Abstract
A number of soil microorganisms can convert insoluble forms of phosphorus (P) to an accessible form to increase plant yields. Phytate is such a large kind of insoluble organic phosphorus that plants cannot absorb directly in soil, so the objectives of this study were to isolate, screen phytate-degrading rhizobacteria (PDRB), and to select potential microbial inocula that could increase the P uptake by plants. In this study, a total of 24 soil samples were collected from natural habitats of eight poplar and pine planting areas from the eastern to southern China. 17 PDRB strains were preliminarily screened from the rhizosphere soil of poplars and pines by the visible decolorization in the phytate selective medium. The highest ratio of the total diameter (colony + halo zone) to the colony diameter of the isolates was JZ-GX1, 3.85. Afterward, 17 PDRB strains were further determined for their abilities to degrade sodium phytate based on the amount of liberated inorganic P in liquid phytate specific medium. The results showed that the phytase ability of the three highest PDRB strains: JZ-GX1, JZ-DZ1 and JZ-ZJ1 were up to 2.58, 2.36 and 2.24 U/mL, respectively, much better than most of the bacteria reported in previous studies. In the soil–plant experiment, compared to CK, the best three strains of PDRB all could significantly promote growth of poplar and Masson pine under container growing. The three efficient PDRB strains were identified as follow: JZ-GX1, Rahnella aquatilis, both JZ-DZ1 and JZ-ZJ1 being autofluorescent, Pseudomonas fluorescens, by 16S rDNA gene sequencing technology, Biolog Identification System and biological characterization. The present study suggests that the three screened PDRB strains would have great potential application as biological fertilizers in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is the second major macronutrient required for the growth and development of plants (Vance 2001). P fertilizers are applied to the soil to alleviate the P deficiency, because most of the soils in the world are P-insufficient (Batjes 1997). However, a large amount of them are rapidly transformed into unavailable forms by forming a complex compound with Fe or Al in acid soil or Ca in calcareous soil before plant roots have a chance to absorb it (Alam and Ladha 2004). Contrasting to the acquisition of nitrogen from the atmosphere through biological fixation, P must be applied to plants through the application of fertilizer (Richardson 2001). Nevertheless, the recovery of P from fertilizer is limited, with only 10–20 % of supplied P being absorbed by plants in the year of application (Mclaughlin et al. 1988). P is invariably the nutrient that is the key limiting factor for plant growth in many agricultural systems (Lewis and Sale 1993).
Phosphorus in soil is in either organic (Po) or inorganic (Pi) forms. Organic phosphorus in soil generally accounts for around 50 % of total soil P, and up to 80 % for pasture soils (Mclaughlin et al. 1990). In soil in China, inositol phosphates constitutes up to 60 % (mean 40 %) of the total Po content, whereas for soil elsewhere, it may account for up to 80 % of Po (Liu 2001). Different kinds of metal-ion derivatives (phytate) represent a primary component of inositol P in the soil. Though, most phytate phosphates are difficult to become soluble, available Pi for plants remains poorly characterized in soil (Richardson and Hadobas 1997). In order to make phytate available as plant nutrition, it must be hydrolyzed into Pi. This process is carried out with phytase. The major source of phytase in soil is considered to be of microbial origin (Rodriguez and Fraga 1999). Various phytases secreted as extracellular enzymes which are able to degrade specific different substrates have been identified in plant roots and soil microorganisms and are likely more important for the acquisition of P from Po substrates in soil (Richardson 1994). Now, many studies provide evidences to support the role of bacteria in hydrolyzing hard-soluble phytate available to plants (Richardson and Hadobas 1997; Richardson et al. 2001).
Poplar and pine are the major planting tree genera in China. By the end of 2010, poplar plantations have exceeded 7.57 million hectares and pine growing areas made up more than 23 % of total forest area in China. In the past decades, poplar and pine plantations deplete a great amount of soil nutrients and subsequently reduce soil fertility. Because of inadequate supply of P in poplar plantations, synthetic P fertilizers have been always applied for plants in the past century (Tang 2006). However, the indiscriminative and excessive applications of synthetic P have led to the reduction in soil fertility and environmental degradation (Liu et al. 2005). At present, poplar plantations in many Chinese regions have led to soil degradation, which will greatly affect the growth of poplar (Liu et al. 2007). Therefore, microbial P fertilizers have been considered as one of possible approaches to increase the yield of plants and maintain a long-term ecological balance of the soil ecosystem (Patel et al. 2008; Mehrpoyan et al. 2010).
To our knowledge, there are few high phytate-mineralizing rhizobacteria (PDRB), which are highly phytase-active and available to forest industry today. Bacteria with high phytase-activities are required to provide raw materials for the preparation of highly efficient bacterial manure. The objectives of this present study were to isolate, screen and identify rhizobacteria from the natural habitats in eight poplar and pine planting areas in China. Phytase activity and growth-promoting effect on poplar and Masson pine of the screened PDRB were evaluated in order to determine effective strains that might be used as biological phosphate fertilizer in the forest industry to promote plant production.
