Abstract
The pathogens are reduced by volatile organic compounds (VOCs) generated by yeasts play an important role in controlling postharvest diseases. The yeast HXMG-1, which works effectively against the grey mould pathogen of grapes (Botrytis cinerea), was evaluated for its potential to generate volatile organic compounds as one of its modes of action. A double Petri dish assay was used to evaluate the effect of VOCs produced by HXGM-1 on mycelial and spore development of the target pathogens. Compared to the control, the VOCs produced by yeast HXMG-1 significantly reduced the growth of mycelium and spore germination of Botrytis cinerea. Specifically, the mycelial growth of Botrytis cinerea was completely restricted and the rate of spore germination of Botrytis cinerea was only 20.11% at a concentration of 1 × 109 CFU/mL. It was also found that the VOCs could significantly inhibit mycelial growth with an inhibition of 82.46% at a concentration of 1 × 108 CFU/mL. The VOCs caused the mycelium to grow curved, resulting in larger mycelial tips, fewer nuclei, and shorter mycelial septum spacing. In vivo tests, noninjure or injure grapes were artificially inoculated with the pathogen hyphal disc followed by biofumigation with VOCs produced by yeast HXMG-1, and the treatments (Wp2 and Wp3) significantly controlled pathogenic infection, confirming the results of in vitro tests. By molecular biological identification based on comparative sequence analysis of the 18S rDNA gene, the HXMG-1 strain was identified as Hanseniaspora uvarum. Through the creation of a phylogenetic tree, HXMG-1 was recognised as a member of the Ascomycota, Hemiascomycota, Yeasts, and Hanseniaspora sp. families. In conclusion, the yeast strain HXMG-1 created VOCs that significantly inhibited the development of Botrytis cinerea on grapes and is expected to be further developed and utilised. This study lays the foundation for the use of Hanseniaspora sp. for biological control of postharvest disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postharvest losses are one of the major problems in grape production, in terms of quality and quantity (Nandhini et al. 2021; Senthil 2011). A 30% cost loss and 39% yield loss in grapes have been recorded as a result of postharvest pathogen infection (Senthil et al. 2011). Grey mould is one of the most harmful postharvest diseases affecting grapes, caused by Botrytis cinerea. The primary strategy for preventing postharvest diseases is currently the use of synthetic fungicides. However, fungicide resistance is a widespread problem with B.cinerea. The discovery of novel, non-toxic bio-antagonists to control postharvest losses of grapes.
The use of new environmentally friendly methods to control postharvest pathogens is at the forefront of various strategies, and the use of biological agents to prevent postharvest diseases would be a potential alternative to synthetic chemicals. Yeasts are found in a wide variety of environments and are considered to be effective antagonists of many plant diseases. Due to their antagonistic ability, minimal culture requirements, and limited biosafety concerns, many of these unicellular fungi have been investigated for biocontrol applications (Freimoser et al. 2019). Many studies around the world have shown that yeast spp. can be used to prevent postharvest diseases (Alvarez et al. 2019; Kasfi et al. 2018; Kheireddine et al. 2021; Perez et al. 2017; Zhang et al. 2012), they have been described as biocontrol yeasts in scientific publication and some of them (C.oleophila, A.pullulans, M.fructicola, C.albidus, and S.cerevisiae) were registered and marketed as plant protection products(Freimoser et al. 2019). Currently, many studies have revealed the mechanism of different yeasts to control plant diseases (Choinska et al. 2020; González-Gutiérrez et al. 2022; Hu et al. 2017; Sangorrín et al. 2007; Zhao et al. 2021): (I) Competition for nutrients or space (González-Gutiérrez et al. 2022; Rosa et al. 2010), this is considered to be the primary mode of action of biocontrol yeasts; (II) Production of extracellular lytic enzymes that can be components in host–pathogen interactions, such as chitinases, glucanases, lipases, or proteases have been regularly reported and highlighted in antagonistic yeasts and implicated in their biocontrol activity (Bar-Shimon et al. 2004; Chan and Tian 2005; Freimoser et al. 2019; Segal et al. 2002); (III) Toleration reactive oxygen species has also been reported as one of the biocontrol mechanisms of yeasts (Castoria et al. 2003); (IV) Formation of biofilms, which is considered to be an apecific and highly successful strategy to compete for space (Desai et al. 2014; Fiori et al. 2012; Maserti et al. 2015); (V) Toxin production, which provides a competitive advantage activity against bacteria or fungi under in dry, oligotrophic conditions, whereas it has no effect on antagonistic in more humid environments (Droby et al. 2002; Jones and Dangl 2006); (VI) Induced resistance. Biocontrol yeasts can induce systemic resistance in plants against a wide range of pathogens and this activity is thought to contribute to their biocontrol activity. Antagonistic yeast, as a bioactivator, can induce the expression of proteins related to disease progression in fruits and vegetables and improve disease resistance (Freimoser et al. 2019; Di Francesco et al. 2015). In recent years, most research on yeast-mediated control of fruit decay has focussed on the induction of defence enzymes against fungal pathogens (Bar-Shimon et al. 2004; Chan and Tian 2005; Freimoser et al. 2019; Segal et al. 2002); (VII) Emission of volatile organic compounds (VOCs). The VOCs produced by some biocontrol yeasts significantly inhibited the growth of postharvest pathogens (Di Francesco et al. 2015; Khunnamwong et al. 2020).
