Abstract
Lactic acid bacteria (LAB) are often used to produce fermented foods. LAB usually grow in “moderate” environmental conditions and commonly encounter stress conditions like changes in pH, temperature, production, storage, and others (Kim NR, Jeong DW, Ko DS, Shim JH, Intl J Biol Macromol 99:594–599. https://doi.org/10.1016/j.ijbiomac.2017.03.009, 1997). These condition changes may lead to poor growth rate or even survival of the bacteria. Stress responses were of great importance for microorganism; they always continually change with temperature and osmotic pressure in the environments (Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, Boches SK, Dewhirst FE, Griffen AL, J Clin Microbiol 40(3):1001–1009. https://doi.org/10.1128/jcm.40.3.1001-1009.2002, 2002). There are various kinds of stress factors, which include physical, chemical, or biological and others. LABs are exposed to these stresses during fermentation, for example, low temperature, high H2O2, and low pH (Kurz M, Saline Syst 4:6. https://doi.org/10.1186/1746-1448-4-6, 2008; Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, Cryan JF, Biol Psychiat 82:472. https://doi.org/10.1016/j.biopsych.2016.12.031, 2017; Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, Boches SK, Dewhirst FE, Griffen AL, J Clin Microbiol 40(3):1001–1009. https://doi.org/10.1128/jcm.40.3.1001-1009.2002, 2002). LABs have the stress-sensing systems to activate defenses, permitting the bacteria to acclimatize the harsh conditions or environmental changes. In LAB, DNA-repairing mechanisms can be also characterized as responding to oxidative stress and acid stress.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Response of Lactic Acid Bacteria to Acid Stress
5.1.1 Introduction
Lactic acid bacteria (LAB) are often used to produce fermented foods. LAB usually grow in “moderate” environmental conditions and commonly encounter stress conditions like changes in pH, temperature, production, storage, and others (Kim et al. 2017). These condition changes may lead to poor growth rate or even survival of the bacteria. Stress responses were of great importance for microorganism; they always continually change with temperature and osmotic pressure in the environments (Becker et al. 2002). There are various kinds of stress factors, which include physical, chemical, or biological and others. LABs are exposed to these stresses during fermentation, for example, low temperature, high H2O2, and low pH (Kurz 2008; Burokas et al. 2017; Becker et al. 2002). LABs have the stress-sensing systems to activate defenses, permitting the bacteria to acclimatize the harsh conditions or environmental changes. In LAB, DNA-repairing mechanisms can be also characterized as responding to oxidative stress and acid stress.
The stress-resistance systems of LAB were sorted into three classes: (1) specifically induced by a sublethal dose of the stress, (2) adapting to one stress condition that allows the cell to resist others in general systems, and (3) stationary-phase-associated stress response, which can induce plenty of regulons that are designed to conquer some stress conditions (Erny et al. 2015; Tsou et al. 2015; Beales 2004; Keijser et al. 2008; Becker et al. 2002).
5.1.2 The Metabolic Responses to Acid Stress by LAB
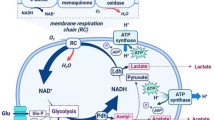
More studies described the mechanisms underlying the capacity of LAB with acidification. Many references concentrated the resistance of LAB to physical damages with acidic condition. For instance, a few of oral bacteria exhibits strikingly differences in the ability to acidification (Ayres 2016; Beales 2004). In these experiments, all streptococcal species found similar release of magnesium at pH 4.0 conditions. However, Lactobacillus casei illustrated damage below of pH 3.0, which had more resistance. Experiments assessed the proton movement on the membrane of L. casei. At the same time, the addition of ATPase inhibitor can cause increase of the proton permeability of L. casei, indicating that the proton entered the cell membrane, while the outflow process involved the proton-translocating ATPase (F1F0-ATPase). F1F0-ATPase can utilize ATP to pump several protons from the cytoplasm in the process of glycolysis. Many studies have shown that some F-ATPase activity in microorganism have their own ability to transfer protons from the cytoplasm to maintain the homeostasis of pH. LABs have the F-ATPase that may have more acid resistance during low pH conditions (Uchihashi et al. 2011). The F-ATPase of LAB is significant because of its good competitiveness relative to other organisms and basic level of inherent acid resistance provided by the enzyme.
LAB could also produce cyclopropane-containing fatty acids (CFAs) in variable conditions, for example, dehydrosterculic acid (C19cyc9) and lactobacilli acid (C19cyc11). In the case of CFA synthase, methyl group can be transformed from S-adenosyl methionine to fatty acids, which can produce CFAs. Bender et al. found that the acid-adapted cells of L. casei ATCC334 are much more than those of control cell, which may increase the levels of CFAs and saturated fatty acids at the cost of UFAs. These results showed that CFA production, occurring in L. helveticus and L. sanfranciscensis, may be part of the rigid response in L. casei. CFAs occur in L. helveticus and L. sanfranciscensis. Meanwhile, a relA gene involves the starvation response of Streptococcus mutans and the production of CFAs under culture condition. In this condition, relA mutant strain showed the larger acid tolerance compared with the parent strain, which was the major effects of the mutation, following growth in biofilms (Bender et al. 1986).
In addition to abovementioned acid tolerance mechanisms of LAB, some other mechanisms of LAB cope with the influence on the damage mediated by acid and improve degressive internal pH values. For example, releasing the ammonia to increase pH value is thought to be a mechanism of acid resistance that distributed evenly (Budin-Verneuil et al. 2006). In common conditions, arginine and amino acid agmatine as parts of sources of ammonia. The release mechanism of ammonia contained the system of arginine deiminase, and it needs to depend on the enzymatic activity: ornithine carbamoyltransferase, arginine deiminase, and carbamate kinase (Champomier Vergès et al. 1999). Arginine is across cells membrane assistances an arginine-specific porter, as part of an arginine-ornithine antiporter system. Arginine can be converted into ammonia and citrulline when it was cleaved by arginine deiminase. Meanwhile, citrulline was delaminated and splitted by ornithine transcarbamylase to ornithine and carbamoyl phosphate. Carbamate kinase can cut carbamoyl phosphate that would become carbon dioxide and ammonia in the substrate-level phosphorylation (Davis et al. 1986; Champomier Vergès et al. 1999). Finally, these involved systems convey the moles of ammonia into cells and an ATP through a series of energy formation reactions.
LAB exploits L-malate, because it can be converted into carbon dioxide by malolactic fermentation pathway (MLF). Within this pathway, L-malic acid will release carbon dioxide by malolactic enzyme and then form one molecule of lactic acid and carbon dioxide, which could be transformed into bicarbonate by carbonic anhydrase (Konings et al. 1997). It is an antiporter system that lactic acid was produced and a molecule of L-malate was used. Since MLF pathway can be found in many LAB, for example, it was used in some wine industry and so on. It is apparent that MLF pathway is associated with protective buffering for the host bacteria. (Konings et al. 1997). MLF is widely distributed among LAB and distinctly within its strain levels. During the fermentation of sugar, LAB could be respect to the ability to grow with alcohol and at low pH (Vrancken et al. 2009).
Numerous studies concerning with LAB have described main growing period of stress response to acid. No well-defined about lactic acid bacteria deploy their acid tolerance, because of significant difference of its species and strain at the same media composition and culture conditions (Beales 2004; Konings et al. 1997). Hence, the transcriptional profiling and proteomics were used in combination to illustrate the changes of LAB under in the acid stress responsive genes. Importantly, the guanidine nucleotide pools of LAB especially affected by starvation, and carbon flow was disrupted by Embden–Meyerhof–Parnas (EMP) system.
5.1.3 Signal Transduction
(p)ppGpp is a perfect position that can influence the variation of cells and the port of conversion, carbon flow, and phosphate pools. Obviously, in different species and strains, protein production is influenced by RelA or its equivalent loss (Magnusson et al. 2005). A fewer reported in the literature about above theory of LAB. For example, Rallu et al. once reported Lactococcus lactis responds rapidly to acidification involving pppGpp (Rallu et al. 1996, 2000). Budin verneuil et al. suggested that there is a deficiency in the protein in relA, guaA(GMP synthase), and pstS(phosphate transporter) with more L. lactis mutant strains compared with the parent strain (MG163) (Budin-Verneuil et al. 2007). For instance, RecA (DNA-repair-recombination protein), pyruvate carboxylase, CTP synthase, glutamyl–tRNA synthetase, R30S ribosomal protein S1, and the subunit of DNA polymerase could overlap the mutant strains, playing important roles at the acid conditions. dnaN, similarly, a whole transcriptional studies about the relA mutant strain of S.mutans refer to 50 transcripts, involving the strict response to RelP- and RelQ-mediated RelA-independent stress response (Nascimento et al. 2009). Certainly, RelA also impact acid tolerance of S.mutans, when forming biofilms and participating in the quorum sensing mediated by AI-2(Lemos et al. 2004).
More literatures show that LAB in an intense area, which could explore two-component signal sensing and response circuits. The general concept about two-component regulatory systems (TCSs) is that it can regulate extensive bacterial reaction under the external stimulus (Gao and Stock 2009; Krell et al. 2010).
More literature indicates that TCSs were related to the control of acid stress response in laboratory (Cui et al. 2012). For example, in the case of S. mutans, multiple TCSs affect the sensitivity to acidification (Li et al. 2002). It has been reported that HK03 and RR03 protein sequences are the basis for BLAST search of S. mutans UA159 genome sequence (Li et al. 2002). Levesque et al. (2007) reported an exhaustive study about 14 recognizable TCS pairs of S. mutans (Levesque et al. 2007). The results illustrated that tcs-2, the homologue of CiaRH system, tcs-3, same as the scnrk-like system, tcs-9, were all involved in a certain degree of acid resistance. The new studies by scholars found that the VicRK TCS of S. mutans was the acid resistance of S. mutans, while the loss of vicK gene affected 89 transcripts in the microarray analysis of the vicK mutant strain (Senadheera et al. 2009). Other studies involved all 14 transducers/kinase pairs of mutations in the stress reaction (Biswas et al. 2008; Kawadamatsuo et al. 2009). These studies also suggest that the expression of the TCS must be carefully explained, because at least some of them do overlap (Chong et al. 2008). With the continuous development of information, TCSs responsiveness - adjustment partner’s DNA binds to theme (Senadheera et al. 2005). There is no significant distinguish about TCSs, which regulate the acid conditions.
