Abstract
The yeasts are microorganisms with great potential for biotechnological applications in diverse areas. The biological control of phytopathogens by yeasts has showed satisfactory results under laboratory conditions, and it has already produced commercial formulations. With this as focus, this work aims to perform in vitro and in vivo evaluations of the action of a Torulaspora globosa yeast strain (1S112), isolated from sugarcane rhizosphere, against the phytopathogenic mold Colletotrichum sublineolum, the causative agent of anthracnose in sorghum. In vitro experiments included the antagonism test in Petri dishes with morphological hyphal evaluation; yeast killer activity; siderophore, volatile compound and hydrolytic enzyme production. In vivo experiments were conducted in greenhouse conditions with a sorghum variety susceptible to C. sublineolum by evaluating the anthracnose disease for 6 weeks. The results indicated that the yeast strain significantly controlled the fungal growth, either in vitro or in vivo. The strain of T. globosa exhibited killer activity against two sensitive strains, which is a novel capacity for this species. The yeast did not produce siderophores, volatile compounds or hydrolytic enzymes, although it has reduced the mycelial growth, resulting in hyphal deformities but not cell death. The yeast controlled the anthracnose disease in sorghum, either inoculated before or after the fungal spores, suggesting that the competition for space and nutrients to dominate the mold and killer toxin production, altering the hyphal morphology, are mechanisms utilized by the yeast in the biocontrol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthracnose in sorghum, caused by the mold Colletotrichum sublineolum Ces (Wils), is one of the most significant diseases in this cereal occurring widely wherever this culture is produced (Pinto 2003). The main symptoms of the disease are observed in any part of the plant, including the root and seeds, although mainly in the leaves and stalk. Necrotic lesions are seen in the leaves but narrow and brownish-reddish lesions occur in the stalks (Shurttleff 1980).
Genetic resistance is one of the main strategy for the disease control but host-specific resistance is often unstable due to the high variability displayed by the mold, rapidly adapting to the resistant cultivars (Casela and Ferreira 1995; Zanette et al. 2009). This fact leads to alternative control methods, especially those employing antagonist species in biological control.
Yeasts are microorganisms that very actively participate in several significant biotechnological processes. One of the most notorious representative species of this group is Saccharomyces cerevisiae of growing interest in industrial processes. Other yeast species having different applications are presented, as biological control has become one of the most promising areas of study. Candida oleophila is available commercially as Aspire® and it produces excellent results in the control of the postharvest decay fungi, allowing less loss and waste thus providing higher profits for producers and traders (Janisiewicz and Korsten 2002; Droby 2006).
Biological control of fungal plant diseases in the field can also use yeast species and several papers have showed positive and encouraging results (Piccinin et al. 2005; Chanchaichaovivat et al. 2008), but very little is known of the function and behavior of this group in the microbial environment. Yeasts isolated from the environment, especially from the agricultural areas, may show better results in the biological control of phytopathogens, as observed by Ahansal et al. (2008). These authors isolated yeasts from the soil, leaves and fruits which significantly controlled Botrytis cinerea.
Some yeast species may produce antimicrobial compounds, like killer toxins, which cause the death of other species of yeast, molds, and bacteria (Walker et al. 1995). The application of killer yeasts in the control of phytopathogenic molds was initially suggested by Jacobs and Van Vuoren (1991) and since then several reports have indicated this approach for fungi in the prevention of food deterioration and in clinical application (Walker et al. 1995). Positive results have also been discovered for the biological control of phytopathogens. Weiler and Schimitt (2003) observed significant control of the fungus Fusarium oxysporum by the yeast Zygosaccharomyces bailii; Santos and Marquina (2004) showed that the yeast Pichia membranifaciens controlled the causal agent of grey mold disease in grapevines (Botrytis cinerea) by producing a killer toxin.
Yeasts possess the capacity to produce hydrolytic enzymes, such as β-glucanases and chitinases, which destroy other fungal cell walls (Jijakli and Lepoivre 1998; Saligkarias et al. 2002). Some yeast species are also able to produce siderophore compounds (chelants of iron (III) ions) that inhibit fungal growth (Calvente et al. 1999; Wang and Chi 2009). Another putative action mode is the production of toxic volatile compounds, as observed in different species (Fialho 2004).
The main form of control, however, is the ability of the yeast to develop quickly on the leaf, fruit and flower surfaces, especially in the presence of sugar, and dominate the whole environment by inhibiting the development of other microorganisms through competition for space and nutrients (Saligkarias et al. 2002; Vero et al. 2002).
The challenge lies in understanding the behavior of yeasts in agricultural areas and in discovering the mechanisms used by this microbial group to assist the plants in production systems, contributing to the balance of the ecosystem and minimizing the application of toxic substances to the environment as fungicides that pollute the soil, groundwater, rivers, and lakes, and cause illness and death of animals and humans.
Considering all these aspects, this study aims to evaluate the action of a yeast strain Torulaspora globosa, isolated from sugarcane rhizosphere, in controlling the phytopathogenic mold Colletotrichum sublineolum, which causes anthracnose in sorghum plants, in vitro and in vivo experiments. The fungal biocontrol mechanisms of the yeast were also approached and discussed.
