Abstract
Starter cultures of Candida tropicalis and Saccharomyces cerevisiae isolated from tchapalo were tested in pure culture and co-culture of four ratios [2:1, 25:4, 1:4, 2:3 (cells/cells)] for their ability to ferment sorghum wort to produce tchapalo. All the starters showed means growth rate between 0.043 and 0.101 h−1. Only C. tropicalis in pure culture showed growth rate lower than that of S. cerevisiae in single culture. During fermentation, according to total soluble solids depletion, yeast starters could be grouped in four different profiles. But in the beer produced, total soluble solids contents were statistically identical. The lowest values were obtained with co-culture C. tropicalis + S. cerevisiae in the ratios of 2:1 and 2:3. Starter cultures with large ratio of C. tropicalis produced a higher organic acids and 2-butanone than S. cerevisiae in pure culture. However, co-culture C. tropicalis + S. cerevisiae (2:1) was the alone starter which produced higher ethanol than S. cerevisiae in pure culture. The beers produced with C. tropicalis + S. cerevisiae (25:4), C. tropicalis + S. cerevisiae (1:4) and C. tropicalis were widely different from those produced with the others starter cultures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several traditional fermented foods exist all over the world. To the raw material, fermentation adds value and enhances nutritional quality and digestibility though biological enrichment. In addition, it provides dietary enrichment through aroma and flavour production (Rolle and Satin 2002). Microorganisms that intervene come from the raw materials, equipment and local environments or from a residue of previous fermentation batch. These microorganisms, by the virtue of their metabolic activities, play an active role in the physical, nutritional and organoleptic modification of the starting material (Aidoo 1994). But, the wide variety of microorganisms present in a spontaneously fermented food gives a product with widely varying quality. The use of starter culture was so suggested as the appropriate approach to alleviate the problems of variations in organoleptic quality and microbiological stability (Sanni 1993; Holzapfel 2002). Today, the use of starter cultures is applied successfully for many products and there are studies in the development of starter cultures for many other fermented foods: kivunde (Kimaryo et al. 2000), ogi (Teniola and Odunfa 2001), togwa (Mugula et al. 2003).
Sorghum beer is a traditional fermented beverage from most of West African countries where sorghum is produced. It is known as tchapalo in Côte d’Ivoire and by various other names in other African countries. Identification studies of yeasts involved in the alcoholic fermentation of the beer have highlighted species of Saccharomyces cerevisiae, Candida tropicalis, Kloeckera apiculata, Hansenula anomala, Torulaspora delbrueckii, Kluyveromyces marxianus and other non-Saccharomyces yeasts according to countries (Sefa-Dedeh et al. 1999; Jespersen 2003). But previous researches aiming to develop starter cultures for sorghum beer production were focused on the use of S. cerevisiae as identification studies have revealed the dominance of this specie (Glover et al. 2005; Maoura et al. 2005). Since literature has reported the contribution of non-Saccharomyces yeasts to the quality of wine and cider, our knowledge on the role of non-Saccharomyces present in sorghum beer needs to be improved.
This study attempted to know the contribution of the specie Candida tropicalis, the major non-Saccharomyces yeast in the alcoholic fermentation of tchapalo, to the quality of the beer produced in Côte d’Ivoire. Microbiological growth, volatile organic compounds, organic acids and total soluble solids were therefore determined during the fermentation.
Materials and methods
Yeasts
Yeast species of Saccharomyces cerevisiae (S. cerevisiae) and Candida tropicalis (C. tropicalis) used as starter cultures in this study were belonged to the culture collection of the Food Technology Department (University of Abobo-Adjame). They were isolated from traditional sorghum beer from the district of Abidjan (Southern Côte d’Ivoire). They were identified by PCR-RFLP of the ITS region and sequencing of D1/D2 domains. Before the production of tchapalo, freeze yeasts of the starter cultures were cultivated in a Sabouraud-Chloramphenicol plate at 30°C for 72 h.
Starter cultures preparation
Yeast culture from Sabouraud-Chloramphenicol plate was harvested with a loop to prepare a dense suspension in 4 ml of sterile distilled water. The suspension was added to 40 ml of sterile final sorghum wort obtained from one randomly selected traditional brewer at Williamsville-Macaci (district of Abidjan). The mixture was incubated at 35°C for 24 h to obtain the starter.
