Abstract
In this study, fennel oil was isolated by hydrodistillation, and the chemical composition was determined by gas chromatography/mass spectral analysis. The antimicrobial activity of fennel oil against Staphylococcus aureus was evaluated by broth microdilution. A haemolysis assay, tumour necrosis factor (TNF) release assay, western blot, and real-time reverse transcription (RT)-PCR were applied to investigate the influence of fennel oil on the production of S. aureus virulence-related exoproteins. The data show that fennel oil, which contains a high level of trans-anethole, was active against S. aureus, with MICs ranging from 64 to 256 μg/ml. Furthermore, fennel oil, when used at subinhibitory concentrations, could dose-dependently decrease the expression of S. aureus exotoxins, including α-toxin, Staphylococcal enterotoxins (SEs) and toxic shock syndrome toxin 1 (TSST-1).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus is a major medical pathogen that causes a wide variety of infections, from simple abscesses to fatal sepsis, as well as toxinoses, such as food poisoning and toxic shock syndrome (Lowy 1998). Its pathogenic versatility is largely attributed to its ability to produce and secrete a number of virulence factors, including superantigen toxins, haemolytic cytotoxins, and surface proteins.
α-toxin is a pore-forming haemolytic toxin that causes membrane damage to many types of mammalian cells. The α-toxin monomers bind to cell membranes and then associate into a heptameric complex to form a pore (Song et al. 1996). Staphylococcal enterotoxins (SEs) and toxic shock syndrome toxin 1 (TSST-1), which are known as pyrogenic toxin superantigens (PTSAgs), share certain structural and biological properties and stimulate the proliferation of T lymphocytes and the release of T-cell-derived cytokines (e.g., TNF-α) (Dinges et al. 2000). These toxins were initially implicated in staphylococcal food poisoning (SEs) and TSS (TSST-1).
Because S. aureus develops resistance to new antibiotics almost as fast as they are introduced, there is a continuous demand to find new and improved antimicrobial agents or to develop alternative therapeutic strategies in both the food and pharmaceutical industries. One of the areas that is currently the subject of considerable interest is plant extracts, and particularly their essential oils, for their potent antimicrobial properties against a broad spectrum of micro-organisms (Smith-Palmer et al. 1998). Fennel (Foeniculum vulgare Miller) is a major aromatic plant belonging to the Apiaceae family, which has long been considered as a medicinal and spice herb. Fennel oil is commonly used as a flavouring agent in food products and as a constituent of cosmetic and pharmaceutical products; furthermore, its antimicrobial properties against a wide range of micro-organisms have been well established (Elgayyar et al. 2001; Aprotosoaie et al. 2008). It has long been known that some plant essential oils could affect the expression of staphylococcal exotoxins (Smith-Palmer et al. 2004). Consequently, the present study aimed to evaluate the influence of subinhibitory fennel oil concentrations on the expression of α-toxin, the two major enterotoxins (SEA and SEB), and TSST-1 in S. aureus.
Materials and methods
Bacterial strains
The methicillin-susceptible S. aureus strain ATCC 29213 was obtained from ATCC. Nineteen S. aureus clinical isolates (6 MSSA and 13 MRSA) were acquired from the First Hospital of Jilin University; the clinical MRSA strains 2985 and 3701, which have the ability to produce α-toxin, SEA and SEB, and TSST-1, were selected for further tests. Bacteria were stored as a 30% glycerol stock at −80°C until testing.
Plant material and essential oil extraction
Fennel (Foeniculum vulgare Miller) was collected from the Guangxi Province of China in July 2009. The plant material was air-dried in a shady and aerated room until the weight was stable. The fennel was crushed, and the fennel oil was extracted by hydrodistillation for 4 h using a Clevenger-type apparatus. The essential oil was dried over anhydrous sodium sulphate.