Materials and methods
Microorganisms
The phytopathogenic mold Colletotrichum sublineolum (kindly provided by EMBRAPA Milho e Sorgo, Sete Lagoas, Minas Gerais, Brazil), the causal agent of anthracnose in sorghum, and the yeast Torulaspora globosa (strain 1S112), isolated from the sugarcane rhizosphere, were utilized throughout the experiments.
The yeast was identified by sequencing the ITS region (including 5.8S gene) in ribosomal DNA according to White et al. (1994), by using the primers ITS-1 and ITS-4. Both fungi are maintained in Potato Dextrose Agar (PDA) and YEPD slants, respectively, for the mold and the yeast, at 4°C.
Evaluation of the in vitro antagonism in solid medium
To evaluate mold growth control by the yeast T. globosa, an assay in which both fungi were grown side by side was done. Petri dishes with PDA medium were inoculated with a 5-mm diameter disk of an actively growing fungal mycelium near the dish edge. A loop of the yeast cells was inoculated at the opposite edge. A control dish was prepared to compare with only inoculation of the mold. The experiment (repeated at least twice) was performed with five replicates, incubating the dishes at 25°C. Mycelial growth was measured every 4 days and the data were statistically analyzed by analysis of variance and Tukey’s test at 5% of significance.
The fungal mycelium was microscopically observed to assess the possible hyphal damage caused by the yeast. Mycelium disks (5 mm in diameter) from different parts were removed from the dish in which the yeast had inhibited the mold growth. They were longitudinally cut to excise the excess culture medium, to obtain thin mycelial layers which were studied microscopically at 400× magnification. The hyphae were photographed and compared with the morphology of the mold growing in the Petri dish solely with PDA.
The mycelium disk samples from the antagonism dishes were also inoculated in the PDA medium to evaluate hyphal viability after cultivation with the yeast. The dishes (five replicates) were incubated at 25°C for 12 days, measuring the diameter of mycelial growth every 4 days. The experiment was repeated at least twice. The data were statistically analyzed by analysis of variance and Tukey’s test at 5% of significance.
Detection of killer activity
The T. globosa strain was characterized by the presence of killer toxin in buffered YEPD-methylene blue medium (pH 4.3–4.7) as described by Ceccato-Antonini et al. (2004). Cell suspensions of the sensitive strains Saccharomyces cerevisiae NCYC1006 and Torulopsis glabrata ATCC15126 were prepared at 4 × 105 cells/ml, after cultivation for 24 h at 30°C in YEPD broth medium. A volume of 100 μl was spread over the culture medium and after drying the yeast T. globosa was inoculated. The dishes were incubated at 30°C for 5 days. The isolate was considered mycocinogenic (producing killer toxin) if an inhibition halo and a blue zone (dead cells) were observed surrounding the T. globosa colony.
Production of antifungal volatile compounds
To evaluate the antifungal volatile compounds produced by the yeast, a new assay in which both yeast and mold were grown side by side was done; however, the Petri dishes now had a division, which prevents the non-volatile exudates produced by the yeast from reaching the fungus through the culture medium. The same amount of PDA medium was poured into each side of the dish, with inoculation of the yeast being done in one side and a 5-mm diameter disk of an actively growing fungal mycelium in the another one. After 12 days of incubation at 25°C, the mycelial growth was measured and compared with that of the dish in which the mold was inoculated without the yeast.
Siderophore production
To detect siderophore production by the yeast, the methodology of Milagres et al. (1999) was followed. The CAS (Chrome Azurol S) Blue Agar was prepared according to Schwyn and Neilands (1987). Although the color change indicates siderophore production, many microorganisms are unable to grow in this culture medium; therefore, one-half part of the medium was removed and replaced by 2% Malt Extract (ME), in which the yeast grew well. The yeast was inoculated only in ME medium and incubated at 25°C for 10 days, in the dark. The yeast growth and the medium color change (from blue to purple or yellow) were then analyzed. As a positive control, a siderophore-producing strain (Aspergillus niger CCT4355) was also assayed, modifying the color of CAS-Blue medium from blue to purple.
Production of hydrolytic enzymes (β-glucanase and chitinase)
Initially, a loop of the yeast cells was transferred to a Falcon tube containing 2.5 ml of YEPD broth and incubated at 32°C for 12 h. Then 1 ml of this cell suspension was inoculated in a 125-ml Erlenmeyer flask with 50 ml of the modified-YEPD broth (with C. sublineolum cell wall at 1% replacing the glucose, according to Saligkarias et al. 2002). A flask with YEPD broth was also inoculated. Triplicates were maintained at 32°C under shaking at 90 rpm. At intervals of 0, 24, 48, 60 and 72 h of incubation, a 2 ml sample was removed and centrifuged at 10,000g for 10 min at 5°C. The supernatant was recovered and analyzed for β-glucanase and chitinase activities, according to Fialho (2004).
To evaluate β-1,3-glucanase production, the colorimetric quantification of glucose (reducing sugars) released from laminarin was determined by the methodology of Miller (1959). The reaction was performed with 50 μl of sodium acetate buffer (0.1 M, pH 5.0), 200 μl of the sample and 250 μl of laminarin (4 mg/ml) and incubated at 37°C for 1 h. Next, the reaction was stopped by adding 250 μl of dinitrosalicylic acid reagent to 250 μl of the reaction and the reducing sugars determined. The enzymatic activity was expressed in U/ml, in which one unit of activity (U) was defined as 1 μg of reducing sugar released from laminarin per minute under the assay conditions.
