Abstract
This study aimed to compare the kinetics of lipopeptide production in solid-state fermentation (SSF) under isothermal and non-isothermal conditions. Models based on the logistic, modified Gompertz and Luedeking–Piret-like equations were developed to describe the time course of fermentation under different conditions. The experiments were conducted in 250 mL flasks and a 50 L fermenter. The results showed that the non-isothermal process had higher levels of product formation rate and substrate utilization rate compared to the isothermal process. The part of substrate carbon to meet microbial maintenance—energy, biomass and lipopeptides formation requirements got increased using the non-isothermal technique. In addition, fermenter conditions positively influenced the lipopeptides formation rate with significantly higher levels of substrate for the microbial growth and product formation, though the product productivity and biomass both decreased as compared to flask. This is the first report that investigates the effects of temperature changing on the kinetics of lipopeptide production by Bacillus amyloliquefaciens strain under SSF condition using soybean flour and rice straw as major substrates in flask and in fermenter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipopeptides are biologically surface active molecules synthesized by microorganisms and can be produced from various low-cost substrates (Mizumoto et al. 2006; Das and Mukherjee 2007). They are effective alternatives to highly used synthetic surfactants with distinct advantages such as low toxicity, biodegradability and biocompatibility (Cameotra and Makkar 2004; Sriram et al. 2011). Over the past few years, solid-state fermentation (SSF) has received increasing interest in the scale-up production of value-added products due to its low capital investment and low operating costs (Holker and Lenz 2005). Therefore, the production of lipopeptides by SSF from flask to fermenter is practically feasible and economically potential for industrial use (Zhu et al. 2013b).
In SSF, the fermentation of products is influenced by the variations in the mass transfer around (Pandey et al. 2000). To gain insight into how the various phenomena within the fermentation system combine to control overall process performance, it is essential to study the relationship among the principal state variables and to explain the behavior of fermentation quantitatively during the fermentation process (Nath and Das 2011). Kinetic modelling is regarded as a useful means for describing a fermentation process, since models help in reflecting and forecasting the microbial growth and product formation (Dodić et al. 2012; Yalcin and Özbas 2005). However, knowledge pertaining to the modeling of the production of secondary metabolites, especially lipopeptides, under SSF conditions is still lacking.
The kinetic models are normally divided into two classes: structured and unstructured models (Votruba et al. 1985; Özilgen 1998). Structured models take metabolic pathways into consideration and are generally complicated, while the unstructured ones are simpler and most frequently employed for modeling microbial systems (Fujikawa et al. 2004; Mu et al. 2006; Mazutti et al. 2010). Recently, a two-temperature-stage process has been proposed for the enhancement of lipopeptide production in SSF (Zhu et al. 2013a). Changing temperature to 37 °C during the productive stage could improve the production of lipopeptides efficiently under SSF condition. However, the mechanism involved in the increased production of lipopeptides by the temperature changing was unknown. The comparative kinetic study of lipopeptide production under different conditions in SSF can give a better understanding of the physiological state and the metabolic behavior of Bacillus amyloliquefaciens XZ-173, which helps to explain the possible mechanism caused by temperature changes and condition scaled during the fermentation of lipopeptides.
The objectives of this study were to model the lipopeptide production by B. amyloliquefaciens XZ-173 in SSF and to provide comprehensive information of differences in kinetics between isothermal and non-isothermal techniques. The unstructured models were used to fit the kinetic processes of cell growth, lipopeptides formation and substrate utilization during the fermentation. Also, mathematical models applied to flasks and fermenter were compared to observe the effect of scale-up on the kinetics of lipopeptide production. The results obtained here were helpful for translating and further development of the process for lipopeptide production to industrial scales.
