Abstract
Solid state anaerobic digestion, as a safe and environment-friendly technology to dispose municipal solid wastes, can produce methane and reduce the volume of wastes. In order to raise the digestion efficiency, this study investigated the pretreatment of yard waste by thermal or chemical method to break down the complex lignocellulosic structure. The composition and structure of pretreated yard waste were analyzed and characterized. The results showed that the pretreatment decreased the content of cellulose and hemicelluloses in yard waste and in turn improved the hydrolysis and methanogenic processes. The thermal pretreatment sample (P1) had the highest methane yield, by increasing 88 % in comparison with digesting the raw material. The maximum biogas production reached 253 mL/g volatile solids (VS). The largest substrate mass reduction was obtained by the alkaline pretreatment (P5). The VS of the alkaline-treated sample decreased about 60 % in comparison with the raw material.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anaerobic digestion (AD) of biomass waste has gained increased attention as a means of producing biogas and solving the problems associated with the organic waste disposal, which has been widely applied to treatment of municipal solid waste (MSW) (Lin et al. 2009; Fernández et al. 2005; Cuetos et al. 2008; Bolzonella et al. 2003). The AD process can be divided into the steps of hydrolysis, acidogenesis, acetogenesis/dehydrogenation and methanation (Hendriks and Zeeman 2009). The first step is the degradation of biomass waste into small molecules through hydrolysis. In the second step, acidogenesis ferments the break-down products to form acetic acid, hydrogen, carbon dioxide and other lower-weight simple volatile organic acids like propionic acid and butyric acid which are, in turn, converted to acetic acid. In the last step, the acetic acid, hydrogen and carbon dioxide are converted into a mixture of methane and carbon dioxide by the methanogenic bacteria (Sreekrishnan et al. 2004; Li et al. 2011). The yard waste is an important part of MSW. However, the cellulose and hemicelluloses in the cell wall are tightly bonded with the lignin, which leads to slow and incomplete degradation (Ellenrieder et al. 2010; Kreuger et al. 2011; Noike et al. 2004). Therefore, an efficient pretreatment is very important for separating cellulose and hemicelluloses from the waste with ease and at a low cost (Park and Kim 2012). The hydrolysis of lignocellulosic biomass is considered as a rate-limiting step in AD (Noike et al. 2004; Lu et al. 2007). Effective pretreatment can degrade the polysaccharide and lignin into smaller molecules, making the cellulose and hemicelluloses more accessible and more readily degradable to anaerobic microorganisms (He et al. 2008).
The pretreatment can be performed by physical methods such as milling, thermal treatment, steam explosion, or by chemical methods such as acid or alkaline treatment, ammonia and carbon dioxide pretreatment (Chandra et al. 2007; Brown and Li 2012; Saritha et al. 2012). The thermal pretreatment can decompose part of lignin, cellulose and hemicelluloses (Lissens et al. 2004). The acid pretreatment can be carried out with concentrated or diluted acid (Kumar et al. 2009). After a pretreatment in high-concentration acid, about 90 % hemicelluloses and cellulose can be converted into sugar. Diluted acid pretreatment employs relatively moderate conditions, and it can change the polymerization, fiber density and crystallinity of the cellulose. Compared with the acid pretreatment, the alkaline pretreatment adopts lower temperature and longer treatment time (Zhu et al. 2010; Monlau et al. 2012; Liang et al. 2011). The alkaline pretreatment can destroy the three-dimensional network of lignin to release cellulose from the lignoncellulosic polymer. Moreover, the alkaline pretreatment can prevent decrease of pH during acetogenesis and promote methanogenesis (Hashimoto 2004). The main problem of the acidic or alkaline pretreatment is the extremely high or low pH value and the large amount of salt by-produced.