Meanwhile, a study with Group B Streptococcus (GBS), strain V/R 2603, has been published, and the global transcriptional analysis of biological growth with a pH of 7.0 and 5.5 is reported (Santi et al. 2009). Similar studies on gene transcription found that about 300 genes were upregulated in pH 5.5 and contrasted with pH 7.0, and 61 genes were downregulated at pH 5.5. Genes expressed in acid growth involve all major metabolic and stress response pathways. In their study, the genes that focused on the pH were controlled by the CsrRS TCS, and about 90% of the downregulated genes and nearly 60% of the upregulated genes were related to the CsrRS. GBS will be from the mother’s vagina (acid) transferred to the baby’s lungs, which may show toxic factor upregulation of invasive phenotype and GBS, including surface protein BibA, this is a kind of GBS vaccine candidates with ph response ability (Santi et al. 2009).
5.2 Responses of Lactic Acid Bacteria to Bile Stress
5.2.1 Introduction
LAB being the most representative probiotics is used for the production of fermented dairy, vegetable-based food, and wine. Bile tolerance, the most crucial property, commonly conformed to the capacity of the bacteria to grow which functions as probiotics (Macpherson et al. 2016; Marchesi et al. 2016; Fetissov 2017; Hamon et al. 2011).
Normally, bile contains cholesterol, bile acids, phospholipids, water, and pigment biliverdin (Patel et al. 2010). It always produces a yellow or green solution in pericentral hepatocytes from the liver of mammals. Bile in the liver could eliminate some biological substances synthesized from cholesterol. The process included the generation of bile flow, and its physiological function is to facilitate the absorption of lipophilic compounds from food (Hu et al. 2015). Bile also has a vital role in the establishment of the intestinal microbiota in humans. Generally, bile could disturb the function of cell membrane in LAB and bifidobacteria (Xiong et al. 2017). Studies on the bile stress response in LAB and bifidobacteria have shown that bile resistance can lead to an integration of multilateral responses, protecting cell membrane resist from bile acids. This process involved in the restoration or degradation of proteins, elimination of the oxidative stress, facilitation of DNA repairing, and enhancement of energy generation by upregulating sugar metabolism (Jie et al. 2016; Patel et al. 2010).
5.2.2 The Mechanism Responses to Bile Stress in Lactic Acid Bacteria
LAB can affect host beneficially by enhancing its intestinal microbial balance. Therefore, LABs have the ability to be resistant to the enzymes and the digestion process. When bacteria enter to the intestinal tract, bile will reach the duodenal section of the small intestine to reduce the bacteria survival. Bile acid is one factor that reduces strain survival in culture conditions. Bile acids can be flip-flopped by lipid bilayer, which increases the tensile strength of the membrane. Hence, plenty of bile acids will bring to bear on cells if it is too much (Bandyopadhyay and Moulik 1988). When the concentration of bile acid becomes higher, the apparent proton conductance and membrane osmosis ability will be disturbed (Martoni et al. 2008).
The ability to survive in bile exposure can be considered as a pivotal factor to select probiotic strains. Membrane characteristics and cells of bacteria will be influenced by bile, which contain dissolution, DNA lesion, acid, oxidative, and osmotic stresses. Therefore, these factors, such as bile, oxidative, acid, detergent, and salt stresses should be cosidered in the studies of bile tolerance. The survival mechanisms of bile tolerance are still indistinct, but the several genes and molecules refer to bile have been identified in lactobacillus (Ai et al. 2008).
Bile salt serves as biological surfactant, which damages the cell membrane of bacteria, leading to cell leakage and cell apoptosis. Hu et al. reported that L. plantarum was resistant to 0.3% bile content and put the bile tolerance into future functional assessment (Hu et al. 2015). More studies suggested that the intact membrane of cell plays an important role in defensing bile (Begley et al. 2005). Lipids play a vital role for keeping the structure of cell membrane, such as fatty acid compound of LAB, which is crucial in the bile tolerance (Küllenberg et al. 2012; Hu et al. 2015).
The hydrolysis of bile salts and the resistance of bile are separated in bile, and bile acids often have potential toxicity and cholesterol metabolites. The use of wild type and bsh gene mutation combination in the bile brine solution and bile tolerance of probiotics can effectively reduce the damage of bile salts. The BSH activity was reduced in L. amylovorus, as well as the the growth rate of bile salts in existence. However, cells are easily affected by bile because of the mutational bsh in Listeria monocytogenes and L. plantarum (Jones et al. 2008).
Bile acids could be conjugated with amino acids to produce the conjugated bile salts (CBA) and then emulsification and solubilization of lipids. CBAs demonstrate antimicrobial activity by interfering with the cell membrane and homeostasis (Begley et al. 2005). However, LABs have in particular defense mechanisms, which resist these harmful behaviors. The hypothesis is that bile salt hydrolase can conjugate bile salts and may improve bile tolerance and bacteria survival in the gut (Lee et al. 2008). Jarocki et al. suggested that bile acid can also be released by bile salt hydrolase reaction, forming micellars on the membrane of Bifidobacterium under bile pressure (Jarocki et al. 2014).
5.2.3 Bile Salt Hydrolases of LAB
Bile salt hydrolase (BSH) is so crucial in the cholesterol-removing effect of LAB. It can hydrolyze conjugated bile salts to amino acid and connect bile salts. L. casei has been reported that lacking of bsh gene may highly sensitive to bile salt stress (Wu et al. 2012). Much more literatures suggested that probiotics have evolved BSH to deal with bile salt stress (Wang et al. 2011; Allain et al. 2017). BSH is a vital enzyme for eliminating cholesterol and catalyzing the conjugated or deconjugated bile salts to free amino acids. Moser and Savage reported that L. buchneri JCM1069 has hydrolase activity metabolizing taurodeoxycholic acid rather than taurocholic acid (Moser and Savage 2001). These acids are usually made of taurine as their amino acid, but there are seven different positions in the steroid. Meanwhile, it’s not relative between BHS activity and resistance to toxicity of conjugated bile salts in LAB (Moser and Savage 2001).
The mechanism of BSH is not well known. Studies have shown that bile salts can form protons, showing toxicity through the cells’ interfaces, while BSH positive cells may protect themselves (Kurdi et al. 2006). The process can eliminate acidification by recycling and exporting the protons. BSH is so specific to certain types of bile that the duration of it can guarantee the survival of bacteria in a changing bile environment. For instance, L. plantarum WCFS1 and L. acidophilus NCFM have four and two bsh genes, respectively, which supported this theory (Patel et al. 2010).
5.2.4 Scope of Bile Salt Hydrolases of LAB
High cholesterol level is a main challenge for human health worldwide. However, probiotic-based oral therapy can efficiently reduce the cholesterol level of blood, and BSH activity has nothing to do with yield. L. plantarum CK102 isolated from human could reduce the levels of blood cholesterol, triglyceride, LDL-cholesterol, and free cholesterol in rats. The supernatant of L. acidophilus ATCC43121 has exhibited cholesterol-removing activity as well (Ahn et al. 2003).
The extract of bile salts produces amino acids, which are then used as carbon, nitrogen, and support sources. For instance, glycine could be hydrolyzed into ammonia and carbon dioxide. However, taurine is hydrolyzed into ammonia, carbon dioxide, and sulfate. Meanwhile, this process has been taken place by BSH-positive strains. The decompression of L. Acidophilus SNUL020 and SNUL01 was reported at a similar speed (Peschel 2002).
BSH cannot display deconjugated activity against the primary salts, when it was deconjugated to the secondary salts. Whether the bacteria express the resistance to bile via the accumulation of BSH remains unclear. However, it is assumed that the protonation of bile salts is toxic through the intracellular interface, while BSH positive cells may be protected by weaker nonconjugated cells (Taranto et al. 1997). This process can be explained that the acidification can be eliminated by restoring and exporting the protons. When the probiotics cells were microencapsulated to achieve the BSH decompression rate, L. reuteri microcapsules metabolize glycol and tauroconjugated bile salts at rates of 10.16 ± 0.46 and 1.85 ± 0.33 μmol/g microcapsule per hour, showing better acid tolerance (Tsou et al. 2015; Kim et al. 2017.
5.3 Responses of Lactic Acid Bacteria to Osmotic Stress
5.3.1 Introduction
LABs have often been exposed to adverse environmental conditions such as industrial processes, natural environment, and human infection. Osmotic stress is a prominent limitation, which can bring about a decrease in survival or growth and affect strains metabolic activities. Osmotic pressure is one of the main stresses encountered by LAB in an industrial environment such as cheese production, beer brewing, and yogurt-making process.
In the manufacture of some foods, LAB is used as a leavening agent. During the process of starter culture preparation, The LAB is exposed to adverse culture, affecting their viability and performance. LABs are confronted with extreme value of pH and osmotic stress conditions, which affect the survival of LAB negatively by disturbing cellular viability. Probiotics such as Lactobacillus and Bifidobacterium are widely employed in yogurts, dietary adjuncts, and other health-related products. To survive and proliferate in gut, LAB may need to elevate the osmotic stress in the upper small intestine. Meanwhile, the osmotic pressure was significantly increased when human was infected with lactic acid bacteria. It is also caused by perspiration in skin infections.
In response to osmotic stress, the development of adaptive strategy is the key to the function of lactic acid bacteria in food fermentation. Therefore, the study of osmoregulation and adaptation of cells to changes in the external osmotic stress is so vital for us to understand its important industrial and medical aspects.