Materials and methods
Microorganisms and culture conditions
Bacillus amyloliquefaciens XZ-173 (CGMCC accession number 4160), capable of producing significant amount of lipopeptide biosurfactants in SSF, was used in this study. The strain was taken from −80 °C frozen stock and transferred onto Luria–Bertani (LB) agar plates for pre-culture. Then a loopful of cells from fresh LB plates were inoculated into liquid LB medium and incubated at 30 °C with agitation at 170 rpm. After growth for 24 h, the medium was used as seed culture for solid substrate fermentations.
Preparation of solid substrate
Agro-industrial byproducts including soybean and rice straw obtained from a local farm were oven-dried (60 °C for 12 h) and subsequently milled and sieved to powder by passing through a 20-mesh screen. Following this, the passed solid substrates were redried (60 °C for 48 h) for use as dry substrates.
Solid state fermentation for lipopopetides
Fermentation in flask
For flask fermentation, 5.58 g of soybean flour and 3.67 g of rice straw supplemented with 1.79 % starch and 1.91 % yeast extract and 1.0 ml of mineral solution (composition in g/L: KH2PO4 1.0; MgSO4·7H2O 0.5; KCl 0.5; l-phenylalanine 2 × 10−3; MnSO4 5 × 10−3; CuSO4 0.16 × 10−3; FeSO4 0.15 × 10−3) were adjusted with initial pH 7.5 and moisture content 55 % in 250 mL flask, and then mixed thoroughly and autoclaved at 115 °C for 30 min. The cooled substrates were inoculated with a level of 10 % (v/w) inoculum, mixed carefully under sterile conditions, and then incubated in a chamber with relative humidity above 80 %. The fermented substrates were sampled every 4 h until 48 h to detect the apparent biomass concentrations, lipopeptide yields and concentrations of total reducing sugars.
Fermentation in fermenter
For large scale fermentation, 5.58 kg of soybean flour and 3.67 kg of rice straw supplemented with 1.79 % starch and 1.91 % yeast extract and 1.0 L of mineral solution were adjusted to initial pH 7.5 and moisture content 55 % in a 50 L fermenter (Biotech-50SS, Shanghai Baoxin Bio-Engineering Equipment Co., Ltd, China), and then mixed thoroughly by rotating the entire vessel for 40 min and autoclaved at 115 °C for 30 min. After cooling, the substrates were inoculated with a level of 10 % (v/w) inoculum and incubated in the fermenter at the assured temperature value with the humidity 80 %, aeration 0.4 vvm, tank pressure 0.03 Mpa. The substrates in the tank were stirred thoroughly for 5 min every 12 h. The fermented substrates were sampled every 4 h until 48 h to detect the apparent biomass concentrations, lipopeptide yields and concentrations of total reducing sugars.
Isothermal and non-isothermal techniques
For the isothermal process, the fermentation was conducted in constant mode at 30 °C for 48 h; For the non-isothermal (two-temperature-stage) process, the fermentation was started in isotherm at 30 °C for the first 24 h and then followed by a temperature shift to 37 °C till 48 h.
Analytical methods
The quantification of bacterial biomass in SSF was conducted according to the method described by Sella et al. (2008) with some modifications. 5 g of fermented substrates were mixed with sterilized water (1:5 w/v) containing several beadings in a 250 mL flask and stirred for 20 min at room temperature. Then the mixture was filtered through two layers of muslin under sterile conditions to obtain cell filtrate. The filtrate was centrifuged at 10,000 rpm for 20 min at 4 °C, and then the cell pellets were washed twice with distilled water and dried to constant weight at 105 °C. We defined the dried solid which mainly consisted of the cells as apparent bacterial biomass, for the description of the biomass changed with fermentation course. And the concentration of apparent biomass was expressed as mg/gdr (per g dry residues).