Chitinase production was evaluated by estimating the release of N -acetylglucosamine (NAG) from the substrate glycol chitin. A volume of 100 μl of the sample was mixed with 200 μl of Mcllvaine buffer (50 mM citric acid; 100 mM sodium phosphate; pH 6.0) and 100 μl of glicol chitin 0.01% (w/v, prepared in the same buffer). After incubation at 37°C for 15 min, the reaction was stopped by adding 250 μl of dinitrosalicylic acid reagent to 250 μl of the reaction and the reducing sugar was determined by the methodology of Miller (1959). The enzymatic activity was expressed in U/ml, in which one unit of activity (U) was defined as 1 μg of reducing sugar (N-acetylglucosamine) released from glicol chitin per minute, under the assay conditions.
Evaluation of the in vitro antagonism in liquid medium
Initially, the yeast inoculum was prepared using the following procedure: a loop of the yeast cells was transferred to a flask containing 25 ml of Potato Dextrose medium (PD) and incubated at 25°C, 160 rpm, until 3 × 107 cells/ml was reached. To obtain the crude extract for the antagonism tests, 100 μl of the yeast (T. globosa) inoculum and a concentration of 105 spores/ml of the mold (C. sublineolum), as required, were placed in 125-ml Erlenmeyer flasks, with 50 ml of PD medium (non-buffered medium, NPD; or buffered medium, pH 4.5, BPD): NPD + yeast; NPD + yeast + mold; BPD + yeast; BPD + yeast + mold. The flasks were incubated at 25°C and 90 rpm, with 2-ml sampling at 24, 48 and 60 h. The samples (crude extracts) were filtered through a 0.22 μm membrane or autoclaved at 121°C, 1 atm, for 20 min. A complete factorial design as 2 × 2 × 2 × 3 (buffered and non-buffered medium; cultivation with or without the mold; filtered or autoclaved sample; sampling periods; respectively) was utilized.
The crude extracts were transferred in equal volumes to glass tubes containing NPD medium inoculated with 105 spores/ml of the mold (final concentration). The control was obtained by inoculating the mold with sterile distilled water replacing the crude extract, in equal volumes. The spore germination was evaluated after 12 h in a Neubauer-counting chamber, in triplicate, counting at least six fields to evaluate the spore germination (%).
The experiment was repeated at least twice and the data were statistically analyzed by analysis of variance and Tukey test at 5% of significance.
In vivo experiment: evaluation of the biological control of C. sublineolum in sorghum by the yeast T. globosa
Greenhouse experiments were conducted with the sorghum variety BR009, susceptible to C. sublineolum, kindly provided by EMBRAPA Milho e Sorgo, Sete Lagoas, Minas Gerais, Brazil. After 30 days of cultivation, 10 ml of a microbial suspension (105 fungal spores/ml and/or 108 yeast cells/ml) were spread directly on the leaves. One week after the inoculation, the disease severity was evaluated using a score from 0 to 9, in which 0 indicated absence of leaf lesions and 9 indicated lesions in 90% of the leaves. Once-a-week evaluations were done for 6 weeks, following the plant harvest to determine the height (cm) and dry mass (mg) of the aerial parts.
The following treatments were used: control, with no inoculation; yeast cells and fungal spores inoculated simultaneously; yeast cells inoculated 24 h after the fungal spore inoculation; yeast cells inoculated 24 h before the fungal spore inoculation; and chemical treatment with systemic fungicide (Metiltiofan® 90 g/100 l), applied 2 days before the fungal spore inoculation. The data were statistically analyzed by non-parametric Fisher’s test at 5% of significance.
Results
Evaluation of the in vitro antagonism
The results revealed that T. globosa (strain 1S112) inhibited mold growth in PDA, exhibiting a clear inhibition halo. The mycelial growth in the Petri dishes inoculated with the yeast side by side differed significantly from the control, i.e., from the dish in which the mold was solely inoculated (Fig. 1).
In vitro antagonism by the yeast T. globosa against the phytopathogenic mold C. sublineolum. a Petri dish with PDA medium inoculated solely with the mold; b petri dish with PDA medium inoculated with the mold and the yeast strain (1S112); c mycelial growth (cm) of the mold growing in PDA medium with (filled diamond) or without (filled square) the yeast strain. Different letters indicate significant difference at 5% by Tukey’s test
Hyphal deformities were observed when mycelial samples were removed from different parts of the PDA medium. The hyphae located nearer to the yeast cells appeared wilted, folded and coiled. The median portion of the mycelium growth showed vesicle formation or granulation along the hyphae. However, the hyphae far away from the yeast cells appeared normal. Hyphae taken from the three positions in PDA medium remained viable (Fig. 2).
Antagonism by the yeast T. globosa against the phytopathogenic mold C. sublineolum. a Petri dish with PDA medium inoculated with the mold and the yeast strain (1S112), showing the places from where samples were taken (1 far away the yeast; 2 median position; 3 closer to the yeast); A-1, A-2 and A-3: hyphal morphology seen under optical microscopy from samples taken from different parts in the PDA medium; b Mycelial growth (cm) of the mold growing in the PDA medium from samples taken in position 1 (filled diamond), position 2 (filled square) and position 3 (filled triangle)
Detection of killer activity
A blue zone surrounding the inhibition halo revealed that the strain of T. globosa killed the sensitive strains (Fig. 3a). This result indicates that the species T. globosa is mycocinogenic, i.e., a yeast with a killer phenotype.