VOCs are small molecules (usually less than 300 Da) that don’t dissolve well in water and have a high gas pressure (Freimoser et al. 2019). Recent experimental evidence suggests that VOCs from yeast play an important role in pathogen interactions (Fialho et al. 2009; Jaibangyang et al. 2020; Khunnamwong et al. 2020). Two Aureobasidium pullulans were evaluated for their ability to induce the production of VOCs for the control of five common postharvest diseases (Di Francesco et al. 2015). Similarly, the citrus black spot pathogen was suppressed in vitro by VOCs produced by Saccharomyces cerevisiae (Fialho et al. 2009). The inhibitory effect of Sporidiobolus pararoseus against B.cinerea spore germination and mycelial development was mainly due to its inhibition of ochratoxin growth and ochratoxin A synthesis in some yeasts during coffee processing. Hua et al. identified 2-phenylethanol as a major component of P.anomala, which produces VOCs that inhibit spore germination, mycelial development, and toxin synthesis. To date, More than 20 different VOCs have been found in various biocontrol yeast strains.
Our previous studies have used different yeasts from different materials were used to control postharvest pathogens. amongst these yeasts, a potentially bioactive yeast called HXMG-1 was found on the surface of the most dominant grape varieties grown in the Tianjin region of China, and biofumigation treatments significantly reduced the incidence of decay in various fruits and vegetables. However, little is known about the ability of the HXMG-1 yeast strain to inhibit postharvest grape rot by producing volatile organic compounds (VOCs). Therefore, the objectives of this research were to determine the biological control activity of VOCs produced by HXMG-1 yeast on grape grey mould through laboratory in vitro experiments and in vivo inoculation, and to determine the taxonomic status of this yeast using molecular biology techniques. All this work will provide the theoretical basis for the further development and exploitation of this strain.
Materials and methods
Yeast
At the Laboratory of Plant Pathology (Tianjin Agriculture University, Tianjin, China), the antagonist yeast HXMG-1 was isolated from the surface of grape fruits for in vitro and in vivo testing. The strain was cultured in YPD medium (prepared with 10 g/L yeast extract, 20 g/L peptone, 20 g/L glucose, 20 g/L agar, 1000 mL H2O). The strain was subcultured on YPD agar at 4-month intervals and kept at 4 °C.
Fruit
Grapes (Jasmine) were harvested in 2020 from an organic farm in Hangu, north China. Samples were selected for homogeneity of shape, size, ripeness, firmness, colour, and absence of mechanical damage or fungal infection. The fruits were then quickly transported to the laboratory in plastic boxes to prevent mechanical damage, under ambient temperature and humidity conditions (18 °C and 75%RH).
Pathogens
B.cinerea, used in this study was isolated from decaying grapes collected in Tianjin, China. It was grown on Potato Dextrose Agar(200 g of potato, 20 g of dextrose, 18 g agar per 1000 mL of distilled water), incubated at 25 °C for 10 days.
Preparation of conidial suspension of the Botrytis cinerea
The surface of the PDA culture was scraped to obtain B.cinerea spore suspension, which was then passed through four layers of sterile cheesecloth to remove any mycelium adhering to the surface. This experimental technique was slightly modified from Zhang’s method (Zhang et al. 2012).
Preparation of suspension of yeast
Yeast was cultured in liquid yeast glucose medium (yeast extract 5 g/L, glucose 10 g/L) for 24 h, before being collected, cleaned and resuspended in sterile distilled water. The cell concentration was adjusted to 1 × 108 CFU/mL using a hemocytometer.