5.3.2 Fundamental Principles of Responses to Osmotic Stress in Lactic Acid Bacteria
In order to insure the direction of water flow enter the cell in the period of growth, the density of solute is much higher than the density demanded to metabolize for these cells. At the same time, all of these growing bacterial cells show high levels of outward expansion pressure, making the membrane close to the expanded polysaccharide wall. It is generally believed that maintaining invariable expansion is the driving power to expanding, growing, and dividing of cell. Changes of water activity in extracellular have a direct influence on the water activity in the cytoplasm, with the water flowing alongside the osmotic gradient. It is generally shown that bacterial cytomembrane shows high water permeability, but as for the majority of solutes, it is an effective barrier. Water could enter into and leave out the cell until the osmosis pressures on both sides of the semipermeable membrane reach equilibrium. The flow of water could cause swelling and bursting of the cell in the condition of hypotonic or dehydrating, shrinking, and plasmolysis in the condition of hypertonic. Water-selective channels, namely, aquaporins, embedded in the membrane, accelerating the transition of water. Such channels regulate the water flux in both directions in order to respond to a sudden penetration or drop. Aquaporins belong to the family of major intrinsic protein (MIP) of transporters ubiquitous. Glycerol facilitators and aquaglyceroporins are also included in the family, which also permits the transition of some small molecules, such as glycerol, other polyols, dihydroxyacetone, CO2, urea, and ammonium.
To avoid undergoing detrimental conditions, microbiology has established an efficient and quick countermoves, in addition to a passive volume regulation. The swelling of bacteria is under control by regulating the osmotic activity of the pool of solutes in the cytoplasm, therefore enabling to adjust the water content via osmosis. This mechanism includes the recovery of swelling, which is one aspect of the most in-depth study of the reaction of LAB to osmotic pressure.
5.3.3 Regulatory Mechanisms in the Osmotic Responses of Lactic Acid Bacteria
The osmotic active compounds in the Lactobacillus family have dual functions in osmotic regulation cells. They not only play a role in keeping cell expansion but also protect biomolecules in vitro under pressure. Many bacteria can protect against osmotic stress through accumulation of glycine betaine, carnitine, and proline (Caldas et al. 1999). Research shows that the beneficial effects of glycine betaine of LAB, which discovered the Rituxan cloning and expression of betaine BetL intake system, significantly improved the B. breve UCC2003, the cloning and expression of liszt bacteria, and acid resistance and salt resistance (Sheehan et al. 2006, 2007). Another important function of compatible solutes is offset due to dry damaging effects of water loss. L. plantarum can be protected by glycine betaine during the drying processes (Kets et al. 1996). When L. plantarum ST-III was cultured in a chemically defined medium with 6% NaCl, glycine betaine significantly improved cell growth (Zhao et al. 2014). Transcriptomics data showed that under the existence of glycine betaine, the gene expression of carbohydrate transport and metabolism was significantly increased. This may be the resistance mechanism of L. plantarum ST-III to salt stress.
The mainly protective agent of sugar is recognized in the preparation of the dried LAB starters (Santivarangkna et al. 2008). It affects the vitality of the starter from the beginning to the end of the process. As mentioned above, some LAB responses to sugar and permeability are affected by the balance of intracellular and extracellular glucose concentrations. As a result, these compounds are present on both sides of the cell membrane and are in contact with the cytoplasmic protein inside. The accumulation of trehalose or the oligosaccharides of Lactobacillus was observed (Kets and Bont 1995; Prasad et al. 2003).
Excepting the regulation of intracellular solutes in hypertonic conditions, LAB rapidly changes the expression of some genes directly refer to the uptake of osmotic agents. Under the high osmotic stress, the stress response protein inhibited the molecular adjoint protein and protease to a certain extent, which was the main stress response system of the protein quality control of LAB. Transcriptional and proteomic studies showed that DnaK, GroEL, and GroES were involved in the reaction of lactate to salt stress (Kilstrup et al. 1997; Xie et al. 2004). The transcription of dnaK gene in Enterococcus faecalis was induced by hyperosmotic conditions too (Flahaut et al. 1996). Analogical observations were found in acidophilic siphonococcus and a variety of Lactobacillus under high salt conditions. (Fukuda et al. 2002; Prasad et al. 2003; De Angelis and Gobbetti 2004).
The variation in bacterial cell wall compositions with external osmolality is another important aspect of the osmoadaptation. High osmotic pressure affects lipid composition of bacterial membranes. These changes affect the osmotic activation curves of osmotic protective agent transporters operated in L. lactis by lipid-protein interactions. Modifications may also affect the permeability of the membrane. The permeability reaction is in connection with the physical properties of the membrane. Compared with the growth of the standard MRS, the cells with the lower growth of polyethylene glycol have a more rigid structure, which refer to the increase of saturated/unsaturated ratio in the total pool of bacterial lipid (Tymczyszyn 2005). The increase of cyclopropane is mainly due to the modification of high-permeable Streptococcus in the composition of membrane fatty acids (Guillot et al. 2000). In L. bulgaricus, the change of membrane performance was also related to the increase of sugar content in the whole lipid pool. In Lactobacillus bulgarian, the changes of membrane properties were also related to the increase of total lipid sugar content (Tymczyszyn 2005). The reaction of Lactobacillus casei to hyperosmotic conditions did not lead to prominent differences in the proportion of glycolipids/phospholipids (Machado et al. 2004). Nevertheless, individual glycolipids and phospholipids revealed several important changes. The small increase in the amount of glycolipids involved in the formation of liposomes may be especially related to the hydrophobicity of the cells shown in Lactobacillus. Some phospholipids were observed to increase significantly. The different content of cardiac phospholipids is considered as a key factor for bacterial infiltration (Romantsov et al. 2009).
Moreover, LAB can react to osmotic pressure by changing the properties of cell walls. The modification plays a major role in the industrial application of LAB under high permeability, because they can modify the sensitivity to cracking (Piuri et al. 2005; Koch et al. 2007). The retardation of Lactobacillus casei in high salinity was related to cell size observed by transmission electron microscopy and modification of cell membrane (Piuri et al. 2005). In addition, in Lactobacillus, short osmotic stress (with 4% NaCl 30 min) caused the induction of murF and murG genes (Xie et al. 2004) involving polysaccharide peptide biosynthesis.
5.4 Responses of Lactic Acid Bacteria to Oxidative Stress
5.4.1 Introduction
The imbalance between production of reactive oxygen species (ROS) and antioxidative mechanisms is oxidative. ROS and oxygen do harm to the biomolecules such as nucleic acid, protein, and lipids, damaging their biological functions. LABs are widely used as a starter culture in food fermentation, and it is vital to secure title of viable cells. During manufacturing process, LAB exposure to oxygen and reactive oxygen species contributes beneficial effects on human health, since it commonly functions as a probiotics. In the gastrointestinal tract, LABs encounter oxidative stress from oxygen gradients and the immune system, reducing viable cell counts (Bermúdezhumarán et al. 2008).
Gene expression, growing, and surviving of LAB were significantly affected by oxidative stresses, since the applications of LAB were restricted. The gene expression of LAB was reprogrammed by the stress; therefore, the physiology adapted to the new to survive.
5.4.2 Metabolic Responses to Oxidative Stress in Lactic Acid Bacteria
In Lactococcus lactis, the conversion of glyceraldehyde-3-phosphate is catalyzed to glycerate-1,3-biphosphate in glycolysis by glyceraldehyde-3-phosphate dehydrogenase. Among the most abundant proteins, the gapB gene is highly expressed by glycolytic pathway. The gapB gene has been probed in 2D gels, and when its cells are exposed to O2, it presents as two spots with different isoelectric points but the same molecular weight (Melchiorsen et al. 2000). It was observed that the relative level of these two spots of Lactococcus lactis has changed in these circumstances: one is in a thioredoxin reductase mutant (trxB1) and another is in respiration metabolism. At the same time, both of these circumstances are related to oxidative stress (Vido et al. 2004, 2005). Thioredoxin–thioredoxin reductase system needs to remove reactive oxygen species (ROS), such as O2, superoxide, H2O2, and hydroxyl radical, in order to avoid cysteine of the GapB protein oxidation before it is attacked. A mass of GapB of Lactococcus lactis, which can avoid glycolysis flux declining via the oxidation of GapB, still keep the whole activity of glycolysis (Boels et al. 2003). What’s more, the growing of without texB1 slowed down under the condition of glutathione, cysteine, and pyruvate (Vido et al. 2005).
In anaerobiosis, pyruvate formate lyase (PFL) was catalyzed from pyruvate to acetyl-CoA. PFL is divided into two fragments in the shift from anaerobiosis to aerobiosis and then lead to its irreversible inactivation. Reducing the glycyl radical into glycine by PFL decativase could avoid cleavage (Melchiorsen et al. 2000). In addition to this, the alternative metabolic pathway induced to sustain acetyl-CoA productions. At the later stages, the gene that encoded pyruvate dehydrogenase compound (PdhABCD, PDHc) expressed (Jensen et al. 2001). The compound also acts as catalyst of the transformation of pyruvate to acetyl-CoA. In aeration conditions, flavoprotein has vital role in the growth of several bacteria. An NADH:H2O of Lactococcus lactis that leads oxidase to overproducing generated a vast of acetate and acetoin, at the exchange of lactate, indicating that the enzyme can effectively promote the transformation of pyruvate to acetate (Hoefnagel et al. 2002).
Researches show that Lactococcus lactis, E. faecalis, and other LAB can activate cytochrome oxidase that is heme-dependent and establish a whole respiration chain (Winstedt et al. 2000; Duwat et al. 2001). The heme-dependent cytochrome oxidase CydAB is usually reported to have more affinity for O2 than other cytochrome oxidases (Rezaïki et al. 2004). Meanwhile, the consumption of O2 in the membrane decreases O2 content in the cytoplasm and restricts the occurrence of ROS. Due to ROS reactivity, Lactococcus lactis and other breathing-permissive LAB demand regulate their heme library, yet permitting enough heme enables to activate the CydAB cytochrome oxidase (Rezaïki et al. 2004).
5.4.3 Regulatory Mechanisms in the Oxidative Stress of Lactic Acid Bacteria
LlrF (DNA-binding regulator) and LlkinF (sensor), involved in oxidative stress, were identified as two TCSs in Lactococcus lactis (O’Connell-Motherway et al. 2000). Inactivation of LlrF is more sensitive to peroxide, and only 9% of mutants survive when exposed to 4 Mm H2O2.It seems that a TCS called ScnRK in Streptococcus mutans could regulate some genes associated with stress, for example, TPX, which could encode mercaptan peroxidase (Chen et al. 2008).
