Abstract
Understanding the mechanisms of stress response and adaptation to stress in the case of lactic acid bacteria (LAB), especially in the case of strains with functional properties, is very important when such strains are potential candidates for starter cultures or probiotics. In this context, our study shows the response of some LAB [four exopolysaccharide (EPS)-producing strains and one strain with potential probiotic effect] to the stresses induced by low and high incubation temperatures, acidity, NaCl, and bile salts, often encountered during the technological processes in food or during the passage through the human gastro-intestinal tract. The strains were able to grow at temperatures up to 40 °C (the mesophilic strains) and 47 °C (the thermophilic strain), in medium with an initial pH of at least 4.0 (Lactobacillus acidophilus IBB801), or in the presence of NaCl up to 10 % (Weissella confusa/cibaria 38.2), or bile salts up to 0.2 % (L. acidophilus IBB801). The protein and isoenzyme patterns of the strains subjected to various stress conditions presented several differences compared with the control patterns, among which the overexpression of some proteins of about 50–60 kDa, differences in the bands intensity in the case of the intracellular enzymes, or the complete loss of some of these bands. The best survival to low pH values and high temperatures was observed for strain L. acidophilus IBB801, the candidate probiotic strain. The EPS production of the four tested strains was, in general, directly related to the growth, the highest yields being obtained when strains were incubated at 24 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactic acid bacteria (LAB) play an important role in the food industry because of their widespread application as starter cultures for the production of many fermented products (Doyle and Beuchat 2007; Mozzi et al. 2010). More recently, other important features of this bacterial group have been described: production of nutraceuticals (vitamins, low-calorie sugars, bioactive peptides, oligosaccharides) with a claimed medical or health benefit, pro- and pre-biotic effect (equilibration of the intestinal microbiota, anti-tumor properties, immunomodulating and cholesterol-lowering effects etc.), life vaccines (Wood 1999; Kneifel 2000; Hugenholtz and Smid 2002; Klaenhamme 2007). During the last years, the so-called “functional food” concept was developed, attracting the attention of both food scientists and health professionals (Hardy et al. 2002; Leroy and DeVuyst 2004; Mozzi et al. 2010). Recently, the use of functional starter cultures in the food fermentation industry is being explored. The functional properties of these starter cultures include food preservation and safety, health advantages (e.g. production of nutraceuticals), technological advantages (e.g., phage-resistance, acceleration of maturation in cheese, prevention of overacidification in yoghurt), but also an organoleptic advantage (production of aroma and flavour, improvement of texture) (Leroy and DeVuyst 2004).
Despite efforts to select new LAB strains with functional properties, only a limited number of them can be used in commercial food products or probiotics, since the performance under optimal laboratory conditions might be difficult to be reached under typical processing conditions (Reina et al. 2005). For instance, the starter strains should resist to adverse conditions encountered in industrial processes (i.e. low and high temperatures, low pH, osmotic stress etc.). Additionally, the formulation and preservation of starter cultures may impose environmental stresses, such as low pH, drying, freezing, thawing, which significantly affect the survival and growth, fermentative capabilities and viability of the cells, decreasing their performance (Zotta et al. 2008).
On the other hand, LAB used as probiotics must endure a number of stresses to ensure they reach the target site in an adequate number to elicit an effect (Mills et al. 2011). These bacteria need to survive in the digestive tract, maybe colonize the digestive mucosa and express their specific functions in these environments with unfavorable conditions. Growth and survival in these environmental niches depend on the ability of the organism to sense and respond to varying conditions such as temperature, pH, nutrient availability and cell population density (Buck et al. 2009).
Lactic acid bacteria, like other bacteria, have evolved stress-sensing systems and defenses against stress, which allow them to withstand harsh conditions and sudden environmental changes. These bacteria respond to stress in a very specific way depending on the species, strain, and the type of stress (Van de Guchte et al. 2002; Serrazanetti et al. 2009; Mills et al. 2011). Bacterial stress responses rely on the coordinated expression of genes which alter different cellular processes (cell division, DNA metabolism, membrane composition, transport etc.) to improve the bacterial stress tolerance (Storz and Hengge-Aronis 2000). The time taken to initiate the stress response is different for different treatments. For example, bacteria respond quickly, in minutes, to heat shock (Yura et al. 1999) compared to cold shock, in hours (Derzelle et al. 2000).