Effects of volatile organic compounds on the mycelial development of Botrytis cinerea
The effect of VOCs on the mycelial development of B.cinerea was investigated using a previous approach with slight modifications (Rouissi et al., 2013). One plate was inoculated with 100 µL of yeast suspension (OD660 = 1.0 × 108 CFU/mL), whilst the other plate, was inoculated with a disc of pathogen mycelium (5 × 5 mm2). The two plates were placed “mouth to mouth” and covered with parafilm. After incubation of the plates at 25 °C for 5 days, mycelial growth was detected. Plates inoculated with pathogens only were used as controls. Radial mycelial growth in two randomly selected vertical directions was evaluated to assess the efficacy of HXMG-1 in inhibiting B.cinerea mycelial growth. The inhibition rate was determined using the following equation:
where T and C respectively, stand for the mean colony diameters of the treated and control samples. The experiment was run twice, and three replicates of each treatment were examined.
The influence of volatile organic compounds on the germination of Botrytis cinerea spores
A dual culture method was used to determine the effect of VOCs on B.cinerea spore germination. 100 µL of B.cinerea spore solution was used to inoculate PDA plates (1 × 106 CFU/mL). YPD plates were coated with 100 µL of yeast suspension at concentrations of 10, 1 × 102, 1 × 104, 1 × 106, 1 × 108 and 1 × 109 CFU/mL respectively. Equivalent volumes of sterile distilled water were used for controls. The two plates were placed “mouth to mouth” and covered with parafilm. After 8 h of incubation at 26 ± 1 °C, the percentage of conidial germination was assessed. The experiment was replicated with three duplicates for each treatment.
Influence of volatile organic compounds on Botrytis cinerea’s hyphal morphology
The influence of VOCs on the hyphal morphology of B.cinerea was studied using a dual culture technique. Mycelial plugs of B.cinerea were placed in the centre of a PDA medium plate, and coverslips were placed diagonally and evenly at a distance of 1 cm from the B.cinerea mycelial plugs to form a circle. A YPD medium plate with 100 µL of yeast cell suspension (1 × 108 cells/mL) was inoculated, and the two plates were put “mouth to mouth” and covered with parafilm. Yeast-free plates were used and samples were collected after 48 h of incubation at 25 °C. A scanning electron microscope (SEM) was used to examine the hyphal morphology of B.cinerea. Each treatment had three replicates, and the experiment was repeated three times to evaluate the effect of VOCs on B.cinerea hyphal morphology.
Influence of volatile organic compounds on the nucleus and septum of Botrytis cinerea’s mycelia
The influence of VOCs on the nucleus and septum of B.cinerea mycelia was investigated using the same approach as previously (Influence of VOCs on B.cinerea hyphal morphology). Mycelia were stained with a solution containing 4′,6-diamidino-2-phenylindole (DAPI, 11 μg/mL) or Calcofluor White (CFW, 85 g/mL) for nuclei or septum distribution, respectively, and observed with a fluorescence microscope (Olympus BX-51, Japan). The technique can also detect B.cinerea hyphal morphology. Each experiment was performed three times, with three replicates of each treatment.
Effects of volatile organic compounds on artificial infection of grapes
The efficacy Efficacy of the VOCs in the management of B.cinerea on grapes was evaluated using the technique proposed by Di Francesco et al. with minor modifications (Di Francesco et al. 2015). Fruits were cleaned with water and disinfected by immersion in sodium hypochlorite solution (0.1 N) for 1 min. The samples were then rinsed twice with distilled and dried for 30 min at room temperature in a sterilised dry air box. The pre-prepared grapes were divided into 8 groups, 20 grapes in each group, of which 4 groups were noninjure, and the other 4 groups with injure. The noninjure treatments of grapes were inoculated directly with a disc of pathogen mycelium (5 × 5 mm2) in the equatorial region without any treatment. The injure treatment of grapes were inoculated with discs of pathogen mycelium (5 × 5 mm2) at the injure sites(2 × 2 mm2 wound was made with sterile inoculum needle) in the equatorial region. Then, the treated grapes are placed on sterile grids(with a sterile and breathable membrane) in closed glass desiccators (10 cm × 18 cm, bottom diameter by up diameter ≈ 5 L). Aliquots (100 µL) of the yeast cell suspension (about 2–8 × 108 CFU/mL) were coated onto 9 cm diameter YPD and cultured at 28 °C for 48 h. The lids of the Petri dishes were removed in the noninjure treatments, and zero (one, two, or three) plates containing yeast cultures were placed in each bottom of the glass dryer labelled Control(Noninjuer), Wp1(Noninjuer), Wp2(Noninjuer), Wp3(Noninjuer). In the injure treatments, the lids of the Petri dishes were removed and zero(one, two or three) plates with the yeast cultures were placed in each bottle of a glass dryer labelled Control(Injuer), Wp1 (Injure), Wp2 (Injure) and Wp3 (Injure) in turn. which were placed in an incubator for 72 h at 25 ℃. Measurement of lesion diameter in two randomly selected vertical directions. A completely randomised design was used for the experiment, with each treatment including three replicates. The experiment was performed three times.