The novel concern of this study is to analyze the effect of major pretreatment methods on yard waste structure and to compare the behavior of untreated and pretreated substrates for effective methane fermentation by solid state digestion. As a matter of fact, in the literature there was almost no report on the comparison of the effects realized by different pretreatments on changing the material structure and dry digestion performance of biomass wastes, although lots of studies have been conducted on the pretreatment for wet digestion (Rughoonundun et al. 2010; Penaud et al. 1999).
Experimental
Materials and pretreatment methods
The yard waste was collected from a lawn in Beijing, China. Before the experiment, the naturally dried yard waste was milled into 5–10 mm. The main properties of the substrate used are shown in Table 1. Both cellulose and hemicelluloses take up about 60 % of the material mass, while the content of lignin is only about 8 wt%.
The tested pretreatment methods were thermal, alkaline and acidic methods according to references (Lin et al. 2013; He et al. 2008; Pang et al. 2008). The parameters of the pretreatment were presented in Table 2. The above-mentioned yard waste was the raw material. The digestion experiments were separated into 2 groups. The Group-I refers to the digestion with pretreatment only via the three methods mentioned above, while the Group-II represents the digestion with pretreatment and further washing using deionized water. In each experiment, 300 g of yard waste (dry basis) were mixed with 1200 mL NaOH or H2SO4 solution. The corresponding loading ratio of the substrate solid matters was 6 % (w/w). The concentration of NaOH was 0.38 mol/L and the concentration of H2SO4 was 0.15 mol/L. According to Fernández et al. (2008), the AD with total solids (TS) of 20 wt% showed a better performance than that of the AD with TS of 30 wt%. The TS was 20 wt% at the beginning of digestion. The amount of water was just right for mixing well the yard waste and reagents, which should be sufficient for chemical reaction (no extra water existed). Hence, the additional treatment of waste water can be avoided.
The alkaline pretreatment (P2 and P5) was carried out at 20 °C for 24 h, while the thermal pretreatment (P1 and P4) and acid pretreatment (P3 and P6) groups were by heating the samples to and then keeping them at 121 °C for 30 min. Extreme pH was shown to be harmful to methanogenesis so that in the three methods the pH value was carefully adjusted. After pretreatment, the pH in the Group-I (P1, P2, P3) was adjusted to 7 ± 0.5 by adding chemical reagents. For the Group-II (P4, P5, P6) the pH was adjusted by washing the samples and then filtering them through an 80-mesh sieve.
Digestion tests and characterization
Anaerobic batch digestion of the treated and untreated yard waste was carried out at 37 °C. The biocatalyst was obtained from municipal wastewater treatment plant under mesophilic operation. Each sample was inoculated with 100 mL seed cultures and digested for 60 days. The working volume of the reactor was 1.5 L. The untreated sample (CK) was adopted as the control test. The biogas production was measured every 24 h. A solid sample was taken every week. The pH value was adjusted every 24 h. The reduction percent of substrate after digestion test was calculated by mass loss as shown in Eq. (1), in which X i refers to the initial mass of substrate, X f denotes the final mass of substrate, and X indicates the amount of volatile solids (VS) or total solids (TS).
The TS and VS were determined at the beginning and end of the digestion according to the Standard Methods for the Examination of Water and Wastewater (APHA 2005). The total carbon (TC) and total nitrogen (TN) were analyzed with an elementary analyzer (VarioEl III). The sample for analysis was centrifuged at 10,000 r/min for 10 min. The resulting liquid was measured by a pH meter (Mettler Toledo FE20), and the solid residue was used to analyze the content of lignin, cellulose and hemicelluloses according to the Van Soest method (Van Soest et al. 1991). The volume of biogas produced was measured by the so-called water displacement method. Its composition was determined in a gas chromatography (Agilent Micro3000-GC) equipped with a thermal conductivity detector (TCD) using argon as the carrier gas. The temperatures of the injector and detector for the GC were 100 and 150 °C, respectively. The content of monomeric sugars was analyzed using an HPLC (Agilent 1260 series) equipped with a Biorad Aminex HPX-87p column and a refractive index detector (RID). The temperatures of the column and the RID were maintained at 80 and 55 °C, respectively (Zieminski et al. 2012).