Materials and methods
Soil samples
A total of 24 soil samples were taken between April and May, 2011 from eight poplar and pine planting regions in four provinces of China. In poplar or pine woods, or at least ensuring no interference from other plants around poplars or pines, dominant trees were selected and surface soil near poplar or pine trees were removed with a shovel at a distance of 15–20 cm from their trunks. Soil samples were collected from a depth of 15–20 cm for obtaining rhizosphere soil. The roots were carefully shaken to separate the soil from the roots. The soil adhering to the roots of poplars or pines was considered to be rhizosphere soil. Then, rhizosphere soil was collected (with three replicates each dominant tree). All soil samples were kept in sterilized paper bags and stored at 4 °C. The basic characteristics of the studied soil samples are listed in Table 1.
Soil analysis
All soil samples (<2 mm) were air-dried for physical and chemical analyses. Soil analysis was routinely done in the Soil Testing Laboratory at Nanjing Forestry University. Chemical analysis, including total P, K, Mg, Ca, Zn, Mn, Cu, Fe, was performed using 9:1 H2SO4–HClO4 (v/v) extract to heat digestion and determined by inductively coupled argon plasma spectrophotometry (ICP-AES Optima 2100DV, Perkin Elmer). Organic matter was determined by the liquid TOC (GE, USA). Soil pHH2O was determined potentiometrically in a 1:2.5 solution (m/v, soil to water) by a pH instrument.
Initial isolation of phytate-degrading rhizobacteria (PDRB)
PDRB were screened following the methods described by Edi Premono et al. (1996) and Liu et al. (2011), with some modification. In brief, 10 g of rhizosphere soil from each sample was aseptically weighed, homogenized in an Erlenmeyer flask with 90 mL sterilized distilled water, and shaken for 30 min at approximate 180 r min−1. After shaking, serial dilutions were immediately prepared, and 0.1 mL aliquots were spread on specific phytate selective solid medium plates (with three replicates each dilution). One liter of medium contained glucose 10.0 g, Phytin 4.0 g, MgCl2 ·6H2O 5.0 g, MgSO4 ·7H2O 0.25 g, KCl 0.2 g, (NH4)2SO4 0.1 g, agar 18.0 g, and distilled water 1,000 mL, 0.1 M NaOH solution was used to adjust the pH to 7.0. Afterward, the Petri dishes were incubated at 28 °C for three days. After the incubation, the plates were examined for the presence of PDRB. Isolates developing clear/halo zone around the colony indicated the presence of PDRB. Then, the ability of the bacteria to degrade insoluble phytin was preliminarily assessed by the mineralization efficiency, expressed as a ratio of the total diameter (colony + halo zone) to the colony diameter, the ratio named HE value. Based on the HE value of each isolate, the best strains of bacteria were selected for showing high phytase activity (Rodriguez and Fraga 1999). Colonies exhibiting different morphological characters were picked up individually and further purified on NA medium plates. After purification, each isolate was stored on NA slant at 4 °C and the isolation result was recorded.
Measurement of enzymatic activity
The initial isolations were inoculated in 50 mL of liquid phytate selective medium without agar and were cultured in a rotary shaker (180 rpm) at 28 °C for 3 days. Culture supernatants, namely crude enzyme fluid were collected via centrifugation (10,000g, 15 min) from the bacterial culture. Then, the supernatant was stored at 4 °C for phytase activity assay (Quan et al. 2001a).
Phytase activity was determined by measuring the amount of liberated inorganic phosphate of phytaye degradation using culture supernatant following the protocol described by Quan et al. (2001b) with a minor modification. The reaction mixture consisted of 1 mL acetate buffer (0.2 M, pH 5.5 containing 1 mM sodium phytate) and 2 mL of culture supernatant (2 mL of autoclaved un-inoculated medium was used as the control treatment). After incubation for 30 min at 37 °C, the reaction was stopped by adding 1 mL of 10 % tricholoroacetic acid. Free phosphate was determined by a modified colorimetric molybdate blue method of Olsen and Sommers (Olsen and Sommers 1982). One unit (U) of phytase activity was defined as the amount of enzyme releasing 1 μmol of Pi equivalent per minute.
Bacterial inoculum preparation
Three strains with the highest phytase activity were incubated for 24 h in NB medium, and 100 μL aliquots of them were respectively transferred into 100 mL flasks containing 50 mL of NB medium. Flasks were cultured in an orbital shaker (180 rpm) at 28 °C. One liter of NB medium contained peptone 10.0 g, beef extract 3 g, NaCl 5.0 g, distilled water 1,000 mL. Until OD600 reached 1.0, bacterial cells were harvested by centrifuging at 10,000×g for 10 min, and the pellet was resuspended in sterilized distilled water. Then the supernatants were adjusted to 107–108 cfu/mL. The cell suspensions were stored at 4 °C and used for the follow-up experiment.