In Lactococcus lactis, genome analysis showed the PerR gene and OhrR gene of Bacillus subtilis, related to peroxide stress response. In Bacillus subtilis, the PerR gene is associated with the iron-containing repressor family (Lee and Helmann 2006). Meanwhile, the PerR reacted with peroxide via iron through Fenton reaction, and what is more, HO˙ oxidized histidine in location 37 or 91 in peptide sequence (Lee and Helmann 2006). Research showed a regulator similar with PerR in E.faecalis could cause oxidative stress response (Verneuil et al. 2005).
In Bacillus subtilis, OHRR protein is a homogeneous dimer belonging to a variety of antibiotic resistance (MarR) families. OhrR protein could regulate ohr genes express that encode thior peroxidase, and then the hydrogen peroxide is reduced to the corresponding alcohol. OHRR is resistant to hydrogen peroxide stress at the location of peptides at 15, through the oxidation of its unique cysteine residue. (Fuangthong and Helmann 2002). Cysteine is oxidized to sulfenic acid (RSH → RSOH) or to more oxidation state in oxidative stress.
Rex, the oxidation of an amino acid, was used to detect stress, and then the expression of cytochrome oxidase gene (cydAB) of Bacillus subtilis and streptomycin was controlled by the pool of NADH (Wang et al. 2008). This protein is a homogeneous dimer that contains two domains: the N-terminal domain is combined with the promoter region of the target gene (such as cydABCD), while the C-terminal identifies the ligand NADH. When the cell is at the stationary stage or the O2 tension reduces, the NADH pool augments, causing it to combine with Rex. The Rex-NADH-DNA complex has been unstable, inhibiting the repression of gene that Rex controls.
5.5 Responses of Lactic Acid Bacteria to Cold Stress
5.5.1 Introduction
LABs play a significant role in the food and biotechnology industry. In the manufacture of foods such as yogurt, cheese (Crow et al. 2001), fermented meat, and vegetables and probiotics, as well as green chemistry applications (Axel et al. 2012), LABs are proverbially used as starters. Freeze-drying or lyophilization shows good reproducibility in preserving bacteria. At supra-zero temperatures, it can be stored for a long time and at low cost as long as the water activity to values is reduced to less than 0.2. At the same time, low water activity to values assures that bacteria have little loss in viability and function. Adding protective agents into the bacterial suspension could keep bacteria stable in freeze-drying. Freeze-drying requires three measures: firstly, concentration frozen and cell suspension protected; secondly, ice removed through sublimation in initial drying; and finally, unfrozen water removed through desorption in secondary drying. In this chapter, a method was described to optimize the process parameters in freeze-drying of LAB, enabling bacteria to achieve the highest survival rates as well as greatest functional recovery.
Freezing is a commonly used technique in preserving, so the fast cooling rates are commonly adopted in food industry. Rapid freezing needs lower storage temperatures and usually results in the degeneration of vitality and acidification when thawing. Bacterial resistance to freezing relies on lots of parameters containing the bacterial species (Rault et al. 2007), protective additives, freezing rate, and final storage temperature. LABs are usually preserved through freeze-drying; however, lower temperatures could harm cells and cause devitalization. Over the years, attempts to improve the vitality of LAB during freeze-drying have been the focus of freeze-drying study.
By comparison, cold shock cannot lead to such clearly defined cell damage. Bacterial species exposed to lower temperatures always happens under different conditions. Food-related bacteria, for example, LAB, are specially and often encountered by lower temperatures in the process of food circulation. This situation that bacteria are exposed to low temperature happens more and more frequently in current few decades because refrigerator is invented to keep food and bacteria in cold storage. It’s found that lots of bacterial species encountered by cold shock are able to temporarily induce arrays of specific proteins called cold-induced proteins (CIPs) (Wouters et al. 2001), as well as repress other synthesized proteins during actively growing or being exposed to other stress state, for instance, heat shock. This reaction is presumed to help cells overcome the physiological stress of cold shock. The downside effect of being encountered by cold stress is mostly due to the physical effect of lower temperature on cell structure and enzyme reaction. The cold shock reaction is a typical manifestation when the exponential growth medium is changed from the most suitable growth temperature to a lower temperature. In most bacteria, such as Bacillus subtilis, temperature reduction leads to the cessation of transient cell growth, during which common protein synthesis is severely suppressed. Nevertheless, these conditions touch off the synthesis of CIPs. Ultimately, this protein synthesis is reduced, and the cells become adapted to low temperature and growth recovery (Jones et al. 1987). The effects of cold shock can be observed at multiple levels: (1) membrane fluidity decreases, affecting membrane-related functions, such as active transport and protein secretion; (2) stable RNA and DNA secondary structure, resulting in reduced mRNA translation and transcriptional efficiency; (3) slow or inefficient folding of some proteins; and (4) in low temperature, the ribosomes have good adaptability under cold conditions (Phadtare 2004). In addition, cold shock greatly disrupts the metabolism of bacteria cells; it is said that L.casei had a good network between metabolic regulation and cold reaction (Beaufils et al. 2007).
The reaction to freezing pressure is often passive, leading to a decrease in survivability and metabolic activity associated with low temperature damage (Guchte et al. 2002). By comparison, cold but positive temperature results in two adaptive responses. First, a subset of CIPs called cold-shock protein (CSP) was synthesized. The second is the change of membrane fatty acid composition, such as increasing the content of unsaturated and circulating fatty acids and allowing the membrane fluidity (Phadtare 2004). Transient physiological modifications of cell proteome patterns and cell membrane properties are generated by these adaptive responses, making bacterial cells better able to face farther pressure.
Application of a slow cooling rate allows for a repeatable freeze protocol, but the biological response of LAB needs characteristics during the freezing process. It is vital to know the basis of cold stress response of molecular mechanisms to improve the selection, storage, and performance of existing industrial stains.
5.5.2 Regulatory Mechanisms in the Cold Responses of Lactic Acid Bacteria
5.5.2.1 Cold-Stress Proteins
During cold stress, the most strongly induced proteins will include a family of CSP proteins. CSP proteins share high degree of sequence identity (45%) founded in many Gram-positive and Gram-negative bacteria (Phadtare 2004). CSPs proteins have been well known as transcriptional and translational regulators. They also act as molecular chaperones because they nonspecifically bind single-stranded nucleic acids and destabilize their secondary structures at low temperature (Gualerzi et al. 2003; Zeeb et al. 2006). CSP proteins are refered to establish a “new” cell balance in cold environment. However, the cell viability of several LABs increased after being sustain to a cold shock prior for freezing. That is to say, cold shock initiates the freezing tolerance, which also called cryotolerance.
The CspA-like protein and CSPs, which are from Psychrobacter sp.B6, have a high sequence homology (43%) with the Y-box factors, which are a family of eukaryotic nucleic acid-binding proteins. In these proteins, the domain involved in the nucleic acid binding is referred to as the cold-shock domain. This domain preferentially binds to the so-called Y-box, a nucleotide sequence element found in the promoter region of mammalian major histocompatibility complex class II genes. The Y-box could be characterized by a high converted sequence ATTGG (Phadtare 2004). This sequence was shown to exist in the promoter regions of at least two cold-shock genes, hns, encoding the nucleoid protein H-NS, and gyrA, encoding a subunit of DNA gyrase. It has suggested that CspA binds to the ATTGG element in the promoter region of gyrA in E. coli (Panoff et al. 1998). It has been shown that the CspB can bind to the single-stranded DNA that contains the ATTGG element as well as the complementary CCAAT sequence (Panoff et al. 1998). Therefore, the result also suggested that CspA and CspB could act as transcriptional enhancers to cold-shock genes by recognizing the putative ATTGG sequence (Phadtare 2004).
The CSPs, which in a cell could be detected by the stability of the proteins. The CSPs of B. subtilis undergo rapidly folding and unfolding transitions and exhibit low conformational stability in solution. Certainly, in vitro conditions, CSPs also are rapidly degraded by proteases but are protected against proteolysis by binding to RNA.
The CSP genes of B.subtilis have been deleted, which induces compensatory effects of the remaining CSPs (Graumann and Marahiel 1997), and a similar response is suggested for L. lactis (Wouters et al. 2000). Interestingly, multiple deletion analysis showed that at least one functional CSP is required for cell viability in B. subtilis, indicating that CSPs play an important role not only during cold-shock adaptation but also during active growth under physiologic temperatures (Graumann and Marahiel 1997). The research found that most familiar LAB genomes have several homologous copies of csp genes. Nevertheless, a large part of them did not make an intensive study. It was found that the chromosome of L. lactis MG1363 had two pairs of cold-induced csp genes (cspA-cspB and cspC-cspD), the cspE gene and the putative cold-shock gene cspD2. L. lactis IL 1403 transformed from 30 to 15 °C, entering into a state of cold shock, meanwhile showing ability of ten times induction of cspB-mediated galactosidase activity (Wouters et al. 2000). It is reported that cspA of E.coli also expressed the activity of cold induction, simultaneously instantaneous induction happening at the level of mRNA and transcription (Goldenberg et al. 1996; Fang et al. 1997).
It is reported that L. plantarum strain NC8 has detected genes of TcspL, cspC, and cspP. At the same time, the overexpression of each CSP gene had different phenotypic effects on Lactobacillus plant (Derzelle et al. 2002, 2003). It is worth noting that L. plantarum cells have a large amounts of cspC transcript during the period of early exponential growth, and the excess expression of cspC improves the growth adaptation at optimum temperature (Derzelle et al. 2003). In science, CSP protein has an important influence on the process of fermentation, which could perform both at low or optimum temperature. Comparing with cold induction, the characteristic of csp gene that noncold induced of L. plantarum and L. Lactis contain a longer 5-UTR.The difference between them is that the transcripts are highly unstable, and the expression level of csp gene between L. plantarum and L.lactis is consistent (Wouters et al. 1999).
5.5.2.2 Membrane Integrity
The resistance of bacteria to freezing or frozen storage depends on many factors, for instance, species of bacteria, growing conditions, CSP protein’s production, etc. The adaptability of Lactobacillus to cold stress is different from bacterial strain and is related to pressure conditions. Nevertheless, in addition to inducing a specific set of proteins, key responses also included major changes in the composition of membrane fatty acids.