Yeast genomic DNA extraction
The yeast strain HXMG-1 on the culture medium was taken up in a mortar and ground under liquid nitrogen next to the flame of an alcohol lamp. The ground yeast strain was placed in a 1.5 mL centrifuge tube bearing the name of the yeast, 0.6 mL Tris–HCl (PH 8.0) buffer containing Ethylene Diamine Tetraacetic Acid (EDTA) (pH 8.0) was added, and the samples were mixed well by sucking and tapping the pipette head. 250 µL of 10% sodium dodecyl sulphate was added to the tube gently inverted and mixed. 3 µL protease K (20 ng/µL) was added and mixed gently and water bathed at 37 ℃ for 1 h. 150 µL 5 mol/L NaCl was added and gently mixed. 150 µL 2% hexadecyl trimethylammonium bromide (CTAB) was added and mixed gently, followed by a water bath at 65 °C, and centrifugation at 12,000 rpm for 20 min at room temperature. The supernatant was carefully drained into a new 1.5 mL centrifuge tube, isopropyl alcohol was added in the same volume, mixed thoroughly, and placed at room temperature for 30 min, Centrifugation was performed at 12,000 rpm for 30 min at 4 ℃. The supernatant was aspirated and the liquid was dried on absorbent paper, 750 µL 70% ethanol was added, the tube wall was gently tapped, the precipitate was suspended and inverted several times, centrifuged at 12,000 RPM for 2 min at 4 ℃. Add 30 µL of purified water to each tube to dissolve the precipitate (Rnase was added to the water, and the final concentration was 10 ng/µL). Gently swirl the tube wall and dissolve overnight at 4 ℃.
Yeast identification
Genomic ribosomal DNA (rDNA) sequences isolated from yeast cells were identified by polymerase chain reaction (PCR). PCR for amplification of LSU rDNA, including the D1/D2 domain, 5.8S rDNA, and internal transcribed spacer (ITS) segments, using universal primers ITS1 (5,-TCCGTAGGTGAA CCTGCGG-3,) and ITS4 (5,-TC CTCCGCTTATTGATATGC-3,). GReaction system: H2O 17.8 µL, buffe 3 µL, NTP 2 µL, primer 13 µL, Primer 23 µL DNA template 1 µL, enzyme 0.2 µL, total volume 30 µL. PCR is performed as follows: initial denaturation at 95 °C for 5 min, repeated denaturation at 95 °C for 30 s (annealing temperature, 48–55 °C for 30 s) and extension at 72 °C for 1 min for 35 cycles and 72 °C for 10 min, 12 °C forever. DNA sequencing of these samples was performed by BGI Sequencing. Nucleotide sequences were BLAST searched using the National Centre for Biotechnology Information database (http://www.ncbi.nlm.nih.gov). A phylogenetic tree based on the 18S rDNA sequence was constructed using the neighbour-joining algorithm in MEGA 2.1,
Statistical analysis
Data are expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to test the significance of differences between each treatment and control. A value of p < 0.05 was considered statistically significant. A randomised design was used for all studies.