Fermentation experiment
The fermentation medium was made from final sorghum wort obtained from randomly identified commercial tchapalo brewer at Williamsville-Macaci. Erlenmeyer flasks containing 400 ml of sterile sorghum wort were inoculated with starter culture to obtain 106 cfu ml−1 and incubated for 12 h at 35°C. Six fermentation systems were constituted as follows: (1) individual pure culture fermentations with C. tropicalis and S. cerevisiae; (2) mixed culture fermentations of both yeast strains, respectively, in the ratios of 2:1, 25:4, 1:4 and 2:3 (cells/cells). At 0, 4, 8 and 12 h of fermentation, the samples were withdrawn for microbial counts, total soluble solids, organic acids and volatile compounds. The experiments were replicated two times.
Analytical determinations
Viable cell counts were evaluated by a traditional plate counting technique using Sabouraud-Chloramphenicol medium to estimate the total yeast populations. Plates were incubated for colony development at 30°C for 72 h.
The Total Soluble Solids (TSS) content, expressed as °Brix value, was determined in each sample using a hand refractometer.
Organic acids were analyzed by high-performance liquid chromatography using an HPLC (Shimadzu Corporation, Japan) apparatus, equipped with a pump (Shimadzu LC-6A Liquid Chromatograph), a detector (Shimadzu SPD-6A UV Spectrophotometric detector) and an Integrator (Shimadzu C-R 6A Chromatopac). Chromatographic separation was performed using an ion-exclusion ORH-801 column (300 × 6.5 mm, Interchrom, France). The eluant was 0.004 N H2SO4 with a flow rate of 0.8 ml min−1 and the detector was set at 210 nm. The samples were microfiltered through a 0.2 μm Millipore membrane filters (Sartorius AG, Goëttingen, Germany) and directly injected (20 μl) onto the chromatographic column. Standard solutions (oxalic, citric, tartaric, malic, lactic and fumaric acids) were prepared by dilution of the individual compounds in distilled water.
Volatile compounds were analyzed in a Shimadzu CG-14A gas chromatograph. Fermenting wort samples (2 μl) were filtered and injected directly. Injector and detector temperatures were 200 and 250°C, respectively. Helium at 2 kg cm−2 was used as the carrier gas. Standard solutions (ethanol, acetaldehyde, 1-propanol, 2-propanol, and 2-butanone) were prepared by dilution of the individual compounds in distilled water.
Statistical analysis
The data obtained were subjected to analysis of variance (Statistica, 99 Edition) and mean differences determined by Duncan’s multiple range test (P < 0.05). Principal component analysis (PCA) was used to establish relationship among variables represented by tchapalo compounds. Only factors with eigenvalues >1 were retained and Varimax rotation was used as rotation type. PCA was performed using XLSTAT software program.
Results
Yeasts growth and TSS evolution
Table 1 gives the growth rate of yeast starters during sorghum wort fermentation. At the beginning of the fermentation (0–4 h), S. cerevisiae in pure culture decreased while the other starter cultures increased. The highest growth rate was obtained with the starter C. tropicalis + S. cerevisiae (2:3) (0.123 h−1). During the following eight hours, S. cerevisiae in pure culture grew faster compared to the other starters and reached higher growth rate. Values were, respectively, 0.141 and 0.134 h−1 at 4–8 h and 8–12 h of fermentation.
C. tropicalis in pure culture started faster (0–4 h) than S. cerevisiae in single culture. But beyond 4 h until the end of fermentation, C. tropicalis population rapidly declined, leading to negative growth rate between 8 and 12 h. Otherwise, C. tropicalis in pure culture presented the lowest mean growth rate values considering the full length of the fermentation. The fastest growth rate was brought about by co-culture of C. tropicalis + S. cerevisiae in the ratio of 2:3 and 1:4. Their growth rates were, respectively, 0.101 and 0.095 h−1.
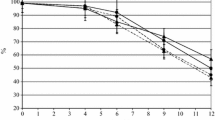
The starter cultures influenced differently TSS evolution as shown in Fig. 1. However, TSS contents were statistically identical in the final product (P > 0.05). The lowest values were obtained with co-culture C. tropicalis + S. cerevisiae in the ratios of 2:1 and 2:3.
