Abstract
Staphylococcus aureus (S. aureus) causes a wide variety of infections, which are of major concern worldwide. S. aureus produces multiple virulence factors, resulting in food infection and poisoning. These virulence factors include hyaluronidases, proteases, coagulases, lipases, deoxyribonucleases and enterotoxins. Among the extracellular proteins produced by S. aureus that contribute to pathogenicity, the exotoxins α-hemolysin, staphylococcal enterotoxin A (SEA) and staphylococcal enterotoxin B (SEB) are thought to be of major significance. Totarol, a plant extract, has been revealed to inhibit the proliferation of several pathogens effectively. However, there are no reports on the effects of totarol on the production of α-hemolysin, SEA or SEB secreted by S. aureus. The aim of this study was to evaluate the effects of totarol on these three exotoxins. Hemolysis assay, western blotting and real-time reverse transcriptase-PCR assay were performed to identify the influence of graded subinhibitory concentrations of totarol on the production of α-hemolysin and the two major enterotoxins, SEA and SEB, by S. aureus in a dose-dependent manner. Moreover, an enzyme linked immunosorbent assay showed that the TNF-α production of RAW264.7 cells stimulated by S. aureus supernatants was inhibited by subinhibitory concentrations of totarol. Form the data, we propose that totarol could potentially be used as a promising natural compound in the food and pharmaceutical industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus (S. aureus), a major pathogen, is a leading cause of both community- and hospital- acquired infections associated with high morbidity and mortality rates (Koszczol et al. 2006; Qiu et al. 2010). This pathogen is capable of causing a wide spectrum of clinical illnesses, including skin and soft tissue lesions, and even food-borne illness (Balaban and Rasooly 2000; Le Loir et al. 2003). In part, the diversity depends on the secretion of a broad spectrum of soluble extracellular proteins. These proteins include enterotoxins, hemolysins, toxic shock syndrome toxin 1, and others (Koszczol et al. 2006). α-hemolysin is secreted by S. aureus as a water-soluble monomer that assembles into a heptamer to form a transmembrane pore on a target membrane (Kawate and Gouaux 2003). The staphylococcal enterotoxins (SEs), a family of nine major serological types of heat stable enterotoxins, are a group of major virulence factors (Larkin et al. 2009), including staphylococcal enterotoxin A (SEA), staphylococcal enterotoxin B (SEB), SEC (SEC1, SEC2, SEC3), SED, SEE, SEG and SEH produced by S. aureus throughout the logarithmic phase of growth and during the transition from the exponential to the stationary phase in a growth-phase-dependent manner (Novick et al. 2010; Koszczol et al. 2010). These toxins cause staphylococcal gastroenteritis, toxic shock-like syndromes, several allergic and autoimmune diseases and food poisoning in humans. Thus, there is a continuing and urgent need to discover new and improved antimicrobial agents to treat S. aureus illnesses, with potential benefits for both the food and pharmaceutical industries (Smith-Palmer et al. 2004).
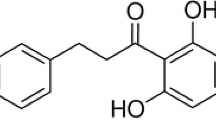
Totarol, a traditional Chinese medicinal herb, is an antibacterial novel phenolic diterpenes, and it is isolated from Podocarpus spp. and a variety of other sources (Jaiswal et al. 2007). Previous studies (Constantine et al. 2001) have shown that totarol could be a potentially effective antimicrobial against S. aureus and could be used to treat clinical illnesses and avoid food spoilage, and it may to be the most potent agent against S. aureus (Muroi and Kubo 1996). And new totarol type diterpenes also showed good antibacterial activity (Sato et al. 2008). Moreover, totarol is approved for use as an antimicrobial additive in several consumer products, including toothpaste and acne treatments (Kim and Shaw 2010).
It has long been known that certain antibiotics can influence the expression of staphylococcal exotoxins. However, to our knowledge, the effects of totarol on the secretion of α-hemolysin and enterotoxins by S. aureus remain uncharacterized. Therefore, in this study, we aimed to assess and investigate the influence of subinhibitory concentrations of totarol on the production of the major enterotoxins, SEA and SEB, and α-hemolysin, by methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA). The importance of the other enterotoxins of SEs are inferior to SEA and SEB, so we didn’t study for the time being. The MRSA strains 1862, 3625, 1980 and the MSSA strain ATCC 29213 were selected for real-time reverse transcriptase-PCR (real-time RT-PCR), western blotting, and tumour necrosises factor alpha (TNF-α) release assays in order to avoid overlooking the potential strain-specific differences and reactivity ranges.