Results and discussion
Substrate structure and composition
In the process of AD, the biomass should be first hydrolyzed because the chemical barriers such as lignin, hemicelluloses and acetyl group inhibit the accessibility of microorganisms to the cellulose substrate (Park and Kim 2012). The chosen pretreatment methods were the thermal, alkaline and acidic treatments. Figure 1 shows the SEM images of the tested yard waste before and after pretreatment. From Fig. 1a, b one can see that there was substantial covering dust or powder on the surface of the raw dry grass, whereas after the treatment with the thermal method most of the dust and powder were removed so that the surface exhibited evident stripe structure. The alkaline pretreatment can destroy the linkages of inter-units and the functional groups (He et al. 2008). The obvious cracks and rough surface shown in Fig. 1c indicate that the lignin experienced severe stripping in alkaline substances, which means that the alkaline pretreatment disrupts the lignin structure and breaks the linkage between lignin and the other carbohydrate fractions in the lignocellulosic biomass. The dilute acid pretreatment hydrolyzes hemicelluloses to its monomeric units, thus rendering the cellulose more available to microorganisms (Agbor et al. 2011). Figure 1d implies that the yard waste was hydrolyzed to form scaly structure after sulfuric acid soaking. The acid pretreatment caused the most serious destruction of biomass. Obviously, the pretreatment increases the specific surface area to make more substrate exposed and accessible to anaerobic microorganisms. It offers more accessible reactive sites for AD and can enhance the biogas yield.
Effective pretreatment of lignocellulosic biomass is characterized by the reduction in particle size, increase in surface area, disruption of cellulose crystallinity, hemicelluloses disruption and lignin redistribution, which can change the composition of the biomass. Therefore, the content of hemicelluloses, cellulose and lignin changed after the pretreatment. The pretreatment decomposed part of the yard waste. Table 3 shows the hemicelluloses, cellulose and lignin contents in yard waste before and after pretreatment. When pretreated with the thermal method, the hemicelluloses, cellulose and lignin contents changed from 32.1, 28.9 and 7.9 to 24.7, 24.3 and 8.5 g/100 g TS, respectively. When pretreated with alkaline solution, the hemicelluloses, cellulose and lignin contents changed from 32.1, 28.9 and 7.9 to 27.7, 26. 9 and 2.7 g/100 g TS, respectively. After the treatment with an acidic solution, the hemicelluloses, cellulose and lignin contents were 17.3, 28. 8 and 1.7 g/100 g TS, respectively. When pretreated with an acidic solution, the loss of hemicelluloses was 17.3 g/100 g TS, which was twice than that for the thermal pretreatment. The loss in cellulose caused by the pretreatment was lower than that for hemicelluloses due to the physical protection of lignocellulosic structure. Cellulose was not directly degraded by the pretreatment but changed its fibers and crystallinity of the lignocellulosic structure. As reported, alkali works on lignin while acid mainly decomposes hemicelluloses (Taherzadeh and Karimi 2008). However, lignin was significantly degraded in both alkaline and acidic pretreatments. The high temperature in the acidic pretreatment resulted in a greater loss of lignin than in the alkaline pretreatment. These results show that the pretreatment could substantially change both the structure and composition of biomass.