After describing successive physical events, we found that the cell membrane is the main target of damage. The barrier properties of cytoplasmic membrane are essential to the energy transduction system of Lactobacillus cells and rely on the physical state of lipid bilayer. As a matter of fact, it was affected by outside temperature. Exactly, it suggests that normal cellular function requires a membrane lipid layer to be in a state of liquid crystal at physiological temperature. At lower temperatures, the lipid bilayer experiences a reversible change that the state of fatty acid chains varies from disordered to ordered array. Therefore, there is an inverse relationship between the ratio of unsaturated fatty acid to saturated fatty acid (U/S) and the growth temperature (Suutari and Laakso 1992). In addition, some certain fatty acids are of great importance to stress response (Li et al. 2009). Fernandez Murga et al. observed a phenomenon that C16:0 and C18:2 fatty acids of Lactobacillus acidophilus growth at 25 °C are increased. The concentration of C18:1 fatty acid increased in the condition of at low temperature in Lactobacillus plantarum and at acidic pH in Streptococcus thermophilus, under osmotic stress in Lactococcus lactis. What’s similar is that in C18:1, some decline happened under the condition of freezing in Lactic streptococci and spray-drying in L. acidophilus (Brennan et al. 1986). A high content of cycC19:0 is beneficial to the cryotolerance in L. bulgaricus, L. helveticus, and L. acidophilus (Gomez et al. 2000). The modulation of membrane lipids among high ratio of CFA/UFA and the concurrent rigidification cause L. lactis TOMSC161 incapable of resisting freeze-drying and storage stress (Velly et al. 2015). In addition, it seems that the membrane fluidity is measured directly by fluorescence anisotropy that is a fast and simple tool to determine the optimal fermentation time, making it possible to acquire antifreeze stem cells.
It is well-known that cold shock is unfavorable to the harmful effects of membrane fluidity on other non-biological stress. This feature was observed in Oenococcus oeni, a wine starter (Chu-Ky et al. 2005). The effects of the combination of cold, acid, and ethanol on membrane physical state and O. oeni survival were analyzed. Ethanol and acid shock induced membrane sclerosis, which was associated with total cell death. By contrast, O. oeni cells restored its ability to survive when suffering first cold shock (8 °C) and then ethanol and acid shock (Chu-Ky et al. 2005). These results indicate that the combination of cold, acid, and ethanol shocks has positive short-term effects on the membrane fluidity and viability of the O. oeni.
5.5.2.3 Freezing and Cryoprotection
Cold stress plays an important role in LAB cooling and freezing and is the primary cause of the loss of LAB activity. Additionally, stress from oxidation and/or hypertonic environments also occurs during the process of freezing and thawing (Stead and Park 2000).
When the bacterial cell mass is transferred from optimum temperature to low temperature, some bacterial strains can survive at extremely low temperatures, and this phenomenon is called freezing tolerance. The freeze-thaw challenge relies on temperature and the durability of cold pre-culture. Of course, it also depends on the initial concentration of bacterial cells. Cryopreservation of cell demands particular optimizations according to the type of microbe, and each type of cell has its own freezing solution. A growing number of studies are trying to develop ways to allow 100% preservation of the freezing–thawing of different cell samples; however, some microbes still do not apply to cryopreservation (Dumont et al. 2004).
During freezing–thawing cycles of LAB, many factors affect them, for instance, the composition and conditions of growth medium, growth stage, fermentation process, and low temperature (Streit et al. 2007; Siaterlis et al. 2009). Presently, a great number of methods have been put forward to maintain the quality of LAB and other probiotics (Panoff et al. 2000; Fonseca et al. 2003; Siaterlis et al. 2009) Among them, the use of cryoprotectant such as betaine, proline, glycerin, and trehalose is considered to be the most effective. These molecules improve cell preservation by reducing the amount of water and/or supporting vitrification and ultimately by preventing cells from forming large molecules (Dumont et al. 2004). It has been reported that Lactobacillus reuteri CICC6226 makes improvement of its membrane integrity and fluidity in the case of 10% trehalose or 10% remodeling of skimmed milk as a protectant in the freeze-drying process (Li et al. 2011). L. sanfranciscensis DSM20451 cells containing GSH than without glutathione showed higher resistance to freeze-drying, freezing–thawing, and cold stress induced by 4 °C cold treatment (Zhang et al. 2010). Cell contained GSH could maintain the integrated structure of the membrane when exposed to freezing–thawing treatment. Additionally, the cells that have GSH exhibited a high proportion of unsaturated fatty acids in the cell membrane during long-term cold treatment. The protective effect of GSH on cryo-damage of cell membrane partly results from the prevention of peroxidation and protection of fatty acids of the membrane. Intracellular accumulation of GSH enhanced the survival and biotechnological properties of L. sanfranciscensis, suggesting that the selection of GSH accumulation strains could improve the robustness of the initial yeast to the fermentation of sourdough.
In order to improve the freezing tolerance of lactic acid bacteria, it is essential to select an appropriate freezing and storage conditions and select resistant strains (Dumont et al. 2004; Monnet et al. 2003). Specific environmental conditions of fermentation, for example, pH, temperature, and centrifugation procedures, should be paid much more attention (Palmfeldt and Hahn-Hägerdal 2000; Beal et al. 2001; Shimrat 2005; Wang et al. 2005a; Streit et al. 2007). At the same time, it has been reported that the pH and temperature of fermentation were intensely affecting the freezing tolerance of Lactobacillus acidophilus. Diacetyl lactis can notably improve cell viability after continuous freezing and thawing (Lee 2004; Panoff et al. 1995; Wang et al. 2005b). Even so, it is innate feature that is crucial for bacterial strain (Fonseca et al. 2001).
The resistance of bacterial cells to freezing might also be improved by genetic engineering. For example, the overproduction of CSPs, CspB, and CspE has been shown to increase the survival of L. lactis after four freeze–thaw cycles of a ten- and fivefold factor, respectively (Wouters et al. 2000). Moreover, the overexpression of sHSPs in L. plantarum enables transformed cells to tolerate heat, solvent, and, importantly, cold stress (Fiocco et al. 2007).
References
Ahn, Y.T., G.B. Kim, K.S. Lim, Y.J. Baek, and H.U. Kim. 2003. Deconjugation of bile salts by Lactobacillus acidophilus isolates. International Dairy Journal 13 (4): 303–311.
Ai, Lianzhong, Hao Zhang, Benheng Guo, Wei Chen, Zhengjun Wu, and Wu. Yan. 2008. Preparation, partial characterization and bioactivity of exopolysaccharides from Lactobacillus casei LC2W. Carbohydrate Polymers 74 (3): 353–357.
Allain, T., S. Chaouch, M. Thomas, I. Vallée, A.G. Buret, P. Langella, P. Grellier, B. Polack, L.G. Bermúdez-Humarán, and I. Florent. 2017. Bile-salt-hydrolases from the probiotic strain Lactobacillus johnsoniiLa1 mediate anti-giardial activityin vitroandin vivo. Frontiers in Microbiology 8: 2707.
Axel, C., E. Zannini, A. Coffey, et al. 2012. Ecofriendly control of potato late blight causative agent and the potential role of lactic acid bacteria: A review. Applied Microbiology & Biotechnology 96 (1): 37–48.
Ayres, J.S. 2016. Cooperative microbial tolerance behaviors in host-microbiota mutualism. Cell 165 (6): 1323–1331. https://doi.org/10.1016/j.cell.2016.05.049.
Bandyopadhyay, A., and S.P. Moulik. 1988. Interaction of bile salts with a nonionic surfactant and their activation energy for conduction as well as calcium and barium ion tolerance in presence of the nonionic surfactant. Indian Journal of Biochemistry & Biophysics 25 (3): 287.
Beal, C., F. Fonseca, and G. Corrieu. 2001. Resistance to freezing and frozen storage of Streptococcus thermophilus is related to membrane fatty acid composition. Journal of Dairy Science 84 (11): 2347–2356.
Beales, N. 2004. Adaptation of microorganisms to cold temperatures, weak acid preservatives, low ph, and osmotic stress: A review. Comprehensive Reviews in Food Science & Food Safety 3 (1): 1–20.
Beaufils, Sophie, Nicolas Sauvageot, Alain Mazé, Jean Marie Laplace, Yanick Auffray, Josef Deutscher, and Axel Hartke. 2007. The cold shock response of Lactobacillus casei: Relation between HPr phosphorylation and resistance to freeze/thaw cycles. Journal of Molecular Microbiology & Biotechnology 13 (1–3): 65–75.
Becker, M.R., B.J. Paster, E.J. Leys, M.L. Moeschberger, S.G. Kenyon, J.L. Galvin, S.K. Boches, F.E. Dewhirst, and A.L. Griffen. 2002. Molecular analysis of bacterial species associated with childhood caries. Journal of Clinical Microbiology 40 (3): 1001–1009. https://doi.org/10.1128/jcm.40.3.1001-1009.2002.
Begley, M., C.G. Gahan, and C. Hill. 2005. The interaction between bacteria and bile. Fems Microbiology Reviews 29 (4): 625–651.
Bender, G.R., S.V. Sutton, and R.E. Marquis. 1986. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infection & Immunity 53 (2): 331.
Bermúdezhumarán, L.G., N.G. Cortesperez, S. Ahleung, F. Lefèvre, G. Yang, Q. Pang, C. Wu, Y. Zeng, K. Adelpatient, and P. Langella. 2008. Current prophylactic and therapeutic uses of a recombinant Lactococcus lactis strain secreting biologically active interleukin-12. Journal of Molecular Microbiology & Biotechnology 14 (1–3): 80–89.
Biswas, Indranil, Laura Drake, Dasha Erkina, and Saswati Biswas. 2008. Involvement of sensor kinases in the stress tolerance response of Streptococcus mutans. Journal of Bacteriology 190 (1): 68.
Boels, I.C., M. Kleerebezem, and W.M. de Vos. 2003. Engineering of carbon distribution between glycolysis and sugar nucleotide biosynthesis in Lactococcus lactis. Applied and Environmental Microbiology 69 (2): 1129–1135.
Brennan, M., B. Wanismail, M.C. Johnson, and B. Ray. 1986. Cellular damage in dried Lactobacillus acidophilus. Journal of Food Protection 49 (1): 47–53.
Budin-Verneuil, A., V. Pichereau, Y. Auffray, D.S. Ehrlich, and E. Maguin. 2006. Proteomic characterization of the acid tolerance response in Lactococcus lactis MG1363. 5 (18): 4794–4807.
