Abstract
Candida sp. strain SY16 produces a glycolipid-type biosurfactant, mannosylerythritol lipid (MEL-SY16), which can reduce the surface tension of a culture broth from 72 to 30 dyne cm−1 and highly emulsify hydrocarbons when cultured in soybean-oil-containing media. As such, laboratory-scale fermentation for MEL-SY16 production was performed using optimized conditions. In batch fermentation, MEL-SY16 was mainly produced during the stationary phase of growth, and the concentration of MEL-SY16 reached 37 g l−1 after 200 h. The effect of pH control on the production of MEL-SY16 was also examined in batch fermentation. The highest production yield of MEL-SY16 was when the pH was controlled at 4.0, and the production was significantly improved compared to batch fermentation without pH control. In fed-batch fermentation, glucose and soybean oil (1:1, w/w) were used in combination as the initial carbon sources for cell growth, and soybean oil was used as the feeding carbon source during the MEL production phase. The feeding of soybean oil resulted in the disappearance of any foam and a sharp increase in the MEL production until 200 h, at which point the concentration of MEL-SY16 was 95 g l−1. Among the investigated culture systems, the highest MEL-SY16 production and volumetric production rate were achieved with fed-batch fermentation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biosurfactants have attracted considerable interest due to their low toxicity, biodegradable nature, and diverse structures (Georgiou et al. 1992; Banat et al. 2000). However, biosurfactants have not yet been employed extensively in industry because of their relatively high production costs. As such, to reduce the costs of biosurfactant production, the important considerations are selecting a strain capable of producing high yields of biosurfactants and optimizing the fermentation and recovery systems (Desai 1987). The current authors previously isolated a yeast strain, Candida sp. SY16, which produces a biosurfactant with a good emulsifying activity against vegetable and crude oils. The biosurfactant (MEL-SY16) was identified as a kind of mannosylerythritol lipid (MEL) and showed excellent surface and interfacial tension reduction at the critical micelle concentration (CMC) (Kim et al. 1999).
MELs are known to exhibit surface-active properties, excellent surface and interfacial tension lowering, high emulsification, and a low CMC (Kitamoto et al. 1993; Kim et al. 2002b; Rau et al. 2004), suggesting their suitability for environmental applications, such as the biosurfactant-enhanced bioremediation of nonaqueous-phase liquid (NAPL) contaminants (Sekelsky and Shreve 1999; Woo et al. 2001). In addition, MELs show antimicrobial activities against Gram-positive bacteria, and their minimum inhibitory concentrations are significantly smaller than those of sucrose and sorbitan monoesters of fatty acids (Kitamoto et al. 1993). Plus, it has been reported that MELs are a potent inducer of apoptosis and differentiation in mouse melanoma cells (Zhao et al. 1999).
However, the production of MELs in fermentors has not yet been fully investigated. Kitamoto et al. (1992a) reported on MEL production by resting cells of Candida antarctica, while Adamczak and Bednarski (2000) investigated the influence of the medium composition and aeration on the synthesis of the MEL from C. antarctica ATCC 20509. Yet, in both cases, the MEL production was not very high. Thus, to realize the commercialization of MELs, more information is necessary to optimize and enhance their production in fermentor systems.
Accordingly, the present study investigated the culture conditions and certain physiological properties for MEL-SY16 production in a jar fermentor, and the most efficient production system for MEL-SY16 was found to be fed-batch fermentation.
Materials and methods
Microorganism
The Candida sp. SY16 (Candida antarctica KCTC 7804) used in this study was isolated in the authors’ laboratory from orchard soil from Daejon using the oil film-collapsing assay method (Kim et al. 1997). The partial 26S rDNA sequence (AY833088) of strain SY16 was closest to Pseudozyma tsukubaensis (99%, 604/605) (Sugita et al. 2003).