For determination of lipopeptide yield, the fermented substrates were mixed with distilled water (1:10 w/v) by stirring for 1 h at room temperature, centrifuging at 10,000 rpm for 10 min to remove the insoluble matter. 6 mol/L HCl was added to the cell-free supernatants to a final pH of 2.0 and stored at 4 °C overnight to precipitate the crude lipopeptides. The crude lipopeptides were recovered by centrifugation at 10,000 rpm for 20 min at 4 °C and extracted with 20 mL of dichloromethane. The extraction was dried using a rotary vacuum evaporator. The residues was re-suspended in distilled water and neutralized, then lyophilized to powder form. The recovery of lipopeptides was expressed as mg/gds (per g dry substrate).
For determination of total reducing sugars, 5 g of sample substrates were hydrolyzed in 100 mL 3 mol/L HCl at 100 °C for 20 min and neutralized with NaOH solution. Then the mixture was filtered through 0.45 μm membranes to obtain filtrate. The cooled extract was used for analysis and the total reducing sugar was measured by the dinitrosalicylic acid (DNS) method described by Miller (1959). The concentration of total reducing sugar was expressed as mg/gdr.
Kinetic modeling
The logistic model is one of the most widely used model for describing microbial growths of biological systems (Wachenheim et al. 2003). It can reflect a circumstance where exponential growth is indicated and where that growth is approaching a fixed limit (Altiok et al. 2006). Although the logistic model does not involve a substrate term, it is suitable for the approximation of the microbial growth in this study, due to constant values of the initial substrate concentrations and the inoculation volume. This model can be described by the following equation:
where X is the apparent biomass concentration (mg/gdr); t is the fermentation time (h); μ m is the maximal specific growth rate (/h); and X m is the maximal apparent biomass concentration (mg/gdr). Integration of Eq. (1) gives a sigmoidal variation of X as a function of t Eq. (2) as follows:
where X 0 is the initial apparent biomass concentration (mg/gdr).
It is known that lipopeptides are mostly accumulated during the stationary phase of the microbial growth in SSF (Zhu et al. 2012). Hence, the modified Gompertz model which gives the lag time and the maximum product formation rate was employed to model the lipopeptide production during the fermentation course in the following equation (Van Ginkel et al. 2001):
where P is the lipopeptide production rate (mg/gds); P m is the potential maximal lipopeptide production rate (mg/gds); R m is the maximal rate of lipopeptides formed [mg/(gds h)]; t L is the lag time to exponential product formed (h).
The fermentation substrate is used to form cell material and metabolic products as well as the maintenance of cells. Thus, in this study the substrate consumption may be expressed as substrate conversion to lipopeptides and substrate consumption for maintenance (Yang et al. 2011):
where S is the total sugar concentration (mg/gdr); S 0 is the initial sugar concentration (mg/gdr); Y X/S is the yield coefficient for cells on substrate used for cell formation; Y P/S is the yield coefficient for lipopeptide product on substrate used for product formation; m is the maintenance constant (/h).
Using the boundary condition at t0 = 0, X = X 0, S = S 0, Eq (4) can be easily integrated to give the following equation:
where S 0 is the initial substrate concentration (mg/gds).
Statistical analysis
All experiments were carried out in triplicate. The average values of experimental results were used for the calculation of kinetic parameters and fitting the kinetic models. The logistic, modified Gompertz and Luedeking–Piret-like models were developed by non-linear regression analysis, using available Origin 8.5 software.
Results
Kinetics of microbial growth
The experimental biomass versus time profiles and estimated growth models are shown in Fig. 1. It can be observed that the apparent biomass reaches its maximum at 32 h (2.06 mg/gdr) for the isothermal process and at 36 h (2.15 mg/gdr) for the non-isothermal process in flask, respectively. Thereafter the apparent biomass concentrations decreased gradually to about 2.00 and 2.08 mg/gdr at 48 h for the two processes. In fermenter, the dynamic changes of biomass concentration were similar to flask but the values of apparent biomass concentration were lower than those in flask at the same time points both for isothermal and non-isothermal processes. Correlation coefficients (R 2) for the model fits were calculated as 0.995, 0.997, 0.993 and 0.996, respectively. These results indicated that the logistical model calculations can explain 99.5, 99.7, 99.3 and 99.6 % of variability in the actual results, respectively. Therefore, the developed mathematical models was able to adequately describe the microbial growth during the different fermentations.