Yield and composition of biogas
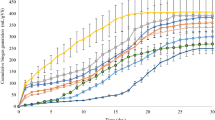
The crystalline cellulosic structure is shielded with lignin and hemicelluloses, which makes it difficult to be converted into biogas by anaerobic bacteria. The pretreatment prior to the biogas production can increase the digestibility of the lignocellulosic materials. It can also raise the biodegradation rate and biogas yield in biological energy conversion processes. Figure 2 presents the effect of pretreatment on the cumulative yield of biogas. It can be seen that the biogas yield was low in the first 20 days. Then, a sharp increase in the biogas production appeared for the Group-I on the 30th day and for the Group-II on the 15th day. This indicates that the start-up time of digestion was shortened by washing with deionized water after pretreatment. The anaerobes had to get fit into the circumstance of high salt density such as Na+ and SO4 2+ caused by more chemical reagents in the Group-I, which can delay the start-up time (Chen et al. 2008). The digestion of the pretreated biomass in the Group-I almost ended after 40 days of fermentation, while the fermentation for the Group-II kept a steady increase rate in gas yield till the end of digestion. Within 60 days, the biogas production of thermally pretreated sample (P1) and acidly pretreated sample (P6) was 253 mL/g VS and 209 mL/g VS, respectively. Both are higher than that of digestion of the raw dry grass. Compared with Fig. 2a, the biogas production with alkaline/acidic pretreatment grew faster in Fig. 2b. The chemically pretreated dry grasses were washed with deionized water to remove phenolic and heterocyclic compounds, for example, vanillin, vanillin alcohol, furfural and HMF that were all inhibitors for the AD. However, some fermentable sugars were also washed out by deinoized water so that the biogas yield was lower for P4 than P1. In the Group-I, the biogas yield of the sample treated with the thermal method (P1) was higher than those treated with the chemical methods (P2 and P3), which proved as well the inhibition effects of salts on digestion. Water under high pressure and high temperature can penetrate into the biomass, and in turn hydrate cellulose and remove hemicelluloses and part of lignin. The major advantage of the thermal pretreatment is that there is no chemical added into the fermentation system (Chandra et al. 2012).
Effect of pretreatment on cumulative biogas yield (the digestion was at 37 °C and lasted for 60 days, and referring to Table 2 for sample conditions)
When pretreated with an alkaline solution, the biogas yield was 55 mL/g VS, while after washing with deionized water the biogas yield increased to 106 mL/g VS. It can be seen that the biogas yield of the samples pretreated by alkaline method was lower than that of the untreated biomass (CK), which was 185 mL/g VS. This was probably attributed to the loss of fermentable sugars in pretreatment. Alkaline may cause reconstruction of cellulose and condensation of lignin (Gregg and Saddler 1996). Another reason may be the accumulation of volatile fatty acids (VFA). Alkaline treatment could prevent decrease of pH, which probably led to a high VFA concentration (Hashimoto 2004). In this study, the peak value of VFA was 45.3 g/L in alkaline pretreatment (P5), 21–36 % higher than the others. The accumulation of VFA was detrimental for methanogens which can lead to the decrease of pH value and also the methane production (Buyukkamaci and Filibeli 2004; Nguyen et al. 2007). The result was similar to the result that the methane production decreased after pretreatment with alkali or acid (Lin et al. 2013).
Figure 3 presents the effect of pretreatment on the methane content in the biogas. It can be seen that the methane content quickly rose to 80 vol% for the samples P2 and P3. Meanwhile, all in the Group-II showed a steady increase from 20 to 80 vol%. The monomeric sugars were generated in alkaline/acid pretreatment, which could be directly used by acetogenic methanogens. But for the samples in the Group-II, the soluble small molecule substances were eliminated by washing, and it took time for the cellulose to be hydrolyzed to produce monosaccharide that could be digested in the fermentation. The methane content decreased at the end of digestion for the samples of P2 and P3. For P1, P4 and P6, the methane content remained above 60 vol% from the 30th day till the end of experiment. The cumulative methane yield was calculated with the biogas yield and concentration of methane. Figure 4 shows the effect of pretreatment on the cumulative methane yield. Compared with Fig. 2, it can be seen that the cumulative yield of methane follows the same trend as the biogas yield does. The methane yield for the samples CK, P1, P2, P3, P4, P5, P6 was 64, 119, 13, 16, 48, 18 and 98 mL/g TS after digestion for 60 days, respectively.