Materials and methods
Bacterial strains and reagents
Nine clinical MRSA isolates were obtained from the First Hospital of Jilin University from the blood samples of infected patients. The quality control strain, the MSSA strain ATCC 29213, was obtained from the China Medical Culture Collection Center. Totarol was purchased from Sigma-Aldrich, and stock solutions at various concentrations were dissolved in dimethyl sulfoxide (DMSO) obtained from Sigma-Aldrich. Mueller–Hinton (MH) broth used to test antimicrobial susceptibility according to Clinical and Laboratory Standards Institute guidelines (CLSI 2009) and lysogeny broth (LB) used to enrich and cultivate S. aureus in order to reached at the post-exponential growth phase were purchased from Qingdao Hope Bio-Technogy Co., Ltd.
Antimicrobial susceptibility testing
The minimal inhibitory concentrations (MICs) of totarol for S. aureus ATCC 29213 and MRSA strains, which have the potency to produce α-hemolysin, SEA and SEB, were evaluated in triplicate by the broth microdilution method adapted from previous researchers and in accordance with the CLSI. The test was performed in 96-well flat-bottomed microtitration plates. In brief, target strains were inoculated on MH agar and grown overnight at 37 °C. Totarol were prepared in MH broth to obtain graded subinhibitory concentrations by serial two-fold dilutions. S. aureus were grown and diluted in MH broth to 105 CFU mL−1. A total of 50 μL of the graded totarol dilutions was added to individual wells of a 96-well microtiter plate in triplicate. Then, the 50 μL of a bacterial culture was added to each well. Finally, the plate was inoculated aerobically at 37 °C for 24 h. The MICs were defined as the lowest concentration of totarol at which no visible growth was observed.
Hemolysis assay
Hemolytic activity was determined based on a method previously described with rabbit erythrocytes (Worlitzsch et al. 2001). The isolated MRSA 1862 selected in this assay was cultured in the presence of graded subinhibitory concentrations of totarol in LB at 37 °C until the post-exponential growth phase was reached (OD600 = 2.5, equivalent to 1.0 × 109 CFU mL−1). Hemolytic activities of bacterial culture supernatants were evaluated according to the method of Rowe and Welch (Rowe and Welch 1994). The bacterial culture supernatants were collected after centrifugation (5500×g, 4 °C, 1 min), and before the addition of 25 μL of defibrinated rabbit blood, a 100 μL of bacterial culture supernatant was added to 875 μL of phosphate-buffered saline (PBS) buffer. And then, they were incubated for 30 min at 37 °C. Following centrifugation (5500×g, 4 °C, 1 min), the hemolytic activity was detected by measuring the optical density at 543 nm of the supernatant. The positive control, totarol-free culture supernatant, served as the 100 % hemolysis control, and the percent hemolysis was calculated by comparison with the positive control culture. The PBS buffer served as a negative control.
Real-time RT-PCR assay
The MRSA 1862, MRSA 3625, MRSA 1980 and ATCC 29213 were cultivated in LB in the presence or absence of graded subinhibitory concentrations of totarol (0.25, 0.5 and 1 μg mL−1) to the post-exponential growth phase for approximately 8 h. The totarol-free culture with DMSO served as control. Total RNA from S. aureus strains treated with graded subinhibitory concentrations of totarol were extracted using the TRIzol RNA isolation kit (Life Technologies) as described in the manufacturer’s manual (Yeh and Yen 2006). The primer pairs which have already been published by Qiu et al. (2010) used in quantitative RT-PCR are listed in Table 1. In brief, RNA was reverse transcribed into cDNA using the Takara RNA PCR kit (Takara, Kyoto, Japan). The PCR reactions were performed in 20 μL total volume and contained SYBR Premix Ex Taq™ (Takara). The reactions were performed using the 7000 Sequence Detection System. The cycling conditions were as follows. Stage 1: cycle at 95 °C for 30 s; Stage 2: 40 cycles at 95 °C for 5 s, 60 °C for 34 s; Stage 3: one dissociation step of 95 °C for 15 s, 60 °C for 30 s, and 95 °C for 15 s. All samples were analyzed in triplicate and normalized against 16S rRNA expression. Relative expression levels were determined by the (ΔΔC T) method described in Applied Biosystems User Bulletin (Livak and Schmittgen 2001). All samples were measured in triplicate.
