Abstract
The persistence of propanil in soil and aquatic environments along with the possible accumulation of toxic degradation products, such as chloroanilines, is of environmental concern. In this work, a continuous small-scale bioprocess to degrade the herbicide propanil, its main catabolic by-product, 3,4-dichloroaniline (3,4-DCA), and the herbicide adjuvants is carried out. A microbial consortium, constituted by nine bacterial genera, was selected. The isolated strains, identified by amplification and sequencing of their 16S rDNA, were: Acidovorax sp., Luteibacter (rhizovicinus), Xanthomonas sp., Flavobacterium sp., Variovorax sp., Acinetobacter (calcoaceticus), Pseudomonas sp., Rhodococcus sp., and Kocuria sp. The ability of the microbial consortium to degrade the herbicide was evaluated in a biofilm reactor at propanil loading rates ranging from 1.9 to 36.8 mg L−1 h−1. Complete removal of propanil, 3,4-DCA, chemical oxygen demand and total organic carbon was obtained at propanil loading rates up to 24.9 mg L−1 h−1. At higher loading rates, the removal efficiencies decayed. Four of the identified strains could grow individually in propanil, and 3,4-DCA: Pseudomonas sp., Acinetobacter calcoaceticus, Rhodococcus sp., and Xanthomonas sp. The Kokuria strain grew on 3,4-DCA, but not on propanil. The first three bacteria have been related to biodegradation of phenyl urea herbicides or chlorinated anilines. Although some strains of the genera Xanthomonas and Kocuria have a role in the biodegradation of several xenobiotic compounds, as far as we know, there are no reports about degradation of propanil by Xanthomonas or 3,4-DCA by Kocuria species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Propanil (3,4-dichloropropananilide; N-(3,4-dichlorophenyl) propamide; 3,4-DCPA) is an anilide herbicide. Its main use is for the control of broad-leaved and grass weeds in rice, and its mode of action is via the inhibition of Hill reaction in photosynthetic electron transfer (photosystem II [PSII]) (Sahid et al. 2011). As other herbicides, propanil affects non-target organisms and presents severe risks for birds, small mammals, and several species of the aquatic biota (Galhano et al. 2011); macro invertebrates (Taylor et al. 1994), plankton communities (Jak et al. 1998), and fishes (Call et al. 1987). In the marine environment, PSII herbicides used in agriculture affect corals and their symbiotic dinoflagellate algae. The herbicide penetrates the coral tissues reducing the photochemical efficiency of the algal symbionts (Jones 2005).
Propanil is an unstable compound, its photodegradation in water and soil leads to the accumulation of 3,4-dichloroaniline (3,4-DCA), which is more toxic than the herbicide (Wendel and Mason 2009). 3,4-DCA is not a readily biodegradable or hydrolysable compound, and it usually accumulates in soils and water by the catabolism of propanil or chlorinated phenylamide herbicides, such as diuron and linuron. Under anaerobic conditions, slow degradation to more refractory monochloroanilines takes place (Hund-Rinke and Simon 2005), and projections of mineralization rates observed in short-term experiments indicate that the residual life in soil of herbicide-derived chloroanilines may extend for up to 10 years (Bartha 1971).
Because of propanil ecotoxicity, its water solubility (130–500 mg L−1 at 25 °C) (Mackay et al. 2006), its persistence in soil and aquatic environments (Danchour et al. 1986), and the possible accumulation of the toxic by-product 3,4-DCA, the complete removal of both compounds from contaminated sites is of environmental concern. The removal of non point source pollutants such as agrochemicals that affect soils and water bodies is difficult. To facilitate their removal, the use of wetlands, runoff holding ponds, or permeable reactive barriers could be a suitable technical choice.
For propanil degradation, photocatalytic and electrochemical oxidation techniques could be used (Garrido et al. 2001; Konstantinou et al. 2001). Physicochemical processes are useful to degrade organic compounds, principally when they are concentrated, but for degradation of micropollutants, these methods could be uneconomic. For micropollutant degradation biological treatments have been recommended (Carvalho et al. 2010; Correa and Steen 1995). In case of agrochemical pollutants, a practical solution to remove them from water could be the use of specialized microbial consortia attached to porous material acting as a permeable reactive barrier.
