Abstract
A Gram-positive rod-shaped bacterium isolated on nutrient agar plates incubated at 28 ± 2°C. The identity of the bacterium was confirmed by sequencing of the 16S rRNA gene and it reveals that it shares highest similarity with Bacillus thioparus CECT 7196T (99.08%). It was capable of growing at temperatures ranging from 4 to 40°C, but optimum growth was observed at 28 ± 2°C. Strain NII-0902 is endowed with multiple plant growth promotion attributes such as phosphate solubilization, Indole acetic acid (IAA), siderophore and HCN production, which were expressed differentially at sub-optimal temperatures (5–40°C). It was able to solubilize phosphate (17.7 μg ml−1), and produce IAA (139.7 μg ml−1) at 28 ± 2°C. Qualitative detection of siderophore production and HCN were also observed. At 5°C it was found to express all the plant growth promotion attributes except HCN production. The ability to colonize roots is a sine qua non condition for a rhizobacteria to be considered a true plant growth-promoting rhizobacteria (PGPR). Bacillus sp. NII-0902 has a potential ability to colonize roots visualized by transparency, bacterial growth (turbid, milky and narrow zone) along and around roots and truly supported by scanning electron micrograph. Hence, it is proposed that, Bacillus thioparus sp. NII-0902 could be deployed as an inoculant to attain the desired results of bacterization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants are constantly involved in interactions with a wide range of bacteria. These plant-associated bacteria colonize the rhizosphere (rhizobacteria), the phyllosphere (epiphytes), and the inside of plant tissues (endophytes). Bacterial strains that have beneficial effects on plant health are referred to as beneficial plant-associated bacteria, plant-growth-promoting bacteria (PGPB), or plant-growth promoting rhizobacteria (PGPR; Andrews and Harris 2000). PGPB can promote plant growth directly or indirectly, via biocontrol of host plant diseases, production of phytohormones, or improvement of plant nutritional status (Glick 1995). Rhizobia are perhaps the best known beneficial plant associated bacteria because of the importance of the nitrogen fixation that occurs during the Rhizobium–legume symbiosis. The co-inoculation of other PGPB with rhizobia is becoming a practical method in the development of sustainable agriculture, because of yield increases seen compared with inoculation with rhizobia alone. PGPB that have been tested as co-inoculants with rhizobia include strains of the following well-known rhizobacteria: Azospirillum, Azotobacter, Bacillus, Pseudomonas, Serratia and Streptomyces (Baudoin et al. 2010; Biari et al. 2008; Ahmad et al.2008). PGPB have been isolated by screening the rhizosphere, phyllosphere, and the tissues of plants showing particularly vigorous growth in the field. In this study we isolated the effective plant-growth-promoting Bacillus thioparans strain NII-0902 from a soil sample collected from dense forest in Western ghat India.
Materials and methods
Isolation
The soil used for bacterial isolation was collected from a root-free soil, rhizosphere, and rhizoplane of Western Ghat in west coast of India, located at an altitude of 900 m above mean sea level. The processed soil sample was serially diluted, spread plated on full strength nutrient agar and incubated at 28°C for 48 h. A cream whitish colored bacterial colony was purified by repeated culturing on nutrient agar (NA) and maintained in 20% glycerol at −80°C. All the subsequent experiments were conducted after raising fresh culture.
Bacterial identification and characterization
Isolated strain was subsequently differentiated by grams reaction, salt tolerance and microscopic observation. Subsample isolates were initially selected on the basis morphology, physiology and were assessed for Gram reaction. The ability of the isolates to grow in diverse temperature range was carried out by growing each bacterial isolate on nutrient broth and incubated separately at four different temperatures i.e., 5–40°C (5, 15, 30 and 40°C). Results were recorded after every 4 h at 600 nm. The ability of the isolates to grow in different salt concentrations was carried out by inoculating bacterial culture on NA plates supplemented with 0–25% (w/v) NaCl and the plates were incubated at 28 ± 1°C for 3 days. The ability of the isolates to grow in alkaline or acid media was tested in nutrient broth in which the pH was adjusted from 4.0 to 12.0 (at a pH 1.0 interval) and incubated at 28 ± 1°C for 3 days. The ability to use different carbon sources was tested using the Biolog GN Microplate method (Biolog, Hayward, CA). Strain NII-0902 was then screened for traits that might be associated with ability to functions as PGPR, each test performed in triplicate.