Results
Effects of volatile organic compounds on the development of B.cinerea mycelium
Since there was no contact between the yeast strain and the pathogen in our study, the antifungal effect of B.cinerea on mycelium development could be attributed to the VOCs produced by yeast HXMG-1. Compared to the control, VOCs produced by yeast HXMG-1 suppressed mycelium development of B.cinerea, although with varying efficacy (Table 1, Fig. 1). With the application of a treatment that increased the concentration of VOCs, the mycelial development of B.cinerea gradually slowed down. At a concentration of 1 × 109 CFU/mL (98.25% inhibition), the mycelial growth B.cinerea was almost completely inhibited. The low inhibitory effect of B.cinerea was also found (43.85% − 1 × 102 CFU/mL; 59.95% − 1 × 104 CFU/mL). There were significant changes between treatments (p < 0.05). B.cinerea mycelial growth was found to be more sensitive to VOCs produced by yeast HXMG-1.
Effects of volatile organic compounds produced by yeast HXMG-1 on the germination of Botrytis cinerea spores
Based on the results of mycelial growth, we found that VOCs had the most pronounced suppression effect on mycelial development. To investigate the effect of VOCs on spore germination, Spore germination of B.cinerea was estimated after 12 h at 25 °C. It was found that different VOC treatments inhibited B.cinerea spore germination (Table 2). B.cinerea spore germination decreased steadily with treatment concentrations and showed significant variation between treatments. The treatment of 1 × 109 CFU/mL treatment had the most significant effect on B.cinerea spore germination, with only 20.11% of conidia germinating. The 1 × 108 CFU/mL treatment also had a good effect on B.cinerea spore germination (44.67% of germinated conidia).
Effect of volatile organic compounds produced by yeast HXMG-1 on hyphae morphology of B.cinerea
SEM and fluorescence microscopy examinations were used to examine morphological changes in the hyphae of treated B.cinerea to further, investigate the influence of VOCs produced by yeast HXMG-1 on B.cinerea. As shown in Fig. 2, untreated control hyphae had normal morphology (Fig. 2a, c and e), whereas treated hyphae had morphological abnormalities with wrinkled and uneven distortions (Fig. 2b, arrows). The average hyphal branching angle reached plateau 82.43°–100.85° in the VOCs (Fig. 2d, double arrows), whereas the average hyphal branching angle in control was 50.24°–70.75° (Fig. 2c, double arrows). Microscopic examination showed that the hyphal tissue was twisted and contracted in samples exposed to VOCs produced by yeast HXMG-1 (Fig. 2f, arrow). In conclusion, treatment of yeast strain HXMG-1 can cause abnormal growth and development of the mycelium of B.cinerea.
Effects of volatile organic compounds on mycelial nuclei and septum of B.cinerea
Figure 3 shows that the mycelial nuclei in B.cinerea were densely distributed in control (Fig. 3b), but the number of mycelial nuclei in B.cinerea treated with yeast strain HXMG-1 was reduced by about 50% at the same distance compared to the control, and the distribution was not more uniform (Fig. 3a). As shown in Fig. 4, the spacing of the control septa was 33.23–69.00 µm, and the spacing of the treated septa was 16.12–28.00 µm, indicating that the septum spacing was significantly reduced. It can be concluded that treatment with yeast strain HXMG-1 inhibited nuclear division and mycelial elongation of B.cinerea, thereby affecting rapid mycelial reproduction and development of B.cinerea.
Effects of yeast strain HXMG-1′s volatile organic compounds in vivo on B.cinerea on grapes
According to the test (Fig. 5), grey mould on grapes was better controlled by the treatments. The diameter of the lesions decreased significantly in noninjure groups than in injure groups (p < 0.05) for the same treatment (Fig. 5a). With increasing VOC concentration, compared to the control, except for the treatment of Wp1 in noninjure groups, the other diameters of lesions decreased significantly (p < 0.05) (Fig. 5b). Especially with the treatment of Wp3, the lesion inhibition was inhibited to 55.75% in the noninjure treatment group and 60.48% in the injure treatment group (Fig. 5b). The results of the treatment experiments showed that the VOCs produced by the yeast strain HXMG-1 were tested and had a good effect on B.cinerea on grapes.