Besides the active compounds, the inclusion of additives considered inert in commercial formulation of pesticides has become a common practice in their production. However, the full extent of the toxicity of the adjuvants used in pesticide formulations to aquatic life has been rarely assessed (Eke et al. 1996). Adjuvants include chemicals with diverse functions in the pesticide formulation. For example, wetting and dispersing agents such as polyoxethylated alkylphenols, polyoxethylated fatty alcohols, tridecyl alcohol polyglycol ether, alkylphenylsulfonates, salts of ligninsulfonic acid, dibutylnaphthalenesulfonic acid, swelling polysaccharides, defoamers including tributylphosphate or dialkylpolysiloxanes, antifreeze agents such as ethyleneglycol, propyleneglycol, or glycerol, oligoester surfactants, polyols such as sorbitol, maltitol, isomaltitol, or lactitol, and preservation agents such as benzoic acid, or formaldehyde (Albrecht and Frisch 1989; Bevinakatti and Waite 2012). Beside pesticides, diverse adjuvants have been found in groundwater and top soils (Hildebrandt et al. 2007). These chemicals may have various toxicological profiles, ranging from possible harmless products, to others that could represent a serious toxicological threat (Cox and Surgan 2006; Tobiassen et al. 2003). Although a pure microbial strain would be able to metabolize a mixture of carbon sources such as propanil and adjuvants, a mixed microbial community would be more reliable to metabolize mixed substrates.
Hence, in this work, a continuous small-scale bioprocess to degrade propanil, its main catabolic by-product, 3,4-dichloroaniline, as well as the adjuvants of the herbicide, using a microbial consortium attached to a porous support, is proposed.
Materials and methods
Herbicide
The main substrate used in this study was a commercial presentation of propanil (N-(3,4-dichlorophenyl) propanamide) provided by Bayer Crop Science, named Surcopur 360 CE (Bayer México). It contains 35.6 % of propanil, and 64.4 % of organic and inorganic adjuvants.
Standards of propanil (PS-356) and 3,4-dichloroaniline (F2506) were provided by Chem Service Inc., PA USA.
Culture media
During the experimental process, a minimal-mineral-salts medium (MS) was prepared, and its formulation was (in g L−1); K2HPO4, 0.4 and MgSO4, 0.1. Five millilitre of an oligo-elemental solution (in mg L−1, FeSO4 7H2O, 2.0; MnSO4·H2O, 0.20; Na2MoO4·2H2O, 0.10; CuSO4, 0.20; H3BO3, 0.02) were added to reach the final concentration.
MS medium plus 50 ppm of equivalent propanil (MSP) was used as a selective medium for microorganisms able to degrade propanil; for the propagation of the inoculum, and as the medium supplied to the biofilm reactor.
MSP-Agar (2 %) was used as selective medium for maintenance of the bacterial consortium and the isolated bacterial strains.
Peptone-yeast extract Agar medium (PY) was used to observe the cultivable microbial diversity.
Selection of a microbial consortium able to degrade propanil
Samples of agricultural soils from the states of Veracruz and Tabasco, México, were used to isolate a microbial community able to use propanil as nitrogen source. A continuous microbial enrichment in a small fixed bed continuous selector was used for microbial enhancement and selection. The selector was packed with alternating layers of soil (≈0.5 cm) and porous support (≈2.5 cm). The selector was filled with MS-propanil medium (V L = 350 mL) and batch-cultivated for 7 days; then, the same medium was supplied at a flow rate of 14 mL h−1. The propanil concentration in the outflowing liquid was evaluated periodically. After 8 weeks of continuous operation, the propanil concentration diminished markedly. Afterward, the selector was drained and dismantled. The porous stone fragments were separated and used to inoculate a fixed bed bioreactor. Samples of stone fragments colonized with the enriched microbial community were cryopreserved in a glycerol-water mixture (70:30) at −80 °C for future use.
Identification of the cultivable strains found in the microbial consortium
Bacterial colonies showing morphological differences in PY agar plates were propagated in liquid PY medium for 24 h. Using the primers U968 and L1401 (Felske et al. 1996), the DNA extracted and purified from recovered cells was PCR-amplified. DNA amplicons of about 500 bp of 16S rDNA were purified and sequenced at the Laboratory of Molecular Biology Institute (UNAM). To identify the bacterial isolates, 16S rDNA sequences were compared with those stored in the NCBI GenBank. Reported species showing the highest similarity were regarded as the isolated strains.