Total soluble solids (TSS) depletion during the fermentation of the sorghum wort carried out by six starter cultures. Sc: S. cerevisiae, Ct: Candida tropicalis, Ct + Sc A: C. tropicalis + S. cerevisiae (2:1), Ct + Sc B: C. tropicalis + S. cerevisiae (25:4), Ct + Sc C: C. tropicalis + S. cerevisiae (1:4), Ct + Sc D: C. tropicalis + S. cerevisiae (2:3)
Organic acids profile
During fermentations, the starters exhibited a low variability in the level of lactic acid, the major organic acid in the fermenting wort (Fig. 2). In the tchapalo produced, the highest content was obtained with C. tropicalis in pure culture (20.7 g l−1) and the lowest content with C. tropicalis + S. cerevisiae (1:4) (14.4 g l−1). On the contrary, significant starter culture variability was observed for the other organic acids, except oxalic acid (results not shown).
The major organic acid content during the fermentation of sorghum wort inoculated with six starter cultures. Sc: S. cerevisiae, Ct: Candida tropicalis, Ct + Sc A: C. tropicalis + S. cerevisiae (2:1), Ct + Sc B: C. tropicalis + S. cerevisiae (25:4), Ct + Sc C: C. tropicalis + S. cerevisiae (1:4), Ct + Sc D: C. tropicalis + S. cerevisiae (2:3)
The total sum of organic acids analyzed during the fermentation, illustrated by Table 2, shows a regular production with C. tropicalis in pure culture. The content evolved from 18.4 g l−1 to 24.4 g l−1. The organic acids content in tchapalo was the highest with C. tropicalis in pure culture and this content decreased more and more for co-culture starters when the ratio of C. tropicalis decreases.
Volatile compounds
For all of the starters used, the major part of the ethanol was produced between 8 and 12 h of fermentation (Fig. 3). After 8 h, C. tropicalis in pure culture produced higher ethanol than S. cerevisiae in pure culture. But after 12 h of fermentation, the results show that tchapalo produced with C. tropicalis in pure culture contained the lowest ethanol rate (0.2%). The highest rate was produced by C. tropicalis + S. cerevisiae (2:1).
Ethanol rate in the fermenting sorghum wort carried out by six starter cultures. Sc: S. cerevisiae, Ct: Candida tropicalis, Ct + Sc A: C. tropicalis + S. cerevisiae (2:1), Ct + Sc B: C. tropicalis + S. cerevisiae (25:4), Ct + Sc C: C. tropicalis + S. cerevisiae (1:4), Ct + Sc D: C. tropicalis + S. cerevisiae (2:3)
Among the analyzed volatile compounds, 1-propanol, 2-propanol and acetaldehyde were not been detected in most of the samples. When they were detected, the contents were less than 1 mg l−1. The only compound analyzed measurable by the gas chromatography method used in this study was 2-butanone. Table 3 gives its content measured according to the used starter cultures. At the end of the process, tchapalo contained between 4.04 and 5.63 mg l−1. Except starter C. tropicalis + S. cerevisiae (2:3), all other starters achieved the fermentation with higher 2-butanone content than S. cerevisiae in pure culture. The highest value (5.63 mg l−1) was obtained with C. tropicalis + S. cerevisiae (25:4).
Principal components of tchapalo produced
The eleven measured variables of tchapalo produced with the different starter cultures were reduced to two main components (F1 and F2) by the PCA. F1 and F2 explained 93.58% of total data variance. The variables which mainly contributed positively (F loadings> 0.8) to F1 were lactic acid and total sum of organic acids, to F2 were oxalic and tartaric acids (Table 4).
As shown fig. 4, beers produced with C. tropicalis in pure culture and co-culture C. tropicalis + S. cerevisiae in the ratios of 25:4 and 1:4 were widely separated from beer produced with S. cerevisiae in pure culture. But those obtained with co-culture C. tropicalis + S. cerevisiae (2:1) and C. tropicalis + S. cerevisiae (2:3) resulted more or less close to that produced with S. cerevisiae in pure culture.