Western blot assay
The MSSA strain ATCC 29213 and MRSA 1862, MRSA 3625, MRSA 1980 were grown in LB ensured that the bacterial could reach at the post-exponential growth phase. After overnight incubation, S. aureus strain ATCC 29213 and MRSA strains were adjusted to an optical density of 0.05 at 600 nm with fresh LB. The culture samples were supplemented with subinhibitory concentrations of totarol (0.5, 1 and 2 μg mL−1), and collected from post-exponential growth-phase cultures (OD600 nm of 2.5, equivalent to 1.0 × 109 CFU mL−1), the bacteria incubated with totarol about 12 h. The totarol-free culture with DMSO served as control. The 100 % of trichloroacetic acid was added to the culture supernatants, with a final concentration of 10 %. After overnight incubation at 4 °C, the precipitate was centrifuged at 8500×g for 30 min at 4 °C. Then, the precipitate was washed with ice-cold ethanol. After recentrifugation and drying, it was dissolved in 0.5 ml of 0.1 M Tris. Western blotting was performed as described previously (Sun et al. 2010). For Western blotting, proteins in equal volumes of culture supernatants (28 μL) were subjected to sodium dodecyl sulphate-polyacrylamide (12 %) gel electrophoresis (SDS-PAGE) at 120 V. Then electrophoretically transferred into polyvinylidene fluoride (PVDF) membranes in transfer buffer using a semidry transfer cell (Bio-Rad, Munich, Germany) at 15 V for 40 min. After blocking with 5 % skimmed milk with shaking slowly, PVDF membranes were incubates with α-hemolysin, SEA, SEB antibodies diluted to 1:8000, 1:10,000 and 1:5000 with 5 % skimmed milk according to the manufacturers were incubated overnight at 4 °C. Then, the membranes were incubated with HRP-conjugated goat-anti rabbit antiserum as the secondary antibody. Finally, the reacted patterns were visualized with ECL substrate (Beyotime), and the images were obtained using a CanoScan LiDE 100 scanner (Canon). Protein blots were measured using the Image-J software in order to obtain accurate results (Huang et al. 2013).
TNF-α release assay
TNF-α release assay was performed according to a modified method described previously (Bernardo et al. 2004). Briefly, the MRSA 1862, MRSA 3625, MRSA 1980 were grown overnight in fresh LB until the optical density at 600 nm reached over 2.5. Then, the S. aureus were diluted 30-fold into 40 mL of DMEM, respectively. After incubation at 37 °C for 30 min with constant shaking, the S. aureus supernatants diluted by DMEM cultures were divided into 10 mL portions in four triangular flasks. The culture samples were supplemented with subinhibitory concentrations of totarol (0.25, 0.5 and 1 μg mL−1), the totarol-free culture with DMSO served as control. Then, the cultures were further incubated at 37 °C for 4 h with constant shaking. Finally, the S. aureus strains were centrifugated at 1000×g for 5 min, and the supernatants containing secreted proteins were filtered through a 0.2-μm pore-size filter. A total of 106 (200 μL) RAW264.7 macrophage cells were seeded into 96-well tissue culture plates. After cell adherence, the 50 μL of MRSA strains culture supernatants with graded subinhibitory concentrations of totarol were added into the fresh DMEM medium and incubated at 37 °C for 16 h. Then, the supernatants were collected and centrifuged at 1000×g for 5 min. TNF-α in the supernatants were measured using mouse TNF-α Platinum ELISA (eBioscience, USA) in accordance with the instructions of the manufacturer, respectively. As a comparison, ATCC 29213 was also performed in TNF-α release assay.
Results
Effects of subinhibitory concentrations of totarol on S. aureus growth
The antibacterial activity of totarol against nine different MRSA strain isolates and the MSSA strain ATCC 29213 were assessed. The MIC values of totarol against S. aureus are shown in Table 2. According to these results, we concluded that the MIC values of totarol against S. aureus strains were 2–4 μg mL−1. Totarol could be a kind of antimicrobial agent, because it has the potential to act against S. aureus, which causes clinical illnesses and food spoilage.