Evaluation of the isolated strains to grow on propanil or 3,4-dichloroaniline
All the isolated strains were seeded on MSP or MS-3,4-DCA agar plates. After incubation at 28 °C for 72 h, the presence of colonies or microcolonies able to use propanil or 3,4-dichloroaniline as carbon and nitrogen sources, was determined.
Fixed bed bioreactor
The fixed bed bioreactor (FBR) was a bubble column packed with fragments of a porous volcanic stone named tezontle in México. The characteristics of the porous material were obtained according to Gómez-De Jesús et al. 2009. A scheme of the bioreactor used is shown in Fig. 1. The FBR nominal volume was 0.9 L. The volume occupied by the porous support (V S ) was 0.498 L, and the interstitial liquid volume (V L ) was 0.402 L. The later was used to calculate the volumetric loading (B V ) and removal rates (R V ) of the herbicide. An aeration rate of 0.15 L min−1 was maintained during the operation of the reactor.
Kinetics of propanil biodegradation
Once the FBR was inoculated with the enriched microbial community, it was batch-operated for 72 h to allow the microbial colonization of the porous bed. Afterward, the FBR was continuously fed with MS medium containing propanil (C R = 48.15 ± 1.38 mg L−1) at known loading rates \( B_{V} = \, \frac{{F(C_{R} )}}{{V_{L} }} \). In all cases, COD, TOC, propanil, 3,4-DCA and chloride concentrations were periodically analyzed in effluent samples.
The following kinetic and stoichiometric terms were used to evaluate the overall bioreactor performance. The term \( R_{V} = \frac{dc}{dt} \) is the volumetric removal rate of the compound c and is equivalent to reactor’s productivity; it can be estimated in continuous systems, operating in steady state condition, as \( R_{V} = \, \frac{{F(C_{R} - c)}}{{V_{L} }} \); the term C R is the concentration of the compound c in the inflowing liquid. The removal rate R V , joined to the removal efficiency \( \eta = \frac{{R_{V} }}{{B_{V} }} \), is useful to evaluate the biodegradation capabilities of the microbial consortium.
Periodic measurements of effluent’s UV spectrograms (λ = 190–300 nm) were made to verify the stability of the continuously operated bioreactor after a change in the flow rate \( F \). Usually, a new steady state was reached after six hydraulic retention times \( HRT = \, \frac{{V_{L} }}{F} \).
Analytical methods
Spectrophotometric determination of propanil
Propanil concentration was determined by its absorption at λ = 245 nm in a Beckman DU 650 spectrophotometer.
Propanil and 3,4-dichloroaniline determination by HPLC
Propanil and 3,4-DCA were determined in an HPLC Beckman–Coulter System Gold 125, equipped with an UV–Vis detector. A reversed-phase C18 column was used. The mobile phase was a methanol–water mixture (70:30). The water was previously acidified to pH 3 with orthophosphoric acid. The flow rate was 0.6 mL min−1, and the run time was 8 min (Carvalho et al. 2010).
Determination of chemical oxygen demand (COD) and total organic carbon (TOC)
The removal of propanil and the organic adjuvants that compose the herbicide formulation was measured by two methods; the chemical oxygen demand (COD) and the total organic carbon (TOC). The former is related to the amount of oxygen required to oxidize the propanil to CO2, ammonia and water (dichromate does not oxidize ammonia into nitrate); and is easily calculated from the next balance equation.
The estimated COD equivalence for the compound was 1.468 mg O2 [mg propanil]−1, and the carbon content of propanil is 0.495 mg C [mg propanil]−1. The differences observed between the values of COD and TOC for propanil pure, and those experimentally obtained, correspond to the oxygen consumed by the oxidizable herbicide adjuvants. The methods Hach 8000 (range 3–150 mg COD L−1) and Hach 10129 (range 0.3–20 mg C L−1), were used for COD and TOC determinations (Water Analysis Handbook 2002).
Chloride released by propanil dehalogenation
For chloride determination, the mercuric thiocyanate method, Hach 8113, range 0.1–25 mg Cl L−1 was used (Water Analysis Handbook 2002).
