tetrazolium medium. Phytopathology 44:693–695
Kempe J, Sequeira L (1983) Biological control of bacterial wilt of potatoes: attempts to induce resistance by treating tubers with bacteria. Plant Dis 67:499–503
Koné SB, Dionne A, Tweddell RJ, Antoun H, Avis TJ (2010) Suppressive effect of non-aerated compost teas on foliar fungal pathogens of tomato. Biol Control 52:167–173
Kuo S, Ortiz-Escobar M, Hue N, Hummel R (2004) Composting and compost utilization for agronomic and container crops. Recent Res Dev Environ Biol 1:451–513
Lee Y-H, Choi C-W, Kim S-H, Yun J-G, Chang S-W, Kim Y-S, Hong J-K (2012) Chemical pesticides and plant essential oils for disease control of tomato bacterial wilt. Plant Pathol J 28:32–39
Lemessa F, Zeller W (2007) Isolation and characterisation of Ralstonia solanacearum strains from Solanaceae crops in Ethiopia. J Basic Microbiol 47:40–49
Li J-G, Dong Y-H (2013) Effect of a rock dust amendment on disease severity of tomato bacterial wilt. Antonie Van Leeuwenhoek 103:11–22
Lindahl BD et al. (2013) Fungal community analysis by high-throughput sequencing of amplified markers—a user’s guide. New Phytol 199:288–299
Md Islam T, Toyota K (2004) Suppression of bacterial wilt of tomato by Ralstonia solanacearum by incorporation of composts in soil and possible mechanisms. Microbes Environ 19:53–60
Moral R, Paredes C, Bustamante M, Marhuenda-Egea F, Bernal M (2009) Utilisation of manure composts by high-value crops: safety and environmental challenges. Bioresour Technol 100:5454–5460
Palmer A, Evans K, Metcalf D (2010) Characters of aerated compost tea from immature compost that limit colonization of bean leaflets by Botrytis cinerea. J Appl Microbiol 109:1619–1631
Pane C, Celano G, Villecco D, Zaccardelli M (2012) Control of Botrytis cinerea, Alternaria alternata and Pyrenochaeta lycopersici on tomato with whey compost-tea applications. Crop Prot 38:80–86. doi:10.1016/j.cropro.2012.03.012
Pane C, Celano G, Zaccardeli M (2014) Metabolic patterns of bacterial communities in aerobic compost teas associated with potential biocontrol of soilborne plant diseases. Phytopathol Mediterr 53:277
Pradhanang P, Ji P, Momol M, Olson S, Mayfield J, Jones J (2005) Application of acibenzolar-S-methyl enhances host resistance in tomato against Ralstonia solanacearum. Plant Dis 89:989–993
SAS Institute I (2011) SAS® 9.3. Software. SAS Institute, Inc. Cary
Scheuerell SJ, Mahaffee WF (2006) Variability associated with suppression of gray mold (Botrytis cinerea) on Geranium by foliar applications of nonaerated and aerated compost teas. Plant Dis 90:1201–1208. doi:10.1094/pd-90-1201
Shiomi Y, Nishiyama M, Onizuka T, Marumoto T (1999) Comparison of bacterial community structures in the rhizoplane of tomato plants grown in soils suppressive and conducive towards bacterial wilt. Appl Environ Microbiol 65:3996–4001
Shrestha K, Adetutu EM, Shrestha P, Walsh KB, Harrower KM, Ball AS, Midmore DJ (2011) Comparison of microbially enhanced compost extracts produced from composted cattle rumen content material and from commercially available inocula. Bioresour Technol 102:7994–8002. doi:10.1016/j.biortech.2011.05.096
Tan S, Jiang Y, Song S, Huang J, Ling N, Xu Y, Shen Q (2013) Two Bacillus amyloliquefaciens strains isolated using the competitive tomato root enrichment method and their effects on suppressing Ralstonia solanacearum and promoting tomato plant growth. Crop Prot 43:134–140
Thomas P, Upreti R (2014) Testing of bacterial endophytes from non-host sources as potential antagonistic agents against tomato wilt pathogen Ralstonia solanacearum. Adv Microbiol 4:656–666
van Elsas JD, Kastelein P, van Bekkum P, van der Wolf JM, de Vries PM, van Overbeek LS (2000) Survival of Ralstonia solanacearum biovar 2, the causative agent of potato brown rot, in field and microcosm soils in temperate climates. Phytopathology 90:1358–1366
van Elsas JD, Chiurazzi M, Mallon CA, Elhottovā D, Krištůfek V, Salles JF (2012) Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc Natl Acad Sci 109:1159–1164
Winstead N, Kelman A (1952) Inoculation techniques for evaluating resistance to Psuedomonas solanacearum. Phytopathology 42:628–634
Wu K, Yuan S, Wang L, Shi J, Zhao J, Shen B, Shen Q (2014) Effects of bio-organic fertilizer plus soil amendment on the control of tobacco bacterial wilt and composition of soil bacterial communities. Biol Fertil Soils 50:961–971
Yabuuchi E, Kosako Y, Yano I, Hotta H, Nishiuchi Y (1995) Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen. nov.: proposal of Ralstonia pickettii (Ralston, Palleroni and Doudoroff 1973) comb. nov., Ralstonia solanacearum (Smith 1896) comb. nov. and Ralstonia eutropha (Davis 1969) comb. nov. Microbiol Immunol 39:897–904
Yaynu H (1989) Characteristics of isolates of Pseudomonas solanacearum in Ethiopia Ethiopian. J Agric Sci 11:7–13
Yuliar YAN, Toyota K (2015) Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ 30:1
Acknowledgements
The authors would like to thank staff members at the bacteriology section of Ambo Plant Protection Research centre for provision of the glasshouse facility used for conducting the research. The Australian Development Scholarship (ADS) provided to the first author is gratefully acknowledged and we thank the School of Land and Food of UTAS for operational support. Thanks to David Ratkowsky for his advice on statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mengesha, W.K., Powell, S.M., Evans, K.J. et al. Diverse microbial communities in non-aerated compost teas suppress bacterial wilt. World J Microbiol Biotechnol 33, 49 (2017). https://doi.org/10.1007/s11274-017-2212-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-017-2212-y