Totarol attenuates hemolysis by S. aureus by decreasing the production of α-hemolysin
The MRSA 1862, a clinical MRSA strain which was chosen to be representative in this assay, was cultured with increasing subinhibitory concentrations of totarol, and the bacterial culture supernatants were subjected to hemolysis assay. Hemolytic ability of S. aureus MRSA 1862 supernatants assay showed totarol could obviously inhibit hemolytic effects with the red cell of rabbit co-incubation for 30 min at 37 °C (Fig. 1a). As shown in Fig. 1b, and the hemolytic percent in positive control for MRSA 1862 served as 100 %. Compared to the positive control, when cultured with 0.25, 0.5 and 1 μg mL−1 of totarol, the percentage of hemolysis was reduced to 95.6, 81.5 and 26.5 %, respectively. For the negative control, the percentage of hemolysis was 5.9 %. As expected, a dose-dependent attenuation of hemolysis was observed in the tested strain. In addition, totarol itself did not cause haemolysis of rabbit erythrocytes at 0.5 or 1 μg mL−1 concentrations (data not shown).
Hemolytic activities of MRSA 1862 culture supernatants grown in the presence or absence of totarol. Qualitative analysis (a) and quantitative analysis (b). Haemolysis of rabbit erythrocytes by culture supernatants of MRSA 1862 in the absence or presence of graded concentrations of totarol. Values represent the mean ± SD for three independent experiments. Student’s paired t test was used to compare each culture with totarol to the untreated culture (*P < 0.05; **P < 0.01)
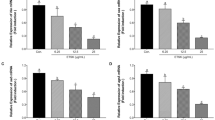
Totarol represses the transcription of agrA, hla, sea and seb by S. aureus
Real-time RT-PCR analysis was used to quantify mRNA levels of hla, sea and seb which regulated the expression of α-hemolysin, SEA and SEB in S. aureus MRSA 1862, MRSA 3625 MRSA 1980 and ATCC 29213 cultures after treatment with different subinhibitory concentrations of totarol. However, the expression of hla, sea and seb are positively regulated by the agr two-component system (Bronner et al. 2004; Sambanthamoorthy et al. 2006). Therefore, the transcription of agrA was also evaluated. As shown in Fig. 2, as expected, totarol markedly decreased the transcription of agrA, hla, sea and seb in MRSA strains and ATCC 29213 in a dose-dependent manner. For example, when cultured with increasing subinhibitory concentrations of totarol against ATCC 29213, the transcriptional levels of hla were reduced to 73.2, 52.3 and 12.5 % compared to the negative control, respectively; and the transcriptional levels of hla in MRSA 1980 were reduced to 74.3, 52.7 and 15.2 % compared to the negative control, respectively. Our data indicate that totarol acts as a potential inhibitor of the transcription of exotoxin genes agrA, hla, sea and seb.
Relative expression levels of agrA, hla, sea and seb in ATCC 29213 (a), MRSA 1862 (b), MRSA 3625 (c) and MRSA 1980 (d) with graded subinhibitory concentrations of totarol. Bars show the mean values of the experiments (n = 3). Error bars show the standard deviation. * Indicates P < 0.05, and ** indicates P < 0.01 when compared with the drug-free group
Totarol represses the expression of α-hemolysin, SEA and SEB
To determine whether the reduced hemolytic activities of S. aureus culture supernatants in the presence of various subinhibitory concentrations of totarol were due to the diminished production of α-hemolysin, the culture supernatants were subjected to western blot analysis, and supernatants were prepared in the same manner as for the haemolysis assay. As exotoxins are secreted during post-exponential growth principally, S. aureus ATCC 29213 and MRSA strains were grown with graded subinhibitory concentrations of totarol to an OD600 of 2.5. As shown in Fig. 3, the results revealed that totarol at subinhibitory concentrations was effective at inhibiting α-hemolysin, SEA and SEB secreted by MRSA 1862, MRSA 3625, MRSA 1980 and ATCC 29213 in a dose-dependent manner. For example, growth in the presence of 0.25 μg mL−1 totarol did not result in a measurable reduction in α-hemolysin, SEA and SEB secreted by ATCC 29213. But at 1 μg mL−1, the production of immunoreactive protein decreased obviously both for MRSA 1862 and ATCC 29213. Exoproteins produced by MRSA 3625 and MRSA 1980 were also decreased obviously, and the amount of SEB was less than SEA and α-hemolysin produced by MRSA 1980. In order to aquire accurate results, we used the Image-J software. As shown in Fig. 3b, d, f, h, the grayscale percentages were decreased obviously compared to the control.