Soil–plant experiment (container growing)
Soil without any fertilization from the Purple Mountain near Nanjing Forestry University, China was selected because of its naturally low effective P content and low level of organic matter. The main characteristics of soil were: pH 5.59; organic content 0.75 g/kg. After surface sterilization, poplar (Populus euramericana San Martino) and Masson pine (Pinus massoniana Lamb.) seeds were planted individually in plant growth containers which consisted of 400 g of a mixture 1:1 (w/w) soil–sand (autoclaved at 121 °C for 90 min to eliminate the native microflora). Plants were grown at 25 °C in a plant growth chamber with 16-h light and 8-h dark and soil moisture was kept between 40 and 60 % of maximum water holding capacity. After 90 days, these container-grown seedlings with a consistent growth were used for treatment. Each seedling’s height (H1) and ground diameter (D1) were measured. Fifteen mL bacterial suspensions were carried into around seedling roots. Seedlings inoculated with 15 mL sterile water alone served as control treatment. Eight replicates were used for each treatment. One hundred and fifty days after the inoculation of bacterial suspensions, heights (H2) and ground diameters (D2) of the seedlings were determined again. The increase rates of heights and ground diameters were calculated by using the follow formula. Increase rate (%) = [H2(D2) − H1(D1)]/H1(D1) × 100. In this experiment, variables were strictly controlled, all materials (the soil and growing conditions) were the same except the the bacteria inoculated.
16S rDNA gene sequencing and phylogenetic analysis
The three selected bacterial strains exhibiting highest phytase ability were characterized by 16S rDNA gene sequence analysis. Genomic DNA of each PDRB strain was extracted and purified according to the method described by Ren et al. (2011). The fragments of 16S rDNA were amplified with the primers: fD1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and rP2 (3′-ACGGCTACCTTGTTACGACT-5′). The PCR amplification was performed as follows: one cycle of 5 min at 94 °C, followed by 30 cycles of 30 s at 94 °C, 30 s at 56 °C, and 1 min at 72 °C, followed by one cycle of 5 min at 72 °C, and then the PCR products were purified and sequenced at the Invitrogen Corporation in Shanghai. 16S rDNA gene sequences of the isolate were compared with 16S rDNA gene sequences available by the BLASTN search in the NCBI, GenBank database (http://www.ncbi.nlm.nih.gov). Multiple sequence alignment was performed using ClustalW, phylogenetic dendogram was constructed by the neighbour-joining method and tree topologies were evaluated by performing bootstrap analysis of 1,000 data sets using MEGA 4.0 (Molecular Evolutionary Genetic Analysis 4.0).
Identification with Biolog Identification System
Three bacterial strains with the highest phytase ability in vitro were tested for C-source utilization pattern and identified using Biolog Identification System. The Biolog GENIII micro plate analyzes a microorganism in 94 phenotypic tests: 71 carbon source utilization assays and 23 chemical sensitivity assays. The test panel provides a phenotypic fingerprint of the microorganism which can be used to identify it at species level. Specific steps refer to the instructions of Biolog Identification System. Briefly, fresh purified cultures were incubated on Biolog Universal Growth (BUG™) Agar medium and 24-h-old cultures were inoculated into inoculation fluid by using sterile cotton swabs. Turbidity of the inoculants was adjusted to 90 % for Inoculation Fluid A (IF-A) by using turbidity meter. The microbial suspensions of 100 μL were inoculated into each well of GENIII micro plate using 8-channel repeating pipette. Plates were incubated at 28 °C and recorded color development at intervals of 12 h for 12–48 h using a micro plate reader (Model EL311, BioTek Instruments, USA) with 590 nm wavelength. Finally readings of the color development of the three plates were compared with Biolog database (GENIII Database, Release 6.0) and isolates were identified at species level.
Biological characterization of the three efficient PDRB
The biological characteristics of PDRB isolates were tested according to the methods described in Bergey’s Manual of Determinative Bacteriology, 8th Edition (Holt et al. 1994), including colony and cell morphology, flagella, spore, capsule production, Gram staining, KOH test. All staining results were observed using a Leica microscope under a 100 × oil lens (Leica, Wetzlar, Germany). All tests were repeated at least three times for each isolate to assess the reliability of the test results.
The cell morphology of the cultured bacterial strains was observed by electron microscopy. For scanning electron microscopy (SEM), PDRB colonies, which had been incubated on NA culture medium for three days, were fixed with glutaraldehyde. After rinsing several times in Na-cacodylate buffer solution, specimens were post fixed for 4.5 h in 1 % osmium tetroxide at 4 °C and washed in Nacacodylate buffer solution. Dehydration through a graded series of ethanol solutions and 100 % acetone was lastly followed by critical point drying with liquid CO2 (EMITECH K850). The specimens were subsequently mounted on stubs, sputtered with gold (HITACH E-1010) and examined using a scanning electron microscope (QUANTA 200, FEI, USA).