Results
Identification of bacterial isolates
Nine bacterial isolates were obtained after the enrichment procedure. By PCR amplification, sequencing, and comparison of bacterial 16S rDNA amplicons with NCBI GenBank sequences, the strains were identified (Table 1).
Growth of the bacterial isolates on commercial herbicide formulation, propanil and 3,4-DCA.
The isolates were individually seeded in agar plates of MS medium containing propanil or 3,4-dichloroaniline. After 24 h of incubation at 28 °C, the ability of each isolate to use the different substrates as carbon and nitrogen source was registered. Four of the identified strains grew individually on propanil, and on 3,4-dichloroaniline; Xanthomonas sp., Acinetobacter calcoaceticus, Pseudomonas sp. and Rhodococcus sp. As expected, the bacterial consortium grew well on both substrates. Growth of the isolated strain of Kocuria was only observed on 3,4-dichloroaniline (Table 2).
Removal of herbicide in the continuously operated FBR
FBR operational conditions
Eleven flow rates F were probed throughout FBR operation. They varied from 14 to 300 mL h−1, corresponding to dilution rates D from 0.035 to 0.746 h−1 and HRT from 28.71 to 1.34 h.
In all cases, the supplied MSP medium contained the commercial herbicide formulation at concentrations corresponding to an equivalent value (C R ) of 48.14 ± 1.38 mg propanil L−1; thus, the volumetric loading rates B V , varied from 1.68 to 36.9 mg propanil L−1 h−1. The TOC and COD values at the FBR entrance were 73.44 ± 1.64 mg C L−1 and 148.4 ± 5.25 mg COD L−1, respectively. The corresponding volumetric loading rates at the FBR entrance varied from 2.52 to 53.62 mg C L−1 h−1 for B V,TOC , and 5.23–116.06 mg COD L−1 h−1 for B V,COD .
Considering the chlorine content of the propanil molecule, the calculated chloride ion concentration at the FBR entrance was 16.45 ± 0.72 mg Cl L−1, and the corresponding volumetric loading rates of chloride varied from 0.60 to 11.81 mg Cl L−1 h−1.
Propanil removal from the herbicide formulation
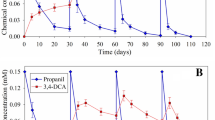
Figure 2 shows the change in the volumetric removal rate R V of propanil when volumetric loading rates B V were increased in the FBR. The removal efficiency η of propanil could be easily observed from the solid line representing the slope η = R V /B V .
At B V values up to 25 mg propanil L−1 h−1, removal efficiencies of 100 % were estimated. However, at higher B V values (36.78 mg propanil L−1 h−1) the volumetric rate R V did not increase proportionally with B V ; in consequence, a decay in the removal efficiency of propanil was observed (η = 66 %). Except the maximum loading rate tested, accumulation of 3,4-DCA, or other metabolic aromatic intermediate, were not detected spectrophotometrically, or by HPLC analysis. The maximum stoichiometric removal rate for 3,4-DCA was estimated in 17.5 mg 3,4-DCA L−1 h−1.
Among the few known processes for propanil biodegradation, Carvalho et al. (2010) describe the use of a microbial consortium for propanil and 3,4-DCA removal in a repeated batch or repeated fed batch suspended cell culture. The estimated R V values for propanil and 3,4-DCA varied from 14.3 to 21.44 mg propanil L−1 h−1, and 1.9–7.56 mg 3,4-DCA L−1 h−1, respectively. The R V values for both compounds obtained in the fixed bed reactor are comparable with those reported by Carvalho et al. (2010).
Propanil dechlorination
Because dechlorination is involved in 3,4-dichloroaniline degradation, where 4-chloroaniline or 4-chlorocatechol could appear as metabolic intermediates (Hongsawat and Vangnai 2011), the chlorine released along the FBR operation was determined. Figure 3 shows the theoretical and experimental chloride release rates obtained at different propanil removal rates. Dechlorination rates stoichiometrically correspond to the complete dechlorination of the propanil degraded. These results are consistent with the absence of chlorinated aromatic by-products in the FBR’s outflowing liquid.