Among the stress-induced responses studied in bacteria, the heat shock response has been described in greater detail (Gouesbert et al. 2002; Castaldo et al. 2006; Corcoran et al. 2006; Di Cagno et al. 2006). The tolerance of LAB to heat treatment is a complex process involving proteins with chaperone activity, temperature sensing and control of ribosomal functions (De Angelis and Gobbetti 2004). The mechanisms of acid resistance have been also reported in lactic acid bacteria. The strategies include changes in the expression levels of acid shock proteins and enzymes, and macromolecular changes (e.g., alteration of cytoplasmic membrane lipid content) that allow bacteria to combat the negative consequences of cytoplasmic acidification (Van de Guchte et al. 2002; Wu et al. 2011). On the other hand, the stress response to bile salts presence was much less studied and the mechanisms of survival in these conditions are not fully understood, although the ability to survive exposure to bile is one of the commonly used criteria to select potential probiotic strains. However, several genes and molecules involved in this process have been recently indentified in lactobacilli (Lebeer et al. 2008; Hamon et al. 2011).
Understanding the stress regulatory networks gives us information to control these responses in order to achieve desirable robustness of bacteria in relation to various industrial processes. The knowledge of the mechanisms involved in stress adaptation is essential for selecting the most efficient strain for a particular product.
The present study aimed to characterize the response to various stresses (i.e. low and high temperatures, acidity, salt, and bile salts) of some functional LAB strains isolated from Romanian artisan dairy products.
Materials and methods
Bacterial strains and culture media
Four exopolysaccharide (EPS)-producing strains, isolated from Romanian artisan dairy products were used in this study. They were previously identified as: Leuconostoc citreum 1.11, L. pseudomesenteroides 20.6, L. mesenteroides 21.2 and Weissella confusa/cibaria 38.2, respectively (Grosu-Tudor et al. 2013). Additionally, a termophilic strain, Lactobacillus acidophilus IBB801, with probiotic potential, was also used. This strain was isolated from fermented milk and it produces acidophilin 801, which was purified and characterized before (Zamfir et al. 1999). All strains were kept in MRS medium (de Man et al. 1960), at −75 °C and transferred twice in the same medium, before the experiments.
Stress treatments
Strains were subjected to different stress conditions: incubation temperatures ranging from 10 to 50 °C, pH values of the growth medium from 2.0 to 6.5 (pH adjusted with 1 N HCl), addition to the growth medium of NaCl up to 10 % (w/v), and bile salts (Sigma-Aldrich Chemie GmbH, Germany) up to 0.4 % (w/v), respectively. Growth/survival was followed by measuring the pH and the optical density of the culture at 600 nm and by counting the colony forming units (CFU/ml) on solid MRS medium (1.5 %, w/v, of agar).
For the acid tolerance evaluation, two ml of overnight cultures of the tested strains were centrifuged (10 min at 11000×g) and the cellular sediment was resuspended in two ml of MRS broth previously adjusted with 1 N HCl to pH values of 2.0, 3.0, and 4.0, respectively. Alternatively, 1 M lactic acid (Merck KGaA, Darmstadt, Germany) was used for the pH adjustment. The initial bacterial concentration was about 1010 CFU/ml (log 10). The suspensions were incubated at 37 °C, for 24 h (for pH adjusted with HCl) and for 3 h (for pH adjusted with lactic acid), respectively. Samples were taken at different time intervals. Simultaneously, a control at pH 6.2 was used.
Heat resistance was tested by the method of Stopforth et al. (2008). Cells were exposed to different temperatures (60, 70 and 80 °C) for 1, 3, or 5 min and immediately cooled on ice, before counting the viable cells.
Protein extraction
The cultures obtained in different conditions were centrifuged and cells were washed twice with Tris–EDTA buffer (20 mM Tris, 0.1 mM EDTA, pH 7.6) and re-suspended in the same buffer, supplemented with protease inhibitors mixture (Sigma-Aldrich). Protein extracts were obtained by sonication using a LabsonicM apparatus (Sartorius, Germany), at 80 % power, on ice, for 3 min. Cell debris were removed by centrifugation (12 000×g, 5 min, at 4 °C). Protein content was measured using the method of Bradford (1976).
Analysis of total proteins by one-dimensional SDS-PAGE
The differences in cytosolic protein patterns were analysed by one-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Portions of the total protein extracts, containing similar amounts of proteins were loaded onto the polyacrylamide gel. The gel and sample preparation were done according to Laemmli (1970). The polyacrylamide concentrations were 12 % in the running gel and 4 % in the stacking gel. Electrophoresis was conducted in a Compact Dual Plate apparatus V20-CDC (Scie-Plas Ltd., UK) at a constant voltage of 90 V in the stacking gel and 180 V in the running gel. Broad range protein molecular weight marker (Promega, USA) was used as reference. Gels were stained with Coomassie Brilliant Blue.
Detection of enzymatic activities
The presence of some intracellular enzymes [lactate dehydrogenase (LDH), alcohol dehydrogenase (ADH), glucose-6-phosphate-dehydrogenase, superoxide dismutase (SOD)] in the protein extract and the changes in their respective isoenzymes patterns were detected after one dimensional native polyacrylamide gel electrophoresis. The separating gel (8 % concentration), the sample buffer and the running buffer were SDS-free. Electrophoresis was conducted at 10 °C in the same apparatus and at the same running voltages as for the protein assay. After electrophoresis, gels were washed for a few seconds in distilled water and covered with the appropriate mixture for the enzymatic activity detection.