Plot of the two principal components in PCA of tchapalo fermented with six starter cultures. Sc: S. cerevisiae, Ct: Candida tropicalis, Ct + Sc A: C. tropicalis + S. cerevisiae (2:1), Ct + Sc B: C. tropicalis + S. cerevisiae (25:4), Ct + Sc C: C. tropicalis + S. cerevisiae (1:4), Ct + Sc D: C. tropicalis + S. cerevisiae (2:3)
Discussion
In this paper, we used C. tropicalis and S. cerevisiae in pure and co-culture cultures in order to know the contribution of this non-Saccharomyces yeast on the sorghum beer quality. The two yeast species showed different characteristics during pure culture fermentations. At onset of the fermentation, S. cerevisiae died off and then grew. Conversely, C. tropicalis grew first and died off at the end of the fermentation. The different yeast behaviour observed at the early hours of fermentation could be attributed to oxygen effect. Indeed, it has been reported that oxygen availability during the first stages of alcoholic fermentation favoured the growth of non-Saccharomyces yeasts (Hansen et al. 2001). Bourgeois and Mafart (1996) also reported a death of S. cerevisiae at the start of fermentation for the modern type beer production. During the course of the alcoholic fermentation, the death of non-Saccharomyces yeasts is usually attributed to the lower ethanol tolerance of these yeasts by comparison to S. cerevisiae (Jolly et al. 2006). But in this study, C. tropicalis death is not led to ethanol concentration as previous reports showed that apiculate yeasts such as Candida and Pichia can tolerate ethanol concentrations higher than 6% (Pallman et al. 2001; Combina et al. 2004). However, the changing environmental conditions of increasing organic acids concentration and nutritional depletion may explain their death (Pretorius 2000). In addition, in fermenting sorghum wort, oxygen can be rapidly depleted due to semi-anaerobic growth conditions. Hansen et al. (2001) reported that non-Saccharomyces yeasts are less tolerant to conditions of low available oxygen than S. cerevisiae.
When C. tropicalis and S. cerevisiae were mixed, the specific growth rates were lower compared with the pure of S. cerevisiae. Ciani et al. (2006) reported similar results for three non-Saccharomyces yeasts (Hanseniaspora uvarum, Torulaspora delbrueckii and kluyveromyces thermotolerans) in co-cultures with S. cerevisiae. These findings suggest interaction between C. tropicalis and S. cerevisiae. The interaction could not result from competition for sugar consumption as beers produced with pure cultures and co-cultures shown similar TSS contents. However, it could result from the diffusion of metabolites between the two species. There are several compounds produced by yeasts during alcoholic fermentation that may become inhibitory to other yeast species or strains. Aside from ethanol, certain metabolites such as short to medium chain fatty acids can reach concentrations leading to cell death of certain yeast species (Ludovico et al. 2001; Fleet 2003). Another reason for the reduced growth rates in mixed cultures could be attributed to amino acid and vitamin consumption by non-Saccharomyces yeasts during the first stages of fermentation which can disable the subsequent growth of S. cerevisiae strains (Fleet 2003).
Interaction among different yeast species can have either stimulatory or inhibitory effects on their metabolic activity. Our results showed that there was a stimulatory effect for ethanol production with C. tropicalis + S. cerevisiae (2:1) which led to higher ethanol concentration than S. cerevisiae pure culture.
Volatile compounds have an important role in determining the flavour profile of beer. Among those analyzed in this study, only 2-butanone was found. This is in agreement with other studies which reported that the synthesis of secondary products by yeasts is an individual and reproducible strain characteristic (Romano et al. 2003). The strains used were not able to produce 1-propanol, 2-propanol and acetaldehyde higher than 1 mg l−1 neither in pure culture nor in co-culture fermentations. Although 2-butanone was found in the fermenting wort, in all the beers produced, concentrations did not exceed 80 mg l−1, the perception threshold (Moll 1991). Therefore, it weakly contributed to the flavour of tchapalo. Moll (1991) reported similar observation for the modern type beer.
The results also showed higher production of organic acids by pure C. tropicalis than pure S. cerevisiae. This is consistent with previous results which reported that non-Saccharomyces yeasts are characterized as secondary compound producers (Lema et al. 1996; Lambrechts and Pretorius 2000). Although S. cerevisiae produced organic acids, a co-culture C. tropicalis + S. cerevisiae led to a decrease of these compounds compared to the content with C. tropicalis in pure culture. The decrease was more important with the increase ratio of S. cerevisiae.
The use of C. tropicalis and S. cerevisiae in pure and in co-culture to achieve the fermentation of the sorghum wort showed that the selected species influence differently the quality of the produced tchapalo. Of the analyzed starter cultures in this study, co-culture C. tropicalis + S. cerevisiae (2:1) has better characteristics to produce tchapalo than S. cerevisiae in pure culture. This may indicates that C. tropicalis contribution to the quality of sorghum beer depends on its ratio. But further investigations are required before the definitive conclusion.