16S rRNA gene sequencing and phylogenetic analysis
Extraction and amplification of genomic DNA for 16S rRNA gene sequence analysis was carried out as described by Cui et al. (2001). The 16S rRNA gene fragment was amplified by using universal primers. Based on 16S rRNA gene sequences, phylogenetically related bacteria were aligned by using a BLAST search (Altschul et al. 1990) against the GenBank database. Multiple alignments with sequences of related taxa of the genus Bacillus were implemented by using CLUSTAL_X (Thompson et al. 1997). The 16S rRNA gene sequence similarity values were calculated by pairwise comparison (Kimura 1980). A neighbour-joining phylogenetic tree was constructed (Saitou and Nei 1987) from evolutionary distances calculated using the Jukes–Cantor coefficient (Jukes and Cantor 1969). The topology of the phylogenetic tree was evaluated by the bootstrap resampling method of Felsenstein (1985) with 1000 replicates. The GenBank/EMBL/DDBJ accession number for the isolate is FJ897475.
Quantitative estimation of phosphate solubilization and IAA production
Initial qualitative estimation of the P-solubilizing activity of the isolate was carried out on Pikovskaya agar (Pikovskaya 1948). Quantitative estimation of tricalcium phosphate (TCP) solubilization and IAA production were carried out at 28°C temperatures. Quantitative estimation of P solubilization was carried out as per standard methodology (Nautiyal 1999), by inoculating 1 ml of bacterial suspension (3 × 107 cells ml−1) in 50 ml of NBRIP broth in Erlenmeyer flasks (150 ml), and incubating the flasks for 7 days. At the end of the incubation period the cell suspension was centrifuged at 10,000 rev min−1 for 10 min and the P content in the supernatant was spectrophotometrically estimated by the ascorbic acid method (Murphy and Riley 1962). Estimation of indole acetic acid (IAA) was done by inoculation of 200 μl of bacterial suspension (3 × 108 cells ml−1) in 10 ml Luria–Bertani (LB) broth amended with L-tryptophan (100 μg ml−1) and incubating it for a period of 48 h. The IAA content in the culture suspension was estimated by the standard procedure (Gordon and Weber 1951). All the studies were repeated on three independent dates to confirm the results.
Qualitative measurement of siderophore and hydrocyanic acid (HCN) production
Siderophore and HCN production by the isolate were estimated qualitatively at 28°C. Siderophore production was detected by the Chrome Azurol-S (CAS) assay (Schwyn and Neilands 1987) in 110 mm Petri dishes, and the diameter of the clearing zone was measured. HCN production was inferred by the qualitative method of Bakker and Schipper (1987). The change in the color of the filter paper previously dipped in 2% sodium carbonate prepared in 0.05% picric acid, from yellow to dark brown was rated visually depending on the intensity of the colour change.
Bioassay-based plant growth promotion ability on wheat
Seed microbiolization
Rhizobacterial cell suspensions were prepared by adding sterile saline (0.85% NaCl) to slants containing bacterial cultures in tryptone soy broth in the exponential phase followed by shacking in order to render a turbid suspension. The concentration was adjusted, in a spectrophotometer, to OD540 = 0.5, what corresponds approximately to 108 c.f.u./ml. Wheat seeds were dipped into the suspension and allowed to imbed overnight at room temperature (Silva et al. 2003).
Root colonization bioassay
Wheat seeds were surface sterilized in 50% ethanol (30 s) followed by 2% NaClO (3 min), washed three times in sterile water, soaked in 5 ml of a suspension of rhizobacteria for 24 h and then transferred to sterile 0.6% water-agar tubes. Control seeds were treated with sterile uninoculated medium. Periodic visual inspections were performed daily in order to detect bacterial growth around arising roots. In case of doubt or difficulty of observation, it proved useful to remove the whole seedling from the agar gel for visual inspection. Assays were carried twice with three replicates per culture.
Scanning electron microscopic studies
Wheat seedling of 21 day old was randomly selected from growth pots of each treatment for scanning electron microscopic examination. Tissue samples from inoculated and non-inoculated seedling roots of cowpea were thoroughly washed in water to remove soil particles and were fixed in 2% glutaraldehyde (made up in 0.1 M cacodylate buffer) in the refrigerator (8°C) for 1.5 h. Samples were washed two times in the same buffer for 10 min, post fixed in 1% OsO4 for 4 h, and dehydrated as follows: 30, 50, 70, 85, and 95% ethanol for 15 min; 100% ethanol, two times for 15 min each. For SEM, sputter coating, and a JEOL–JSM 5600LV model operating at 20 kV were used. Root vascular systems and rhizobacteria colonization patterns were observed by SEM.