For LDH detection, gels were immersed into a mixture containing Tris A buffer (0.2 M Tris, 1 mM EDTA, pH 8.0), 0.5 M D,L-lactic acid (Merck), NAD+ (1 % solution), NBT (1 % solution) and PMS (1 % solution) (all three from Sigma-Aldrich) and they were incubated in the dark until blue bands appear (Whitt 1970).
For ADH detection, gels were incubated in the dark with the mixture containing Tris A buffer, 0.5 M MgCl2, ethanol (95°), NAD+, NBT, MTT (Sigma-Aldrich), PMS (all as 1 % solutions in water), untill blue bands appear (Tanksley 1979).
For glucose-6-phosphate dehydrogenase detection, gels were incubated in the dark in a mixture of 0.1 M Tris, pH 7.5, 1 M MgCl2, glucose-6-P, NADP+ (Sigma-Aldrich), NBT and PMS, untill blue bands appear (Tanksley 1983).
Finally, for superoxide dismutase detection, gels were incubated under a neon tube in a mixture containing Tris A buffer, 0.5 M MgCl2, NAD+, NBT and PMS (all as 1 % solutions in water). SOD isozymes appear as light bands on a blue background.
Exopolysaccharide (EPS) production
EPS-producing strains were grown in filtrated mMRS (Van der Meulen et al. 2007) at 10, 24, 37 and 40 °C to estimate their EPS yields. The isolation and quantification of the EPS was carried out as described previously (Degeest and De Vuyst 1999). Total EPS yields were determined gravimetrically by measuring the polymer dry mass (PDM) after 48 h of drying at 37 °C. EPS isolation was done in duplicate and results are given as the mean values of the individual measurements.
Results and discussion
Understanding the mechanisms of stress response and adaptation to stress in the case of LAB, especially in the case of strains with functional properties, is very important from a scientific and technological point of view. Strains to be used as starter cultures require metabolic activity to contribute to the taste and texture of the fermentation end-products, while strains to be used as probiotics require vitality to exert their health-beneficial effect (Mills et al. 2011). The subject is very actual and many newly developed techniques contribute to a better understanding of the way bacteria react to various environmental changes. In this context, our study shows the response of some LAB strains with potential functional properties (EPS biosynthesis, potential probiotic effect) to the stresses induced by low and high incubation temperatures, acidity, NaCl, and bile salts. Cold/heat, salt, and low pH-induced stresses are often encountered during the technological processes in food, while environments with low pH and bile salts are usually encounterred during the passage through the human gastro-intestinal tract.
Growth/survival under stress conditions
All EPS-producing strains used throughout this study grew well at temperatures between 24 °C and 40 °C (Table 1). This was expected, since Leuconostoc sp. and Weissella sp. are mesophilic, with optimal growth temperatures between 20 and 30 °C (Wood and Holzapfel 1995). Although the growth was much slower at 10 °C, the cells kept their viability for a long incubation period (the CFU/ml remained almost the same for at least 24 h after reaching the maximum, results not shown). When incubated at 40 °C, these strains showed a very fast growth in the first 3–6 h, sometimes even faster then strains incubated at 24 and 37 °C, but the viability of the cells was quikly lost afterwards. For instance, in the case of L. pseudomesenteroides 20.6, the CFU number decreased with 3 logs at 24 h comparing to the maximum value reached at 6 h of incubation (results not shown). High temperatures (45 °C and over) were lethal to all these strains. We have previously shown that L. acidophilus IBB801, which is a thermophilic strain, still grows at 47 °C, but does not grow at temperatures higher then 50 °C (Zamfir and Grosu-Tudor 2009). This strain was also able to grow in the presence of 0.2 % of bile salts, in the presence of NaCl up to 3 % and in the medium with an initial pH of at least 4.0. The three Leuconostoc strains were able to grow in MRS with an initial pH of 5.0-5.5 and in the presence of NaCl up to 6 % (strain L. pseudomesenteroides 20.6), values within the normal limits for this genus (Wood and Holzapfel 1995). The presence of bile salts in the medium did not allow the growth of these strains (results not shown).
Weissella confusa/cibaria 38.2 showed a very good tolerance to NaCl. After 24 h of incubation in the presence of 6 and 7 % of NaCl, the OD at 600 nm were 1.3 and 0.9, respectively. In the presence of higher concentrations of NaCl, the growth was slower, but after 48 h of incubation, OD were 1.5, 0.7 and 0.6 for the cultures obtained in the presence of 8, 9 and 10 % NaCl, respectively. This strain was also able to grow in MRS medium with an initial pH of at least 4.5 and even in the presence of low concentrations (0.1 %) of bile salts.