COD and TOC removal
Besides propanil, other carbon sources are present in the herbicide formulation as organic co-formulants; thus, COD and TOC determinations are indicative of the removal of the mixed substrates, propanil and organic adjuvants. Figure 4 (COD removal) and Fig. 5 (TOC removal), show that at loading rates up to 73 mg COD L−1 h−1 and 36.7 mg TOC L−1 h−1, respectively, the mixed substrates were simultaneously, and almost entirely removed by the microbial consortium. Nevertheless, at higher loading rates, decays in the COD and TOC removal efficiencies were estimated. The η COD and η TOC values were, respectively, 62.8 and 59.3 %.
Removal rates R V,COD of the mixed substrates (propanil and organic adjuvants) at different COD loading rates B V,COD . (Filled circle) R V,COD of mixed substrates, (Gray circle) Estimated R V,COD of propanil. The 100% removal efficiency of COD is the slope η = R V,COD /B V,COD represented by the solid line
Removal rates R V,TOC of the mixed substrates (propanil and organic adjuvants) at different TOC loading rates B V,TOC . (Filled triangle) R V,TOC of mixed substrates, (Gray triangle) Estimated R V,TOC of propanil. The 100% TOC removal efficiency is the slope η = R V,TOC /B V,TOC represented by the solid line
By determining the values of the chemical oxygen demand and the TOC content of propanil, the plots shown in gray in Figs. 4 and 5, corresponding to the removal rates of propanil pure, were obtained. The difference with the upper curves (total COD and TOC removal rates) is due to the biodegradation of the organic adjuvants originally present in the herbicide formulation.
Propanil degradation
A final run was made by replacing the commercial presentation of the herbicide (containing adjuvants) with propanil (99 % purity), feeding the reactor at a volumetric loading rate of 5.98 mg propanil L−1 h−1. By liquid chromatography, the disappearance of propanil was observed, and accumulation of aromatic by-products was not detected (data not shown). These results are indicative that the microbial consortium did not require the presence of cosubstrates for propanil catabolism.
Discussion
Only four of the identified strains grew individually on propanil, and on 3,4-dichloroaniline; Xanthomonas sp., Acinetobacter calcoaceticus, Rhodococcus sp. and Pseudomonas sp.
Strains of Xanthomonas in bacterial consortia able to degrade o-chloroaniline and 2,4-dichloroaniline, have been reported (Wang et al. 2007), and in several papers, the last three bacteria have been related to biodegradation of phenylurea herbicides (Correa and Steen 1995; Carvalho et al. 2010; Kaufman and Blake 1973) or chlorinated anilines (Janke et al. 1984; Vangnai and Petchkroh 2007). Several strains of Variovorax also degrade phenylurea herbicides such as diuron (Sørensen et al. 2008) and linuron (Dejonghe et al. 2003).
The role of the genus Kocuria in the biodegradation of keratin (Vidal et al. 2000) and several xenobiotic compounds such as polycyclic aromatic hydrocarbons (Ahmed et al. 2010), chlorophenols (Karn et al. 2011), trinitrotoluene (Solyanikova et al. 2012) and synthetic dyes (Parshetti et al. 2010), is well documented. Nevertheless, as far as we know there are no reports about biodegradation of propanil or 3,4-DCA by species of the genus Kocuria. The isolated Kocuria strain degrade 3,4-DCA, but could not grow on propanil. These results suggest that this strain lacks aryl acylamidase, enzyme responsible for the initial degradation of propanil to 3,4-DCA and propionic acid. This enzyme is found in rhizosphere microflora, including the bacterial genera Pseudomonas, Alcaligenes, Rhizobium and Bradyrhizobium (Hoagland et al. 1994).
It is known that the other cultivable strains present in the bacterial consortium are involved in the biodegradation of other xenobiotics. By example, species of Acidovorax have been found dominant in microbial communities able to degrade aromatic hydrocarbons (Aburto and Peimbert 2011), in microbial communities isolated from oil sands (Golby et al. 2012), in benzene-contaminated groundwater (Aburto et al. 2009), and also in ampicillin-bearing wastewater (Shen et al. 2011). Members of the genus Acidovorax could assimilate synthetic nitroarene compounds such as nitrotoluene (Ju and Parales 2011). Species of the genus Flavobacteriun have been reported as members of microbial consortia involved in the biodegradation of dioxane (Sun et al. 2011), methyl parathion and chlorpyrifos (Pino and Peñuela 2011). Strains of Luteibacter degrade petroleum hydrocarbons (Zhang et al. 2011), polychlorinated biphenyls (Leigh et al. 2006), and organophosphate insecticides such as methamidophos (Wang et al. 2011). The presence of bacteria in the biofilm consortium, unable to degrade propanil or chloroanilines could suggest a role carried out by these strains in biofilm integrity or biodegradation of herbicide adjuvants.