In vivo antagonistic effect of VOCs produced by HXMG-1on B.cinerea in grapes (Diameters of lesions–cm) Fruits were artificially inoculated for 72 h at 25 °C and 80% RH.Control consisted of NYDA without yeast inoculation.each value is the mean ± standard deviation of three replicates per treatment. Different letters represent significant differences amongst the yeast VOCs concentrations according to one-way analysis of variance(ANOVA).A value of p < 0.05
Results of the identification of the yeast strain HXMG-1
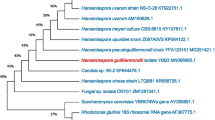
By molecular biological identification based on comparative sequence analysis of the 18S rDNA gene, the HXMG-1 strain was identified as Hanseniaspora uvarum. The 18S rDNA sequence of yeast strain HXMG-1 was submitted to GenBank, and a sequence similarity search was performed by BLAST. Eight strains with high homology were selected, and the phylogenetic tree was constructed by using MEGA 2.1 software. Figure 6 shows that yeast strain HXMG-1 and members of the genus Hanseniasperu are on the same branch. The homology was 100%. Therefore, the yeast strain HXMG-1 was identified as Ascomycota, Hemiascomycetes, Saccharomycetales, and the yeast family (Saccharomycetaceae), a member of the genus Hanseniasperu.
Discussion
Humans are interested in biological control agents due to concerns about chemical resistance and environmental damage. The use of natural enemies to reduce the number of plant diseases is known as biological control (Chen et al. 2018). Plant protection in the twenty-first century should include biological control as an important component wherever possible. Biological control has been widely used in the process of postharvest disease control of crops (Prendes et al. 2018; Ruiz-Moyano et al. 2016; Tang et al. 2015). The problem of chemical residues can be avoided by using of biological control methods for postharvest fruit diseases, thus eliminating the toxic effect of chemical agents on the human body and improving food safety. The technology of biological control of postharvest fruit and vegetable diseases is being perfected and will eventually replace conventional chemicals. The use of antagonistic yeast in the biological control of postharvest diseases has attracted increasing interest in recent years (Liu et al. 2013; Lutz et al. 2013; Zhang et al. 2019). Currently, research is mainly focussed on the mechanism of biocontrol yeasts, improving the disease control effect of biocontrol yeasts, and using genetic engineering technology to construct new antagonistic yeasts (Cheng et al. 2019; Shao et al. 2019). Yeasts are abundant in nature and widely used in the food industry. Many studies have demonstrated that yeast is safe and non-toxic as a biocontrol bacterium, and no genetic toxicity has been found. These have been used as evidence that it can be used as a biocontrol bacterium for fruit and vegetable diseases and is safe for the human body. It can also be demonstrated that the yeast is safe to use as a source of biocontrol factor as it was isolated from the surface of the grape fruit for this investigation.
The history of using biocontrol yeasts for biological control of postharvest fruit diseases is relatively short. Currently, more than 10 antagonistic yeasts have been reported, and these yeasts are mainly used to control penicillium, green mould, grey mould, etc. during storage. However, there is no published report using Hanseniasperu to inhibit B.cinerea in grapes. The results of this investigation showed that yeast strain HXMG-1 significantly inhibited B.cinerea. The preliminary study on the inhibitory mechanism of VOCs produced by yeast strain HCXMG-1 on B.cinerea, and other agents needs further research and confirmation. The effects of VOCs produced by yeast HXMG-1 on B.cinerea in grapes were presented in detail. The results of this article indicated that the VOCs were successful in effectively preventing grape grey mould. Numerous recent investigations have shown that yeast-produced VOCs produced by yeast can significantly inhibit the development of several pathogenic microbes (Choinska et al. 2020; Di Francesco et al. 2015; Oro et al. 2018). At the same time, VOCs have potential application value in agriculture, industry and pharmacology due to the relatively small molecular weight of volatiles and their ease of diffusion. However, whether VOCs produced by other yeasts can affect the grey mould of grapes, how yeast-produced VOCs can be effectively applied to the current production practise, and whether it can also have a good control effect on other fruit and vegetable diseases still need to be further studied and confirmed.
In this study, the effects of VOCs produced by yeast HXMG-1 on B.cinerea were investigated in vitro and in vivo experiments without contact between pathogenic bacteria and yeast. The results showed that VOCs by yeast HXMG-1 could significantly inhibit B.cinerea. The method rules out antimicrobial activity, nutrient competition, and other modes of action that involve contact. Therefore, this study focuses on the inhibition of volatile compounds produced by yeast on pathogenic bacteria, which may be the primary mode of action of yeast. The study of the active components of volatile compounds of yeast HXMG-1 will be our next important work.