Media and cultivation
The cells were maintained as a 20% (v/v) glycerol stock at −80°C after growing in a YM medium composed of (per liter distilled water, pH 6.5) peptone, 5.0 g; yeast extract, 3.0 g; malt extract, 3.0 g; and glucose 5.0 g. Seed cultures were carried out in the YM medium at 30°C with reciprocal shaking at 120 rpm for 24 h. The laboratory-scale fermentation was carried out at 30°C, stirred at 500 rpm, and aerated at a rate of 1.0 vvm in a jar fermentor (5.0 l) (KFC; Korea Fermentor Company, Inchon, Korea) containing 2.0 l medium. The seed cultures prepared in the YM medium were inoculated at 5% (by volume). An optimized flask culture medium (Kim et al. 2002a) was used to produce the MEL-SY16 in the batch fermentation and had the following composition (per liter distilled water): 15 g of glucose; 15 g of soybean oil; 10 g of NH4NO3; 2.5 g of K2HPO4; 0.1 g of NaH2PO4; 0.5 g of MgSO4·7H2O; 0.1 g of CaCl2·2H2O; 0.02 g of MnSO4·H2O; and 1 g of peptone. Soybean oil as the feeding carbon source was added using a feed pump (5 g oil min−1) to 70 (w/v) and 100 g l−1 (w/v) at 44 and 104 h, respectively. The culture pH was maintained at 4.0 by the addition of 2N NaOH after 24 h of culture. The dissolved oxygen concentration (DOC) was monitored and maintained above 20% of air saturation by varying the airflow rate (0.2–2.0 vvm) and agitation speed (500–750 rpm). Foam-stat fed-batch fermentation was also carried out in the same medium as used for the above fed-batch fermentation. The oil feeding strategy for the foam-stat fermentation was based on the foam that resulted with the depletion of the oil substrate. As such, when foam was detected by an antifoam sensor, the oil substrate was automatically added to the culture broth up to 5 g l−1 using the feed pump (5 g oil min−1) attached to the jar fermentor. Total feed volume of soybean oil was calculated from the number counted in the antifoam control panel.
Analyses
The cell growth in the oil substrate was measured based on the optical density at 660 nm after washing the cells with ethanol/butanol/chloroform (10:10:1) and more washing with distilled water, then represented using the dry cell mass determined as a function of A 660; dry cell mass (g l−1)=0.25×A 660. The concentration of MEL-SY16 in the medium was determined using the anthrone method (Kitamoto et al. 1992a). The MEL-SY16 purified as previously reported (Kim et al. 1999) was used as the standard in the anthrone method. The residual vegetable oils were determined as reported by Kawashima et al. (1983). The glucose concentration was determined with a glucose kit using glucose oxidase (Sigma, St. Louis, MO, USA). The surface and interfacial tensions were measured using the ring method with a Digital-Tensiometer K10ST (Krüss, Hamburg, Germany) after ultrasonic treatment of the biosurfactant solution. The nitrogen concentration (ammonium ion) in the cultures was determined photometrically by the phenate method (Alleman et al. 1995). A TLC analysis of the MEL was developed using a solvent system of chloroform/methanol/water/acetic acid (85:15:1:1) on a silica gel plate (No.5715, Merck, Darmstadt, Germany) and visualized using 30% sulfuric acid.
Results
Batch fermentation for production of MEL-SY16
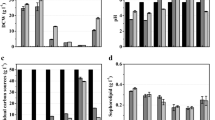
A fermentation study of the biosurfactant (MEL-SY16) production from Candida sp. SY16 was performed. Figure 1 shows the typical time course of cell growth and MEL-SY16 production with a batch mode in a 5-l jar fermentor using an optimized flask culture medium (Kim et al. 2002a). The MEL-SY16 was extracellularly produced from the initial stationary phase of cell growth (50 h) and rapidly produced after a culture time of 100 h. The maximum biosurfactant production by strain SY16 was in the stationary phase of cell growth. The production of MEL-SY16 was not growth-associated. The ammonium nitrogen was almost consumed after 30 h of culture. The MEL-SY16 and cell concentrations reached 37 and 10 g l−1 after 200 h, respectively, while the residual oil was 25 g l−1 at the same time. The pH of the culture broth decreased from 7.8 to 6.0 during the log phase of cell growth and then rapidly decreased again to about 4.5 when the biosurfactant production sharply increased after 100 to 150 h.
To increase the product (MEL) concentration, first, the cell concentration was increased by increasing the concentrations of the nitrogen sources. As such, the organic and inorganic nitrogen source concentrations were respectively increased and the improvement in the cell growth and biosurfactant production in the jar fermentor compared (Fig. 2). The concentration of peptone, the organic nitrogen source, was increased to 5.0 g l−1, which was five times higher than that in the optimized flask culture medium. With 5.0 g l−1 peptone, the cell concentration reached 23 g l−1, which was twice than that in the previous optimized medium (Fig. 1), yet the MEL-SY16 concentration was only about 20 g l−1 after 190 h, which was about 60% of that obtained from the batch fermentation described above. Thus, although the pH did not go below 4 and the specific growth rate was increased, the biosurfactant production was not improved.