Mechanisms of action by the yeast T. globosa against C. sublineolum. a Blue halo of cell death of the sensitive strain (S. cerevisiae NCYC 1006), indicating that the yeast strain of T. globosa (1S112) produces the killer toxin (C, T. globosa colony; H, halo of cell death of the sensitive strain; SY, sensitive strain growth); b, c petri dishes with central division for testing the production of antifungal volatile compounds, with PDA medium inoculated solely with the mold (b) and with the mold and yeast (c); d, e test for siderophore production, the positive control with a strain of Aspergillus niger showing the color change of the medium (d) and the dish with the yeast T. globosa (e)
Production of volatile compounds
The results indicated no difference between the mycelia grown in the absence of yeast and with the inoculated yeast cells using Petri dishes with a central division that prevented any contact between the mold and the yeast (Fig. 3b, c). No volatile compounds were then produced by T. globosa strain.
Siderophore production
The T. globosa (1S112) strain did not alter the color of the CAS-Blue Medium (Fig. 3d, e), indicating no siderophore production.
Production of hydrolytic enzymes (β-glucanase and chitinase)
The enzymatic assays revealed that the yeast did not produce significant amounts of β-glucanase and chitinase within the parameters tested.
Evaluation of the in vitro antagonism in liquid medium
This experiment aimed to evaluate some parameters that influence the antagonism of T. globosa against C. sublineolum in a liquid medium, as the yeast induction by the mold to produce antagonistic factors (growing yeast in the presence of the mold); the effect of yeast-generated acids on the mold (buffering the medium); time of yeast growth; and the effect of thermal treatment on the yeast crude extracts (by autoclaving or filtration) in a complete factorial experiment. The results of the complete statistical analysis are presented in Table 1.
The yeast inhibited mold spore germination as the percentage of mold germination was lower than the control treatment. The crude extracts which were not autoclaved but filtered exhibited a greater effect in controlling fungal spore germination, indicating the action of temperature-sensitive compounds on the phytopathogenic mold (Fig. 4).
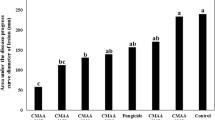
Effect of the crude extracts (autoclaved or filtered) from the cultivation of the yeast (T. globosa) over the spore germination of the mold (C. sublineolum). Legend: With mold—supernatant from the mixed cultivation (yeast-mold); Without mold—supernatant from the pure cultivation (yeast); filled diamond non-buffered medium; filled square buffered medium; filled triangle control (distilled water). Diferent letters in the bars indicate significant difference at 5% by Tukey’s test
No significant difference was observed between the crude extracts obtained from pure (yeast) and mixed cultures (yeast + mold) on spore germination as well as the use of buffered medium to cultivate the yeast (Table 1). Bar graphics in Fig. 4 illustrate the main effects with significant results over the spore germination (time of cultivation and treatment of the crude extract) regardless the other effects, considering the overall average for the effect. The supernatant sampled after 60 h of cultivation and filtered crude extract presented significant results (P < 0.05) for mold germination control (Fig. 4).
In vivo experiment: evaluation of the biological control of C. sublineolum in sorghum by the yeast T. globosa
Considering the results of anthracnose severity after 6 weeks of sorghum evaluation, the use of the fungicide reduced significantly the disease progress. The treatments in which the yeast was inoculated either before or after the mold also controlled the disease progress, similar statistically from the treatment with the fungicide but significantly different from the treatment where only the fungal spores were inoculated (Fig. 5a).
In vivo experiment: evaluation of the biological control of C. sublineolum in sorghum by the yeast T. globosa. a The score relative to the disease progress (0–9) after 6 weeks of observation in the greenhouse, with six treatments, as (T1) with no inoculation; (T2) yeast cells inoculated simultaneously with the mold; (T3) yeast cells inoculated 24 h after the fungal spore inoculation; (T4) inoculation of fungal spores; (T5) yeast cells inoculated 24 h before the fungal spore inoculation; and (T6) chemical application of the systemic fungicide Metiltiofan® (90 g/100 l), 2 days before the fungal spore inoculation; b height (cm) of the sorghum plants harvested at the end of the experiment in greenhouse conditions; c dry mass (mg) of the aerial plant of the sorghum plants harvested at the end of the experiment in greenhouse conditions. Different letters in the bars indicate significant difference at 5% by Tukey’s test
Regarding the height and dry mass of the aerial parts of the sorghum plants, no significant differences were observed among the treatments (Fig. 5b, c).
Discussion
The antagonism observed in in vitro assay indicates the potential of applying the yeast T. globosa in the biological control of C. sublineolum. The mycelium from the antagonism zone growing normally in PDA medium suggested that the injury caused by the yeast T. globosa was neither irreversible nor to a great extent. The yeast does act on the mycelium but causing non-lethal hyphal deformities, which could affect the hyphal penetration into the host tissues. Ferreira et al. (1991) evaluated the bacterial action of Bacillus subtilis in the biocontrol of Eutypa lata in grapes observing the differences in hyphal morphology, with tip narrowing and vesicle formation throughout the hyphal length. Chaurasia et al. (2005) also studied the antagonism of Bacillus subtilis against Fusarium oxysporum, with abnormal hyphal development, cell wall lysis, vacuolization and granulation. In both reports, the irregularities in hyphal morphology were credited to the production of bacteria-induced antibiotic substances; however, neither the nature and the method of action of these substances nor the microscopic analysis of the yeast-controlled mold mycelia were described.