Autofluorescence detection of the three best PDRB strains
In order to detect the fluorescent bacteria, the three best PDRB strains were cultured on King’s B medium (King et al. 1954). After three days, isolates that could be observed a change at the medium surrounding the bacterial growth to fluorescent blue under the excitation of UV light were considered positive (Schwyn and Neilands 1987). Meanwhile, the isolated strains were collected from NA culture medium for three days, spread in one drop of sterilized distilled water on a slide glass, and air dried. Visualization was performed using a Leica DM5000B fluorescent microscope with an FITC filter (maximum excitation = 490 nm and maximum emission = 520 nm green fluorescence) (Leica, Wetzlar, Germany).
Data analysis and photomicrographs and photos preparation
Dates in the study (three or more replicates for each treatment) were expressed as mean ± standard deviation (SD). Data analysis was carried out using SPSS (SPSS Version 18.0, SPSS Inc.). All dates reported were analyzed by one-way analysis of variance and Tukey’s HSD post hoc test. Differences with P ≤ 0.05 were considered significant. Photomicrographs and photos were prepared using Adobe Photoshop CS 5.0 software.
Results
Soil analysis
The soil analysis results showed as the following parameters (Table 1). The organic matter content of the soil samples collected from Nanning, Dezhou and Zhenjiang, were more than 55 mg/kg and those of the four soil samples from Nanjing were 25–39 mg/kg, including Purple Mountain, Nanjing Forestry University, Jiangsu Academy of Forestry (poplar) and Jiangsu Academy of Forestry (pine). The lowest one was Huangshan Mountain soil samples with organic matter 22.1 mg/kg. The total P content from soil samples collected from Nanning was the highest, 29.6 mg/kg and the next ones were those from Dezhou and Zhenjiang. The remaining soil samples were obviously lower than the ones of aforementioned samples. The total P content in soil sample from Purple Mountain was the lowest, 3.2 mg/kg. Among the determined metal elements, the contents of K, Mn were close in all samples except one from Purple Mountain and the other metal elements (Ca, Mg, Fe) varied widely. The pH range of soil samples was 4.2–6.9.
Initial isolation of phytate-degrading rhizobacteria
Seventeen strains that can solubilize Phytin and form peripheral halo zone on phytase specific agar medium around colonies were isolated from soil samples and named as in Table 2. Total 17 strains of PDRB were obtained preliminarily. Respectively, six promising strains of PDRB were isolated from Guangxi Academy of Forestry soil, from which the most isolates were got among these 24 soil samples, the next were the soil samples from Lingxian and Shiye, four promising strains were gained. A strain with higher HE value is, as a role, a strain having stronger phytate degradation potential based on the results of plate screening, JZ-GX1, JZ-DZ1 and JZ-ZJ1 were better strains. Meanwhile, JZ-GX1 was the best strain and could produce approximately 22.52 mm clear halo around colony and the HE value was up to 3.85, followed by JZ-DZ1 and JZ-ZJ1 (Table 2; Fig. 1).
Phytase activity of the 17 initial isolations
Phytase activity is an important index for degradation potential evaluation, the higher the phytase activity, the stronger the degradation potential. Phytase assay used for quantitative screening (Fig. 2) also showed JZ-GX1, JZ-DZ1 and JZ-ZJ1 exhibited the highest phytase activity in the supernatants, up to 2.58, 2.36 and 2.24 U/mL, respectively. They were significantly higher (P ≤ 0.05) than the other PDRB strains. JZ-GX1 was found to be the most efficient strain.
Effect of inoculating PDRB on the growth of poplar and Masson Pine
Growth accumulation of poplar and Masson pine seedlings inoculated with bacterial suspension of JZ-GX1, JZ-DZ1 and JZ-ZJ1 planted in soil for 150 days were depicted in Table 3 and Fig. 3. There were highly significant effects both on heights and ground diameters of the seedlings among all the three inoculations (Table 3). Compared with CK, the best effect of growth promotion was found in inoculation with JZ-GX1, which could efficiently promote poplar and Masson pine growth. To poplars, increase rates of height and ground diameter were up to 368.42, 177.82 % by inoculating with bacterial suspension of JZ-GX1, to Masson pines, which were 68.92, 96.20 %, respectively. Followed by JZ-DZ1 and JZ-ZJ1, both in poplars and Masson pines, they were much better than CK. It was also found that the increase rate of height was significantly higher than ground diameter’s in poplar, but it did the reverse in Masson pine.
Identification of the three efficient PDRB strains by phylogenetic analysis
Total DNA of each strain was extracted. As the template, each strain’s 16SrDNA were used for PCR amplification with bacterial universal primer. Electrophoresis was used to detect amplified fragment and the result of electrophoresis was showed that at approximate 1,500 bp, there were three obvious and stabilized bands, which conformed to expected DNA bands. The sequences of the three efficient strains were deposited in GenBank nucleotide sequence database, their accession numbers are as follow: JZ-GX1, KC351183, JZ-DZ1, KC351184, JZ-ZJ1, KC351185.