Comparison of nitrogen source effects on the MEL production from Candida sp. SY16 in batch fermentation. The concentration of peptone was 5.0 g l−1 (open symbols), and NH4NO3 was 2.5 g l−1 (closed symbols). Fermentation was performed with 500 rpm and 1 vvm, at 30°C in 5-l jar fermentor (working volume of 2 l). Cell growth (○, •), MEL concentration (□, ▪), and pH (▵, ▴) were monitored for 190 h
Meanwhile, when the NH4NO3 inorganic nitrogen source was increased 2.5 times to 2.5 g l−1, the cell growth and biosurfactant production stopped after 80 h, probably because the pH fell to 2.0. However, MEL production was observed from the relatively short culture time (48 h), and the MEL production rate was higher than that with the peptone increase until the initial stationary growth phase (72 h), while the pH was higher than 3.0. These facts suggest that the MEL production was continued and improved when maintaining a pH above 3.
Therefore, the effect of pH control on the production of MEL-SY16 was examined in batch fermentation (Fig. 3). The fermentation was performed with the optimized medium containing NH4NO3 at 2.5 g l−1 for 200 h, and the pH control, started after 24 h of culture, was maintained at 3.0, 4.0, and 5.0 using 2N NaOH. The highest production of MEL-SY16 was 48 g l−1, with the pH controlled at 4.0, where the maximum cell concentration was almost the same as that in the batch fermentation without any pH control (10 g cell l−1). Controlling the pH at 4.0 significantly improved the MEL production compared to that from the batch fermentation without pH control. However, when the pH was controlled at 5.0, the MEL production significantly decreased in spite of an increased cell growth. The following fermentation experiments were performed when controlling the pH at 4.0 using a 2N NaOH solution.
Fed-batch fermentation for MEL-SY16 production
The above batch fermentation results showed that Candida sp. SY16 produced MEL-SY16 mainly in the stationary phase of growth, suggesting the feasibility of separating the MEL production phase from the cell growth phase and MEL production by fed-batch fermentation using a two-stage culture system under suitable growth-limiting conditions. In a previous study (Kim et al. 2002a), a higher yield of MEL-SY16 was produced by a two-stage culture compared to a batch mode under nitrogen-limited conditions in a flask culture.
Figure 4 shows the fed-batch fermentation for the production of MEL-SY16 from Candida sp. SY16 using the optimized culture conditions, with a combination of glucose and soybean oil (1:1, w/w) as the initial carbon sources during the cell growth phase, soybean oil as the feeding carbon source during the MEL production phase, and the pH controlled at 4.0 (±0.2). The pH was decreased from 7.5 to 4.0 when initiating the pH control after 24 h, then maintained at 4.0 until the end of the fermentation. For convenience, the fermentation process was divided into two phases, active cell growth phase and active MEL-SY16 production phase, as described above. The glucose was assimilated first in the initial carbon substrates and almost consumed after 20 h, while the initial 15 g l−1 addition of soybean oil was exhausted after 44 h, whereupon further soybean oil was fed to a concentration of 70 g l−1 as the carbon source for the production phase (arrow 1 in Fig. 4). The further feeding of soybean oil resulted in a rapid increase in MEL-SY16 production, and the cell concentration reached 22 g l−1 after 104 h, at which point the soybean oil was totally consumed and vigorous foaming occurred. Consequently, more soybean oil was fed to a concentration of 100 g l−1 (arrow 2 in Fig. 4), which resulted in the disappearance of the foam. Thereafter, the MEL concentration sharply increased again until 200 h, when the remaining soybean oil was about 20 g l−1. The maximum concentrations of MEL-SY16 and cells were 95 and 25 g l−1, respectively.