The corresponding kinetic parameters for the biomass growth are summarized in Table 1. It can be seen that the non-isothermal process had higher levels of X m (2.166 vs. 2.055 mg/gds) and lower values of μ m (0.156 vs. 0.165/h) as compared to the isothermal process in flask, indicating that the temperature changes had a positive effect on the accumulation of biomass but it would decrease the specific growth rate of apparent biomass as a result of the extended growth time in SSF. For the isothermal process, the maximal specific growth rate increased as the fermentation scaled, while the trend for non-isothermal process was different. Under non-isothermal process, the specific growth rate of cells remained relatively stable from flask to fermenter.
Kinetics of lipopeptides formation
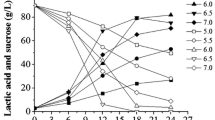
The behaviors of lipopeptides formation at different scales of growth, as shown in Fig. 2a–d, reveal that the microorganism began to synthesize lipopeptides after 4 h of fermentation and the highest levels of lipopeptide yields were obtained at 48 h (50.01 mg/gds) for the isothermal process and at 44 h (55.83 mg/gds) for the non-isothermal process in flask, respectively. After 24 h, the non-isothermal process had higher lipopeptide yields than isothermal process at the same time point. In fermenter, the lipopeptide formation trend coincided with that in flasks for the two processes with lower maximal values of lipopeptide production (42.68 vs. 50.01 mg/gds for isothermal process and 50.05 vs. 55.83 mg/gds for non-isothermal process, respectively) as compared to flask. For the proposed kinetic models, the values of R 2 were in the range of 0.986–0.998, suggesting that the modified Gompertz equation was found to be an appropriate model for successfully reflecting the production of lipopeptides during fermentation of agro-industrial byproducts in SSF.
The effects of temperature changing on the productive parameters of lipopeptide fermentation were clearly evident (Table 2). It was found that the values of potential maximal lipopeptide production (P m) were increased by 20.53 % in flask and by 13.10 % in fermenter under the non-isothermal process, respectively. For the maximal lipopeptide formation rate (R m), the non-isothermal process had a significantly higher value [2.540 vs. 2.085 mg/(gds h)] in flask, while the value in fermenter increased slightly [2.635 vs. 2.570 mg/(gds h)] as compared to isothermal process. These changes confirmed the positive influence of temperature changing on the lipopeptide production. However, the non-isothermal process showed higher levels of lag time to exponential product formed (t L) than the isothermal process, both in flask and in fermenter, meaning that higher temperature would delay the synthesis of lipopeptides during the early stage of fermentation. For the same process, the values of P m and t L decreased significantly as the fermentation scaled. And the fermenter conditions increased the lipopeptide formation rates with higher values of R m than flask conditions.
Comparison of the specific product formation rate
To compare the microbial capability of producing lipopeptides as functions of temperature changing and scale-up, the apparent specific formation rates of lipopeptides [mg/(gdb h), mg product/(g dry biomass)/h] during different fermentations were taken into account and the results are shown in Fig. 3. After 24 h of fermentation, the non-isothermal process showed higher levels of apparent specific lipopeptide production rates than the isothermal process in flask and in fermenter. For experiments conducted in fermenters the peak occurred at 28 h for both of the two processes, which was 8 h ahead as compared to flasks. And the maximal apparent specific lipopeptide production rate gap between the two processes in flask [0.13 (mg/(gdb·h))] was higher than that in fermenter [0.02 (mg/(gdb·h))]. In other words, the scaled conditions in fermenter accelerated the formation rates of lipopeptides in SSF.