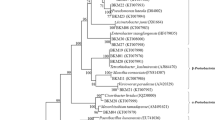
The identification based on 16S rDNA sequences and their phylogeny was presented in Fig. 4. The 16S rDNA gene sequences of the three PDRB strains exhibited that JZ-GX1 had a high confidence value 95 % with Rahnella aquatilis, both JZ-DZ1 and JZ-ZJ1 had 94, 99 % confidence to Pseudomonas fluorescens, respectively.
Neighbour-joining tree of JZ-GX1, JZ-DZ1 and JZ-ZJ1 based on 16S rDNA sequences. It showed the phylogenetic relationship between the best three strains and other representative bacterial strains. Bootstrap values (50 %) based on 1,000 replications were shown at nodes of the tree. Bar was 0.02 substitutions per nucleotide position (values <50 not included)
BIOLOG identification of bacterial isolates
Based on the capability of consuming carbon-based nutrition determined by Biolog Identification System, JZ-GX1 was identified as R. aquatilis and both JZ-DZ1 and JZ-ZJ1 were identified as P. fluorescens (Table 4). In general, in every index, when the biolog similarity is ≥0.5, the identification is credible. In this Biolog identification, the biolog similarities were up to 0.758, 0.697, and 0.647, respectively, which were significantly higher than 0.5. Thus, the identification results were reliable. And it was consistent with the 16S rDNA gene sequencing results.
Biological characterization of the three efficient PDRB strains
Strains JZ-GX1, JZ-DZ1, and JZ-ZJ1 were incubated on NA medium, their colony characteristics were recorded and photographed after three days (Table 5; Fig. 5a–c).
Biological characterization of the three efficient PDRB strains. Colony characteristics of JZ-GX1 (a), JZ-DZ1 (b), JZ-ZJ1 (c), the three strains were incubated on NA medium for three days and photographed under the same magnification. SEM microphotographs of cell morphology of the three efficient PDRB strains, d JZ-GX1, ×20,000, e and f JZ-DZ1 and JZ-ZJ1, respectively, ×10,000. The results of staining and aerobism experiments, g and i JZ-GX1 and JZ-ZJ1 with a single polar flagellum, h JZ-DZ1 with multiple polar flagella, Scale bar = 5 μm. j The capsule staining of JZ-DZ1, the white arrow pointed to capsule. Three strains were all aerobic bacteria, k JZ-GX1, l. JZ-ZJ1, m JZ-DZ1
Cell morphology and size of each strain could be clearly observed by scanning electron microscopy (Fig. 5d–f). The cells of JZ-GX1, JZ-DZ1 and JZ-ZJ1 were all rod-shaped, the cell size of JZ-GX1 was the smallest, 0.3–1.0 × 0.1–0.2 μm, JZ-DZ1, JZ-ZJ1 were 0.8–1.3 × 0.4–0.5 μm, 1.0–1.3 × 0.4–0.5 μm in size, respectively.
Through spore staining, flagella staining and capsule staining, the biological characteristics of the three efficient strains had been understood more profoundly. All three strains did not form spores, but were motile. JZ-GX1 and JZ-ZJ1 had a single long polar flagellum. JZ-DZ1 had long multiple polar flagella (Fig. 5g–i arrows showed). JZ-DZ1 was the only one with a discernible capsule (Fig. 5j white arrow showed). The results of aerobism experiments showed that all the three strains were oxic bacteria, their growth without cover glass were better than these under cover glass (Fig. 5k–m).
Auto-fluorescence detection of the three best PDRB strains
In the three efficient PSB strains, only JZ-DZ1 and JZ-ZJ1 showed positive for fluorescence on King’s B solid medium (Fig. 6a–b), while the auto-fluorescence can be detected in single cell of the two strains from fluorescence microscope by Leica DM5000B (Fig. 6c–d).
Up to this point, the biological and auto-fluorescence characterizations of the three best isolates were consistent with the identification results of 16S rDNA gene sequencing and Biolog Identification System. The three best isolates were correctly identified. JZ-GX1 was R. aquatilis. Both JZ-DZ1 and JZ-ZJ1 were P. fluorescens.
Discussion
Several studies have reported the isolation of phytate degrading bacteria from the rhizospheric soil (Hussin et al. 2007; Baharak et al. 2009; Jorquera et al. 2011), but few reported in China. This study showed not only the occurrence of phytate degrading bacteria in the poplar or pine rhizosphere from different ecological places in China, but also the differences in their phytate degrading abilities. These differences might be related to the soil region, soil type, soil nutrient, and the age of the plants (Tables 1). It has been reported that the percentage of PDRB may be affected by soil organic matter, fertility (Liu et al. 2011), types (Reyes et al. 2006), the ways of farming (Lin et al. 2000), and the age of plants (Baby et al. 2001). In this study, we obtained more efficient isolates from these soil samples collected from Nanning, Zhenjiang and Dezhou, with higher contents of organic matter, total P, Ca, Mg and lower Fe ingredient, pH were 6.7–6.8 (Table 1).