Fed-batch fermentation for MEL production by Candida sp. SY16. The fermentation was performed at 30°C with an agitation of 500 rpm and aeration of 1 vvm in a 5-l jar fermentor (initial working volume of 2 l). The culture pH was controlled at 4.0 (±0.2) using a 2N NaOH solution. Arrow 1 indicates the feeding of soybean oil to a concentration of 70 g l−1, and arrow 2 indicates the soybean oil feeding to 100 g l−1 and changes in the aeration and agitation to 0.3 vvm and 700 rpm, respectively, from the initial conditions (1 vvm and 500 rpm)
The raw lipid mixture isolated from the above fed-batch fermentation principally contained MEL-SY16, identified at an R f of 0.4 on the TLC (Fig. 5). In the previous reports (Kim et al. 1999, 2002a), the MEL-SY16 was simply confirmed from the R f value of 0.4 on TLC analysis. The intensity of the MEL-SY16 spot increased with the culture time. It was also found that the fatty acids increased with the decomposition of the substrate oil until 140 h of culture, then decreased with their incorporation into the MEL. The compound extracted from the MEL-SY16 spot on the TLC showed a minimum surface tension of 28.0 dyne cm−1 and interfacial tension of 1.0 dyne cm−1 against kerosene at 20 mg extracts l−1 DW.
TLC patterns of lipid mixture isolated from fed-batch fermentation of Candida sp. SY16. Samples were extracted from the fed-batch culture (Fig. 4) using ethyl acetate, and the organic solvent fraction spotted onto a TLC plate. The spots were visualized with 30% H2SO4. Lanes 1 to 9 are the samples cultured after 44, 68, 104, 128, 140, 152, 164, 176, and 200 h, respectively
An attempt was also made to perform foam-stat fed-batch fermentation, using soybean oil as both an antifoam agent to extinguish the foam and carbon substrate for the production of MEL-SY16. This was based on the above results, where foaming occurred when the soybean oil was consumed, while the foam disappeared when soybean oil was fed. The foam-stat feeding strategy was related to the occurrence of foam as a result of the oil substrate depletion. As such, when foam was detected by an antifoam sensor, an appropriate volume of the oil substrate was automatically added to the culture broth. The oil feeding was initiated at 78 h in the stationary phase, and the total concentration of soybean oil fed was about 80 g l−1 until 200 h of culture, at which point the concentrations of MEL-SY16 and cells were only 40 and 14 g l−1, respectively (data not shown). The soybean oil was totally fed to the culture broth at a lower concentration than the above fed-batch fermentation, which resulted in the lower production of MEL. However, the product yield coefficient (Y p/s) in the foam-stat fed-batch fermentation was higher than that of the fed-batch fermentation (Table 1).
Discussion
This fermentation study investigated the production system of MEL-SY16. As such, the extracellular production of the glycolipid-type biosurfactant MEL-SY16 by Candida sp. SY16 only occurred when the strain was cultured in a vegetable-oil-containing medium. The laboratory-scale fermentation performed for MEL production used an optimized flask culture medium as the basal medium (Kim et al. 2002a). Table 1 summarizes the results of the MEL production by Candida sp. SY16 according to certain fermentation parameters.
In the batch fermentation, the MEL-SY16 was mainly produced during the stationary phase of cell growth when the nitrogen was nearly exhausted, similar to other yeast strains producing glycolipid biosurfactants (Hommel et al. 1987; Spoeckner et al. 1999). When comparing an organic and inorganic nitrogen source for the MEL production, the MEL synthesis was repressed, in spite of abundant cells and a high cell growth rate, with more organic nitrogen (peptone at 5 g l−1), which was preferentially used for cell growth rather than the MEL production. Hommel et al. (1987) also previously reported that higher specific growth rates resulted in reduced biosurfactant production, while Guerra-Santos et al. (1984) provided additional evidence that biosurfactant production is associated with slow cell growth. Conversely, the MEL production was initiated after a relatively short culture time when the concentration of inorganic nitrogen was increased, and the MEL production continued as long as the culture pH was maintained above 3.
Therefore, the effect of pH control on the MEL production was examined in batch fermentation. The production of MEL-SY16 significantly improved when the pH was controlled at 4.0, yet became repressed at 5.0. Stüwer et al.(1987) also reported that a pH range between 3.5 and 4.5 was suited for sophorolipid formation, whereas a pH value of about 5 prevented glycolipid production. As such, the results of the batch fermentation and a previous investigation of the optimum medium composition for MEL-SY16 production in a flask culture all suggest that the production of MEL-SY16 is sensitive to the pH of the culture. The optimized concentrations of nitrogen and phosphate for the flask culture also prevented the pH from going below 3 during the culture.