Results
Characterization of strain NII-0902
The strain NII-0902 was a mesophilic, aerobic, Gram-positive motile bacterium that formed groups of 2–4 cell chains. Cells were rod-shaped measuring 0.5–0.7 μm wide and 1.0–1.7 μm long in 48 h in NA at 28 ± 2°C by SEM. Furthermore, colonies grown overnight on NA at 28°C were circular, with smooth margins, cream-white in color and 0.8–1 mm in diameter. Strain NII-0902 grew in 5% (w/v) NaCl but did not grew in the presence of 7, 10 and 15% (w/v) NaCl. Growth was observed at 5, 15, 30 and 40°C but not at 45°C (Fig. 1). Optimal growth temperature on NA was 28 ± 2°C at pH 7.0. Strain NII-0902 was positive for catalase, nitrate reduction, hydrogen sulphide, gelatin hydrolysis, citrate utilization, hydrolysis of aesculine, lysine decarboxylase and ornithine decarboxylase. Negative to indole, voges Proskauer’s, methyl red, urease, phenyl deamination and β-galactosidase. Positive for assimilation of arabinose, adonitol, cellobiose, malonate, arabinose, glucose, lactose, malonate, melibiose, raffinose, rhamnose, saccharose, trehalose and xylose but negative for oxidase reaction, Table 1 shows other features of strain NII-0902 compared with closely related species of genus Bacillus.
Phylogenetic position of the isolate
Strain NII-0902 was assigned to the genus Bacillus based on biochemical and morphological criteria. To confirm these results, the 16S rRNA gene sequence was obtained and phylogenetic analysis was carried out. Neighbour-joining tree placed Bacillus NII-0902 nested in a clade with some species of the genus Bacillus, with a 56% bootstrap value, the clade that contained both strains of Bacillus thioparus CECT 7196T and Bacillus selenatarsenatis DSM 18680T (Fig. 2). Strain NII-0902 showed above 99% 16S rRNA gene similarity with both the strains. High degree of similarity among strains of the16S rRNA gene suggests that all of them may belong to the same species. Nevertheless, there are examples of strains and species with very high 16S rRNA gene sequences identity that differ markedly in their DNA–DNA hybridization values as well as in their morphological and biochemical characteristic that are frequently used to separate one species from the other (Martínez-Murcia et al. 1992; Jaspers and Overmann 2004). High sequence identity could be expected in Bacillus as the rate of evolution of the 16S rRNA gene is exceptionally low due a bottle neck in its evolution (Palys et al. 2000).
Neighbour-joining tree derived from 16S rRNA gene sequences showing the relationships between strain NII-0902 and related Bacillus species. Bootstrap percentages (based on 1,000 replications) greater than 50% are given at branching points. Bar 0.005 substitutions per nucleotide position. Bacillus kribbensis DSM 17871T was used as the outgroup to root the tree. Accession numbers are given in parentheses
Plant growth attributes
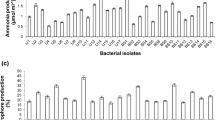
The different plant growth promotion traits of the isolate were determined at different incubation temperatures from 5 to 40°C (Table 2). At 5°C the isolate was able to solubilize phosphate (8.0 μg of ml−1 day−1), and produce IAA (38.68.0 μg of ml−1 day−1). The P-solubilizing ability of the isolate was evidently visible on plates of Pikovskaya agar, were it produced a clear halo zone (Fig. 3a). Although the zone of solubilization around the bacterial colony on Pikovskaya agar after varies from 0 to 72 h. The pH of the P-solubilization in broth was found to decline, in each case, due to bacterial activity; lowering of pH coincided with increase in the efficiency of phosphate-solubilizing activity. The pH was found to decline from 7.0 to 4.0–3.0 (Fig. 3b). Qualitative detection of siderophore production and HCN were also observed in all tested temperature. It was interesting to observe that the isolate was able to retain its functional traits even at 5°C, which was the lower temperature extreme for its growth, while higher values for all parameters were recorded at 28 ± 2°C. The plant growth promotion potential of Bacillus thioparus sp. NII-0902 was determined by a root colonization bioassay in wheat seeds. It was observed that the bacterized seedlings recorded 71.4 and 47.4% higher root and shoot lengths compared to uninoculated control (Table 3). A corresponding increase in the root and shoot biomass was also observed in bacterized seedlings. It was observed that seed bacterization resulted in greater enhancement of the root growth, as compared to the shoot growth.