Budin-Verneuil, A., V. Pichereau, Y. Auffray, D. Ehrlich, and E. Maguin. 2007. Proteome phenotyping of acid stress-resistant mutants of Lactococcus lactis MG1363. Proteomics 7 (12): 2038–2046.
Burokas, A., S. Arboleya, R.D. Moloney, V.L. Peterson, K. Murphy, G. Clarke, C. Stanton, T.G. Dinan, and J.F. Cryan. 2017. Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biological Psychiatry 82: 472. https://doi.org/10.1016/j.biopsych.2016.12.031.
Caldas, T., N. Demont-Caulet, A. Ghazi, and G. Richarme. 1999. Thermoprotection by glycine betaine and choline. Microbiology 145 (Pt 9): 2543.
Champomier Vergès, M.C., M. Zuñiga, F. Morel-Deville, G. Pérez-Martínez, M. Zagorec, and S.D. Ehrlich. 1999. Relationships between arginine degradation, pH and survival in Lactobacillus sakei. Fems Microbiology Letters 180 (2): 297.
Chen, Pm, Hc Chen, Ct Ho, Ht Cjlien Jung, Jy Chen, and Js Chia. 2008. The two-component system ScnRK of Streptococcus mutans affects hydrogen peroxide resistance and murine macrophage killing. Microbes & Infection 10 (3): 293.
Chong, P., L. Drake, and I. Biswas. 2008. LiaS regulates virulence factor expression in Streptococcus mutans. Infection & Immunity 76 (76): 3093–3099.
Chu-Ky, S., R. Tourdot-Marechal, P.A. Marechal, and J. Guzzo. 2005. Combined cold, acid, ethanol shocks in Oenococcus oeni: Effects on membrane fluidity and cell viability. Biochimica Et Biophysica Acta 1717 (2): 118–124.
Crow, V., B. Curry, and M. Hayes. 2001. The ecology of non-starter lactic acid bacteria (NSLAB) and their use as adjuncts in New Zealand Cheddar.[J]. International Dairy Journal 11 (4): 275–283.
Cui, Y., W. Liu, X. Qu, Z. Chen, X. Zhang, T. Liu, and L. Zhang. 2012. A two component system is involved in acid adaptation of Lactobacillus delbrueckii subsp.bulgaricus. Microbiological Research 167 (5): 253–261.
Davis, C.R., D.J. Wibowo, T.H. Lee, and G.H. Fleet. 1986. Growth and metabolism of lactic acid bacteria during and after malolactic fermentation of wines at different pH. Applied & Environmental Microbiology 51 (3): 539.
De Angelis, M., and M. Gobbetti. 2004. Environmental stress responses in Lactobacillus: A review. Proteomics 4 (1): 106–122.
Derzelle, Sylviane, Bernard Hallet, Thierry Ferain, Jean Delcour, and Pascal Hols. 2002. Cold shock induction of the cspL gene in Lactobacillus plantarum involves transcriptional regulation. Journal of Bacteriology 184 (19): 5518–5523.
Derzelle, Sylviane, Bernard Hallet, Theirry Ferain, Jean Delcour, and Pascal Hols. 2003. Improved adaptation to cold-shock, stationary-phase, and freezing stresses in Lactobacillus plantarum overproducing cold-shock proteins. Applied & Environmental Microbiology 69 (7): 4285.
Dumont, F., P.A. Marechal, and P. Gervais. 2004. Cell size and water permeability as determining factors for cell viability after freezing at different cooling rates. Applied & Environmental Microbiology 70 (1): 268.
Duwat, P., S. Sourice, B. Cesselin, G. Lamberet, K. Vido, P. Gaudu, Y. Le Loir, F. Violet, P. Loubière, and A. Gruss. 2001. Respiration capacity of the fermenting bacterium Lactococcus lactis and its positive effects on growth and survival. Journal of Bacteriology 183 (15): 4509–4516.
Erny, D., A.L. Hrabe de Angelis, D. Jaitin, P. Wieghofer, O. Staszewski, E. David, H. Keren-Shaul, T. Mahlakoiv, K. Jakobshagen, T. Buch, V. Schwierzeck, O. Utermohlen, E. Chun, W.S. Garrett, K.D. McCoy, A. Diefenbach, P. Staeheli, B. Stecher, I. Amit, and M. Prinz. 2015. Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience 18 (7): 965–977. https://doi.org/10.1038/nn.4030.
Fang, L., W. Jiang, W. Bae, and M. Inouye. 1997. Promoter-independent cold-shock induction of cspA and its derepression at 37 degrees C by mRNA stabilization. Molecular Microbiology 23 (2): 355.
Fetissov, S.O. 2017. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nature Reviews. Endocrinology 13 (1): 11–25. https://doi.org/10.1038/nrendo.2016.150.
Fiocco, D., V. Capozzi, P. Goffin, P. Hols, and G. Spano. 2007. Improved adaptation to heat, cold, and solvent tolerance in Lactobacillus plantarum. Applied Microbiology & Biotechnology 77 (4): 909–915.
Flahaut, S., Abdellah Benachour, Jean Christophe Giard, Philippe Boutibonnes, and Yanick Auffray. 1996. Defense against lethal treatments and de novo protein synthesis induced by NaCl in Enterococcus faecalis ATCC 19433. Archives of Microbiology 165 (5): 317–324.
Fonseca, F., C. Béal, and G. Corrieu. 2001. Operating conditions that affect the resistance of lactic acid bacteria to freezing and frozen storage. Cryobiology 43 (3): 189–198.
Fonseca, F., C. Beal, F. Mihoub, M. Marin, and G. Corrieu. 2003. Improvement of cryopreservation of Lactobacillus delbrueckii subsp. bulgaricus CFL1 with additives displaying different protective effects. International Dairy Journal 13 (11): 917–926.
Fuangthong, M., and J.D. Helmann. 2002. The OhrR repressor senses organic hydroperoxides by reversible formation of a cysteine-sulfenic acid derivative. Proceedings of the National Academy of Sciences of the United States of America 99 (10): 6690–6695.
Fukuda, D., M. Watanabe, S. Sonezaki, S. Sugimoto, K. Sonomoto, and A. Ishizaki. 2002. Molecular characterization and regulatory analysis of dnaK operon of halophilic lactic acid bacterium Tetragenococcus halophila. Journal of Bioscience and Bioengineering 94 (4): 388–394.
Gao, Rong, and Ann M. Stock. 2009. Biological insights from structures of two-component proteins. Annual Review of Microbiology 63 (1): 133.
Goldenberg, D., I. Azar, and A.B. Oppenheim. 1996. Differential mRNA stability of the cspA gene in the cold-shock response of Escherichia coli. Molecular Microbiology 19 (2): 241.
Gomez, Zavaglia A., E.A. Disalvo, and G.L. De Antoni. 2000. Fatty acid composition and freeze-thaw resistance in lactobacilli. Journal of Dairy Research 67 (2): 241.
Graumann, P., and M.A. Marahiel. 1997. Effects of heterologous expression of CspB, the major cold shock protein of Bacillus subtilis, on protein synthesis in Escherichia coli. Molecular and General Genetics 253 (6): 745–752.
Gualerzi, C.O., A.M. Giuliodori, and C.L. Pon. 2003. Transcriptional and post-transcriptional control of cold-shock genes. Journal of Molecular Biology 331 (3): 527–539.
Guillot, A., D. Obis, and M.Y. Mistou. 2000. Fatty acid membrane composition and activation of glycine-betaine transport in Lactococcus lactis subjected to osmotic stress. International Journal of Food Microbiology 55 (1–3): 47–51.
Hamon, E., P. Horvatovich, E. Izquierdo, F. Bringel, E. Marchioni, D. Aoudéwerner, and S. Ennahar. 2011. Comparative proteomic analysis of Lactobacillus plantarum for the identification of key proteins in bile tolerance. BMC Microbiology 11 (1): 63.
Hoefnagel, M.H., M.J. Starrenburg, D.E. Martens, J. Hugenholtz, M. Kleerebezem, I.I. Van Swam, R. Bongers, H.V. Westerhoff, and J.L. Snoep. 2002. Metabolic engineering of lactic acid bacteria, the combined approach: Kinetic modelling, metabolic control and experimental analysis. Microbiology 148 (4): 1003–1013.
Hu, B., F. Tian, G. Wang, Q. Zhang, J. Zhao, H. Zhang, and W. Chen. 2015. Enhancement of bile resistance in Lactobacillus plantarum strains by soy lecithin. Letters in Applied Microbiology 61 (1): 13.
Jarocki, P., M. Podleśny, P. Glibowski, and Z. Targoński. 2014. A new insight into the physiological role of bile salt hydrolase among intestinal bacteria from the genus Bifidobacterium. Plos One 9 (12): e114379.
Jensen, Niels Bang Siemsen, Claus Rix Melchiorsen, Kirsten Væver Jokumsen, and John Villadsen. 2001. Metabolic behavior of Lactococcus lactis MG1363 in microaerobic continuous cultivation at a low dilution rate. Applied & Environmental Microbiology 67 (6): 2677.
Jie, Bi, Liu Song, Du Guocheng, and Chen Jian. 2016. Bile salt tolerance of Lactococcus lactis is enhanced by expression of bile salt hydrolase thereby producing less bile acid in the cells. Biotechnology Letters 38 (4): 659–665.
Jones, P.G., R.A. Vanbogelen, and F.C. Neidhardt. 1987. Induction of proteins in response to low temperature in Escherichia coli. Journal of Bacteriology 169 (5): 2092.
Jones, Brian V., Máire Begley, Colin Hill, Cormac G.M. Gahan, and Julian R. Marchesi. 2008. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proceedings of the National Academy of Sciences of the United States of America 105 (36): 13580.
Kawadamatsuo, M., Y. Shibata, and Y. Yamashita. 2009. Role of two component signaling response regulators in acid tolerance of Streptococcus mutans. Oral Microbiology & Immunology 24 (2): 173.
Keijser, B.J.F., E. Zaura, S.M. Huse, J.M.B.M. van der Vossen, F.H.J. Schuren, R.C. Montijn, J.M. Ten Cate, and W. Crielaard. 2008. Pyrosequencing analysis of the oral microflora of healthy adults. Journal of Dental Research 87 (11): 1016–1020.