Western blot analysis of α-hemolysin, SEA and SEB secretion by S. aureus strain ATCC 29213 (a, b), MRSA 1862 (c, d), MRSA 3625 (e, f) and MRSA 1980 (g, h) after growth with increasing subinhibitory concentrations of totarol. The proteins were subjected to SDS-PAGE, and probed with the indicated antibodies against SEA and SEB after transfer to PVDF membranes. A horseradish peroxidase-conjugated goat anti-rabbit antiserum was used as the secondary antibody, and the blots were developed using the ECL substrate, and the Image-J software was used to analyse the results of western blot for the accuracy
Totarol reduces TNF-α release
It has been shown that SEs secreted by S. aureus stimulate cells of the immune system such as macrophages resulting in the release of TNF and other proinflammatory cytokines (Balaban and Rasooly 2000; Bernardo et al. 2004; Dinges et al. 2000). Therefore, TNF-α release assay was carried out to elucidate the biological relevance of the reduction in α-hemolysin, SEA and SEB secretion induced by totarol. As shown in Fig. 4, the levels of TNF-α release were reduced when RAW264.7 cells were cultured with supernatant from S. aureus treated with increasing subinhibitory concentrations of totarol (0.25–1 μg mL−1, totarol-free culture with DMSO served as control). When subjected to 0.25, 0.5, 1 μg mL−1 of totarol, the amount of TNF-α released from RAW264.7 macrophage cells stimulated by S. aureus strain ATCC 29213 was decreased to 85.88, 59.15 and 49.75 % compared to control cultures, respectively; for MRSA 1862, it was decreased to 66.62, 37.42, and 32.56 %, respectively; for MRSA 3625, it was decreased to 71.77, 40.05 and 37.14 %, respectively; and for MRSA 1980, it was decreased to 80.56, 42.25 and 39.37 %, respectively. However, treatment with totarol alone did not cause the release of TNF-α at concentrations of 0.5 or 1 μg mL−1 (data not shown). Totarol diminished the TNF-α activity of S. aureus supernatants in a dose-dependent manner. The result was in accordance with western blot assay and real-time RT-PCR assay.
TNF-α release from RAW264.7 subjected to supernatants of S. aureus strains ATCC 29213 and MRSA 1862, MRSA 3625, MRSA 1980 grown to an OD600nm of 2.5 in the presence or absence of increasing concentrations of totarol in DMEM. 24 h later, TNF-α release was measured by ELISA. Values represent the mean ± SD for three independent experiments. Student’s paired t test was used to compare each culture with totarol to the untreated culture (*P < 0.05; **P < 0.01)
Discussion
Staphylococcus aureus is a major cause of hospital acquired (nosocomial) infection of surgical wounds and infections associated with indwelling medical devices. A large number of virulence factors produced by S. aureus may contribute to its pathogenesis and play a significant role. As a consequence, the ability of S. aureus to cause food poisoning depends on the secretion of virulence factors including SEs and others (Dinges et al. 2000). Moreover, staphylococcal gastroenteritis and food poisoning do not result from the ingestion of S. aureus itself but rather from enterotoxins that are preformed within the food (Smith-Palmer et al. 2004). Consequently, the clinical performance with respect to food poisoning of antibiotics used for the treatment of S. aureus infections lies not only on their bacteriostatic/bactericidal effects but also on their ability to prevent the release of virulence factors from dying or stressed bacteria (Bernardo et al. 2004). Among the extracellular proteins produced by S. aureus, α-hemolysin is the key factor responsible for the hemolysis, while SEs are the major toxins that can act as superantigens, inducing macrophages to release proinflammatory cytokines, including TNF-α (Balaban and Rasooly 2000; Dinges et al. 2000; Bernardo et al. 2004). Like most staphylococcal exoproteins, α-hemolysin and SEs are not expressed constitutively, but are primarily secreted during the post-exponential growth phase (Ohlsen et al. 1997). α-hemolysin has an essential influence on S. aureus pneumonia, as strains lacking this toxin are attenuated virulent in a murine model of lung disease (Wardenburg et al. 2007; Qiu et al. 2012). Up to now, a few published reports on the biological activities of totarol have referred to its antimicrobial properties. Totarol has been found to exhibit potent antibacterial activity against a number of Gram-positive bacteria, including S. aureus strains, both the penicillin-susceptible and penicillin-resistant strains (Kubo et al. 1992). Dufour et al. (2003) have previously reported that although the combination of totarol and nisin or lactoperoxidase system did not present the potent antimicrobial activity, it did show a modest enhancement of activity against a number of organisms, particularly the Gram-negative organisms. Smith et al. (2007) have suggested that totarol would be a good lead candidate for further development in the search for effective drugs against resistant S. aureus, but the combination of totarol and other compouds could be more effective. Therefore, the synergistic action