Kinetics of substrate consumption
As can be observed from Fig. 4a–d, the total sugar concentration reduced as the fermentation proceeded under various conditions. The non-isothermal process caused a larger reduction of the total sugar concentration after 24 h of fermentation as compared to the isothermal process in both flask and fermenter. For the same process, the total sugar utilization in fermenter was lower than that in flask throughout the incubation time. A reasonable fit (R 2 value >0.985) to the total sugar-time profile was achieved by using the Luedeking–Piret-like equation [Eq. (5)] for each fermentation. Therefore, the proposed mathematical model provided a suitable simulation of the total sugar consumption during the lipopeptide fermentation in SSF.
The coefficients for total sugars used for product formation (Y P/S ) of non-isothermal process reached 2.870 in flask and 9.639 in fermenter, respectively, which were higher than those obtained from the isothermal process (Table 3). The non-isothermal process could increase the coefficients for total sugars used for the cell formation (Y X/S ) by 10.34 % in flask and by 38.46 % in fermenter as compared to the isothermal process. And the maintenance of cells (m) increased largely under the non-isothermal process with values of 0.426/h in flask and 0.718/h in fermenter, respectively. As the fermentation scaled, the values of Y P/S and Y X/S under the two processes both increased significantly. The non-isothermal process had a higher value of m in fermenter than in flask, while the isothermal process showed the reverse.
Discussion
An increase in temperature results in more kinetic energy of the enzyme and the substrate (Ikasari et al. 1999). The temperature changing to a higher value stimulated the bacterial growth to further utilize the substrates and caused a longer period (4 h) for the microbial growth in flask and in fermenter (Fig. 1). Consequently, the apparent biomass concentration obtained under non-isothermal process was higher than that under isothermal process after 24 h of fermentation time. The kinetics of microbial growth showed that the apparent biomass concentrations under different conditions did not always increase with the fermentation time. The decrease of the apparent biomass concentrations after a period of fermentation could be explained by the observation that the high concentration of lipopeptides produced by the microorganism might lyse the membranes and in some degree cause a degradation of the cell (Heerklotz and Seelig 2007).
After 44 h of non-isothermal fermentation, the yields of lipopeptides began to decrease in both flask and fermenter. This was mainly due to the bacterial self-resistance to accumulation of lipopeptides (Tsuge et al. 2001). It was reported that the problems of difficulty in heat transfer and diffusion limitation of nutrients in scale-up fermentation negatively influence the yields of metabolite product under SSF conditions (Raghavarao et al. 2003). Although the fermenter system used in this study had stirring and temperature control devices, such problems still existed in lipopeptide production and decreased the productivity of lipopetides in fermenter. The increased specific lipopeptide production rates under non-isothermal process from 24 to 48 h of fermentation in flask and in fermenter indicated the increased levels of lipopeptide production during this stage, ensuring the productive improvement of non-isothermal process over isothermal process.
Introducing a practicable approach to modeling the product formation kinetics in SSF is valuable owing to the difficulty in monitoring and controlling different involved variables of the fermentation systems (Mitchell et al. 2004; Hashemi et al. 2011). The increased growth of bacteria increased the lipopeptide production, resulting in a higher value of potential maximum lipopeptide production than the isothermal process. The differences in environmental factors such as agitation speed and oxygen supply would influence the cell metabolism and product formation (Prabhakar et al. 2005). Thus, the productivity of lipopeptides in the fermenter was lower than in the flask for the same process. A longer lag was found under non-isothermal process, suggesting that the temperature changing delayed the overall kinetics of lipopeptide formation and accelerated the synthetic rates of lipopeptides during the fermentation. Higher temperature was not conducive for the lipopeptide production as it might not be suitable for the growth of microorganism in the initial fermentation (Zhu et al. 2013a).