The use of yeasts in phytopathogen control is still the focus of several reports, as it presents potential in field application. Some mechanisms were earlier observed in yeasts as biocontrol agents of plant pathogenic molds in leaves and fruits, as competitors for nutrients and space (Fokkema 1984; Droby et al. 1989; Filonow 1998; Janisiewicz et al. 2000); production of cell-wall degradable enzymes, like β-1,3 glucanase and chitinase (Castoria et al. 2001; Masih and Paul 2002; Urquhart and Punja 2002); production of antifungal metabolites (Walker et al. 1995; Masih et al. 2001; Hofte et al. 2004); resistance induction in the host (Wilson et al. 1994; Droby et al. 2002; El Ghaouth et al. 2003); and mycoparasitism (Wisniewski et al. 1991; Arras et al. 1998; El Ghaouth et al. 1998).
Thus far, no report is available indicating the use of T. globosa as a biocontrol agent. As this yeast strain was isolated from an agricultural area (cultivated with sugarcane), further studies are warranted to understand its role and significance in the rhizosphere and its action on phytopathogen control and in plant growth promotion.
No report has been also made on the killer activity exhibited by T. globosa. This fact confirms that neither are the mycocinogenic species identified nor are the factors involved in the expression of these characteristics known. The frequency of the killer phenomenon in yeasts appears to be high in natural environments and industrial processes (Stumm et al. 1977; Young 1982; Shimizu et al. 1985; Ceccato-Antonini et al. 2004) and the toxins have been suggested as antifungal agents in the phytopathogen control (El-Tarabily and Sivasithamparam 2006).
The killer toxin may be responsible for the hyphal deformation, as evidenced in the antagonism tests in the Petri dishes. Studies have revealed that the toxin attacks the cell membrane, decreasing the intracellular pH and causing an overflow of potassium ions and ATP (Skipper and Bussey 1977; De La Pena et al. 1981; Martinac et al. 1990); or the action include glycoprotein-induced toxin groups responsible for the proton formation, which generates ion channels without the stabilization of the electrochemical potential of the membrane, resulting in eventual cell death (Martinac et al. 1990; Golubev 1998; Marquina et al. 2002).
The deformities observed in the hyphae of the mold C. sublineolum when growing side by side with the yeast could have been caused by the hydrolytic enzymes released by the yeast. This is a characteristic observed in some antagonist yeasts, which produce enzymes able to degrade the cell wall of molds (Jijakli and Lepoivre 1998; Masih and Paul 2002; Urquhart and Punja 2002). The results of the enzymatic assays however showed that this yeast was not capable to produce significant amounts of hydrolytic enzymes in the conditions used.
The results probably imply yeast killer toxin action on the mold, as the sensitivity of the killer proteins to temperature is ascertained. However, other mechanisms may be present in this yeast-mold interaction because even when the crude extract was autoclaved, a significant control was noted when compared with the control (without the crude extract). The production of indole-3-acetic acid (IAA) is a candidate mechanism once it has been proposed for the biocontrol of phytopathogenic molds. Indeed, the strain of T. globosa here utilized was able to produce this plant regulator (data not shown) as already observed for other species of yeasts (Nakamura et al. 1991; Xin et al. 2009). Brown and Hamilton (1993) have observed the inhibition of spore germination and mycelial growth of molds by IAA. The disease severity caused by Phytophtora infestans have decreased with the addition of IAA in excised potato leaves (Martinez Noel et al. 2001). Moreover, Yu et al. (2009) have recently described the enhanced efficacy of the yeast antagonist Cryptococcus laurentii against the blue mold rot caused by Penicillium expansum in apple fruit when IAA was added to the yeast suspensions.
The siderophore production is a mechanism involved with the competition for nutrients because the molecules of this substance are capable of “stealing” the iron (III) ions into the medium, making them unavailable to other microorganisms (Calvente et al. 1999). This mechanism of action was discarded for the yeast strain used due to its inability to produce siderophore. Similarly, the T. globosa strain was not able to produce volatile compounds which could inhibit the mold growth.
The experiment designed to survey the antagonism ‘yeast against mold’ in liquid medium revealed that the yeast-produced antagonist substances were not stimulated by the mold. Levy (2003) also found no difference between the activities of both supernatants (resulted from the cultivation of yeast with or without the mold), however Coelho (2005) found more efficient results on the biocontrol with the pure cultivation supernatant of Pichia ohmeri on the Penicillium expansum germination. Neither the use of buffered medium to cultivate the yeast influenced the spore germination, which implies that the yeast-produced antagonistic compounds are not sensitive to pH variations or are either inactivated or stimulated by it. This result ruled out the action of acids produced by the yeast as a mechanism to control the mold germination/growth. The supernatant sampled after 60 h presented better results for mold germination control probably because of the accumulation of antagonist substances, as killer toxins and others. Buzzini et al. (2004) observed a constant increase in toxin production during exponential growth with a peak during early stationary phase for two killer strains.