References
Aidoo KE (1994) Application of biotechnology to indigenous fermented foods. Proc Technol Dev Ctries 12:83–94
Bourgeois CM, Mafart P (1996) La brasserie. In: Bourgeois CM, Larpent JP (eds) Microbiologie alimentaire: aliments fermentés et fermentation alimentaire. Technique & Documentation-Lavoisier, Paris, pp 91–109
Ciani M, Beco L, Cornitini F (2006) Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int J Food Microbiol 108:239–245
Combina M, Elía A, Mercado L, Catania C, Ganga A, Martínez C (2004) Dynamics of indigenous yeast populations during spontaneous fermentation of wines from Mendoza, Argentina. Int J Food Microbiol 99:237–243
Fleet GH (2003) Yeast interactions and wine flavour. Int J Food Microbiol 86:11–22
Glover RLK, Abaidoo RC, Jakobsen M, Jespersen L (2005) Biodiversity of Saccharomyces cerevisiae isolated from a survey of pito production sites in various parts of Ghana. Syst Appl Microbiol 28:755–761
Hansen EH, Nissen P, Sommer P, Nielsen JC, Arneborg N (2001) The effect of oxygen on the survival of non-Saccharomyces yeasts during mixed culture fermentations of grape juice with Saccharomyces cerevisiae. J Appl Microbiol 91:541–547
Holzapfel WH (2002) Appropriate starter culture technologies for small-scale fermentation in developing countries. Int J Food Microbiol 75:197–212
Jespersen L (2003) Occurrence and taxonomic characteristics of strains of Saccharomyces cerevisiae predominant in African indigenous fermented foods and beverages. FEMS Yeast Res 3:191–200
Jolly NP, Augustyn OPH, Pretorius IS (2006) The role and use of non-Saccharomyces yeasts in wine production. S Afr J Enol Vitic 27:15–39
Kimaryo VM, Massawe GA, Olasupo NA, Holzapfel WH (2000) The use of a starter culture in the fermentation of cassava for the production of “kivunde”, a traditional Tanzanian food product. Int J Food Microbiol 56:179–190
Lambrechts MG, Pretorius IS (2000) Yeast and its importance to wine aroma. A review. S Afr J Enol Vitic 21:97–129
Lema C, Garcia-Jares C, Orriols I, Angulo L (1996) Contribution of Saccharomyces and non-Saccharomyces populations to the production of some components of Albariño wine aroma. Am J Enol Vitic 47:206–216
Ludovico P, Sousa MJ, Silva MT, Leao C, Corte-Real M (2001) Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiol 147:2409–2415
Maoura N, Mbaiguinam M, Nguyen HV, Gaillardin C, Pourquie J (2005) Identification and typing of the yeast strains isolated from bili bili, a traditional sorghum beer of Tchad. Afr J Biotechnol 4:646–656
Moll M (1991) Bières et coolers. Technique & Documentation-lavoisier, Paris, p 515p
Mugula JK, Narvhus JA, Sørhaug T (2003) Use of starter cultures of lactic acid bacteria and yeasts in the preparation of togwa, a Tanzanian fermented food. Int J Food Microbiol 83:307–318
Pallman C, Brown J, Olineka T, Cocolin L, Mills D, Bisson L (2001) Use of WL medium of profile native flora fermentations. Am J Enol Vitic 52:198–203
Pretorius IS (2000) Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 16:675–729
Rolle R, Satin M (2002) Basic requirements for the transfer of fermentation technologies to developing countries. Int J Food Microbiol 75:181–187
Romano P, Fiore C, Paraggio M, Caruso M, Capece A (2003) Function of yeast species and strains in wine flavour. Int J Food Microbiol 86:169–180
Sanni AI (1993) The need for process optimization of African fermented foods and beverages. Int J Food Microbiol 18:85–95
Sefa-Dedeh S, Sanni AI, Tetteh G, Sakyi-Dawson E (1999) Yeasts in the traditional brewing of ‘pito’ in Ghana. World J Microbiol Biotechnol 15:593–597
Teniola OD, Odunfa SA (2001) The effects of processing methods on the level of lysine, methionine and the general acceptability of ogi processed using starter cultures. Int J Food Microbiol 63:1–9
Acknowledgments
The authors greatly appreciate the financial support of International Foundation for Science, Sweden (E/4167-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
N’Guessan, F.K., N’Dri, D.Y., Camara, F. et al. Saccharomyces cerevisiae and Candida tropicalis as starter cultures for the alcoholic fermentation of tchapalo, a traditional sorghum beer. World J Microbiol Biotechnol 26, 693–699 (2010). https://doi.org/10.1007/s11274-009-0224-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-009-0224-y