Gas chromatography/mass spectral analysis
The chemical composition of the fennel oil was detected by GC/MS performed on a GCMS-QP2010 Plus Gas Chromatograph/Mass Spectrometer (Shimazu Co., Ltd., Kyoto, Japan) equipped with a fused silica capillary column (Rix-5 ms; 30 m × 0.25 mm × 0.25 μm; Shimadzu, Kyoto, Japan). The carrier gas was helium (1 ml/min). The oven temperature was maintained at 60°C for 10 min and programmed to reach 250°C at a rate of 10°C/min. The split ratio was adjusted to 100:1, and the injector temperature was set at 280°C. Mass spectra were obtained by electronic impact at 70 V, and the mass range was from m/z 50 to 500. Identification of the constituents was performed by a computer-based library search.
MIC determination
Stock solutions of fennel oil at different concentrations were prepared in dimethyl sulphoxide (DMSO) (Sigma-Aldrich). The MICs (minimum inhibitory concentrations) of fennel oil against 20 S. aureus strains were evaluated using a broth microdilution method as described by Carson et al. (1995), with minor modifications. All tests were performed in Mueller–Hinton broth (MHB) (BD Biosciences, Inc., MD, USA) supplemented with Tween-80 (Sigma-Aldrich) at a final concentration of 0.5%. Serial doubling dilutions of fennel oil were prepared in a 96-well plate over the range of 8–1,024 μg/ml. Following the inoculation of 5 × 105 cfu/ml of overnight broth cultures into each well, the plates were incubated aerobically at 37°C for 24 h. The MIC was defined as the lowest concentration of fennel oil at which the bacteria do not demonstrate visible growth.
Growth curve assay
An overnight culture of S. aureus was diluted into 1000 ml fresh MHB supplemented with 0.5% Tween-80 and grown at 37°C with shaking at 200 rpm to obtain a starting OD 600 value of 0.3, and 100-ml volumes of the culture were aliquoted into six 250-ml Erlenmeyer flasks. Five of the flasks were supplemented with fennel oil (dissolved in DMSO) at concentrations of 6.25, 12.5, 25, 50 and 100% MIC. Bacteria were further cultured at 37°C with agitation at 200 rpm under aerobic conditions, and the growth of the cells was monitored by measuring the OD 600 values at the indicated time points.
Haemolysis assay
The haemolysis assay was performed as previously described (Qiu et al. 2010b) with rabbit erythrocytes. In brief, bacteria were grown in MHB supplemented with 0.5% Tween-80 at 37°C with graded subinhibitory concentrations of fennel oil until reaching the post-exponential growth phase (OD600 nm of 2.5). Culture supernatants were collected and were filter sterilised with a 0.22-μm (pore size) acetate syringe filter. A 0.1-ml volume of culture supernatant was mixed with 2.5% defibrinated rabbit blood in PBS buffer. After 15 min at 37°C, the unlysed blood cells were pelleted by centrifugation (5,500 × g, room temperature, 1 min). The haemolytic activity was determined by measuring the optical density (at 543 nm) of the cell-free supernatant. The control culture supernatant served as the 100% hemolysis control, and the % hemolysis was calculated by comparison to the control culture.
Tumour necrosis factor (TNF) release assay
The TNF release assay was performed by an established method described by Qiu et al. (2010a). In short, overnight bacterial cultures grown in RPMI-1640 (Invitrogen, CA, USA) were incubated into fresh, prewarmed RPMI-1640 medium (500 ml) supplemented with 0.5% Tween-80. Following incubation at 37°C for 30 min with aeration, cultures were divided into aliquots of 100 ml. Increased concentrations of fennel oil were added to bacterial suspensions, and the cultures were further incubated for 4 h at 37°C with constant shaking. S. aureus supernatants were collected by centrifugation, filtered through a 0.2-μm filter and immediately analysed as described below.
The animal studies were conducted in accordance with the experimental practices and standards approved by the Animal Welfare and Research Ethics Committee at Jilin University. Specific-pathogen-free BALB/c mice (male, 6–8 weeks old, weighing 18–22 g) were supplied by the Experimental Animal Center of Jilin University (Changchun, China). Mice were euthanised by cervical dislocation. Single spleen cell suspensions were prepared in RPMI-1640, washed and resuspended in complete RPMI-1640 medium. A total of 106 (150 μl) cells were added to wells of 96-well plat tissue culture plates and then supplemented with 50 μl of S. aureus culture supernatants. After incubation for 16 h at 37°C, the supernatants were harvested by centrifugation (1,000 × g for 5 min). TNF in the supernatants was measured using the Mouse TNF-α ELISA MAXTM Standard Set (Biolegend, Inc., San Diego, USA).