Effect of pretreatment on methane content in generated biogas (the digestion was at 37 °C and lasted for 60 days, and referring to Table 2 for sample conditions)
Effect of pretreatment on cumulative methane yield (the digestion was at 37 °C and lasted for 60 days, and referring to Table 2 for sample conditions)
Degradation efficiency
The reduction of cellulose and hemicelluloses represents the digestion efficiency. Figure 5 shows the reduction of cellulose and hemicelluloses in the process of the tested solid state digestion. The decrease of cellulose amount in the substrate was higher than that of hemicelluloses, which means that cellulose was the main source and better substrate for methane production in AD. The decrease in the amount of cellulose and hemicelluloses for the Group-II samples (P4, P5, P6) was higher than that for the Group-I samples (P1, P2, P3). It can be concluded that the digestion efficiency of washed samples after pretreatment was higher than the unwashed ones. The result suggested that washing after pretreatment was an effective measure to remove the inhibitive small molecular compounds yielded in the acidic/alkaline pretreatment.
In general, the higher degradation ratio was correlated with the higher biogas yield (Liew et al. 2011). The reduction of cellulose and hemicelluloses amount was higher when the raw material was pretreated with the thermal and acidic methods. But both cellulose and hemicelluloses were substantially degraded in the alkaline pretreatment with a relatively low methane yield during digestion. The COD of the digestion leachate showed that the soluble hemicelluloses and lignin were dramatically increased in alkaline circumstance. The COD in the alkaline pretreatment (P2) was 68 g/L while the ones for the thermal (P1) and acidic (P3) pretreatments were 33 and 28 g/L, respectively. Apparently, the alkaline pretreatment increased the content of soluble substances in the substrate (Penaud et al. 1999). However, a large part of the organic compounds was not utilized by methanogens to generate biogas in the AD process.
The percentage of VS indicates the fraction of biodegradable matters containing in the substrate. The pretreatment raised the VS percent to provide more biodegradable substance for anaerobes. The VS content of raw grass was 64.9 wt% (Table 1). After the thermal, alkaline and acidic pretreatments, the VS content of the grass changed to 72.0, 66.4 and 64.8 wt%, respectively. With deionized water washing after the thermal, alkaline and acidic pretreatments, the VS content was increased to 79.1, 86.9 and 91.0 wt%, respectively. The increase in the VS content followed the order of P6 > P5 > P4 > P1 > P2 > CK > P3. Overall, the VS content increased after the thermal, acidic or alkaline pretreatments. Comparing the samples in Group-I and Group-II, the washing with deionized water increased the VS content, as a result of washing out of some salts.
Figure 6 shows the degradation efficiency of substrate in AD. The total solids (TS) of P1, P2, and P3 in the Group-I decreased by 54.8, 30.1 and 42.8 %, respectively. Meanwhile, the TS of P4, P5, and P6 in the Group-II decreased by 37.5, 55.3 and 40.7 %, respectively. The reduction percentage for VS was 59.9, 34.8 and 46.3 % when the dry grass was pretreated with the thermal, alkaline and acidic methods, respectively. These reduction percentages were 48.4, 60.1 and 53.6 % for the thermal, alkaline and acidic pretreatments with an afterward washing. The loss of VS was higher than that of TS. Degradation efficiency of the Group-II samples was higher than that of the Group-I samples after AD. The reduction in VS followed the following order from high to low: P5 > P1 > P6 > P4 > P3 > CK > P2, the same sequence as the biogas production except for P5 and CK. Most of cellulose and hemicelluloses were digested for the sample P5 so that the highest removal efficiency was obtained by P5. The reduction of P1 and P6 was high because most of the substrate had been consumed in yielding methane. The removal efficiency was higher than the results reported by Chanakya et al. (1999), implying a better digestion result due to pretreatment.