Some positive interactions among the parameters studied were found, implying that there are differences in the effects of yeast crude extracts on mold germination considering the time of cultivation and type of supernatant, for instance, with different tendencies along the time, depending on whether the supernatant was autoclaved or filtered.
The remarkable antagonism in vitro exhibited by the yeast T. globosa against the phytopathogenic mold C. sublineolum stimulated the in vivo experiment in a greenhouse to assess the biological control of anthracnose in sorghum. The ability of the yeast to reproduce and dominate the leaves more quickly than the phytopathogenic mold could explain the efficiency of the biological control regardless the yeast was inoculated before or after the mold, because the last one is a slow-growing microorganism. The genus Colletotrichum also requires an energy source external to the conidia to germinate (Windels and Lindow 1985) and in this respect, the competition for nutrients with the yeast is expensive since the latter is an effective competitor for sugar in the phylloplane.
Taqarort et al. (2008) have surveyed yeasts isolated from the surface of citric fruits and observed that the species with the best results for the biocontrol of Penicillium digitatum were also excellent plant-surface colonizers. Results by Capdeville et al. (2007) showed that Cryptococcus magnus was able to colonize wound surfaces of papaya fruit much faster than the pathogen (Colletotrichum gloeosporioides) outcompeting for space and nutrients. Additionally, the yeast produced a flocculent matrix, which affected hyphae integrity.
Different results were observed by Piccinin et al. (2005) using S. cerevisiae against C. sublineolum in sorghum, which required a weekly application (and not a single application) of the yeast to obtain better results in fungal growth control.
The absence of plant growth promotion verified in the treatments where the yeast was inoculated not necessarily means that this yeast is not a plant growth promoter (although it is a indole-3-acetic acid producer), because it was applied over the leaves not reaching the soil and rhizosphere, as these are places where the yeast would express characteristics of growth promotion.
To the extent of our knowledge, no report concerning the usage of the yeast T. globosa in plant disease control is available, but some Indian industries use a commercial formulation of biofertilizers including this yeast in their composition, especially because of its capacity in solubilizing phosphate. Vora and Shelat (1998) have verified that T. globosa is capable of dissolving tricalcium phosphate due to its ability to produce acetic acid. The inoculation of this species in different cultures in the field showed a significant increase in the productivity of the plant cultures inoculated. In fact, the strain 1S112 here utilized is a potassium-solubilizing microorganism from rocks (data not shown), reinforcing the potential application of this yeast as a commercial formulation.
In brief, the yeast T. globosa was able to significantly control the phytopathogenic mold C. sublineolum, either in vitro or in vivo experiments in greenhouse with sorghum. The mechanisms of action involved the production of the killer toxin and competition for space and nutrients. The yeast strain did not produce either siderophore, volatile compounds or hydrolytic enzymes, which indicated that the hyphal deformities may be due to the killer toxin. Despite causing hyphal abnormalities, no cell death was observed. A profound investigation upon the chemical composition of the yeast extract is required to identify the antagonistic substances. Further studies are warranted to evaluate other mechanisms of action to get a potential product that can be used in the biological control of anthracnose in the field.
References
Ahansal L, Sassi AB, Martini A, Vaughan-Martini A, Walker G, Boussaid A (2008) Biodiversity of yeasts isolated from the indigenous forest of Argan (Argania spinosa (L.) Skeels) in Morocco. World J Microbiol Biotechnol 24:777–782
Arras G, De Cicco V, Arru S, Lima G (1998) Biocontrol by yeast of blue mould of citrus fruits and the mode of action of an isolate of Pichia guilliermondii. J Hortic Sci Biotech 73:413–418
Brown AE, Hamilton JTG (1993) Indole-3-ethanol produced by Zygorrhynchus moelleri and indole-3-acetic acid analogue with antifungal activity. Mycol Res 96:71–74
Buzzini P, Corazzi L, Turchetti B, Buratta M, Martini A (2004) Characterization of the in vitro antimycotic activity of a novel killer protein from Williopsis saturnus DBVPG4561 against emerging pathogenic yeasts. FEMS Microbiol Lett 238:359–365
Calvente V, Benuzzi D, Tosetti MIS (1999) Antagonistic action of siderophores from Rhodotorula glutinis upon the postharvest pathogen Penicillium expansum. Int Biodeter Biodegr 43:167–172
Capdeville G, Souza Junior MTS, Santos JRP, Miranda SP, Caetano AR, Falcão R, Gomes ACMM (2007) Scanning electron microscopy of the interaction between Cryptococcus magnus and Colletotrichum gloeosporioides on papaya fruit. Pesqui Agropecu Bras 42(11):1537–1544