Western blot assay
Bacteria were cultured, and supernatants were collected in the same fashion as for the haemolysis assay. Proteins in equal volumes of culture supernatants (20 μl) were subjected to sodium dodecyl sulphate (SDS)-polyacrylamide (12%) gel electrophoresis at 120 V. Proteins were transferred to polyvinylidene fluoride membranes (Wako Pure Chemical Industries, Ltd, Osaka, Japan) using a semidry transfer cell (Bio-Rad, Munich, Germany). Membranes were incubated overnight at 4°C in 10% milk powder as a blocking reagent. The production of α-toxin, SEA, SEB, and TSST-1 in S. aureus was detected by incubation with the indicated antibodies. Antibodies to SEA, SEB, and α-hemolysin were purchased from Sigma-Aldrich and diluted to 1:10000, 1:5000 and 1:8000, respectively; then, a horseradish peroxidase-conjugated anti-rabbit antiserum (Sigma-Aldrich) diluted to 1:4000 was used as the secondary antibody. The antibody to TSST-1 (Santa Cruz Biotechnology, California, USA) was diluted to 1:200 according to the manufacturer’s recommendations; then, a horseradish peroxidase-conjugated anti-mouse antiserum (Sigma-Aldrich) diluted to 1:5000 was used as the secondary antibody. The blots were developed using ECL substrate (GE Healthcare, Buckinghamshire, UK).
Proteolytic activity assay
Samples of culture supernatant (100 μl) were added to 1 ml of azocasein (Sigma-Aldrich; 1 mg/ml in 100 mmol Tris–HCl, PH 7.2) and incubated at 37°C for 1 h. The reaction was terminated by addition of 1 ml of trichloroacetic acid (5%, w/v) and mixing; undigested azocasein was allowed to precipitate for 30 min. The mixture was then centrifuged at 10,000×g for 10 min and the absorbance of the supernate read at 328 nm. One unit of protease activity was defined as giving an absorbance of 0.001 after incubation for 1 h at 37°C.
RNA isolation and real-time RT-PCR
The S. aureus strain ATCC 29213 was incubated with or without the addition of 50% MIC of fennel oil to the post-exponential growth phase (OD600 nm of 2.5), as described in the haemolysis assay. Total RNA was isolated using the RNeasy Mini kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s directions. The contaminating DNA was removed using the optional on-column RNase-free DNase I step (Qiagen, Hilden, Germany). RNA was reverse transcribed into cDNA using the Takara RNA PCR kit (AMV) Ver. 3.0 (Takara, Kyoto, Japan), according to the manufacturer’s protocol. The genes and primer sequences employed for the real-time RT-PCR analysis are listed in Table 1. The real-time PCR was performed using the 7000 Sequence Detection System (Applied Biosystems, Courtaboeuf, France) and SYBR Premix Ex Taq (Takara). Reaction mixtures were initially incubated for 30 s at 95°C, followed by 35 cycles of 5 s at 95°C, 30 s at 55°C, and 20 s at 72°C. A melt-curve analysis was also carried out to assess PCR specificity and resulted in single primer-specific melting temperatures. All samples were analysed in triplicate, and the housekeeping gene 16S rRNA was used as an endogenous control. In this study, relative quantification based on the relative expression of a target gene versus the 16S rRNA gene was utilised to determine the transcript level changes between samples.
Statistical analysis
Statistical analysis was performed with SPSS 12.0 statistical software. The data are presented as the mean values ± SD (n = 3). An independent Student’s t-test was used to analyse the data. Differences were considered statistically significant when the p value was less than 0.05.
Results
The chemical composition of fennel oil is shown in Table 2. Fennel oil contains a high level of trans-anethole (88.91%).