The paper showed that VOCs produced by yeast HXMG-1 could strongly limit B.cinerea mycelium development at an inhibition rate of 82.46% with a concentration of 1 × 108 CFU/mL. It caused mycelium bending growth, mycelium tip enlargement, nucleus reduction, and septum spacing increase. According to a study, the volatile chemical produced by Saccharomyces cerevisiae 0939–5 prevented the development of grape wilt by 77.67% at a dosage of 1 × 108 CFU/mL, which can also cause the mycelia of B.cinerea to appear distorted and deformed, but the distance between the septa increases. These results showed that VOCs generated by yeast HXMG-1 could suppress B.cinerea. This research lays the groundwork for future research and applications of VOCs produced by yeast HXMG-1 in control.
References
Alvarez A et al (2019) Role of Antarctic yeast in biocontrol of Penicillium expansum and patulin reduction of apples. Environ Sustain 2(3):277–283
Bar-Shimon M et al (2004) Characterization of extracellular lytic enzymes produced by the yeast biocontrol agent Candida oleophila. Curr Genet 45(3):140–148
Castoria R, Caputo L, De Curtis F, De Cicco V (2003) Resistance of postharvest biocontrol yeasts to oxidative stress: a possible new mechanism of action. Phytopathology 93(5):564
Chan Z, Tian S (2005) Interaction of antagonistic yeasts against postharvest pathogens of apple fruit and possible mode of action. Postharvest Biol Technol 36(2):215–223
Chen PH, Chen RY, Chou JY (2018) Screening and Evaluation of Yeast Antagonists for Biological Control of Botrytis cinerea on Strawberry Fruits. Mycobiology 46(1):33–46
Cheng L, Nie X, Jiang C, Li S (2019) The combined use of the antagonistic yeast Hanseniaspora uvarum with β-aminobutyric acid for the management of postharvest diseases of kiwifruit. Biol Control 137:104019
Choinska R, Piasecka-Jozwiak K, Chablowska B, Dumka J, Lukaszewicz A (2020) Biocontrol ability and volatile organic compounds production as a putative mode of action of yeast strains isolated from organic grapes and rye grains. Antonie Van Leeuwenhoek 113(8):1135–1146
Desai JV, Mitchell AP, Andes DR (2014) Fungal biofilms, drug resistance, and recurrent infection. Cold Spring Harb Perspect Med 4(10):a019729
Di Francesco A, Ugolini L, Lazzeri L, Mari M (2015) Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol Control 81:8–14
Droby S, Vinokur V, Weiss B, Cohen L, Porat R (2002) Induction of resistance to Penicillium digitatum in grapefruit by the yeast biocontrol agent Candida oleophila. Phytopathology 92(4):393–399
Fialho MB, Toffano L, Pedroso MP, Augusto F, Pascholati SF (2009) Volatile organic compounds produced by Saccharomyces cerevisiae inhibit the in vitro development of Guignardia citricarpa, the causal agent of citrus black spot. World J Microbiol Biotechnol 26(5):925–932
Fiori S et al (2012) Identification of differentially expressed genes associated with changes in the morphology of Pichia fermentans on apple and peach fruit. FEMS Yeast Res 12(7):785–795
Freimoser FM, Rueda-Mejia MP, Tilocca B, Migheli Q (2019) Biocontrol yeasts: mechanisms and applications. World J Microbiol Biotechnol 35(10):154
González-Gutiérrez KN, Ragazzo-Sánchez JA, Barros-Castillo JC, Narváez-Zapata JA, Calderón-Santoyo M (2022) Yeasts with potential biocontrol of Colletotrichum gloeosporioides in avocado (Persea americana Mill. cv. Hass) and characterization of Yamadazyma mexicana mechanisms. Eur J Plant Pathol 165(3):525–543
Hu H, Wisniewski ME, Abdelfattah A, Zheng X (2017) Biocontrol activity of a cold-adapted yeast from Tibet against grey mould in cherry tomato and its action mechanism. Extremophiles 21(4):789–803
Jaibangyang S, Nasanit R, Limtong S (2020) Biological control of aflatoxin-producing Aspergillus flavus by volatile organic compound-producing antagonistic yeasts. Biocontrol 65(3):377–386
Jones JD, Dangl JL (2006) The plant immune system. Nature 444(7117):323–329
Kasfi K, Taheri P, Jafarpour B, Tarighi S (2018) Identification of epiphytic yeasts and bacteria with potential for biocontrol of grey mould disease on table grapes caused by Botrytis cinerea. Span J Agric Res 16(1):e1002
Kheireddine A et al (2021) Characterization of new yeast isolates collected from different fruits in Tunisia and biocontrol activity against Penicillium expansum on apples. J Plant Pathol 103(4):1169–1184