Weissella strains have been isolated from a variety of sources, including fresh vegetables, silage fermentation, meat products, traditionally fermented foods and occasionally from raw milk and sewage (Dellaglio and Torriani 1986; Hammes and Vogel 1995; Kandler et al. 1983; Ampe et al. 1999; Paludan-Muller et al. 1999). During our studies, Weissella strains have been isolated from Romanian traditionally fermented dairy products (Zamfir et al. 2006; Van der Meulen et al. 2007) and from samples collected from the initial stages of spontaneous vegetables fermentations (Wouters et al. 2013). The salt resistance of these strains is expected (Lee et al. 2002); however, the ability to grow at such high salt concentrations is not usual for strains belonging to this genus (Collins et al. 1993).
When exposed to high temperatures (80 °C), cells of the tested strains were killed completely after 3 min of exposure, except strain L. acidophilus IBB801, which was killed after 5 min of exposure. Exposure to 60 and 70 °C resulted in a slight decrease of the viable cells numbers. For instance, for the EPS producing strains, after 5 min at 60 °C the decrease was about 1–2 logs, and after 5 min at 70 °C about 4–5 logs, depending on the strain (results not shown). As expected, the thermophilic strain L. acidophilus IBB801 showed a higher resistance to heat, the viable cell number remaining almost the same after 5 min of exposure to 60 °C and decreasing with 3 logs after 5 min of exposure to 70 °C. A significant portion (about log 5 CFU/ml, comparing with log 11 for the control) of this strain still survived after 3 min of exposure to 80 °C.
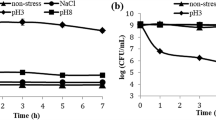
The best acid tolerance was shown by L. acidophilus IBB801 strain, especially when the pH was adjusted with HCl. In this case, after 3 h of incubation at pH 2.0, the viable cells concentration decreased with 3 logs as compared with the initial value (Fig. 1c). W. confusa/cibaria showed a very good tolerance to pH 3.0 and 4.0, the viable cells concentration decreasing with only 2 and 1 log, respectively, after 24 h of incubation. At pH 2.0, the viable cells concentration decreased very fast, after 1 h of incubation being with 8 logs lower, while after 2 h the viability was completely lost. Similar results were obtained for the three Leuconostoc strains incubated at pH 2.0. At pH values of 3.0 and 4.0, the viable cells concentrations of these strains slightly decreased in the first 6–8 h of incubation, but after 24 h a significant decrease was seen at pH 4.0 and the viability was completely lost at pH 3.0 (Fig. 1a, b).
When the pH was adjusted with lactic acid, a more dramatic effect was observed. At pH 2.0, the viability of all tested strains was lost immediately after the contact. Again, L. acidophilus IBB801 was the most tolerant strain to low pH values, the viable cells concentration slightly decreasing (1–2 CFU logs) during 6 h of incubation at pH 3.0 and 4.0. Similar results were obtained for all the other strains when incubating at pH 4.0. At pH 3.0, the viability was completely lost after 10 min in the case of W. confusa/cibaria 38.2 and L. citreum 1.11, 1 h in the case of L. pseudomesenteroides 20.6, and 2 h in the case of L. mesenteroides 21.2.
It was previously shown that LAB can generate pH gradients across the cell membrane, such than when the medium pH is low, the cytoplasmatic pH is always higher. In the presence of lactate or acetate, however, somewhat lower pH gradients are maintained (McDonald et al. 1990). This is because at low pH, organic acids diffuse in their undissociated form across the cytoplasmatic membrane causing a fast acidification of the cytoplasm and collapse of the proton motive force, resulting in inhibition of nutrient transport. A lower sensitivity to low pH adjusted with HCl was also observed for sourdough lactobacilli (De Angelis et al. 2001).
Analysis of total proteins by one-dimensional SDS-PAGE
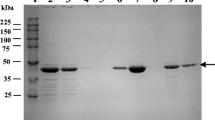
SDS-PAGE of the whole protein extracts from the four EPS-producing strains cultivated at different temperatures showed several changes of the protein patterns. The most evident was the overexpression at high incubation temperatures (37 and 40 °C) of the proteins corresponding to about 60 kDa, most probably heat shock or chaperone proteins, while at low incubation temperatures (10 °C) we could detect an overexpression of the proteins corresponding to about 45, 50, and 90 kDa, respectively (Fig. 2).
A similar overexpression of the proteins corresponding to about 60 kDa was also detected (Fig. 3) in the case of strains L. citreum 1.11 and L. mesenteroides 21.2 when subjected to low pH (both at pH 3.0 ajusted with lactic acid and pH 2.0 and 3.0 adjusted with HCl). The protein patterns of samples obtained from strains cultivated in the presence of various concentrations of NaCl showed several differences compared with the control patterns, including an overexpression of proteins corresponding to 50–60 kDa (results not shown).