The MIC of fennel oil for each of the 20 S. aureus strains was evaluated and ranged from 64 to 256 μg/ml. The MIC values of fennel oil against S. aureus ATCC 29213, MRSA 2985 and MRSA 3701 were 128 μg/ml. As shown in Fig. 1, fennel oil at concentrations from 6.25 to 50% MIC had no significant influence on the growth of S. aureus strain ATCC 29213. However, when supplemented with 100% MIC of fennel oil, the growth rate was significantly decreased; after 30, 180 and 360 min of fennel oil treatment, the OD600 values were 54.5, 53.1 and 58.9% of the fennel oil-free culture, respectively. Although the growth kinetics can vary greatly between strains, the growth of MRSA 2985 and MRSA 3701 were affected by these concentrations of fennel oil in a similar manner. In other words, the addition of 6.25, 12.5, 25, and 50% MIC of fennel oil had no significant effects on MRSA 2985 and MRSA 3701 growth (data not shown).
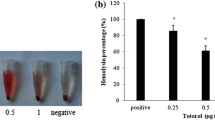
As shown in Table 3, when supplemented with 6.25% MIC of fennel oil, the haemolysis of S. aureus strains ATCC 29213, MRSA 2985 and MRSA 3701 culture supernatants were 66.7, 55.8 and 42.5% of their drug-free cultures, respectively. Remarkably, no haemolytic activities were observed when cultured with 50% MIC of fennel oil. This dose-dependent inhibition of haemolysis was observed in all of the investigated strains. As shown in Fig. 2, the culture supernatants of S. aureus grown in the presence of graded subinhibitory concentrations of fennel oil stimulated significantly lower levels of TNF-α release. In addition, fennel oil itself did not stimulate or inhibit TNF-α production at 100% MIC (data not shown). Apparently, fennel oil repressed the TNF-inducing activity of S. aureus culture supernatants in a dose-dependent fashion.
Figure 3 shows the α-toxin, SEA and SEB, and TSST-1 levels in the S. aureus culture supernatants after exposure to fennel oil. Treatment with increasing concentrations of fennel oil resulted in a dose-dependent decrease in the production of α-toxin, SEA and SEB, and TSST-1. Growth with 6.25% MIC of fennel oil resulted in a recognisable reduction in the secretion of these toxins, whereas at 50% MIC, no or little immunoreactive proteins could be detected in all of the strains tested.
There was no significant influence on protease secretion by ATCC 29213, MRSA 2985 or MRSA 3701 cultured with graded subinhibitory concentrations of fennel oil (Fig. 4).
Table 4 shows that treatment with 50% MIC of fennel oil significantly decreases the transcription levels of hla, sea, seb, tst, and agrA of the S. aureus strain ATCC 29213.
Discussion
The increasing emergence of multi-drug-resistant pathogens has intensified the need to find novel antimicrobial agents for the prevention and treatment of bacterial infections. In recent decades, plant essential oils have been gaining great attention, and their antibacterial activities against a broad spectrum of micro-organisms have been well established (Smith-Palmer et al. 1998, Smith-Palmer 1999; Valero and Salmeron 2003). Due to their multi-component nature, plant essential oils are more difficult for bacteria to develop resistance to than many common used antibiotics, which have a single target site (Smith-Palmer et al. 2004). In the last few years, many studies have been conducted in different countries to demonstrate the significance of these oils in therapeutic treatments (Benoit-Vical et al. 2006; Senatore et al. 2007). In the present study, fennel oil was active against both MSSA and MRSA, with MICs ranging from 64–256 μg/ml. The results were consistent with previous studies (Mohsenzadeh 2007; Aprotosoaie et al. 2008), indicating that fennel oil is a potentially effective antimicrobial agent against S. aureus, and it may deserve further investigation for its potential therapeutic efficacy in S. aureus infections.