Conclusion
Experimental studies on digestion behavior of untreated and pretreated yard waste showed that the pretreatment can accelerate hydrolysis and improve the performance of solid state AD of biomass waste for biogas production. The pretreatment can destroy the linkage between lignin and cellulose and decrease the cellulose and hemicelluloses content in the substrate. The biogas yield of yard waste treated with the thermal method was much higher than those with the alkaline or acidic pretreatments. Because the inorganic salts, phenolic and heterocyclic compounds generated in chemical pretreatment can inhibit the digestion, the biogas yield in this case was lower. This inhibition from salts can be removed by washing the treated sample using deionized water. However, the waste water generated from washing are remaining to be treated for suitable applications. Among the tested pretreatment methods, the thermal pretreatment had the highest methane yield, which reached 119 mL/g TS and was 88 % relatively higher than that for the raw yard waste. The greatest biomass reduction due to digestion was obtained by the sample pretreated with alkaline. Its amount of volatile solids decreased about 60 % compared to the raw dry grass.
References
Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: fundamentals toward application. Biotechnol Adv 29(6):675–685
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington DC
Bolzonella D, Innocenti L, Pavan P, Traverso P, Cecchi F (2003) Semi-dry thermophilic anaerobic digestion of the organic fraction of municipal solid waste: focusing on the start-up phase. Bioresour Technol 86(2):123–129
Brown D, Li Y (2012) Solid state anaerobic co-digestion of yard waste and food waste for biogas production. Bioresour Technol 127:275–280
Buyukkamaci N, Filibeli A (2004) Volatile fatty acid formation in an anaerobic hybrid reactor. Process Biochem 39(11):1491–1494
Chanakya HN, Srikumar KG, Anand V, Modak J, Jagadish KS (1999) Fermentation properties of agro-residues, leaf biomass and urban market garbage in a solid phase biogas fermenter. Biomass Bioenerg 16(6):417–429
Chandra R, Bura R, Mabee W, Berlin A, Pan X, Saddler J (2007) Substrate pretreatment: the key to effective enzymatic hydrolysis of lignocellulosics? Biofuels 108:67–93
Chandra R, Takeuchi H, Hasegawa T, Kumar R (2012) Improving biodegradability and biogas production of wheat straw substrates using sodium hydroxide and hydrothermal pretreatments. Energy 43:273–282
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Bioresour Technol 99(10):4044–4064
Cuetos MJ, Gómez X, Otero M, Morán A (2008) Anaerobic digestion of solid slaughterhouse waste (SHW) at laboratory scale: influence of co-digestion with the organic fraction of municipal solid waste (OFMSW). Biochem Eng J 40(1):99–106
Ellenrieder J, Schieder D, Mayer W, Faulstich M (2010) Combined mechanical enzymatic pretreatment for an improved substrate conversion when fermenting biogenic resources. Eng Life Sci 10(6):544–551
Fernández A, Sanchez A, Font X (2005) Anaerobic co-digestion of a simulated organic fraction of municipal solid wastes and fats of animal and vegetable origin. Biochem Eng J 26(1):22–28
Fernández J, Pérez M, Romero L (2008) Effect of substrate concentration on dry mesophilic anaerobic digestion of organic fraction of municipal solid waste (OFMSW). Bioresour Technol 99(14):6075–6080
Gregg D, Saddler JN (1996) A techno-economic assessment of the pretreatment and fractionation steps of a biomass-to-ethanol process. Appl Biochem Biotech 57(1):711–727
Hashimoto AG (2004) Pretreatment of wheat straw for fermentation to methane. Biotechnol Bioeng 28(12):1857–1866
He Y, Pang Y, Liu Y, Li X, Wang K (2008) Physicochemical characterization of rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energy Fuel 22(4):2775–2781
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100(1):10–18