Casela CR, Ferreira AS (1995) Virulence associations in the sorghum anthracnose fungus Colletotrichum graminicola. Fitopathol Bras 20:33–38
Castoria R, De Curtis F, Lima G, Caputo L, Pacífico S, De Cicco V (2001) Aureobasidium pullulans (LS-30) an antagonist of postharvest pathogens of fruits: study on its modes of action. Postharvest Biol Tech 22:7–17
Ceccato-Antonini SR, Tosta CD, Silva AC (2004) Determination of yeast killer activity in fermenting sugarcane juice using selected ethanol-making strains. Braz Arch Biol Tech 47:13–23
Chanchaichaovivat A, Panijpan B, Ruenwongsa P (2008) Putative modes of action of Pichia guilliermondii strain R13 in controlling chilli anthracnose after harvest. Biol Control 47:207–215
Chaurasia B, Pandey A, Palni LMS, Trivedi P, Kumar B, Colvin N (2005) Diffusible and volatile compounds produced by an antagonistic Bacillus subtilis strain cause structural deformations in pathogenic fungi in vitro. Microbiol Res 160:75–81
Coelho AR (2005) Controle de Penicillium expansum/Biodegradação de Patulina: perfil cromatográfico de composto bioativo de leveduras killer visando aplicação pós-colheita. Ph.D. thesis, Universidade Estadual de Londrina
De La Pena P, Barros F, Gascón S, Lazo PS, Ramos S (1981) Effect of yeast killer toxin on sensitive cells of Saccharomyces cerevisiae. J Biol Chem 256:10420–10425
Droby S (2006) Yeast Metschnikowia fructicola NRRL Y-30752 for inhibiting deleterious microorganisms on plants. US Patent 6994849
Droby S, Chalutz E, Wilson CL, Wisniewski M (1989) Characterization of the biocontrol activity of Debaryomyces hansenii in the control of Penicillium digitatum on grapefruit. Can J Microbiol 35:794–800
Droby S, Vinokur V, Weiss B, Cohen L, Daus A, Goldschmidt EE, Porat R (2002) Induction of resistance to Penicillium digitatum in grapefruit by the yeast biocontrol agent Candida oleophila. Phytopathology 92:393–399
El Ghaouth A, Wilson CL, Wisniewski M (1998) Ultrastructural and cytochemical aspects of the biological control of Botrytis cinerea by Candida saitoana in apple fruit. Phytopathology 88:282–291
El Ghaouth A, Wilson CL, Wisniewski M (2003) Control of postharvest decay of apple fruit with Candida saitoana and induction of defense responses. Phytopathology 93:344–348
El-Tarabily KA, Sivasithamparam K (2006) Potential of yeasts as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Biom Life Sci 47:25–35
Ferreira JHS, Matthee FN, Thomas AC (1991) Biological control of Eutypa lata on grapevine by an antagonistic strain of Bacillus subtilis. Phytopathology 81:283–287
Fialho MB (2004) Efeito in vitro de Saccharomyces cerevisiae sobre Guignardia citricarpa, agente causal da pinta preta dos citros. Dissertation, Escola Superior de Agricultura. “Luiz de Queiroz”, Universidade de São Paulo
Filonow AB (1998) Role of competition for sugars by yeast in the biocontrol of gray mold of apple. Biocontrol Sci Tech 8:243–256
Fokkema NJ (1984) Competition for endogenous and exogenous nutrients between Sporobolomyces roseus and Cochliobolus sativus. Can J Bot 62:2463–2468
Golubev WI (1998) Mycocins (killer toxins). In: Kurtzman CP, Fell JW (eds) The yeasts, a taxonomic study. Elsevier, Amsterdam, pp 55–62
Hofte M, Poppe L, Druvefors U, Schnurer J, De Cal A, Melgarejo P, Stèpien V, Jijakli MH (2004) Modes of action of Candida sake CPA-1, Pantoea agglomerans CPA-2, Epicoccum nigrum 282 and Pichia anomala J121, effective antagonists of postharvest pathogens. In: International workshop disease biocontrol in food production: development of biocontrol agents of fungal diseases for commercial applications in food production systems, Seville, Spain, March 24–27, p 27
Jacobs CJ, Van Vuoren HJJ (1991) Effects of different killer yeast on wine fermentations. J Amst Soc Brew 42:295–299
Janisiewicz WJ, Korsten L (2002) Biological control of postharvest diseases of fruits. Annu Rev Phytopathol 40:411–441
Janisiewicz WJ, Tworkoski TJ, Sharer C (2000) Characterizing the mechanism of biological control of postharvest diseases on fruits with a simple method to study competition for nutrients. Phytopathology 90:1196–1200
Jijakli MH, Lepoivre P (1998) Characterization of an exo-β-1, 3-glucanase produced by Pichia anomala strain K, antagonist of Botrytis cinerea on apples. Phytopathology 88:335–343
Levy RM (2003) Aplicação de leveduras no controle de Penicillium expansum. Ph.D. Thesis, Universidade Estadual de Londrina
Marquina D, Santos A, Peinado JM (2002) Biology of killer yeasts. Int Micrbiol 5:65–72
Martinac B, Zhu H, Kubalski A, Zhou X, Culbertson M, Bussey H, Kung C (1990) Yeast K1 killer toxin forms ion channels in sensitive yeast spheroplasts and in artificial liposomes. Cell Biol 87:6228–6232
Martinez Noel GMA, Madrid EA, Botin R, Lamattina L (2001) Indole acetic acid attenuates disease severity in potato-Phytophtora infestans interaction and inhibits the pathogen growth in vitro. Plant Physiol Biochem 39:815–823
Masih EI, Paul B (2002) Secretion of beta-1, 3-glucanase by the yeast Pichia membranifaciens and its possible role in the biocontrol of Botrytis cinerea causing mold disease of the grapevine. Curr Microbiol 44:391–395