The substrate consumption was associated to the cell growth and the product formation, and the higher values of biomass and lipopeptide production obtained under non-isothermal process caused a higher requirement for substrate, which decreased the total sugar concentration more largely in the culture as compared to the isothermal process. And the higher yields of biomass and lipopeptides in flask indicated a higher level of substrate utilization and lowered the total sugar concentration than in fermenter. As higher temperature could enhance the microbial activity in the fermentation, the non-isothermal process accelerated the transformation of substrate and consequently increased the parts of substrate carbon used for the cell formation, product formation and maintenance purposes. The values of of Y P/S and Y X/S in fermenter were higher than in flask, which evidenced that the scaled conditions increased the part of substrate carbon to meet the specific biomass growth and product formation (Hensing et al. 1995).
Conclusion
Through these models, theoretical predictions were made and they matched well with the experimental results. The non-isothermal process was found to increase the kinetic parameters related to the lipopeptide productivity but decrease the specific growth rate of biomass and lipopeptide synthesis in the initial fermentation. The production of lipopeptides in fermenter was lower than in flask, but the scaled conditions had positive effects on the formation rates of lipopeptides. Hence, the productive differences between flask and fermenter constituted an important topic for a further study. This information will be useful in the temperature changing condition and the process scaling up.
References
Altiok D, Tokatli F, Harsa S (2006) Kinetic modeling of lactic acid production from whey by Lactobacillus casei (NRRL B-441). J Chem Technol Biotechnol 81:1190–1197
Cameotra SS, Makkar RS (2004) Recent applications of biosurfactants biological and immunological molecules. Curr Opin Microbiol 7:262–266
Das K, Mukherjee AK (2007) Comparison of lipopeptide biosurfactants production by Bacillus subtilis strains in submerged and solid state fermentation systems using a cheap carbon souce: some industrial applications of biosurfactants. Process Biochem 42:1191–1199
Dodić JM, Vučurović DG, Dodić SN, Grahovac JA, Popov SD, Nedeljković NM (2012) Kinetic modelling of batch ethanol production from sugar beet raw juice. Appl Energy 99:192–197
Fujikawa H, Kai A, Morozumi SA (2004) A new logistic model for Escherichia coli growth at constant and dynamic temperatures. Food Microbiol 21:501–509
Hashemi M, Mousavi SM, Razavi SH, Shojaosadati SA (2011) Mathematical modeling of biomass and α-amylase production kinetics by Bacillus sp. in solid-state fermentation based on solid dry weight variation. Biochem Eng J 53:159–164
Heerklotz H, Seelig J (2007) Leakage and lysis of lipid membranes induced by the lipopeptide surfactin. Eur Biophys J 36:305–314
Hensing MCM, Rouwenhorst RJ, Heijnen JJ, van Dijken JP, Pronk JT (1995) Physiological and technological aspects of large-scale heterologous-protein production with yeasts. Antonie Van Leeuwenhoek 67:261–279
Holker U, Lenz J (2005) Solid-state fermentation—are there any biotechnological advantages? Curr Opin Microbiol 8:301–306
Ikasari L, Mitchell DA, Stuart DM (1999) Response of Rhizopus oligosporus to temporal temperature profiles in a model solid-state fermentation system. Biotechnol Bioeng 64:722–728
Mazutti MA, Zabot G, Boni G, Skovronski A, de Oliveira D, Di Luccio M, Rodrigues MI, Mauqeri F, Treichel H (2010) Mathematical modeling of Kluyveromyces marxianus growth in solid-state fermentation using a packed-bed bioreactor. J Ind Microbiol Biotechnol 37:391–400
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Mitchell DA, von Meien OF, Krieger N, Dalsenter FDH (2004) A review of recent developments in modeling of microbial growth kinetics and intraparticle phenomena in solid-state fermentation. Biochem Eng J 17:15–26