Fed-batch fermentation was carried out for the high production of MEL-SY16 based on the results of the batch culture, where the production phase of MEL-SY16 was found to be completely separated from the cell growth phase. The highest concentration and the volumetric production rate of MEL-SY16 were achieved in the fed-batch fermentation when glucose and soybean oil were used in combination as the initial carbon sources during the cell growth phase, and soybean oil was used as the feeding carbon source during the MEL production phase. In a previous flask culture study (Kim et al. 2002a), the current authors selected a carbon source for the cell growth and secondary feeding carbon source for the MEL production in a two-stage culture. In the production phase, the nitrogen was nearly exhausted (data not shown). Some other reports have also described that nitrogen exhaustion may switch on biosurfactant formation (Guerra-Santos et al. 1984) or initiate the production of glycolipids containing predominantly MEL from Ustilago maydis (Spoeckner et al. 1999). In the current study, it is supposed that the MEL-SY16 was initially accumulated in the cells from the water-soluble carbon substrate, then excreted extracellularly by the water-insoluble inducer, fatty acids, and lipids, which also play a role as a fatty-acid precursor of the hydrophobic domain in glycolipid biosurfactants. Kitamoto et al. (1992b) reported that Candida sp. strain T-34 accumulated a significant amount of MEL intracellularly as a storage material from water-soluble carbon sources such as glycerol and glucose. Plus, Meesters et al. (1996) reported that lipids were accumulated intracellularly in the form of oil droplets by the oleaginous yeast Cryptococcus curvatus using glycerol as the carbon source.
In the fed-batch fermentation, the soybean oil feeding resulted in the disappearance of any foam and the rapid increase in MEL-SY16 production, which reached 95 g l−1 after 200 h. However, the further addition of soybean oil after 200 h of culture did not induce any extra MEL production.
The fact that foam appeared when the soybean oil was consumed and disappeared when the oil was added revealed the possibility of foam-stat fermentation, using soybean oil as both the antifoam agent to eliminate foam and the carbon substrate for biosurfactant production.
The foam-stat fed-batch culture for biosurfactant production was firstly carried out using a laboratory-scale fermentor. The total amount of soybean oil added was about 80 g l−1 during the fermentation, yet the concentration of MEL only reached 40 g l−1. The oil feeding was limited, in this case, and more frequent oil feeding showed no improvement in the MEL-SY16 formation. Rau et al. (2004) also reported that batchwise addition of the carbon substrate essentially led to decreased MEL formation from Pseudozyma aphidis. Therefore, these results would seem to indicate an additional regulatory mechanism for the biosynthesis of MELs, which is the subject of further investigation. Plus, an optimized feeding strategy for the substrate oil is also needed to improve the MEL production in a foam-stat fed-batch culture.
Several biosurfactants show low CMC values and significantly reduce the surface tension of water or a fermentation broth to less than 30 dyne cm−1. These surface activities compare favorably to those obtained with commercial synthetic surfactants. However, the production yields of these biosurfactants in a culture medium are too low for extensive use in industrial fields. The main limiting factor to the commercialization of biosurfactants is the economics of large-scale production. Considering these economic limitations, MELs may be promising biosurfactants, as they can be produced at about 100 g l−1; plus, additional physiological and fermentation studies may further enhance their production yield.
References
Adamczak M, Bednarski W (2000) Influence of medium composition and aeration on the synthesis of biosurfactants produced by Candida antarctica. Biotechnol Lett 22:313–316
Alleman JE, Jones MN, Kamhawy SM, Keefe CW (1995) 4500-NH3 F. Phenate method. In: Eaton AD, Clesceri LS, Greenberg AE (eds) Standard methods for the examination of water and wastewater. APHA-AWWA-WEF, Washington DC, pp 4-80–4-81
Banat IM, Makkar RS, Cameotra SS (2000) Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol 53:495–508