In conclusion, the selected consortium immobilized in the biofilm reactor could degrade propanil and the adjuvants present in a commercial presentation of the herbicide. Determinations of propanil dechlorination, as well as TOC and COD removal, suggest the complete degradation of propanil, its chlorinated by-products, and the organic additives of the herbicide formulation. Furthermore, the microbial consortium did not require the presence of cosubstrates to use propanil as the sole source of carbon and nitrogen, and no metabolic intermediaries were detected by HPLC at loading rates up to 25 mg L−1 h−1.
To carry out the biological removal of ecotoxic compounds present in agricultural wastewaters, the technical and economical feasibility of the process should be considered. Permeable biological barriers (biobarriers) that use inexpensive biofilm-supporting materials with good mechanical strength could be a feasible option for treating agricultural wastewaters. The porous volcanic stone (tezontle) used as packing material in the biofilm reactor is a vesicular basaltic-andesitic-scoria accumulated in the trans-mexican volcanic belt. It is broadly distributed in central México and is widely used as building and construction material. The characteristics of this support material; size, irregular shape, and porosity, joined to its mechanical resistance, made it a good candidate for biobarriers construction.
As a final point, among the few known processes for propanil biodegradation, Carvalho et al. (2010) described the use of a microbial consortium for propanil and 3,4-DCA removal in a repeated batch or repeated fed batch suspended cell culture. In this work, the R V values obtained for both compounds are similar or higher to those reported in the aforesaid paper.
References
Aburto A, Peimbert M (2011) Degradation of benzene-toluene mixture by hydrocarbon adapted bacterial communities. Ann Microbiol 61:553–562
Aburto A, Fahy A, Coulon F, Lethbridge G, Timmis KN, Ball AS, McGenity TJ (2009) Mixed aerobic and anaerobic microbial communities in benzene-contaminated groundwater. J Appl Microbiol 106:317–328
Ahmed RZ, Ahmed N, Gadd GM (2010) Isolation of two Kocuria species capable of growing on various polycyclic aromatic hydrocarbons. Afr J Biotechnol 9:3611–3617
Albrecht K, Frisch G (1989) Liquid pesticidal compositions in the form of suspension concentrates. US Patent 4, 804, 399
Bartha R (1971) Fate of herbicide-derived chloroanilines in soil. J Agr Food Chem 19:385–387
Bevinakatti HS, Waite AG (2012) Surfactant compounds. US Patent 8, 097, 564
Call DJ, Poirier SH, Knuth ML, Harting SL, Lindberg CA (1987) Toxicity of 3,4-dichloroaniline to fathead minnows, Pimephales promelas, in acute and early life-stage exposures. Bull Environ Contam Toxicol 38:352–358
Carvalho G, Marques R, Lopes AR, Faria C, Noronha JP, Oehmen A, Nunes OC, Reis MAM (2010) Biological treatment of propanil and 3,4-dichloroaniline: kinetic and microbiological characterisation. Water Res 44:4980–4991
Correa IE, Steen WC (1995) Degradation of propanil by bacterial isolates and mixed populations from a pristine lake. Chemosphere 30:103–116
Cox C, Surgan M (2006) Unidentified inert ingredients in pesticides: implications for human and environmental health. Environ Health Persp 114:1803–1806
Danchour A, Bitton G, Coste CM, Bastide J (1986) Degradation of the herbicide propanil in distilled water. Bull Environ Contam Toxicol 36:556–562
Dejonghe W, Berteloot E, Goris J, Boon N, Crul K, Maertens S, Höfte M, De Vos P, Verstraete W, Top EM (2003) Synergistic degradation of linuron by a bacterial consortium and isolation of a single linuron-degrading Variovorax strain. Appl Environ Microbiol 69:1532–1541