Mizumoto S, Hirai M, Shoda M (2006) Production of lipopeptide antibiotic iturin A using soybean curd residue cultivated with Bacillus subtilis in solid-state fermentation. Appl Microbiol Biotechnol 72:869–875
Mu Y, Wang G, Yu HQ (2006) Kinetic modeling of batch hydrogen production process by mixed anaerobic cultures. Bioresour Technol 97:1302–1307
Nath K, Das D (2011) Modeling and optimization of fermentative hydrogen production. Bioresour Technol 102:8569–8581
Özilgen M (1998) Kinetics of multiproduct acidogenic and solventogenic batch fermentations. Appl Microbiol Biotechnol 29:536–543
Pandey A, Soccol CR, Mitchell D (2000) New developments in solid state fermentation. I-Bioprocess-products. Process Biochem 35:1153–1169
Prabhakar A, Krishnaiah K, Janaun J, Bono A (2005) An overview of engineering aspects of solid-state fermentation. Malays J Microbiol 1:10–16
Raghavarao KSMS, Ranganathan TV, Karanth NG (2003) Some engineering aspects of solid-state fermentation. Biochem Eng J 13:127–135
Sella SR, Dlugokenski RE, Guizelini BP, Vandenberghe LP, Medeiros AB, Pandey A, Soccol CR (2008) Selection and optimization of Bacillus atrophaeus inoculum medium and its effect on spore yield and thermal resistance. Appl Biochem Biotechnol 151:380–392
Sriram MI, Kalishwaralal K, Deepak V, Gracerosepat R, Srisakthi K, Gurunathan S (2011) Biofilm inhibition and antimicrobial action of lipopeptide biosurfactant produced by heavy metal tolerant strain Bacillus cereus NK1. Colloid Surf B 85:174–181
Tsuge K, Ohata Y, Shoda M (2001) Gene yerP, involved in surfactin self-resistance in Bacillus subtilis. Antimicrob Agents Chemother 45:3566–3573
Van Ginkel S, Sung S, Lay JJ (2001) Biohydrogen production as a function of pH and substrate concentration. Environ Sci Technol 35:4726–4730
Votruba J, Volesky B, Yerushalmi L (1985) Mathematical model of batch acetone–butanol fermentation. Biotechnol Bioeng 28:247–255
Wachenheim DE, Patterson JA, Ladisch MR (2003) Analysis of the logistic function model: derivation and applications specific to batch cultured microorganisms. Bioresour Technol 86:157–164
Yalcin SK, Özbas ZY (2005) Determination of growth and glycerol production kinetics of a wine yeast strain Saccharomyces cerevisiae Kalecik 1 in different substrate media. World J Microbiol Biotechnol 21:1303–1310
Yang JS, Rasa E, Tantayotai P, Scow KM, Yuan HL, Hristova KR (2011) Mathematical model of Chlorella minutissima UTEX2341 growth and lipid production under photoheterotrophic fermentation conditions. Bioresour Technol 102:3077–3082
Zhu Z, Zhang GY, Luo Y, Ran W, Shen QR (2012) Production of lipopeptides by Bacillus amyloliquefaciens XZ-173 in solid state fermentation using soybean flour and rice straw as the substrate. Bioresour Technol 112:254–260
Zhu Z, Li R, Yu GH, Ran W, Shen QR (2013a) Enhancement of lipopepitdes production in a two-temperature-stage process under SSF conditions and its bioprocess in the fermenter. Bioresour Technol 127:209–215
Zhu Z, Zhang FG, Wei Z, Ran W, Shen QR (2013b) The usage of rice straw as a major substrate for the production of surfactin by Bacillus amyloliquefaciens XZ-173 in solid-state fermentation. J Environ Manag 127:96–102
Acknowledgments
This work was supported by National Key Technology Support Program (Grant No. 2013BAD08B04-7), Innovative Research Team Develoment Plan of the Ministry of Education of China (IRT256), the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions, and the 111 Project (B12009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Z., Sun, L., Huang, X. et al. Comparison of the kinetics of lipopeptide production by Bacillus amyloliquefaciens XZ-173 in solid-state fermentation under isothermal and non-isothermal conditions. World J Microbiol Biotechnol 30, 1615–1623 (2014). https://doi.org/10.1007/s11274-013-1587-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1587-7