Khunnamwong P et al (2020) Evaluation of antagonistic activity and mechanisms of endophytic yeasts against pathogenic fungi causing economic crop diseases. Folia Microbiol (praha) 65(3):573–590
Liu J, Sui Y, Wisniewski M, Droby S, Liu Y (2013) Review: Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int J Food Microbiol 167(2):153–160
Lutz MC, Lopes CA, Rodriguez ME, Sosa MC, Sangorrin MP (2013) Efficacy and putative mode of action of native and commercial antagonistic yeasts against postharvest pathogens of pear. Int J Food Microbiol 164(2–3):166–172
Maserti B et al (2015) Proteome changes during yeast-like and pseudohyphal growth in the biofilm-forming yeast Pichia fermentans. Amino Acids 47(6):1091–1106
Nandhini M, Harish S, Aiyanathan KEA, Durgadevi D, Beaulah A (2021) Glycerol-based liquid formulation of the epiphytic yeast Hanseniaspora guilliermondii isolate YBB3 with multiple modes of action controls postharvest Aspergillus rot in grapes. J Plant Pathol 103(4):1253–1264
Oro L, Feliziani E, Ciani M, Romanazzi G, Comitini F (2018) Volatile organic compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae inhibit growth of decay causing fungi and control postharvest diseases of strawberries. Int J Food Microbiol 265:18–22
Perez MF et al (2017) Antagonistic yeasts for the biological control of Penicillium digitatum on lemons stored under export conditions. Biol Control 115:135–140
Prendes LP et al (2018) Isolation, identification and selection of antagonistic yeast against Alternaria alternata infection and tenuazonic acid production in wine grapes from Argentina. Int J Food Microbiol 266:14–20
Rosa MM, Tauk-Tornisielo SM, Rampazzo PE, Ceccato-Antonini SR (2010) Evaluation of the biological control by the yeast Torulaspora globosa against Colletotrichum sublineolum in sorghum. World J Microbiol Biotechnol 26(8):1491–1502
Ruiz-Moyano S et al (2016) Yeasts isolated from figs (Ficus carica L.) as biocontrol agents of postharvest fruit diseases. Food Microbiol 57:45–53
Sangorrín MP, Lopes CA, Jofré V, Querol A, Caballero AC (2007) Spoilage yeasts from Patagonian cellars: characterization and potential biocontrol based on killer interactions. World J Microbiol Biotechnol 24(7):945–953
Segal E, Yehuda H, Droby S, Wisniewski M, Goldway M (2002) Cloning and analysis of CoEXG1, a secreted 1,3-beta-glucanase of the yeast biocontrol agent Candida oleophila. Yeast 19(13):1171–1182
Senthil L (2011) Efficacy of different biological control agents against major postharvest pathogens of grapes under room temperature storage conditions. Phytopathol Mediterr 50(1):55–65
Shao Y-Z, Zeng J-K, Tang H, Zhou Y, Li W (2019) The chemical treatments combined with antagonistic yeast control anthracnose and maintain the quality of postharvest mango fruit. J Integr Agric 18(5):1159–1169
Tang J et al (2015) Combining an antagonistic yeast with harpin treatment to control postharvest decay of kiwifruit. Biol Control 89:61–67
Zhang C, Chen K, Wang G (2012) Combination of the biocontrol yeast Cryptococcus laurentii with UV-C treatment for control of postharvest diseases of tomato fruit. Biocontrol 58(2):269–281
Zhang Q et al (2019) Screening and identification of an antagonistic yeast controlling postharvest blue mould decay of pears and the possible mechanisms involved. Biol Control 133:26–33
Zhao L et al (2021) Efficacy of Wickerhamomyces anomalus yeast in the biocontrol of blue mould decay in apples and investigation of the mechanisms involved. Biocontrol 66(4):547–558
Acknowledgements
This research was funded by a project of the Key Laboratory of Storage of Agricultural Products, Ministry of Agriculture and Rural Affairs P. R. China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they have no conflicts of interest. Each of the study’s authors is aware of and consents to the submission of this report.
Ethical approval
The authors earnestly guarantee that the study is honest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, N., Wang, B., Cui, X. et al. Biocontrol activities of grey mould of grapes with the volatile organic compounds generated by yeast HXMG-1 isolated from grapes. J Plant Dis Prot (2024). https://doi.org/10.1007/s41348-024-00920-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41348-024-00920-2