Kets, Edwin P.W., and Jan A.M. De Bont. 1995. Protective effect of betaine on survival of Lactobacillus plantarum subjected to drying. Fems Microbiology Letters 116 (3): 251–255.
Kets, E., P. Teunissen, and J. De Bont. 1996. Effect of compatible solutes on survival of lactic acid bacteria subjected to drying. Applied & Environmental Microbiology 62 (1): 259–261.
Kilstrup, M., S. Jacobsen, K. Hammer, and F.K. Vogensen. 1997. Induction of heat shock proteins DnaK, GroEL, and GroES by salt stress in Lactococcus lactis. Applied & Environmental Microbiology 63 (5): 1826–1837.
Kim, N.R., D.W. Jeong, D.S. Ko, and J.H. Shim. 2017. Characterization of novel thermophilic alpha-glucosidase from Bifidobacterium longum. International Journal of Biological Macromolecules 99: 594–599. https://doi.org/10.1016/j.ijbiomac.2017.03.009.
Koch, S., G. Oberson, E. Eugster-Meier, L. Meile, and C. Lacroix. 2007. Osmotic stress induced by salt increases cell yield, autolytic activity, and survival of lyophilization of Lactobacillus delbrueckii subsp. lactis. International Journal of Food Microbiology 117 (1): 36–42.
Konings, W.N., J.S. Lolkema, H. Bolhuis, H.W. van Veen, B. Poolman, and A.J.M. Driessen. 1997. The role of transport processes in survival of lactic acid bacteria. Energy transduction and multidrug resistance. Antonie Van Leeuwenhoek 71 (1–2): 117–128.
Krell, T., J. Lacal, A. Busch, H. Silvajiménez, M.E. Guazzaroni, and J.L. Ramos. 2010. Bacterial sensor kinases: Diversity in the recognition of environmental signals. Annual Review of Microbiology 64 (1): 539–559.
Küllenberg, Daniela, Lenka A. Taylor, Michael Schneider, and Ulrich Massing. 2012. Health effects of dietary phospholipids. Lipids in Health & Disease 11 (1): 3.
Kurdi, Peter, Koji Kawanishi, Kanako Mizutani, and Atsushi Yokota. 2006. Mechanism of growth inhibition by free bile acids in lactobacilli and Bifidobacteria. Journal of Bacteriology 188 (5): 1979.
Kurz, M. 2008. Compatible solute influence on nucleic acids: Many questions but few answers. Saline Systems 4: 6. https://doi.org/10.1186/1746-1448-4-6.
Lee, Ki Beom. 2004. Cold shock response in Lactococcus lactis ssp. diacetylactis: A comparison of the protection generated by brief pre-treatment at less severe temperatures. Process Biochemistry 39 (12): 2233–2239.
Lee, J.W., and J.D. Helmann. 2006. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature 440 (7082): 363–367.
Lee, K., H.G. Lee, and Y.J. Choi. 2008. Proteomic analysis of the effect of bile salts on the intestinal and probiotic bacterium lactobacillus reuteri. Journal of Biotechnology 137 (1–4): 14–19.
Lemos, J.A., T.A. Brown Jr., and R.A. Burne. 2004. Effects of RelA on key virulence properties of planktonic and biofilm populations of Streptococcus mutans. Infection & Immunity 72 (3): 1431–1440.
Levesque, Cm, Rwperry Mair, Pcy Lau Ja, Yh. Li, and Dg Cvitkovitch. 2007. Systemic inactivation and phenotypic characterization of two-component systems in expression of Streptococcus mutans virulence properties. Letters in Applied Microbiology 45 (4): 398.
Li Y H, Lau P C Y, Tang N, et al. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans[J]. Journal of bacteriology, 2002, 184(22): 6333–6342.
Li, C., J.L. Zhao, Y.T. Wang, X. Han, and N. Liu. 2009. Synthesis of cyclopropane fatty acid and its effect on freeze-drying survival of Lactobacillus bulgaricus L2 at different growth conditions. World Journal of Microbiology & Biotechnology 25 (9): 1659–1665.
Li, B., F. Tian, X. Liu, J. Zhao, H. Zhang, and W. Chen. 2011. Effects of cryoprotectants on viability of Lactobacillus reuteri CICC6226. Applied Microbiology Biotechnology 92: 609–616.
Machado, M. Cecilia, Claudia S. López, Horacio Heras, and Emilio A. Rivas. 2004. Osmotic response in Lactobacillus casei ATCC 393: Biochemical and biophysical characteristics of membrane. Archives of Biochemistry & Biophysics 422 (1): 61.
Macpherson, A.J., M. Heikenwalder, and S.C. Ganal-Vonarburg. 2016. The liver at the nexus of host-microbial interactions. Cell Host and Microbe 20 (5): 561–571. https://doi.org/10.1016/j.chom.2016.10.016.
Magnusson, Lisa U., Anne Farewell, and Thomas Nyström. 2005. ppGpp: a global regulator in Escherichia coli. Trends in Microbiology 13 (5): 236–242.
Marchesi, J.R., D.H. Adams, F. Fava, G.D. Hermes, G.M. Hirschfield, G. Hold, M.N. Quraishi, J. Kinross, H. Smidt, K.M. Tuohy, L.V. Thomas, E.G. Zoetendal, and A. Hart. 2016. The gut microbiota and host health: A new clinical frontier. Gut 65 (2): 330–339. https://doi.org/10.1136/gutjnl-2015-309990.
Martoni, Christopher, Jasmine Bhathena, Aleksandra Malgorzata Urbanska, and Satya Prakash. 2008. Microencapsulated bile salt hydrolase producing Lactobacillus reuteri for oral targeted delivery in the gastrointestinal tract. Applied Microbiology and Biotechnology 81 (2): 225–233.
Melchiorsen, C.R., K.V. Jokumsen, J. Villadsen, M.G. Johnsen, H. Israelsen, and J. Arnau. 2000. Synthesis and posttranslational regulation of pyruvate formate-lyase in Lactococcus lactis. Journal of Bacteriology 182 (17): 4783.
Monnet, C., C. Béal, and G. Corrieu. 2003. Improvement of the resistance of Lactobacillus delbrueckii ssp. bulgaricus to freezing by natural selection. Journal of Dairy Science 86 (10): 3048–3053.
Moser, S.A., and D.C. Savage. 2001. Bile salt hydrolase activity and resistance to toxicity of conjugated bile salts are unrelated properties in lactobacilli. Applied & Environmental Microbiology 67 (8): 3476–3480.
Nascimento, M.M., V.V. Gordan, C.W. Garvan, C.M. Browngardt, and R.A. Burne. 2009. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiology & Immunology 24 (2): 89.
O’Connell-Motherway, M., P. Morel, and Sinderen D. Van. 2000. Six putative two-component regulatory systems isolated from Lactococcus lactis subsp. cremoris MG1363. Microbiology 146 (4): 935–947.
Palmfeldt, J., and B. Hahn-Hägerdal. 2000. Influence of culture pH on survival of Lactobacillus reuteri subjected to freeze-drying. International Journal of Food Microbiology 55 (1–3): 235.
Panoff, Jean Michel, Bouachanh Thammavongs, Jean Marie Laplace, Axel Hartke, Philippe Boutibonnes, and Yanick Auffray. 1995. Cryotolerance and cold adaptation in Lactococcus lactis subsp. lactis IL1403. Cryobiology 32 (6): 516–520.
Panoff, J.M., B. Thammavongs, M. Guéguen, and P. Boutibonnes. 1998. Cold stress responses in mesophilic bacteria. Cryobiology 36 (2): 75–83.
Panoff, J.M., B. Thammavongs, and M. Guéguen. 2000. Cryoprotectants lead to phenotypic adaptation to freeze-thaw stress in Lactobacillus delbrueckii ssp. bulgaricus CIP 101027T. Cryobiology 40 (3): 264–269.
Patel, Anil K., Reeta R. Singhania, Ashok Pandey, and Sudhir B. Chincholkar. 2010. Probiotic bile salt hydrolase: Current developments and perspectives. Applied Biochemistry and Biotechnology 162 (1): 166–180.
Peschel, Andreas. 2002. How do bacteria resist human antimicrobial peptides? Trends in Microbiology 10 (4): 179–186.
Phadtare, S. 2004. Recent developments in bacterial cold-shock response. Current Issues in Molecular Biology 6 (2): 125.
Piuri, M., C. Sanchez-Rivas, and S.M. Ruzal. 2005. Cell wall modifications during osmotic stress in Lactobacillus casei. Journal of Applied Microbiology 98 (1): 84–95.
Prasad, Jaya, Paul Mcjarrow, and Pramod Gopal. 2003. Heat and osmotic stress responses of probiotic Lactobacillus rhamnosus HN001 (DR20) in relation to viability after drying. Applied & Environmental Microbiology 69 (2): 917–925.
Rallu, F., A. Gruss, and E. Maguin. 1996. Lactococcus lactis and stress. Antonie Van Leeuwenhoek 70 (2–4): 243–251.
Rallu, F., A. Gruss, S.D. Ehrlich, and E. Maguin. 2000. Acid- and multistress-resistant mutants of Lactococcus lactis: Identification of intracellular stress signals. Molecular Microbiology 35 (3): 517.
Rault, A., C. Béal, S. Ghorbal, et al. 2007. Multiparametric flow cytometry allows rapid assessment and comparison of lactic acid bacteria viability after freezing and during frozen storage.[J]. Cryobiology 55 (1): 35–43.
Rezaïki, Lahcen, Bénédicte Cesselin, Yuji Yamamoto, Karin Vido, Evelien Van West, Philippe Gaudu, and Alexandra Gruss. 2004. Respiration metabolism reduces oxidative and acid stress to improve long-term survival of Lactococcus lactis. Molecular Microbiology 53 (5): 1331–1342.
Romantsov, T., Z. Guan, and J.M. Wood. 2009. Cardiolipin and the osmotic stress responses of bacteria. Biochimica et Biophysica Acta 1788 (10): 2092–2100.