Kreuger E, Sipos B, Zacchi G, Svensson SE, Bjornsson L (2011) Bioconversion of industrial hemp to ethanol and methane: the benefits of steam pretreatment and co-production. Bioresour Technol 102(3):3457–3465
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48(8):3713–3729
Li Y, Park SY, Zhu J (2011) Solid-state anaerobic digestion for methane production from organic waste. Renew Sust Energ Rev 15(1):821–826
Liang Y, Zheng Z, Hua R, Luo X (2011) A preliminary study of simultaneous lime treatment and dry digestion of smooth cordgrass for biogas production. Chem Eng J 174(1):175–181
Liew LN, Shi J, Li Y (2011) Enhancing the solid-state anaerobic digestion of fallen leaves through simultaneous alkaline treatment. Bioresour Technol 102(19):8828–8834
Lin Y, Wang D, Wu S, Wang C (2009) Alkali pretreatment enhances biogas production in the anaerobic digestion of pulp and paper sludge. J Hazard Mater 170(1):366–373
Lin Y, Liang J, Wu S, Wang B (2013) Was pretreatment beneficial for more biogas in any process? Chemical pretreatment effect on hydrogen–methane co-production in a two-stage process. J Ind Eng Chem 19(1):316–321
Lissens G, Thomsen AB, Baere LD, Verstraete W, Ahring BK (2004) Thermal wet oxidation improves anaerobic biodegradability of raw and digested biowaste. Environ Sci Technol 38(12):3418–3424
Lu S, Imai T, Ukita M, Sekine M (2007) Start-up performances of dry anaerobic mesophilic and thermophilic digestions of organic solid wastes. J Environ Sci 19(4):416–420
Monlau F, Barakat A, Steyer JP, Carrere H (2012) Comparison of seven types of thermo-chemical pretreatments on the structural features and anaerobic digestion of sunflower stalks. Bioresour Technol 120:241–247
Nguyen PHL, Kuruparan P, Visvanathan C (2007) Anaerobic digestion of municipal solid waste as a treatment prior to landfill. Bioresour Technol 98(2):380–387
Noike T, Endo G, Chang JE, Yaguchi JI, Matsumoto JI (2004) Characteristics of carbohydrate degradation and the rate-limiting step in anaerobic digestion. Biotechnol Bioeng 27(10):1482–1489
Pang YZ, Liu YP, Li XJ, Wang KS, Yuan HR (2008) Improving biodegradability and biogas production of corn stover through sodium hydroxide solid state pretreatment. Energy Fuel 22(4):2761–2766
Park YC, Kim JS (2012) Comparison of various alkaline pretreatment methods of lignocellulosic biomass. Energy 47:31–35
Penaud V, Delgenes J, Moletta R (1999) Thermo-chemical pretreatment of a microbial biomass: influence of sodium hydroxide addition on solubilization and anaerobic biodegradability. Enzyme Microb Tech 25(3):258–263
Rughoonundun H, Granda C, Mohee R, Holtzapple MT (2010) Effect of thermochemical pretreatment on sewage sludge and its impact on carboxylic acids production. Waste Manag 30(8–9):1614–1621
Saritha M, Arora A, Lata (2012) Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J Microbiol 52(2):122–130
Sreekrishnan T, Kohli S, Rana V (2004) Enhancement of biogas production from solid substrates using different techniques—a review. Bioresour Technol 95(1):1–10
Taherzadeh MJ, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9(9):1621–1651
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74(10):3583–3597
Zhu J, Wan C, Li Y (2010) Enhanced solid-state anaerobic digestion of corn stover by alkaline pretreatment. Bioresour Technol 101(19):7523–7528
Zieminski K, Romanowska I, Kowalska M (2012) Enzymatic pretreatment of lignocellulosic wastes to improve biogas production. Waste Manag 32(6):1131–1137
Acknowledgments
The authors acknowledged the financial support from National Natural Science Foundation of China (21161140329), Hi-Tech Research and Development Program of China (863 Program, 2012AA021401) and National Key Technology R&D Program (2012BAC03B05).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, Z., Li, W., Zhang, G. et al. Impact of pretreatment on solid state anaerobic digestion of yard waste for biogas production. World J Microbiol Biotechnol 30, 547–554 (2014). https://doi.org/10.1007/s11274-013-1473-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1473-3