One-dimensional SDS-PAGE analysis of total proteins extracted from L. citreum 1.11, L. pseudomesenteroides 20.6, L. mesenteroides 21.2, and Weissella confusa/cibaria 38.2. The four lanes for each strain correspond to: control, pH 3.0 (adjusted with lactic acid), pH 2.0, and pH 3.0 (both adjusted with HCl)
Several compounds produced or overproduced by LAB under stress conditions, most probably involved in the protection of producing cells, have been described and characterized (Van de Guchte et al. 2002; Champomier-Verges et al. 2010). Among these, a major part is represented by proteins and various enzymes. Heat shock and chaperone proteins, including 60-kDa chaperonin (GroL), chaperone protein dnaJ (DnaJ), chaperone protein dnak (DnaK), 33-kDa chaperonin (HslO), and 10-kDa chaperonin (GroS), are recognized as “general stress response proteins”, since their upregulation has been detected as a response to various stresses, including heat, low pH, high pressure, salt, and osmotic stress (Van de Guchte et al. 2002; Champomier-Verges et al. 2010; Mills et al. 2011; Wu et al. 2012). In the case of low pH, chaperone proteins exert great impact on interacting with the glycolytic enzymes and increase the stability of proteins in the presence of acid challenge (De Angelis et al. 2004; Hormann et al. 2006).
Detection and measurements of enzymatic activities
In the isoenzymatic spectrum of LDH for the different variants, we observed that for the mesophilic strains L. pseudomesenteroides 20.6 and L. mesenteroides 21.2, a second band, very weak (probably with a low specificity for the substrate) was detected in the cultures obtained at 10 and 24 °C, comparing with the other two temperatures, where only one band was present (Fig. 4a, arrows). Conformational LDH isoenzymes have been observed long ago with the leuconostoc enzyme preparations; the corresponding bands can be of different intensity, especially when both L(+)- and D(−)-lactates are used separately as substrates in the electrophoresis gel (Garviae 1969). For strain L. acidophilus IBB801, the same phenomenon was observed, but when cultivated at 37 and 40 °C (Fig. 4a, arrows). The presence of the second LDH band was correlated with the higher specific LDH activity, spectrophotometrically measured (results not published).
The differences in the isoenzymatic spectrum of glucose-6-phosphate dehydrogenase and ADH for the strains incubated at different temperatures were not significant (results not shown). The staining method used for SOD detection resulted in a large variety of bands, proving the low specificity of the method. We could observe, however, some differences in the intensity of some bands in the upper part of the gel (Fig. 4b) for the strains incubated at different temperatures. For the mesophilic strains, the bands were more intense when incubated at 37 and 42 °C. For L. acidophilus IBB801, the bands were more intense for the variants obtained at lower temperatures (10 and 24 °C). SOD belong to a group of antioxidant enzymes and are known to be involved in the protection against oxidative stress in various organisms, including LAB (Bruno-Bárcena et al. 2004). Our results show the potential involvment of this enzyme in other types of stresses, such as high or low incubation temperatures.
For the strains subjected at low pH values, no significant differences were detected in the isoenzymes patterns of SOD between the tested variants (results not shown). In the case of LDH, a second band (apart from the major band detected for the control) was detected for strain L. pseudomesenteroides 20.6 incubated for 30 min at pH 2.0 (adjusted with HCl), while for L. mesenteroides 21.2, a second band was detected for the variants incubated for 1 h at pH 2.0 (adjusted with HCl) and pH 3.0 (adjusted with lactic acid), respectively (Fig 5a). In the electrophoretic pattern of ADH isoenzymes we could also detect some differences in the intensity of the major band in the middle of the gel (Fig. 5b). For instance, this band was very weak or absent for the strains subjected to lactic acid (in the case of strains 1.11, 20.6 and 21.2). In the case of strain 38.2, we could only detect the ADH bands for the control, but not for the strain subjected to low pH values. The absence of this band might be explained by the total inactivation of the enzyme by the low pH.
Isoenzyme electrophoretic patterns of L. citreum 1.11, L. pseudomesenteroides 20.6, L. mesenteroides 21.2, W. confusa/cibaria 38.2, and L. acidophilus IBB801. The four lanes for each strain correspond to: control, pH 3.0 (adjusted with lactic acid), pH 2.0, and pH 3.0 (both adjusted with HCl). A—lactate dehydrogenase, B—alcohol dehydrogenase
Previous results showed a good correlation of growth and the metabolic activity of these strains incubated in different conditions (different incubation temperatures, pH values, NaCl) as shown by the specific activity of several intracellular enzymes (LDH, ADH, malate dehydrogenase) (results not published). On the contrary, SOD activities were the highest for the strains cultivated under stress conditions. The present study confirm these findings by the corresponding isoenzyme electrophoretic patterns.