Santi, I., R. Grifantini, S.M. Jiang, C. Brettoni, G. Grandi, M.R. Wessels, and M. Soriani. 2009. CsrRS regulates group B Streptococcus virulence gene expression in response to environmental pH: A new perspective on vaccine development. Journal of Bacteriology 191 (17): 5387–5397.
Santivarangkna, C., B. Higl, and P. Foerst. 2008. Protection mechanisms of sugars during different stages of preparation process of dried lactic acid starter cultures. Food Microbiology 25 (3): 429–441.
Senadheera, M. Dilani, Bernard Guggenheim, Grace A. Spatafora, Yi Chen Cathy Huang, Jison Choi, David C.I. Hung, Jennifer S. Treglown, Steven D. Goodman, Richard P. Ellen, and Dennis G. Cvitkovitch. 2005. A VicRK signal transduction system in Streptococcus mutans affects gtfBCD, gbpB, and ftf expression, biofilm formation, and genetic competence development. Journal of Bacteriology 187 (12): 4064.
Senadheera, D., K. Krastel, R. Mair, A. Persadmehr, J. Abranches, R.A. Burne, and D.G. Cvitkovitch. 2009. Inactivation of VicK affects acid production and acid survival of Streptococcus mutans. Journal of Bacteriology 191 (20): 6415–6424.
Sheehan, Vivien M., Roy D. Sleator, Gerald F. Fitzgerald, and Colin Hill. 2006. Heterologous expression of BetL, a betaine uptake system, enhances the stress tolerance of Lactobacillus salivarius UCC118. Applied & Environmental Microbiology 72 (3): 2170–2177.
Sheehan, Vivien M., Roy D. Sleator, Colin Hill, and Gerald F. Fitzgerald. 2007. Improving gastric transit, gastrointestinal persistence and therapeutic efficacy of the probiotic strain Bifidobacterium breve UCC2003. Microbiology 153 (10): 3563–3571.
Shimrat, M. 2005. Influence of fermentation time, cryoprotectant and neutralization of cell concentrate on freeze-drying survival, storage stability, and acid and bile exposure of Bifidobacterium animalis ssp. lactis cells produced without milk-based ingredients. Journal of Applied Microbiology 99 (6): 1330.
Siaterlis, A., G. Deepika, and D. Charalampopoulos. 2009. Effect of culture medium and cryoprotectants on the growth and survival of probiotic lactobacilli during freeze drying. Letters in Applied Microbiology 48 (3): 295.
Stead, D., and S.F. Park. 2000. Roles of Fe superoxide dismutase and catalase in resistance of Campylobacter coli to freeze-thaw stress. Applied & Environmental Microbiology 66 (7): 3110–3112.
Streit, F., G. Corrieu, and C. Béal. 2007. Acidification improves cryotolerance of Lactobacillus delbrueckii subsp. bulgaricus CFL1. Journal of Biotechnology 128 (3): 659–667.
Suutari, M., and S. Laakso. 1992. Changes in fatty acid branching and unsaturation of Streptomyces griseus and Brevibacterium fermentans as a response to growth temperature. Applied & Environmental Microbiology 58 (7): 2338.
Taranto, M.P., F. Sesma, A. Pesce De Ruiz Holgado, and G.F. De Valdez. 1997. Bile salts hydrolase plays a key role on cholesterol removal by Lactobacillus reuteri. Biotechnology Letters 19 (9): 845–847.
Tsou, Chih-Chiang, Dmitry Avtonomov, Brett Larsen, Monika Tucholska, Hyungwon Choi, Anne-Claude Gingras, and Alexey I. Nesvizhskii. 2015. DIA-Umpire: Comprehensive computational framework for data-independent acquisition proteomics. Nature Methods 12 (3): 258–264. https://doi.org/10.1038/nmeth.3255.
Tymczyszyn, E.E. 2005. Influence of the growth at high osmolality on the lipid composition, water permeability and osmotic response of Lactobacillus bulgaricus. Archives of Biochemistry & Biophysics 443 (1): 66–73.
Uchihashi, T., R. Iino, T. Ando, and H. Noji. 2011. High-speed atomic force microscopy reveals rotary catalysis of rotorless F1-ATPase. Science 333 (6043): 755.
Van De Guchte, Maarten, Pascale Serror, Christian Chervaux, Tamara Smokvina, Stanislav D. Ehrlich, and Emmanuelle Maguin. 2002. Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 82 (1–4): 187.
Velly, H., M. Bouix, S. Passot, C. Penicaud, H. Beinsteiner, S. Ghorbal, P. Lieben, and F. Fonseca. 2015. Cyclopropanation of unsaturated fatty acids and membrane rigidification improve the freeze-drying resistance of Lactococcus lactis subsp. lactis TOMSC161. Applied Microbiology & Biotechnology 99 (2): 907.
Verneuil, N., A. Rincé, M. Sanguinetti, B. Posteraro, G. Fadda, Y. Auffray, A. Hartke, and J.C. Giard. 2005. Contribution of a PerR-like regulator to the oxidative-stress response and virulence of enterococcus faecalis. Microbiology 151 (12): 3997–4004.
Vido, Karin, Dominique Le Bars, Michel Yves Mistou, Patricia Anglade, Alexandra Gruss, and Philippe Gaudu. 2004. Proteome analyses of heme-dependent respiration in Lactococcus lactis: Involvement of the proteolytic system. Journal of Bacteriology 186 (6): 1648–1657.
Vido, K., H. Diemer, A. Van Dorsselaer, E. Leize, V. Juillard, A. Gruss, and P. Gaudu. 2005. Roles of thioredoxin reductase during the aerobic life of Lactococcus lactis. Journal of Bacteriology 187 (2): 601–610.
Vrancken, G., T. Rimaux, S. Weckx, L. De Vuyst, and F. Leroy. 2009. Environmental pH determines citrulline and ornithine release through the arginine deiminase pathway in Lactobacillus fermentum IMDO 130101. International Journal of Food Microbiology 135 (3): 216–222.
Wang, Y., G. Corrieu, and C. Béal. 2005a. Fermentation pH and temperature influence the cryotolerance of Lactobacillus acidophilus RD758. Journal of Dairy Science 88 (1): 21–29.
Wang, Y., J. Delettre, A. Guillot, G. Corrieu, and C. Béal. 2005b. Influence of cooling temperature and duration on cold adaptation of Lactobacillus acidophilus RD758. Cryobiology 50 (3): 294–307.
Wang, E., M.C. Bauer, A. Rogstam, S. Linse, D.T. Logan, and C. von Wachenfeldt. 2008. Structure and functional properties of the Bacillus subtilis transcriptional repressor Rex. Molecular Microbiology 69 (2): 466–478.
Wang, G., S. Yin, H. An, S. Chen, and Y. Hao. 2011. Coexpression of bile salt hydrolase gene and catalase gene remarkably improves oxidative stress and bile salt resistance in Lactobacillus casei. Journal of Industrial Microbiology & Biotechnology 38 (8): 985–990.
Winstedt, L., L. Frankenberg, L. Hederstedt, and C. Von Wachenfeldt. 2000. Enterococcus faecalis V583 contains a cytochrome bd-type respiratory oxidase. Journal of Bacteriology 182 (13): 3863–3866.
Wouters, J.A., B. Jeynov, F.M. Rombouts, W.M. de Vos, O.P. Kuipers, and T. Abee. 1999. Analysis of the role of 7 kDa cold-shock proteins of Lactococcus lactis MG1363 in cryoprotection. Microbiology 145 (11): 3185.
Wouters, Jeroen A., Frank M. Rombouts, Oscar P. Kuipers, Willem M. De Vos, and T. Abee. 2000. The role of cold-shock proteins in low-temperature adaptation offood-related bacteria. Systematic & Applied Microbiology 23 (2): 165–173.
Wouters, J.A., H. Frenkiel, W.M.D. Vos, et al. 2001. Cold shock proteins of Lactococcus lactis MG1363 are involved in Cryoprotection and in the production of cold-induced proteins[J]. Applied and Environmental Microbiology 67 (11): 5171–5178.
Wu, C., J. Zhang, M. Wang, G. Du, and J. Chen. 2012. Lactobacillus casei combats acid stress by maintaining cell membrane functionality. Journal of Industrial Microbiology & Biotechnology 39 (7): 1031–1039.
Xie, Y., L.S. Cutler, A. Chou, and B. Weimer. 2004. DNA macroarray profiling of Lactococcus lactis subsp. lactis IL1403 gene expression during environmental stresses. Applied & Environmental Microbiology 70 (11): 6738–6747.
Xiong, Zhi Qiang, Qiao Hui Wang, Ling Hui Kong, Xin Song, Guang Qiang Wang, Yong Jun Xia, Hui Zhang, Yong Sun, and Lian Zhong Ai. 2017. Short communication: Improving the activity of bile salt hydrolases in Lactobacillus casei based on in silico molecular docking and heterologous expression. Journal of Dairy Science 100 (2): 975–980.
Zeeb, Markus, Klaas E.A. Max, Ulrich Weininger, Christian Löw, Heinrich Sticht, and Jochen Balbach. 2006. Recognition of T-rich single-stranded DNA by the cold shock protein Bs-CspB in solution. Nucleic Acids Research 34 (16): 4561–4571.
Zhang, Juan, Du Guo Cheng, Yanping Zhang, Xian Yan Liao, Miao Wang, Yin Li, and Jian Chen. 2010. Glutathione protects Lactobacillus sanfranciscensis against freeze-thawing, freeze-drying, and cold treatment. Applied & Environmental Microbiology 76 (9): 2989–2996.
Zhao, Shanshan, Qiuxiang Zhang, Guangfei Hao, Xiaoming Liu, Jianxin Zhao, Yongquan Chen, Hao Zhang, and Wei Chen. 2014. The protective role of glycine betaine in Lactobacillus plantarum ST-III against salt stress. Food Control 44: 208–213.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Chen, W., Lu, W. (2018). Environmental Stress Responses of Lactic Acid Bacteria. In: Lactic Acid Bacteria in Foodborne Hazards Reduction. Springer, Singapore. https://doi.org/10.1007/978-981-13-1559-6_5
Download citation
DOI: https://doi.org/10.1007/978-981-13-1559-6_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1558-9
Online ISBN: 978-981-13-1559-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)