Strains of PDRB were routinely screened by a plate assay method, in which insoluble phytate (e.g. phytin phosphate)was used as the only sources of phosphorus. The strains which can produce halo/clear zone around the colonies are selected (Rodriguez and Fraga 1999). However, the reliability of this screening method is not absolutely credible all the time, as some strains that have lower HE value, but higher phytase activity (Table 2; Fig. 2), such as JZ-ZJ3, JZ-GX5, and JZ-GX6. The reasons for this phenomenon may due to the fact that some bacteria in the medium containing glucose can produce a large number of organic acids, which cannot be diluted immediately, and also can produce transparent rings around the colonies. So they lead to misperception which makes flat initial screening method lack of specificity and with reduced sensitivity. Thus, it is necessary to use the liquid phytate specific medium to assay phytase activity of the initially screened strains as the second screening. In this study, 17 strains showed reproducibly phytate degrading ability on phytate specific medium. Three of them (JZ-GX1, JZ-DZ1, JZ-ZJ1) showed a greater phytate degrading capacity and phytase activity (2.58, 2.36 and 2.24 U/mL respectively) than the other PDRB strains under in vitro conditions. Which were much better than most of the other reported bacterial strains (Edi Premono et al. 1996; Hussin et al. 2007; Gulati et al. 2007).
At present, Biolog Identification System and 16S rDNA gene sequencing technology have become the simple, rapid technical methods for bacteria classification and identification field on an international scale (Barbieri et al. 2000; Unno et al. 2005). In this study, we combined Biolog Identification System and 16S rDNA gene sequencing technology to systematically identify the three PDRB in order to ensure the reliability of our results. Results of the two methods were completely consistent. Therefore, the identification results were conclusive. At the same time, through the traditional tests, bacterial structures (flagella, capsule) and characters (aerobism, autofluorescence) of the three best strains were characterized well. In addition, all the morphology, biological, and auto-fluorescence characterizations of the three strains were consistent with the identification results, which made the identification results more reliable.
Numerous reports indicated that species of the genera, Bacillus (Choi et al. 2001), Citrobacter (Kim et al. 2003), Enterobacter (Yoon et al. 1996), Escherichia (Golovan, 2000), Klebsiella (Sajidan et al. 2004), Lactobacillus (De Angelis et al. 2003), Megasphaera (Yanke et al. 1998), Mitsuokella (Yanke et al. 1998), Prevotella (Yanke et al. 1998), Pseudomonas (Richardson and Hadobas 1997), Selenomonas (Yanke et al. 1998) etc. possess the ability of solubilizing and mineralizing phytate. In relation to PDRB characterized as Pseudomonas, members of this genus had been isolated from the rhizosphere soil and their capacity to degrade Phytate had been estimated, for example P. fluorescens (Patel et al. 2010; Park and Cho 2011). P. fluorescens is an important plant growth promoting rhizobacterium, which is known as one of plant beneficial rhizosphere microorganism species having larger populations in soil (Hayat et al. 2010). P. fluorescens strains need relatively simple nutrition, they are able to use most root exudates nutrition to rapidly colonize plant rhizosphere (Pineda et al. 2010). The strains play roles to promote plant growth and to improve resistance to diseases (García et al. 2012). In the present study, a higher number of identified efficient isolations were P. fluorescens. The result was consistent with previous reports. Furthermore, in present study, Rahnella strain JZ-GX1 (R. aquatilis) showed the highest phytase activity and efficiently degraded phytate among all isolation strains. In previous literature, it was reported that R. aquatilis had a suppressive effect on the parasitic nematode Xiphinema index of grapevine (Aballay et al. 2012), it was a plant growth-promoting, disease-suppressive rhizobacterium (Guo et al. 2012). However, there were few related studies reported that the strains of R. aquatilis were able to efficiently degrade (mineralize) phytate, this study is a supplement of the plant rhizosphere phosphate-solubilizing microorganisms species.
Application of bacterial inocula as bio-fertilizers has been reported to improve plant growth and increase yields of plants (Compant et al. 2005; Mehrpoyan et al. 2010). In this study, the beneficial effect of the three efficient PDRB (R. aquatilis JZ-GX1, P. fluorescens JZ-DZ1 and JZ-ZJ1) on poplar and pine growth under container growing was evaluated, and all of them could significantly promote poplar and Masson pine growth. It could be owned to their phytate-degrading capacity to promote P nutrition uptake and plant growth in soils with low content of available phosphorus. So, these three effective PDRB strains would have great potential application as biological phosphate fertilizer in the future. Caution should be taken. Before developing these strains as bio-fertilizer in forest industry in the future, their biological safety must be evaluated and only the strains safe to human beings, animals, and other organisms in the environments can be used in agricultural production.
More research should be conducted to determine their optimal fermentation conditions and the beneficial effects on plant growth under field conditions. At the same time, their mechanisms on plant growth promotion could be further studied.