of totarol and more compouds is necessary to research in our further investigations. Currently in the food industry, there is a tendency in food processing to avoid the addition of chemical preservatives. The traditional use of plants provides a basis for identifying types of plant extracts useful for specific food purposes. Historically, many plant extracts have been reported to have antimicrobial properties (Hoffman 1987). In addition, the renewal of interest in the food industry and the increasing consumer demand for effective and safe natural products means that quantitative data on plant extracts are required (Bajpai et al. 2008). According to our study, our research has determined that the MIC values of totarol against several S. aureus strains ranged from 2 to 4 μg mL−1. Previous study have shown that totarol exhibited good antibacterial activity, with an MIC of 2 μg mL−1 against S. aureus (Smith et al. 2007), and it is in accordance with our results. Compared with previous data, we found that the antimicrobial activity of totarol was better than that of licochalcones (the MICs of licochalcones A and B are 16 and 128 μg mL−1, respectively) (Hatano et al. 2000), fennel oil (the MIC of fennel oil for S. aureus strains was evaluated and ranged from 64 to 256 μg mL−1) (Qiu et al. 2012), and farrerol (4–16 μg mL−1) (Qiu et al. 2011). Moreover, it has been demonstrated that totarol possessed low toxicity, the half maximal inhibitory concentration (IC50) of totarol was 7.5 μg mL−1 (Gordien et al. 2009). In the present study, through transcriptional, expressional and phenotypic analyses, we concluded that subinhibitory concentrations of totarol reduce α-hemolysin, SEA and SEB secretion in S. aureus ATCC 29213 and MRSA 1862, 3625, 1980 in a dose-dependent manner. Based on the result of the qualitative analysis by hemolysis assay, according to the naked eye, the hemolytic activity of S. aureus MRSA 1862 culture supernatant was decreased by totarol in a dose-dependent manner, consistent with the results of quantitative analysis. The real-time RT-PCR showed that the genes agrA, sea, seb, and hla were notably inhibited at the transcriptional level by totarol in a dose-dependent manner. Previous studies (Oliveira et al. 2006) showed that agrA, hla, sea and seb were the most commonly used real-time RT-PCR amplification targets for the detection of S. aureus isolated from humans and food. Our data indicate that totarol acts as a potential inhibitor of the transcription of the exotoxin genes agrA, hla, sea and seb. From the results of western blot assay, we concluded that totarol was effective at inhibiting α-hemolysin, SEA and SEB secreted by both MRSA 1862, MRSA 3625, MRSA 1980 and ATCC 29213 in a dose-dependent manner. All in all, the results showed the capacity of totarol at subinhibitory concentrations to decrease the production of key virulence factors secreted by S. aureus in a dose-dependent manner. TNF release assay was performed to elucidate the biological relevance of the reduction in SEA and SEB secretion induced by totarol. Our results indicated that TNF-α production by RAW264.7 cells stimulated with S. aureus supernatants containing SEA and SEB was inhibited by subinhibitory concentrations of totarol.
Although the antimicrobial activity of totarol against S.aureus has been demonstrated previously, and in this research, the inhibition of α-hemolysin, SEA and SEB production by totarol was also proved, the mode of antimicrobial action of totarol and inhibitory effect of totarol on exotoxin have not known yet. Several studies on the mechanism of antibacterial activity of totarol claimed cell wall biosynthesis as a possible target. Micol et al. (2001) have indicated that totarol may act by disrupting the phospholipid membrane of bacteria, which led to loss of membrane integrity. Haraguchi et al. (1996) have presented the inhibition of bacterial respiratory transport, but Shapiro and Guggenheim (1998) have found that totarol inhibits growth of anaerobic bacteria. Although different mechanisms for the antibacterial action of totarol have been proposed by several investigators, its antibacterial mechanism of action is far from clear. Thus, further studies about the mode of antimicrobial action of totarol should be performed. In addition, the mechanism of inhibitory effect of totarol on exotoxin remains to be determined, and it is worth to do further experiments.
The research is an area of growing interest, especially as the antimicrobial properties of totarol against a wide range of pathogens are becoming increasingly recognized and their potential application to foods is investigated. Taken together, it is uncommon that totarol have such powerful antimicrobial activities on both MSSA and MRSA, and in view of its antimicrobial properties and antitoxin activity, totarol has the potential to be used as a food preservative and contributes to ensure the safety of foods, and furthermore, it also could be used as an important compound for the design of potent antibacterial agents to fight drug-resistant S. aureus strains.