The biochemical changes (electrophoretic patterns of total cell proteins and of some intracellular enzymes) induced in the presence of bile salts could not be evaluated since the presence of bile salts in the protein extract interferred significantly with the electrophoretic assays.
Exopolysaccharide production
The EPS yields varied significantly with the incubation temperature. As a general rule, the highest yields were obtained when strains were incubated at 24 °C, followed by the yields obtained for strains incubated at 37 °C. When the producing strains were grown at 40 °C we could not isolate any EPS material, except for strain W. confusa/cibaria 38.2. The yields obtained at 10 °C were much smaller, except for strain L. citreum 1.11, for which we obtained similar amounts with the ones obtained at 37 °C (Table 2).
EPS biosynthesis has been shown to be growth-related, the optimal growth conditions being favourable to the production (Torino et al. 2001; Zhang et al. 2011). However, there were also reports that optimal conditions for EPS production by some LAB strains might be different from those for their optimal growth (Gamar et al. 1997) and EPS production has been considered by some authors as a mechanism of bacterial self-protection against unfavorable conditions (Ruas-Madiedo et al. 2002). On the other hand, the growth stop of the producing strain might result in a decrease of EPS concentration, as observed, for instance, for strain S. thermophilus LY03 (Degeest and De Vuyst 1999). This phenomenon might also explain why we could not isolate any EPS material from the four EPS-producing strains incubated at 40 °C, in which case the cells viability decreased very quickly after 6–9 h.
Conclusions
Our study brings information about the response to various stress conditions in the case of some potential functional LAB strains isolated from artisan dairy products. L. acidophilus IBB801 was the most tolerant strain to heat, low pH and bile salts, which makes it suitable for further application as a probiotic strain. The other tested strains were moderately heat tolerant, they tolerate pH values of 3.0 or above and, except W confusa/cibaria 38.2, they were not able to grow in the presence of bile salts. Our results also confirm the involvment of various proteins and enzymes in the stress responses, but also the correlation between the metabolic activity of the tested strains grown in optimal and stressful environments with their functionality (i.e. EPS production).
Abbreviations
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NADP+ :
-
Nicotinamide adenine dinucleotide phosphate
- NBT:
-
Nitro blue tetrazolium
- PMS:
-
Phenazine methosulfate
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium bromide
References
Ampe F, Omar NB, Moizan C, Wacher C, Guyot JP (1999) Polyphasic study of the spatial distribution of microorganisms in Mexican pozol, a fermented maize dough, demonstrates the need for cultivation-independent methods to investigate traditional fermentations. Appl Environ Microbiol 65:5464–5473
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bruno-Bárcena JM, Andrus JM, Libby SL, Klaenhammer TR, Hassan HM (2004) Expression of a heterologous manganese superoxide dismutase gene in intestinal lactobacilli provides protection against hydrogen peroxide toxicity. Appl Environ Microbiol 70:4702–4710
Buck BL, Azcarate-Peril MA, Klaenhammer TR (2009) Role of autoinducer-2 on the adhesiona ability of Lactobacillus acidophilus. J Appl Microbiol 107:269–279
Castaldo C, Siciliano RA, Muscariello L, Marasco R, Sacco M (2006) CcpA affects expression of the groESL and dnaK operons in Lactobacillus plantarum. Microb Cell Fact 5:35–41
Champomier-Verges MC, Zagorec M, Fadd S (2010) Proteomics: a tool for understanding lactic acid bacteria adaptation to stressful environments. In: Mozzi F, Raya RR, Vignolo GM (eds) Biotechnology of lactic acid bacteria—novel applications. Wiley, Iowa, pp 57–72
Collins MD, Samelis J, Metaxopoulos J, Wallbanks S (1993) Taxonomic studies on some leuconostoc-like organisms from fermented sausages: description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J Appl Bacteriol 75:595–603
Corcoran BM, Ross RP, Fitzgerald GF, Dockery P, Stanton C (2006) Enhanced survival of GroESL-overproducing Lactobacillus paracasei NFBC 338 under stressful conditions induced by drying. Appl Environ Microbiol 72:5104–5107