aBacillus thioparus NII-0902 was inoculated on Pikovskaya agar media plates, and incubated for 5 days at 28°C. The clarification halos show P-solubilization and the maximum size of the clarification halos was reached after 96 h. b In a separate experiment using liquid National Botanical Research Institute’s Phosphate (NBRIP) medium for bacterial growth, P-solubilization was confirmed by checking pH of the media on daily basis. The pH of the medium was observed to fall continuously up to 72 h. Values given are means of three replicates. Error bars indicate standard deviation and are shown when larger than the symbol
Scanning electron microscopic observations
Primary root sections of cowpea bacterized with NII-0902 which showing potential plant growth ability was examined by SEM. The results revealed that cells of isolates NII-0902 were consistently distributed on the surface of roots (Fig. 4b, c). Surface furrows appeared to be located at epidermal cell junctions. Root seedlings free of inoculant bacteria typically revealed a smooth, undamaged epidermal root surface (Fig. 4a). Root surfaces from isolate NII-0902 inoculated seedlings were colonized with many clusters of cells associated with fibrillar material, which contributed to the formation of microcolonies. Micro colony formation by deleterious bacteria on root surfaces frequently occurs with effective colonization.
Discussion
Phosphate is abundant in several soils and is one of the major nutrients limiting the plant growth. The overall phosphate use efficiency following phosphate fertilizer application is low because of the formation of insoluble complexes (Vassilev and Vassileva 2003). Hence, frequent application of soluble forms of inorganic phosphate is necessary for crop production and which leaches to the ground water and results in eutrophication of aquatic systems (Del Campillo et al. 1999). In view of environmental concerns and current developments in sustainability, research efforts are concentrated on elaboration of techniques that involve the use of less expensive, though less bio-available sources of plant nutrients such as rock phosphate and by application of phosphate solubilizing bacteria and the agronomic effectiveness can be enhanced (Whitelaw 2000). The present study deals with the cold tolerance and plant growth promotion potential of Bacillus thioparans sp. NII-0902 isolated from the Western ghat forest soil. As the isolate grew at temperatures ranging from 5 to 40°C, it would be appropriate to call it as cold tolerant. Many cold tolerant plant growth promoting rhizobacteria have been reported from different habitats including Rhodococcus, Pantoea, Serratia and Pseudomonas species (Pankaj et al. 2007; Selvakumar et al. 2008a, b, 2009). In this study, an increase in the nutrient uptake as a consequence of seed bacterization has been demonstrated. This phenomenon can be attributed to the ability of the isolate to produce IAA, as IAA positively influences root growth and development, thereby enhancing nutrient uptake (Khalid et al. 2004). Another contributing factor could be the increased availability of phosphorous in the rhizospheric region, as a result of P solubilization by the isolate. It is a well-established fact that improved phosphorous nutrition influences overall plant growth and root development (Jones and Darrah 1994). Further Plant growth promotion activity of the isolate were demonstrated through a plant-based bioassay in tomato seeds. Its use as inoculant resulted in statistically significant increment in root and shoot biomass of wheat seedlings after 21 days of growth. These findings will strongly supported by the previous finding. (Selvakumar et al. 2008a, b, 2009).
From the present study, we demonstrate that the P-solubilizing soil bacteria could serve as efficient biofertilizer candidates for improving the P-nutrition of crop plants. The advantage of using natural soil isolates over the genetically manipulated or the one which has been isolated from a different environmental set up is the easier adaptation and succession when inoculated into the plant rhizosphere. In conclusion the bacterium-induced plant growth promotion observed in this study was attained under different temperature conditions. Hence, it is proposed that strains NII-0902 can be deployed as a bioinoculants to increase the available phosphorous in soil, helps to minimize the P-fertilizer application, reduces environmental pollution and promotes sustainable agriculture. Future studies are required to prove the nature of these isolates and to harness their potential as bio-inoculants in agriculture.