Conclusions
In conclusion, totarol exhibits obvious inhibitory action against S. aureus growth and secretion of α-hemolysis, SEA and SEB. In view of the broad spectrum of antimicrobial activities of totarol reported previously and the findings reported in this study, we propose that totarol could be used in the food and pharmaceutical industries.
References
Bajpai VK, Rahman A, Kang SC (2008) Chemical composition and inhibitory parameters of essential oil and extracts of Nandina domestica Thunb. to control food-borne pathogenic and spoilage bacteria. Int J Food Microbiol 15:117–122
Balaban N, Rasooly A (2000) Staphylococcal enterotoxins. Int J Food Microbiol 61:1–10
Bernardo K, Pakulat N, Fleer S, Schnaith A, Utermöhlen O, Krut O (2004) Subinhibitory concentrations of linezolid reduce Staphylococcus aureus virulence factor expression. Antimicrob Agents Chemother 48:546–555
Bronner S, Monteil H, Prévost G (2004) Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol Rev 28:183–200
Clinical and Laboratory Standards Institute (CLSI) (2009) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, vol 8., Approved Standard M7-A8CLSI, Wayne, PA
Constantine GH, Karchesy JJ, Franzblau SG, LaFleur LE (2001) (+)-Totarol from Chamaecyparis nootkatensis and activity against Mycobacterium tuberculosis. Fitoterapia 72:572–574
Dinges MM, Orwin PM, Schlievert PM (2000) Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 13:16–34
Dufour M, Simmonds RS, Bremer PJ (2003) Development of a method to quantify in vitro the synergistic activity of “natural” antimicrobials. Int J Food Microbiol 85:249–258
Gordien AY, Gray A, Franzblau SG, Seidel V (2009) Antimycobacterial terpenoids from Juniperus communis L. (Cuppressaceae). J Ethnopharmacol 126:500–505
Haraguchi H, Ishikawa H, Kubo I (1996) Mode of antibacterial action of totarol, a diterpene from Podocarpus nagi. Planta Med 62:122–125
Hatano T, Shintani Y, Aga Y, Shiota S, Tsuchiya T, Yoshida T (2000) Phenolic constituents of licorice. VIII. Structures of glicophenone and glicoisoflavanone, and effects of licorice phenolics on methicillin-resistant Staphylococcus aureus. Chem Pharm Bull (Tokyo) 48:1286–1292
Hoffman D (1987) The herb user’s guide. Thorsons Publishing Group, Wellingborough
Huang H, Kang R, Wang J, Luo G, Yang W, Zhao Z (2013) Hepatitis C virus inhibits AKT-tuberous sclerosis complex (TSC), the mechanistic target of rapamycin (MTOR) pathway, through endoplasmic reticulum stress to induce autophagy. Autophagy 9:175–195
Jaiswal R, Beuria TK, Mohan R, Mahajan SK, Panda D (2007) Totarol inhibits bacterial cytokinesis by perturbing the assembly dynamics of FtsZ. Bioche 46:4211–4220
Kawate T, Gouaux E (2003) Arresting and releasing Staphylococcal α-hemolysin at intermediate stages of pore formation by engineered disulfide bonds. Protein Sci 12:997–1006
Kim MB, Shaw JT (2010) Synthesis of antimicrobial natural products targeting FtsZ: (+)-totarol and related totarane diterpenes. Org Lett 12:3324–3327
Koszczol C, Bernardo K, Kronke M, Krut O (2006) Subinhibitory quinupristin/dalfopristin attenuates virulence of Staphylococcus aureus. J Antimicrob Chemother 58:564–574
Koszczol C, Bernardo K, Kronke M, Krut L (2010) Staphylococcal enterotoxins, toxic shock syndrome toxin and streptococcal pyrogenic exotoxins: a comparative study of their molecular biology. Chem Immunol 55:1–35
Kubo I, Muroi H, Himejima M (1992) Antibacterial activity of totarol and its potentiation. J Nat Prod 55:1436–1440
Larkin E, Carman R, Krakauer T, Stiles B (2009) Staphylococcus aureus: the toxic presence of a pathogen extraordinaire. Curr Med Chem 16:4003–4019
Le Loir Y, Baron F, Gautier M (2003) Staphylococcus aureus and food poisoning. Genet Mol Res 2:63–76
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Micol V, Mateo CR, Shapiro S, Aranda FJ, Villalain J (2001) Effects of (+)-totarol, a diterpenoid antibacterial agent, on phospholipid model membranes. Biochim Biophys Acta 1511:281–290