De Angelis M, Gobbetti M (2004) Environmental stress responses in Lactobacillus: a review. Proteomics 4:106–122
De Angelis M, Bini L, Pallini V, Cocconceli PS, Gobbetti M (2001) The acid-stress response in Lactobacillus sanfranciscensis CB1. Microbiology 147:1863–1873
de Man JC, Rogosa M, Sharpe ME (1960) A medium for the cultivation of lactobacilli. J Appl Bacteriol 23:130–135
Degeest B, De Vuyst L (1999) Indication that the nitrogen source influences both amount and size of exopolysaccharides produced by Streptococcus thermophilus LY03 and modelling of the bacterial growth and exopolysaccharide production in a complex medium. Appl Environ Microbiol 65:863–870
Dellaglio F, Torriani S (1986) DNA–DNA homology, physiological characteristics and distribution of lactic acid bacteria from maize silage. J Appl Bacteriol 60:83–93
Derzelle S, Hallet B, Francis K, Ferain T, Delcour J, Hols P (2000) Changes in cspL, cspP, and cspC mRNA abundance as a function of cold shock and growth phase in Lactobacillus plantarum. J Bacteriol 182:5105–5113
Di Cagno R, De Angelis M, Limitone A, Fox PF, Gobbetti M (2006) Response of Lactobacillus helveticus PR4 to heat stress during propagation in cheese whey with a gradient of decreasing temperatures. Appl Environ Microbiol 72:4503–4514
Doyle MP, Beuchat LR (2007) Food microbiology: fundamentals and frontiers. ASM Press, Washington
Gamar L, Blondeau K, Simonet JM (1997) Physiological approach to extracellular polysaccharide production by Lactobacillus rhamnosus strain C83. J Appl Microbiol 83:281–287
Garviae EI (1969) Lactic dehydrogenases of strains of the genus leuconostoc. J Gen Microbiol 58:85–94
Gouesbert G, Jan G, Boyaval P (2002) Two-dimensional electrophoresis study of Lactobacillus delbrueckii subsp. bulgaricus thermotolerance. Appl Environ Microbiol 68:1055–1063
Grosu-Tudor SS, Zamfir M, Van der Meulen R, De Vuyst L (2013) Isolation of novel homopolysaccharide producing lactic acid bacteria from Romanian raw milk and fermented dairy products. Eur Food Res Technol. doi:10.1007/s00217-013-2038-2
Hammes WP, Vogel RF (1995) The genus Lactobacillus. In: Wood BJB, Holzapfel W (eds) The genera of lactic acid bacteria. Blackie Academic and Professional, Glasgow, pp 19–54
Hamon E, Horvatovich P, Izquierdo E, Bringel F, Marchioni E, Aoudé-Werner D, Ennahar S (2011) Comparative proteomic analysis of Lactobacillus plantarum for the identification of key proteins in bile tolerance. BMC Microbiol 11:63–68
Hardy G, Hardy I, McElroy B (2002) Nutraceuticals: a pharmaceutical viewpoint: i. Curr Opin Clin Nutr Metab Care 5:671–677
Hormann S, Scheyhing C, Behr J, Pavlovic M, Ehrmann M, Vogel R (2006) Comparative proteome approach to characterize the highpressure stress response of Lactobacillus sanfranciscensis DSM 20451T. Proteomics 6:1878–1885
Hugenholtz J, Smid EJ (2002) Nutraceutical production with food-grade microorganisms. Curr Opin Biotechnol 13:497–507
Kandler O, Schillinger U, Weiss N (1983) Lactobacillus halotolerans sp. nov., nom. rev. and Lactobacillus minor sp. nov., nom. rev. Syst Appl Microbiol 4:280–285
Klaenhamme T (2007) Probiotics and prebiotics. In: Doyle MP, Beuchat LR (eds) Food microbiology: fundamentals and frontiers. ASM Press, Washington, pp 891–907
Kneifel W (2000) Functional foods with lactic acid bacteria: probiotics—prebiotics—nutraceuticals. Prog Biotechnol 17:101–107
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lebeer S, Vanderleyden J, De Keersmaecker SCJ (2008) Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev 72:728–764
Lee JS, Lee KC, Ahn JS, Mheen TI, Pyun YR, Park YH (2002) Weissella koreensis sp. nov., isolated from kimchi. Int J Syst Evol Microbiol 52:1257–1261
Leroy F, DeVuyst L (2004) Lactic acid bacteria as functional starter cultures for the food fermentation Industry. Trends Food SciTechnol 15:67–78
McDonald LC, Fleming HP, Hassan HM (1990) Acid Tolerance of Leuconostoc mesenteroides and Lactobacillus plantarum. Appl Environ Microbiol 56:2120–2124
Mills S, Stanton C, Fitzgerald GF, Ross P (2011) Enhancing the stress responses of probiotics for a lifestyle from gut to product and back again. Microb Cell Fact 10(suppl):S19
Mozzi F, Raya RR, Vignolo GM (2010) Biotechnology of lactic acid bacteria—Novel applications. Wiley, Iowa