Previous studies have indicated that the expression of S. aureus exotoxins could be influenced by subinhibitory concentrations of antimicrobial agents. Therefore, the antibiotic-induced modulation of virulence factors may result in either aggravation or attenuation of the infection. For example, some β-lactam antibiotics strongly induce the production of virulence-related exoproteins (Ohlsen et al. 1998), suggesting that the symptoms of S. aureus infections may be intensified when patients are treated with these antibiotics. In contrast, some protein-synthesis-suppressing antibiotics, such as clindamycin, linezolid, and quinupristin/dalfopristin, have been shown to disrupt the expression of S. aureus virulence factors (Herbert et al. 2001; Bernardo et al. 2004; Koszczol et al. 2006). As a consequence, these antibiotics are recommended for the management of S. aureus-produced toxic syndromes. Furthermore, it has also been demonstrated that some plant essential oils (e.g., oils of cinnamon, bay and clove) can influence production of exotoxins when used at sub inhibitory concentrations (Smith-Palmer et al. 2004). The data presented here show the ability of subinhibitory concentrations of fennel oil to cause a significant decrease in the production of major exotoxins by S. aureus, indicating that fennel oil may be useful for the treatment of S. aureus infections when used in combination with β-lactam antibiotics. Furthermore, as fennel oil contains extraordinarily high amount of trans-anethole, it might be possible that the effects seen are due mainly to trans-anethole.
Considering the tendency of consumers to avoid foods containing chemical and artificial preservatives, investigators are pursuing natural antimicrobial substances from plant sources, especially their essential oils (Lee et al. 2002). The potential application of essential oils to foods has been well investigated in recent years (Smith-Palmer et al. 2001; Valero and Salmeron 2003). In addition to the inhibition of growth of bacterial cells, researchers are also interested in the inhibition of toxin production. For example, food-borne staphylococcal poisoning is caused by the ingestion of one or more enterotoxins that were pre-formed in foods contaminated with S. aureus. More importantly, SEs are resistant to treatment with heat, strong acid and alkali. Therefore, the ability of fennel oil to inhibit the production of staphylococcal enterotoxins may increase the likelihood of fennel oil being applied as a novel natural food preservative.
Exoprotein expression by S. aureus is co-ordinately controlled by numerous global regulators that act at the transcriptional level (Booth et al. 1997). Previous studies have indicated that sublethal concentrations of antibiotics could affect the translation of certain regulatory gene products in S. aureus, which, in turn, influence the transcription of toxin-encoding genes (Herbert et al. 2001; Qiu et al. 2010b). Therefore, it would be reasonable to infer that the fennel oil-induced inhibition of global regulators might lead to the decreased production of exotoxins. The agr locus is one of the well-characterised staphylococcal global regulators and enhances the post-exponential-phase expression of secreted proteins (e.g., α-toxin, SEB and TSST-1) (Arvidson and Tegmark 2001). The real-time RT-PCR data indicated that the transcriptional level of agrA in S. aureus strain ATCC 29213 was significantly decreased after treatment with 50% MIC of fennel oil. The regulatory mechanism by which S. aureus controls the expression of virulence factors is extremely complicated, involving an interactive, hierarchical regulatory cascade among the gene products of agr and sar as well as other regulators (Chan and Foster 1998). Therefore, we presume that the reduced production of α-toxin, SEB and TSST-1 may, in part, depend on the inhibition of the agr locus induced by fennel oil. The expression of SEA is not controlled by the agr regulatory system, and the action of regulatory genes on SEA production in S. aureus is still unclear (Arvidson and Tegmark 2001). Therefore, it is definite that the impact of fennel oil on SEA production cannot be mediated through influence on agr, and the regulatory mechanism that governs fennel-induced reduction of SEA production remains to be determined.