Muroi H, Kubo I (1996) Antibacterial activity of anacardic acid and totarol, alone and in combination with methicillin, against methicillin-resistant Staphylococcus aureus. J Appl Bacteriol 80:387–394
Novick RP, Christie GE, Penadés JR (2010) The phage-related chromosomal islands of gram-positive bacteria. Nat Rev Microbiol 8:541–551
Ohlsen K, Koller K-P, Hacker J (1997) Analysis of expression of the alpha-toxin gene (hla) of Staphylococcus aureus by using a chromosomally encoded hla:lacZ gene fusion. Infect Immun 65:3606–3614
Oliveira DC, Milheirico C, Vinga S, de Lencastre H (2006) Assessment of allelic variation in the ccrAB locus in methicillin-resistant Staphylococcus aureus clones. J Antimicrob Chemother 58:23–30
Qiu J, Wang D, Xiang H, Feng H, Jiang Y, Xia L et al (2010) Subinhibitory concentrations of thymol reduce enterotoxins A and B and alpha-hemolysin production in Staphylococcus aureus isolates. PLoS ONE 5:e9736
Qiu J, Xiang H, Hu C, Wang Q, Dong J, Li H (2011) Subinhibitory concentrations of farrerol reduce alpha-toxin expression in Staphylococcus aureus. FEMS Microbiol Lett 315:129–133
Qiu J, Niu X, Dong J, Wang D, Wang J, Li H (2012) Baicalin protects mice from Staphylococcus aureus pneumonia via inhibition of the cytolytic activity of α-hemolysin. Infect Immun 75:1040–1044
Rowe GE, Welch RA (1994) Assays of hemolytic toxins. Methods Enzymol 235:657–667
Sambanthamoorthy K, Smeltzer M, Elasri M (2006) Identification and characterization of msa (SA1233), a gene involved in expression of SarA and several virulence factors in Staphylococcus aureus. Microbiology 152:2559–2572
Sato K, Sugawara K, Takeuchi H, Park HS, Akiyama T, Koyama T et al (2008) Antibacterial novel phenolic diterpenes from Podocarpus macrophyllus D. Don. Chem Pharm Bull (Tokyo) 56:1691–1697
Shapiro S, Guggenheim B (1998) Inhibition of oral bacteria by phenolic compounds.1. QSAR analysis using molecular connectivity. Quant Struct Act Relationsh 17:327–337
Smith EC, Kaatz GW, Seo SM, Wareham N, Williamson EM, Gibbons S (2007) The phenolic diterpene totarol inhibits multidrug efflux pump activity in Staphylococcus aureus. Antimicrob Agents Chemother 51:4480–4483
Smith-Palmer A, Stewart J, Fyfe L (2004) Influence of subinhibitory concentrations of plant essential oils on the production of enterotoxins A and B and α-toxin by Staphylococcus aureus. J Med Microbiol 53:1023–1027
Sun SQ, Guo HC, Sun DH, Yin SH, Shang YJ, Cai XP (2010) Development and validation of an ELISA using a protein encoded by ORF2 antigenic domain of porcine circovirus type 2. Virol J 7:274–280
Wardenburg JB, Patel RJ, Schneewind O (2007) Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun 75:1040–1044
Worlitzsch D, Kaygin H, Steinhuber A, Dalhoff A, Botzenhart K, Döring G (2001) Effects of amoxicillin, gentamicin, and moxifloxacin on the hemolytic activity of Staphylococcus aureus in vitro and in vivo. Antimicrob Agents Chemother 45:196–202
Yeh CT, Yen GC (2006) Induction of hepatic antioxidant enzymes by phenolic acids in rats is accompanied by increased levels of multidrug resistance-associated protein 3 mRNA expression. J Nutr 136:11–15
Acknowledgments
Financial support for this work came from the following sources: the National Nature Science Foundation of China (No. 31271951 and No. 31172364), China Postdoctoral Science Foundation (2013M530142), the Important National Science and Technology Specific Projects (2012ZX10003002), the Program for New Century Excellent Talents in University (NCET-09-0434; NCET-13-0245).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, C., Zhao, X., Li, W. et al. Inhibitory effect of totarol on exotoxin proteins hemolysin and enterotoxins secreted by Staphylococcus aureus . World J Microbiol Biotechnol 31, 1565–1573 (2015). https://doi.org/10.1007/s11274-015-1905-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1905-3