Desai JD (1987) Microbial surfactants: evaluation, types, production and future applications. J Sci Ind Res 46:440–449
Georgiou G, Lin SC, Sharma MM (1992) Surface-active compounds from micro-organisms. Bio/Technology 10:60–65
Guerra-Santos LH, Käppeli O, Fiechter A (1984) Pseudomonas aeruginosa biosurfactant production in continuous culture with glucose as carbon source. Appl Microbiol Biotechnol 48:301–305
Hommel R, Stüwer O, Stuber W, Haferburg D, Kleber HP (1987) Production of water-soluble surface-active exolipids by Torulopsis apicola. Appl Microbiol Biotechnol 26:199–205
Kawashima H, Nakahara T, Oogaki M, Tabuchi T (1983) Extracellular production of a mannosylerythritol lipid by a mutant of Candida sp. from n-alkanes and triacylglycerols. J Ferment Technol 61:143–149
Kim HS, Lee CH, Suh HH, Ahn KH, Oh HM, Kwon KS, Yang JW, Yoon BD (1997) A lipopeptide biosurfactant produced by Bacillus subtilis C9 selected through the oil film-collapsing assay. J Microbiol Biotechnol 7:180–188
Kim HS, Yoon BD, Choung DH, Oh HM, Katsuragi T, Tani Y (1999) Characterization of a biosurfactant, mannosylerythritol lipid produced from Candida sp. SY16. Appl Microbiol Biotechnol 52:713–721
Kim HS, Jeon JW, Lee HW, Park YI, Seo WT, Oh HM, Katsuragi T, Tani Y, Yoon BD (2002a) Extracellular production of a glycolipid biosurfactant, mannosylerythritol lipid, from Candida antarctica. Biotechnol Lett 24:225–229
Kim HS, Jeon JW, Kim SB, Oh HM, Kwon TJ, Yoon BD (2002b) Surface and physico-chemical properties of a glycolipid biosurfactant, mannosylerythritol lipid, from Candida antarctica. Biotechnol Lett 24:1637–1641
Kitamoto D, Fuzishiro T, Yanagishita H, Nakane T, Nakahara T (1992a) Production of mannosylerythritol lipids as biosurfactants by resting cells of Candida antarctica. Biotechnol Lett 14:305–310
Kitamoto D, Nakane T, Nakao N, Nakahara T, Tabuchi T (1992b) Intracellular accumulation of mannosylerythritol lipids as storage materials by Candida antarctica. Appl Microbiol Biotechnol 36:768–772
Kitamoto D, Yanagishita H, Shinbo T, Nakane T, Kamisawa C, Nakahara T (1993) Surface active properties and antimicrobial activities of mannosylerythritol lipids as biosurfactants produced by Candida antarctica. J Biotechnol 29:91–96
Meesters PAEP, Huijberts GNM, Eggink G (1996) High-cell-density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl Microbiol Biotechnol 45:575–579
Rau U, Nguyen LA, Schulz S, Wray V, Nimtz M, Roeper H, Koch H, Lang S (2004) Formation and analysis of mannosylerythritol lipids secreted by Pseudozyma aphidis. Appl Microbiol Biotechnol 66:551–559
Sekelsky AM, Shreve GS (1999) Kinetic model of biosurfactant-enhanced hexadecane biodegradation by Pseudomonas aeruginosa. Biotechnol Bioeng 63:401–409
Spoeckner S, Wray V, Nimtz M, Lang S (1999) Glycolipids of the smut fungus Ustilago maydis from cultivation on renewable resources. Appl Microbiol Biotechnol 51:33–39
Stüwer O, Hommel R, Haferburg D, Kleber HP (1987) Production of crystalline surface-active glycolipids by a strain of Torulopsis apicola. J Biotechnol 6:259–269
Sugita T, Takashima M, Poonwan N, Mekha N, Malaithao K, Thungmuthasawat B, Prasarn S, Luangsook P, Kudo T (2003) The first isolation of ustilaginomycetous anamorphic yeasts, Pseudozyma species, from patients' blood and a description of two new species: P. parantarctica and P. thailandica. Microbiol Immunol 47:183–190
Woo SH, Park JM, Rittmann BE (2001) Evaluation of the interaction between biodegradation and sorption of phenanthrene in soil-slurry systems. Biotechnol Bioeng 73:12–24
Zhao X, Wakamatsu Y, Shibahara M, Nomura N, Geltinger C, Nakahara T, Murata T, Yokoyama K (1999) Mannosylerythritol lipid is a potent inducer of apoptosis and differentiation of mouse melanoma cells in culture. Cancer Res 59:482–486
Acknowledgements
This work was supported by a grant from the Korean Molecular and Cellular BioDiscovery Research Program and the MOST/KOSEF to the Environmental Biotechnology National Core Research Center (grant #: R15-2003-012-02001-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, HS., Jeon, JW., Kim, BH. et al. Extracellular production of a glycolipid biosurfactant, mannosylerythritol lipid, by Candida sp. SY16 using fed-batch fermentation. Appl Microbiol Biotechnol 70, 391–396 (2006). https://doi.org/10.1007/s00253-005-0092-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0092-9