Masih EI, Slezack-Deschaumes S, Marmaras I, Ait Barka E, Vernet G, Charpentier C, Adholeya A, Paul B (2001) Characterization of the yeast Pichia membranifaciens and its possible use in the biological control of Botrytis cinerea. FEMS Microbiol Lett 202:227–232
Milagres AMF, Machuca A, Napoleão D (1999) Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (CAS) agar plate assay. J Microbiol Methods 37:1–6
Miller GH (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–429
Nakamura TT, Murakami M, Saotome K, Tomita T, Kitsuwa P, Meyers SP (1991) Identification of indole-3-acetic acid in Pichia spartinae, an ascosporogenous yeast from Spartina alterniflora marshland environments. Mycologia 83:662–664
Piccinin E, Di Piero RM, Pascholati SF (2005) Efeito de Saccharomyces cerevisiae na produtividade de sorgo e na severidade de doenças foliares no campo. Fitopatol Bras 30:5–9
Pinto NFJA (2003) Controle químico da antracnose (Colletotrichum graminicola) do sorgo. Rev Bras Milho e Sorgo 2:148–152
Saligkarias ID, Gravanis FT, Eptona HAS (2002) Biological control of Botrytis cinerea on tomato plants by use of epiphytic yeasts Candida guilliermondii strains 1001 and US 7 and Candida oleophila strain I-182:II. A study on mode of action. Biol Control 25:151–161
Santos A, Marquina D (2004) Killer toxin of Pichia membranifaciens and its possible use as a biocontrol agent against grey mould disease of grapevine. Microbiology 150:2527–2534
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:46–56
Shimizu K, Adachi T, Kitano K, Shimazaki T, Totsuka A, Hara S, Dittrich HH (1985) Killer properties of wine yeast and characterization of killer wine yeasts. J Ferment Technol 63:421–429
Shurttleff MC (1980) A compendium of corn diseases, 2nd edn. St Paul, Am Phytopathol Soc, p 105
Skipper N, Bussey H (1977) Mode of action of yeast toxins: energy requirement for Saccharomyces cerevisiae killer toxin. J Bacteriol 129:668–677
Stumm C, Hermans JMH, Middelbeek EJ, Croes AF, De Vries GJM (1977) Killer sensitive relationships in yeasts from natural habitats. Antonie Leeuwenhoek 43:125–128
Taqarort N, Echairi A, Chaussod R, Nouaim R, Boubaker H, Abdellah A, Benaoumar AA, Boudyach E (2008) Screening and identification of epiphytic yeasts with potential for biological control of green mold of citrus fruits. World J Microbiol Biotechnol 24:3031–3038
Urquhart EJ, Punja ZK (2002) Hydrolytic enzymes and antifungal compounds produced by Tilletiopsis species, phyllosphere yeasts that are antagonists of powdery mildew fungi. Can J Microbiol 48:219–229
Vero S, Mondino P, Burgueno J, Soubes M, Wisniewski M (2002) Characterization of biocontrol activity of two yeast strains from Uruguay against blue mold of apple. Postharvest Biol Tech 26:91–98
Vora MS, Shelat HN (1998) Torulospora globosa: a unique solubilizing tricalcium phosphate. Indian J Agric Sci 68:630–631
Walker GM, Mcleod AH, Hodgson VJ (1995) Interactions between killer yeasts and pathogenic fungi. FEMS Microbiol Lett 127:213–222
Wang WL, Chi ZM (2009) Siderophore production by the marine-derived Aureobasidium pullulans and its antimicrobial activity. Bioresource Technol 100:2639–2641
Weiler F, Schmitt MJ (2003) Zygocin, a secreted antifungal toxin of yeast Zygosaccharomyces bailii, and its effect on sensitive fungal cells. FEMS Yeast Res 3:69–76
White TJ, Bruns T, Lee S, Taylor J (1994) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JS, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 129–141
Wilson CL, Ghaouth A, Chalutz E, Droby S, Stevens C, Lu JY, Khan V, Arul J (1994) Potential of induced resistance to control postharvest diseases of fruits and vegetables. Plant Dis 78:837–844
Windels CE, Lindow SE (1985) Biological control on the phylloplane, 2nd edn. The American Phytopathological Society, Saint Paul
Wisniewski ME, Biles C, Droby S, Mclaughlin R, Wilson CL, Chalutz E (1991) Mode of action of the postharvest biocontrol yeast, Pichia guilliermondii. 1. Characterization of attachment to Botrytis cinerea. Physiol Mol Plant Pathol 39:245–258
Xin G, Glawe D, Doty SL (2009) Characterization of three endophytic, indole-3-acetic acid-producing yeasts occurring in Populus trees. Mycol Res 113(9):973–980
Young TW (1982) The properties of brewing performance of brewing yeast possessing killer character. J Am Soc Brew Chem 42:1–4
Yu T, Chen J, Lu H, Zheng X (2009) Indole-3-acetic acid improves postharvest biological control of blue mold rot of apple by Cryptococcus laurentii. Phytopathology 99(3):258–264
Zanette GF, Nóbrega GMA, Meirelles LDP (2009) Morphogenetic characterization of Colletotrichum sublineolum strains, causal agent of anthracnose of sorghum. Trop Plant Pathol 34(3):146–151
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosa, M.M., Tauk-Tornisielo, S.M., Rampazzo, P.E. et al. Evaluation of the biological control by the yeast Torulaspora globosa against Colletotrichum sublineolum in sorghum. World J Microbiol Biotechnol 26, 1491–1502 (2010). https://doi.org/10.1007/s11274-010-0324-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0324-8