References
Aprotosoaie AC, Hăncianu M, Poiată A, Tuchiluş C, Spac A, Cioană O, Gille E, Stănescu U (2008) In vitro antimicrobial activity and chemical composition of the essential oil of Foeniculum vulgare Mill. Rev Med Chir Soc Med Nat Iasi 112:832–836
Arvidson S, Tegmark K (2001) Regulation of virulence determinants in Staphylococcus aureus. Int J Food Microbiol 291:159–170
Benoit-Vical F, Grellier P, Abdoulaye A, Moussa I, Ousmane A, Berry A, Ikhiri K, Poupat C (2006) In vitro and in vivo antiplasmodial activity of Momordica balsamina alone or in a traditional mixture. Chemotherapy 52:288–292
Bernardo K, Pakulat N, Fleer S, Schnaith A, Utermöhlen O, Krut O, Müller S, Krönke M (2004) Subinhibitory concentrations of linezolid reduce Staphylococcus aureus virulence factor expression. Antimicrob Agents Chemother 48:546–555
Booth MC, Cheung AL, Hatter KL, Jett BD, Callegan MC, Gilmore MS (1997) Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect Immun 65:1550–1556
Carson CF, Cookson BD, Farrelly HD, Riley TV (1995) Susceptibility of methicillin-resistant Staphylococcus aureus to the essential oil of Melaleuca alternifolia. J Antimicrob Chemother 35:421–424
Chan PF, Foster SJ (1998) Role of SarA in virulence determinant production and environmental signal transduction in Staphylococcus aureus. J Bacteriol 180:6232–6241
Dinges MM, Orwin PM, Schlievert PM (2000) Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 13:16–34
Elgayyar M, Draughon FA, Golden DA, Mount JR (2001) Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms. J Food Prot 64:1019–1024
Herbert S, Barry P, Novick RP (2001) Subinhibitory clindamycin differentially inhibits transcription of exoprotein genes in Staphylococcus aureus. Infect Immun 69:2996–3003
Koszczol C, Bernardo K, Krönke M, Krut O (2006) Subinhibitory quinupristin/dalfopristin attenuates virulence of Staphylococcus aureus. J Antimicrob Chemother 58:564–574
Lee JY, Kim YS, Shin DH (2002) Antimicrobial synergistic effect of linolenic acid and monoglyceride against Bacillus cereus and Staphylococcus aureus. J Agric Food Chem 50:2193–2199
Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339:520–532
Mohsenzadeh M (2007) Evaluation of antibacterial activity of selected Iranian essential oils against Staphylococcus aureus and Escherichia coli in nutrient broth medium. Pak J Biol Sci 10:3693–3697
Ohlsen K, Ziebuhr W, Koller KP, Hell W, Wichelhaus TA, Hacker J (1998) Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother 42:2817–2823
Qiu J, Feng H, Xiang H, Wang D, Xia L, Jiang Y, Song K, Lu J, Yu L, Deng X (2010a) Influence of subinhibitory concentrations of licochalcone A on the secretion of enterotoxins A and B by Staphylococcus aureus. FEMS Microbiol Lett 2:135–141
Qiu J, Wang D, Xiang H, Feng H, Jiang Y, Xia L, Dong J, Lu J, Yu L, Deng X (2010b) Subinhibitory concentrations of thymol reduce enterotoxins A and B and alpha-hemolysin production in Staphylococcus aureus isolates. PLoS One 5:e9736
Senatore F, Rigano D, Formisano C, Grassia A, Basile A, Sorbo S (2007) Phytogrowth-inhibitory and antibacterial activity of Verbascum sinuatum. Fitoterapia 78:244–247
Smith-Palmer A (1999) The antimicrobial properties of plant essential oils against foodborne pathogens. PhD thesis, Queen Margaret University College, Edinburgh, UK
Smith-Palmer A, Stewart J, Fyfe L (1998) Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett Appl Microbiol 26:118–122
Smith-Palmer A, Stewart J, Fyfe L (2001) The potential application of plant essential oils as natural food preservatives in soft cheese. Food Microbiol 18:463–470
Smith-Palmer A, Stewart J, Fyfe L (2004) Influence of subinhibitory concentrations of plant essential oils on the production of enterotoxins A and B and alpha-toxin by Staphylococcus aureus. J Med Microbiol 53:1023–1027
Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE (1996) Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 274:1859–1866
Valero M, Salmeron MC (2003) Antibacterial activity of 11 essential oils against Bacillus cereus in tyndallized carrot broth. Int J Food Microbiol 85:73–81
Acknowledgments
This work was supported by the National Nature Science Foundation of China (No. 30972212) and the State Key Laboratory for molecular virology and genetic engineering (No. 2011KF02).
Conflict of interest
The authors declare that there have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jiazhang Qiu and Hongen Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Qiu, J., Li, H., Su, H. et al. Chemical composition of fennel essential oil and its impact on Staphylococcus aureus exotoxin production. World J Microbiol Biotechnol 28, 1399–1405 (2012). https://doi.org/10.1007/s11274-011-0939-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-011-0939-4