Eke KR, Barnden AD, Tester DJ (1996) Impact of agricultural pesticides on water quality. In: Hester RE, Harrison RM (eds) Agricultural chemicals and the environment. Issues in environmental science and technology, vol 5. The Royal Society of Chemistry, UK, pp 43–56
Felske A, Engelen B, Nübel U, Backhaus H (1996) Direct ribosome isolation from soil to extract bacterial rRNA for community analysis. Appl Environ Microbiol 62:4162–4167
Galhano V, Gomes-Laranjo J, Fernández-Valiente E, Videira R, Peixoto F (2011) Impact of herbicides on non-target organisms in sustainable irrigated rice production systems: state of knowledge and future prospects. In: Kortekamp A (ed) Herbicides and environment. InTech, Rijeka, pp 45–72
Garrido EM, Lima JLFC, Delerue-Matos C, Borges F, Silva AMS, Oliveira Brett AM (2001) Electrochemical oxidation of propanil and related N-substituted amides. Anal Chim Acta 434:35–41
Golby S, Ceri H, Gieg LM, Chatterjee I, Marques LL, Turner RJ (2012) Evaluation of microbial biofilm communities from an Alberta oil sands tailings pond. FEMS Microb Ecol 79:240–250
Gómez-De Jesús A, Romano-Baez FJ, Leyva-Amezcua L, Juárez-Ramírez C, Ruiz-Ordaz N, Galíndez-Mayer J (2009) Biodegradation of 2,4,6-trichlorophenol in a packed-bed biofilm reactor equipped with an internal net draft tube riser for aeration and liquid circulation. J Hazard Mater 161:1140–1149
Hildebrandt A, Lacorte S, Barceló D (2007) Assessment of priority pesticides, degradation products, and pesticide adjuvants in groundwaters and top soils from agricultural areas of the Ebro river basin. Anal Bioanal Chem 387:1459–1468
Hoagland RE, Zablotowicz RM, Locke MA (1994) Propanil metabolism by rhizosphere microflora. In: Anderson TA, CoatsJR (eds) Bioremediation through rhizosphere technology. ACS Symposium Series, vol 563. American Chemical Society, USA, pp 160–183
Hongsawat P, Vangnai AS (2011) Biodegradation pathways of chloroanilines by Acinetobacter baylyi strain GFJ2. J Hazard Mater 186:1300–1307
Hund-Rinke K, Simon M (2005) Terrestrial ecotoxicity of eight chemicals in a systematic approach. J Soils Sedim 5:59–65
Jak RG, Maas JL, Scholten MCTH (1998) Ecotoxicity of 3,4-dichloroaniline in enclosed freshwater plankton communities at different nutrient levels. Ecotoxicol 7:49–60
Janke D, Baskunov BP, Nefedova MY, Zyakun AM, Golovleva LA (1984) Incorporation of 18O2 during cometabolic degradation of 3-chloroaniline by Rhodococcus sp. An 117. J Basic Microbiol (Zeitschrift für allgemeine Mikrobiologie) 24(4):253-259. doi:10.1002/jobm.19840240411
Jones R (2005) The ecotoxicological effects of photosystem II herbicides on corals. Mar Pollut Bull 51:495–506
Ju K-S, Parales RE (2011) Evolution of a new bacterial pathway for 4-nitrotoluene degradation. Mol Microbiol 82:355–364
Karn SK, Chakrabarti SK, Reddy MS (2011) Degradation of pentachlorophenol by Kocuria sp. CL2 isolated from secondary sludge of pulp and paper mill. Biodegradation 22:63–69
Kaufman DD, Blake J (1973) Microbial degradation of several acetamide, acylanilide, carbamate, toluidine and urea pesticides. Soil Biol Biochem 5:297–308
Konstantinou IK, Sakkas VA, Albanis A (2001) Photocatalytic degradation of the herbicides propanil and molinate over aqueous TiO2 suspensions: identification of intermediates and the reaction. Appl Catal B-Environ 34:227–239
Leigh MB, Prouzová P, Macková M, Macek T, Nagle DP, Fletcher JS (2006) Polychlorinated biphenyl (PCB)-degrading bacteria associated with trees in a PCB-contaminated site. Appl Environ Microbiol 72:2331–2342