References
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth-promoting activities. Microbiol Res 163:173–181
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Andrews JH, Harris RF (2000) The ecology and biogeography of microorganisms on plant surfaces. Annu Rev Phytopathal 38:145–180
Bakker AW, Schipper B (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp. mediated plant growth stimulation. Soil Biol Biochem 19:451–457
Baudoin E, Lerner A, Sajjad Mirza M, El Zemrany H, Prigent-Combaret C, Jurkevich E, Spaepen S, Vanderleyden J, Nazaret S, Okon Y, Moënne-Loccoz Y (2010) Effects of Azospirillum brasilense with genetically-modified auxin biosynthesis gene ipdC on the diversity of the indigenous microbiota of the wheat rhizosphere. Res Microbiol (in Press)
Biari A, Gholami A, Rahmani HA (2008) Growth promotion and enhanced nutrient uptake of maize (Zea mays L.) by application of plant growth promoting rhizobacteria in Arid region of Iran. J Boil Sci 8:1015–1020
Cui XL, Mao PH, Zeng M, Li WJ, Zhang LP, Xu LH, Jiang CL (2001) Streptomonospora salina gen. nov., sp. nov., a new member of the family Nocardiopsaceae. Int J Syst Evol Microbiol 51:357–363
Del Campillo SE, Van der Zee SEATM, Torrent J (1999) Modelling long-term phosphorous leaching and changes in phosphorous fertility in selectively fertilized acid sandy soils. Eur J Soil Sci 50:391–399
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:109–117
Gordon AS, Weber RP (1951) Colorimetric estimation of indole acetic acid. Plant Physiol 26:192–195
Jaspers E, Overmann J (2004) Ecological Significance of Microdiversity: Identical 16S rRNA Gene Sequences Can Be Found in Bacteria with Highly Divergent Genomes and Ecophysiologies. App Environ Microbiol 70(8):4831–4839
Jones DL, Darrah PR (1994) Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 166:247–257
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HN (ed) Mammalian Protein Metabolism, vol 3. Academic Press, New York, pp 21–132
Khalid A, Arshad M, Zahir ZA (2004) Screening plant growth promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol 96:473
Martínez-Murcia A, Benlloch S, Collins D (1992) Phylogenetic interrelationships of members of the genera Aeromonas and Plesiomonas as determined by 16 s ribosomal DNA sequencing: lack of congruence with results of DNA-DNA hybridizations. Int J Syst Bacteriol 42:412–421
Mónica Pérez-Ibarra B, Flores ME, Varela MG (2007) Isolation and characterization of Bacillus thioparus sp. nov., chemolithoautotrophic, thiosulfate-oxidizing bacterium. FEMS Microbiol Lett 271:289–296
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate-solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Palys T, Berger E, Mitrica I, Nakamura LK, Cohan FM (2000) Protein-coding genes as molecular markers for ecologically distinct populations: the case of two Bacillus species. Int J Syst Evol Microbiol 50(3):1021–1028
Pankaj T, Anita P, Tongmin S (2007) Chromate reducing and plant growth promoting activies of psychrotrophic Rhodococcus erythropolis MtCC 7905. J Basic Microbiol 47:513–517
Pikovskaya RI (1948) Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Mikrobiologiya 17:362–370
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophore. Anal Biochem 160:47–56
Selvakumar G, Kundu S, Joshi P, Nazim S, Gupta AD, Mishra PK, Gupta HS (2008a) Characterization of a cold-tolerant plant growthpromoting bacterium Pantoea dispersa 1A isolated from a sub-alpine soil in the North Western Indian Himalayas. World J Microbiol Biotechnol 24:955–960
Selvakumar G, Mohan M, Kundu S, Gupta AD, Joshi P, Nazim S, Gupta HS (2008b) Cold tolerance and plant growth promotion potential of Serratia marcescens strain SRM (MTCC 8708) isolated from flowers of summer squash (Cucurbita pepo). Lett Appl Microbiol 46:171–175
Selvakumar G, Joshi P, Nazim S, Mishra PK, Bisht JK, Gupta HS (2009) Phosphate solubilization and growth promotion by Pseudomonas fragi CS11RH1 (MTCC 8984) a psychrotolerant bacterium isolated from a high altitude Himalayan rhizosphere. Biologia 64:239–245
Silva HSA, Reginaldo da Silva R, Mounteer A (2003) Development of a Root Colonization Bioassay for rapid screening of rhizobacteria for potential biocontrol agents. J Phytopathol 151:42–46
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Vassilev N, Vassileva M (2003) Biotechnological solubilization of rock phosphate on media containing agro-industrial wastes. Appl Microbiol Biotechnol 61:435–440
Whitelaw MA (2000) Growth promotion of plants inoculated with phosphate solubilizing fungi. Adv Agron 69:99–151
Yamamura S, Yamashita M, Fujimoto N, Kuroda M, Kashiwa M, Sei K, Fujita M, Ike Michihiko (2007) Bacillus selenatarsenatis sp. nov., a selenate- and arsenate-reducing bacterium isolated from the effluent drain of a glass-manufacturing plant. Int J Syst Evol Microbiol 57:1060–1064
Acknowledgments
The authors would like to thank CSIR Task force network programme on Exploration of India’s Rich Microbial Diversity (NWP 0006) for providing the financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
C. K. Deepa and Syed G. Dastager contributed equally to the work.
Rights and permissions
About this article
Cite this article
Deepa, C.K., Dastager, S.G. & Pandey, A. Plant growth-promoting activity in newly isolated Bacillus thioparus (NII-0902) from Western ghat forest, India. World J Microbiol Biotechnol 26, 2277–2283 (2010). https://doi.org/10.1007/s11274-010-0418-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0418-3