References
Aballay E, Prodan S, Martensson A, Persson P (2012) Assessment of rhizobacteria from grapevine for their suppressive effect on the parasitic nematode Xiphinema index. Crop Prot 42:36–41
Alam MM, Ladha JK (2004) Optimizing phosphorus fertilization in an intensive vegetable-rice cropping system. Biol Fert Soil 40:277–283
Baby U, Tensingh I, Baliah N, Ponmurugan P, Premkumar R (2001) Population dynamics of nitrogen fixing and phosphate solubilizing bacteria in tea soil. UPASI Tea Found Inst Newslett 10:8–10
Baharak HK, Giti E, Iraj N (2009) Analysis of phytase producing bacteria (Pseudomonas sp.) from poultry faeces and optimization of this enzyme production. Afr J Biotechnol 8:4229–4232
Barbieri E, Potenza L, Rossi I, Sisti D, Giomaro G et al (2000) Phylogenetic characterization and in situ detection of a Cytophaga-Flexibacter-Bacteroides phylogroup bacteriumin Tuber borchii Vittad. ectomycorrhizal mycelium. Appl Environ Microb 66:5035–5042
Batjes NH (1997) A world data set of derived soil properties by FAO-UNESCO soil unit for global modeling. Soil Use Manag 13:9–16
Choi YM, Suh HJ, Kim JM (2001) Purification and properties of extracellular phytase from Bacillus sp. KHU-10. J Protein Chem 20:287–292
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microb 71:4951–4959
De Angelis M, Gallo G, Corbo MR, McSweeney PLH, Faccia M et al (2003) Phytase activity in sourdough lactic acid bacteria: purification of a phytase from Lactobacillus sanfranciscensis CB1. Int J Food Microbiol 87:259–270
Edi Premono J, Moawad AM, Vlek PLG (1996) Effect of phosphate solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indones J Crop Sci 11:13–23
García GL, Romero D, Zeriouh H, Cazorla FM, Torés JA et al (2012) Isolation and selection of plant growth-promoting rhizobacteria as inducers of systemic resistance in melon. Plant Soil 358:201–212
Golovan S, Wang G, Zhang J, Forsberg CW (2000) Characterization and overproduction of the Escherichia coli appA encoded bifunctional enzyme that exhibits both phytase and acid phophatase activities. Can J Microbiol 46:59–71
Gulati HK, Chadha BS, Saini HS (2007) Production and characterization of thermostable alkaline phytase from Bacillus laevolacticus isolated from rhizosphere soil. J Ind Microbiol Biotechnol 34:91–98
Guo YB, Jiao ZW, Li L, Wu D, Crowley DE, Wang YJ, Wu WL (2012) Draft genome sequence of Rahnella aquatilis Strain HX2, a plant growth-promoting rhizobacterium isolated from vineyard soil in Beijing, China. J Bacteriol 194:6646–6647
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60:579–598
Holt JG, Kreig NR, Sneath PHA, Staley JT, Williams ST (1994) Bergey’s manual of determinative bacteriology. Lippincott Williams and Wilkins, Baltimore
Hussin ASMH, Farouk AE, Greiner R, Salleh HM, Ismail AF (2007) Phytate-degrading enzyme production by bacteria isolated from Malaysian soil. World J Microbiol Biotechnol 23:1653–1660
Jorquera MA, Crowley DE, Marschner P, Greiner Fernández MT et al (2011) Identification of β-propeller phytase-encoding genes in culturable Paenibacillus and Bacillus spp. from the rhizosphere of pasture plants on volcanic soil. FEMS Microbiol Ecol 75:163–172
Kim HW, Kim YO, Lee JH, Kim KK, Kim YJ (2003) Isolation and characterization of a phytase with improved properties from Citrobacter braakii. Biotechnol Lett 25:1231–1234
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med 44:301–307
Lewis DC, Sale PWG (1993) Management of nutrients for pastures. In: Kemp DR, Michalk DL (eds) Pasture management technology for the 21st century. CSIRO Publishing, Melbourne, pp 38–50
Lin QM, Zhao XR, Sun YX, Yao J (2000) Community characters of soil phosphobacteria in four ecosystems. Soil Environ Sci 9:34–37
Liu YZ (2001) Soil nutrients. In: Xiong SG, Wang YT, Cui DJ (eds) Fundamental soil science. China Agricultural University Press, Beijing, pp 219–222
Liu FD, Jiang YZ, Wang HT, Kong LG, Wang Y (2005) Effect of continuous cropping on poplar plantation. J Soil Water Conserv 19:102–105
Liu FD, Jiang YZ, Wang HT, Wang Y, Kong LG (2007) Soil productivity maintenance technique of poplar plantation under continuous cropping. Sci Silvae Sin 43:58–64
Liu H, Wu XQ, Ren JH, Ye JR (2011) Isolation and identification of phosphobacteria in poplar rhizosphere from different regions of China. Pedosphere 21:90–97
Mclaughlin MJ, Alston AM, Martin JK (1988) Phosphorus cycling in wheat-pasture rotations. II. The role of the microbial biomass in phosphorus cycling. Aust J Soil Res 26:333–342