Mackay D, Shiu WY, Ma K-C, Lee SC, (2006) Handbook of physical-chemical properties and environmental fate for organic chemicals, 2nd ed vol IV, Nitrogen and sulfur compounds and pesticides. CRC Press Boca Raton FL, USA, pp 3639–3641
Parshetti GK, Telke AA, Kalyani DC, Govindwa SP (2010) Decolorization and detoxification of sulfonated azo dye methyl orange by Kocuria rosea MTCC 153. J Hazard Mater 176:503–509
Pino N, Peñuela G (2011) Simultaneous degradation of the pesticides methyl parathion and chlorpyrifos by an isolated bacterial consortium from a contaminated site. Int Biodeter Biodegr 65:827–831
Sahid IB, Carso J, Chuah TS (2011) Resistance mechanism of Leptochloa chinensis Nees, to propanil. Weed Biol Manag 11:57–63
Shen L, Xu H, Liu Y (2011) Microbial characterization of the biofilms developed for treating ampicillin-bearing wastewater. J Environ Sci Health A 46:314–322
Solyanikova IP, Baskunov BP, Baboshin MA, Saralov AI, Golovleva LA (2012) Detoxification of high concentrations of trinitrotoluene by bacteria. Appl Biochem Microbiol 48:21–27
Sørensen SR, Albers CN, Aamand J (2008) Rapid mineralization of the phenylurea herbicide diuron by Variovorax sp. strain SRS16 in pure culture and within a two-member consortium. Appl Environ Microbiol 74:2332–2340
Sun B, Ko K, Ramsay JA (2011) Biodegradation of 1,4-dioxane by a Flavobacterium. Biodegradation 22:651–659
Taylor EJ, Maund SJ, Bennet D, Pascoe D (1994) Effects of 3,4-dichloroaniline on the growth of two freshwater macroinvertebrates in a stream mesocosm. Ecotox Environ Safe 29(1):80–85
Tobiassen LS, Nielsen E, Nørhede P, Ladefoged O (2003) Report on the health effects of selected pesticide coformulants. Pesticides Research Nr. 80, Danish Veterinary and Food Administration, Institute of Food Safety and Nutrition, Danish Environmental Protection Agency
Vangnai AS, Petchkroh W (2007) Biodegradation of 4-chloroaniline by bacteria enriched from soil. FEMS Microbiol Lett 268:209–216
Vidal L, Christen P, Coello MN (2000) Feather degradation by Kocuria rosea in submerged culture. World J Microbiol Biotech 16:551–554
Wang C, Lu G-H, Zhou Y-J (2007) Biodegradablilty of chlorinated anilines in waters. Biomed Environ Sci 20:141–145
Wang L, Wang G-L, Li S-P, Jiang J-D (2011) Luteibacter jiangsuensis sp. nov.: a methamidophos-degrading bacterium isolated from a methamidophos-manufacturing factory. Curr Microbiol 62:289–295
Water Analysis Handbook, 4th Edition (2002) Hach Company, Loveland, Co., USA, pp 221–224, 731–736, 743–749
Wendel C, Mason T (2009) Risks of propanil use to federally threatened California red-legged frog. Environmental fate and effects. Division office of pesticides program, Washington DC http://www.epa.gov/espp/litstatus/effects/redleg-frog/#propanil. Accessed 12 July 2012
Zhang LJ, Chen L, Thring RW (2011) Remediation of refinery oily sludge using isolated strain and biosurfactant.In: Proceedings of 2011 International symposium on water resource and environmental protection, Xi’an China, 20–22 may 2011, vol 3, pp 1649–1653
Acknowledgments
The authors wish to thank COFAA-IPN and SIP-IPN for financial support for fellowships to C. J-R, N. R-O, and J. G-M, and to Conacyt for graduate scholarships to V.E. H-G.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herrera-González, V.E., Ruiz-Ordaz, N., Galíndez-Mayer, J. et al. Biodegradation of the herbicide propanil, and its 3,4-dichloroaniline by-product in a continuously operated biofilm reactor. World J Microbiol Biotechnol 29, 467–474 (2013). https://doi.org/10.1007/s11274-012-1200-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-012-1200-5