Paludan-Muller C, Huss HH, Gram L (1999) Characterization of lactic acid bacteria isolated from a Thai low-salt fermented fish product and the role of garlic as substrate for fermentation. Int J Food Microbiol 46:219–229
Reina LD, Breidt F Jr, Fleming HP, Kathariou S (2005) Isolation and selection of lactic acid bacteria as biocontrol agents for nonacidified, refrigerated pickles. J Food Sci 70:M7–M11
Ruas-Madiedo P, Hugenholtz J, Pieternela Z (2002) An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int Dairy J 12:163–171
Serrazanetti DI, Guerzoni ME, Corsetti A, Vogel R (2009) Metabolic impact and potential exploitation of the stress reactions in lactobacilli. Food Microbiol 26:700–711
Stopforth JD, Suhalim R, Kottapalli B, Hill WE, Samadpour M (2008) Thermal inactivation of D- and Z- values of multidrug-resistant and non-multidrug-resistant Salmonella serotypes and survival in ground beef exposed to consumer-style cooking. J Food Protect 71:509–515
Storz G, Hengge-Aronis R (2000) Bacterial stress responses. ASM Press, Washington
Tanksley SD (1979) Linkage, chromosomal association, and expression of Adh-1 and Pgm-2 in tomato. Biochem Genet 17:1159–1167
Tanksley SD (1983) Isozymes in plant genetics and breeding. Elsevier, Philadelphia
Torino MI, Taranto MP, Sesma F, de Valdez GF (2001) Heterofermentative pattern and exopolysaccharide production by Lactobacillus helveticus ATCC 15807 in response to environmental pH. J Appl Microbiol 91:846–852
Van de Guchte M, Serror P, Chervaux C, Smokvina T, Ehrlich SD, Maguin E (2002) Stress response in lactic acid bacteria. Antonie Leeuwenhoek 82:187–216
Van der Meulen R, Grosu-Tudor SS, Mozzi F, Vaningelgem F, Zamfir M, de Valdez GF, De Vuyst L (2007) Screening of lactic acid bacteria isolates from dairy and cereal products for exopolysaccharide production and genes involved. Int J Food Microbiol 118:250–258
Whitt GS (1970) Developmental genetics of the lactate dehydrogenase isozymes of the fish. J Exp Zool 175:1–7
Wood BJB (1999) The lactic acid bacteria Vol. 1–The lactic acid bacteria in health and disease, Part II: Lactic acid bacteria and health. Aspen Publishers, USA, pp 151–339
Wood BJB, Holzapfel WH (1995) The genera of lactic acid bacteria, vol 2. Blackie Academic and Professional, Glasgow
Wouters D, Grosu-Tudor SS, Zamfir M, De Vuyst L (2013) Bacterial community dynamics, lactic acid bacteria species diversity and metabolite kinetics of traditional Romanian vegetable fermentations. J Sci Food Agric 93:749–760
Wu R, Zhang W, Sun T, Wu J, Yue X, Meng H, Zhang H (2011) Proteomic analysis of responses of a new probiotic bacterium Lactobacillus casei Zhang to low acid stress. Int J Food Microbiol 147:181–187
Wu C, Zhang J, Chen W, Wang M, Du G, Chen J (2012) A combined physiological and proteomic approach to reveal lactic-acid-induced alterations in Lactobacillus casei Zhang and its mutant with enhanced lactic acid tolerance. Appl Microbiol Biotechnol 93:707–722
Yura T, Nakahigashi K (1999) Regulation of the heat-shock response. Curr Opin Microbiol 2:153–158
Zamfir M, Grosu-Tudor SS (2009) Impact of stress conditions on the growth of Lactobacillus acidophilus IBB 801 and production of acidophilin 801. J Gen Appl Microbiol 55:277–282
Zamfir M, Callewaert R, Cornea CP, Savu L, Vatafu I, De Vuyst L (1999) Purification and characterization of a bacteriocin produced by Lactobacillus acidophilus IBB801. J Appl Microbiol 87:923–931
Zamfir M, Vancanneyt M, Makras L, Vaningelgem F, Lefebvre K, Pot B, Swings J, De Vuyst L (2006) Biodiversity of lactic acid bacteria in Romanian dairy products. Syst Appl Microbiol 29:487–495
Zhang T, Zhang C, Li S, Zhang Y, Yang Z (2011) Growth and exopolysaccharide production by Streptococcus thermophilus ST1 in skim milk. Braz J Microbiol 42:1470–1478
Zotta T, Ricciardi A, Ciocia F, Rossano R, Parente E (2008) Diversity of stress responses in dairy thermophilic streptococci. Int J Food Microbiol 124:34–42
Acknowledgments
The authors acknowledge their financiar support from the Project No. RO1567-IBB05/2013 from the Institute of Biology Bucharest of Romanian Academy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zamfir, M., Grosu-Tudor, SS. Stress response of some lactic acid bacteria isolated from Romanian artisan dairy products. World J Microbiol Biotechnol 30, 375–384 (2014). https://doi.org/10.1007/s11274-013-1454-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1454-6