Mclaughlin MJ, Baker TG, James TR, Rundle JA (1990) Distribution and forms of phosphorus and aluminium in acidic topsoil under pastures in south-eastern Australia. Aust J Soil Res 28:371–385
Mehrpoyan M, Jaefari A, Askari O (2010) Agronomic benefits of PGRPs on grain yield and nutrients uptake in different cultivars of common bean (Phaseolus vulgaris L.) to reduce the chemical fertilizers. Proceedings of 2010 international conference on environmental science and development, pp 207–211
Olsen SR, Sommers LE (1982) Phosphorus. In: Miller RH, Keeney DR (eds) Methods of soil analysis. American Society of Agronomy, Madison, pp 403–430
Park I, Cho J (2011) The phytase from antarctic bacterial isolate, Pseudomonas sp. JPK1 as a potential tool for animal agriculture to reduce manure phosphorus excretion. Afr J Agr Res 6:1398–1406
Patel MR, Sadhu AC, Patel JC (2008) Effect of irrigation, nitrogen and bio-fertilizer inoculation on N, P and K content and uptake of forage oat (Avena sativa L.). Res Crops 9:544–546
Patel KJ, Vig S, Nareshkumar G, Archana G (2010) Effect of transgenic rhizobacteria overexpressing Citrobacter braakii appA on phytate-P availability to mung bean plants. J Microbiol Biotechn 20:1491–1499
Pineda A, Zheng SJ, van Loon JJ, Pieterse CMJ, Dicke M (2010) Helping plants to deal with insects: therole of beneficial soil-borne microbes. Trends Plant Sci 15:507–514
Quan CS, Fan SD, Zhang LH, Wang YJ, Ohta Y (2001a) Purification properties of a phytase from Candida krusei WZ-001. J Biosci Bioeng 94:419–425
Quan CS, Zhang LH, Wang YJ, Ohta Y (2001b) Production of phytase in a low phosphate medium by a novel yeast candida krusei. J Biosci Bioeng 92:154–160
Ren JH, Ye JR, Liu H, Xu XL, Wu XQ (2011) Isolation and characterization of a new Burkholderia pyrrocinia strain JK-SH007 as a potential biocontrol agent. World J Microbiol Biotechnol 27:2203–2215
Reyes I, Valery A, Valduz Z (2006) Phosphate-solubilizing microorganisms isolated from rhizospheric and bulk soil of colonizer plants at an abandoned rock phosphate mine. Plant Soil 287:69–75
Richardson AE (1994) Soil microorganisms and phosphorus availability. In: Pankhurst CE, Doube BM, Gupta VVSR, Grace PR (eds) Soil biota management in sustainable farming systems. CSIRO Publishing, Melbourne, pp 50–62
Richardson AE (2001) Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust J Plant Physiol 28:897–906
Richardson AE, Hadobas PA (1997) Soil isolates of Pseudomonas sp. that utilize inositol phosphates. Can J Microbiol 43:509–516
Richardson AE, Hadobas PA, Hayes JE, Hara JE, Simpson RJ (2001) Utilization of phosphorus by pasture plants supplied with myo-inositol hexaphosphate is enhanced by the presence of soil microorganisms. Plant Soil 229:47–56
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Sajidan A, Farouk A, Greiner R, Jungblut P, Müller EC, Borris R (2004) Molecular and physiological characterization of a 3-phytase from the Rhizobacterium Klebsiella pneumonia ASRI. Appl Microbiol Biotechnol 65:110–118
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Tang J (2006) Studies on promotion of bacterial fertilizers on poplar growth and its mechanism. Doctoral dissertation, Chinese Academy of Forestry
Unno Y, Okubo K, Wasaki J, Shinano T, Osaki M (2005) Plant growth promotion abilities and microscalebacterial dynamics in the rhizosphere of Lupin analysed by phytate utilization ability. Environ Microbiol 7:396–404
Vance CP (2001) Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol 127:390–397
Yanke LJ, Bae HD, Selinger LB, Cheng KJ (1998) Phytase activity of anaerobic ruminal bacteria. Microbiology 144:1565–1573
Yoon SJ, Choi YJ, Min HK, Cho KK, Kim JW et al (1996) Isolation and identification of phytase producing bacterium, Enterobacter sp., and enzymatic properties of phytase enzyme. Enzyme Microbiol Technol 18:449–454
Acknowledgments
We are indebted to Chinese Special Research Program for Forestry Sectors Beneficial to Public (No. 201004061), the Program for Science and Technology Development of Jiangsu Province in China (No. BE2008393), and a Project Founded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) for the financially supports. We also sincerely thank Dr. De-Wei Li for reviewing the manuscript, Shi-Bo Ma for preparing the photographs with us.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, GE., Wu, XQ., Ye, JR. et al. Isolation and identification of phytate-degrading rhizobacteria with activity of improving growth of poplar and Masson pine. World J Microbiol Biotechnol 29, 2181–2193 (2013). https://doi.org/10.1007/s11274-013-1384-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1384-3