Abstract
Lack of access to potable water has forced many inhabitants of informal settlements in South Africa to rely on surface water sources for their daily water needs, thus exposing these communities to microbial contamination that can result in water-borne diseases. These water sources also serve as natural habitats of pathogenic E. coli strains which harbour virulence factors, which could play a role in the disease process, as well as various multi-drug resistant water-borne pathogens. This study investigated the microbiological quality of two river waters in Durban, South Africa, using total coliform and faecal coliform population as indices. The virulence markers and antibiogram profiles of the E. coli isolates from these rivers were also determined. The results indicated that water from these river sources were of poor microbiological quality and unfit for human consumption. Antibiotic Resistance Profiles of the isolates revealed that 97.1% of the Palmiet River isolates and 71.15% of the Umgeni River isolates were multi-resistant to the antibiotics tested, with all the isolates found to be resistant to novobiocin. Characterization of the virulence markers revealed the presence of stx1, cnf1 and eaeA genes, indicating the possible health risk associated with the ingestion of water from these rivers. The inherent health risks associated with the use of these river water emphasises the need for safe water supply and provision of proper sanitation facilities for the inhabitants of the informal settlements along these river banks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is essential to all known forms of life and is required; for hydration to sustain health, sanitation needs, as a solvent to wash everyday items, for industrial applications and as a thermal transfer agent, among others. Inadequate water treatment has a significant and devastating impact on public health. About 1.2 billion people, worldwide, lack access to safe drinking water, and twice that many lack adequate sanitation. As a result, the World Health Organization (WHO) estimates that 3.4 million people, mostly children, die every year from water-related diseases (Wilkes et al. 2009). Major proportions of these people live in 49 developing countries, which according to the United Nations report, are unfortunately experiencing increasing cases of water-related diseases such as cholera, diarrhoea and dysentery (Pandey 2006). In developing countries such as South Africa, almost 30% of the population lack access to an adequate supply of potable water, which implies that most communities, especially in rural areas, rely mainly on river, stream, well and pond water sources for their daily water needs (Venter 2001). These water sources are frequently exposed to microbial contamination from humans, animals and the environment (Nevondo and Cloete 1999; Lehloesa and Muyima 2000). Potential sources of these pathogens in water include wastewater effluents, combined sewer overflows, runoff from urban land, animal waste, and municipal waste sludges disposed off on land or in water (Kroening 1999; Ram et al. 2008a). A significant proportion of inhabitants of these rural communities are exposed to water-borne diseases (Obi et al. 2002; Ram et al. 2008b).
Previous reports on the microbial quality of river water in some rural communities of South Africa showed that the water sources were unsafe for human consumption and Escherichia coli (E. coli) was one of the predominant potential pathogens isolated (Obi et al. 2002). E. coli strains range from highly pathogenic strains causing disease of the gastrointestinal, urinary or central nervous system in even the most robust human hosts (Falagas and Gorbach 1995), to avirulent isolates which constitute part of the non-pathogenic facultative flora of the human intestine (Nataro and Kaper 1998). The high prevalence of virulent E. coli strains as the primary causative agent of acute diarrhoeal diseases has been extensively reported (El-Sheikh and El-Assouli 2001) and remains a major public health problem for children and young infants (Galane and Le Roux 2001). Over 500 million cases of acute diarrhea have been reported to occur yearly in children aged less than 5 years, worldwide (Snyder and Merson 1982). The pathogenicity of a particular E. coli strain is primarily determined by specific virulence factors which include adhesins, invasins, haemolysins, toxins, effacement factors, cytotoxic necrotic factors and capsules (Kuhnert et al. 2000; Galane and Le Roux 2001). Additional genes that were detected in pathogenic E. coli encode various virulence factors which directly indicate their virulence and pathotype (Kuhnert et al. 2000). During evolution, bacterial species have become capable of transferring virulence genes not only between members of a particular species but also between different bacterial species, creating new pathotypes with new combinations of different virulence genes (Schubert et al. 1998). The detection of specific virulence attributes of a given strain allows for the determination of potential reservoirs of virulence genes which are expected to play a key role as the origin of emerging diseases caused by pathogenic E. coli and is a useful tool in the analysis and detection of new strains (Kuhnert et al. 2000; Ram et al. 2009).
In addition to the problem of the detection of pathogens in water samples in population above the recommended standards is the wide antibiotics resistance commonly demonstrated by these pathogens (Engberg et al. 2001; Ash et al. 2002). Several pathogens have been shown to demonstrate a significant increase in resistance to some specific antibiotics over a short period of time (Coker and Adefeso 1994; Hoge et al. 1998), either as a result of selective pressure, antibiotic abuse by humans or over use in animals (White et al. 2000). Previous studies indicate a direct correlation between antimicrobial use and the extent of antimicrobial resistance (Mellon et al. 2001). Several rivers in the United States have therefore been indicated as reservoirs of antibiotic resistant bacteria (Ash et al. 2002). South Africa’s water resources have been under increasing threat of pollution in recent years (Fatoki et al. 2001; Igbinosa and Okoh 2009). Informal settlements that lack appropriate sanitary infrastructure and proper water supply are likely to have serious consequences for health care management and disease prevention within the local communities (Lo Presti et al. 2000). The potential outbreaks of water-borne diseases therefore, continue to grow with the increasing demands for potable water (White et al. 2000).
Recently, many rivers across Durban are continuously subjected to domestic waste runoff, industrial effluents and leakages from sewage pipes and storm water drains due to poor maintenance and improper water resource management, which results in an alarming extent of pollution. Also, the qualities of surface waters in Durban have not been adequately investigated, despite the fact that the microbiological qualities of river water sources in other provinces in the country have been reported. This study was therefore undertaken; to determine the level of contamination of two river water sources in Durban, South Africa using total coliform and fecal coliform population as indices, to detect the presence of virulence markers among the E. coli strains from these river water sources, as well as determine the Antibiotic Resistance Profiles (ARPs) of the E. coli strains. This is expected to assist in the determination of the extent of pollution of these rivers, the pathogenic potential of the E. coli strains residing in these water sources and the health risks associated with human consumption and domestic use of these contaminated river water sources.
Materials and methods
Description of the collection sites
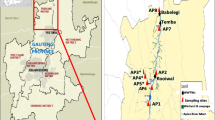
The two river water sources used in this study are Umgeni and Palmiet River, located in KwaZulu-Natal region of South Africa. The Umgeni River is the largest catchment, occupying vast majority of the central portion of the region, and provides water to over 3.5 million people. The Albert Falls, Midmar and Nagle Dams are situated within this catchment, supporting an area which is responsible for approximately 65% of the total economic production in KwaZulu-Natal province. Both Durban and Pietermaritzburg are reliant on this catchment for most of their water supply; however, the sampling points were located in Durban. The Palmiet River is a small catchment area of 500 km2, with 5 irrigation and hydropower dams along its length, and provides water for agricultural and industrial uses. The sampling points for the river sources were located near informal settlements.
Collection of water samples
Water samples were collected from the river water sources in plastic containers along designated collection points. The containers were sterilized using 70% (v/v) alcohol and thereafter rinsed with water from the source prior to collection. The water sample was collected by holding the bottle at the bottom and plunging it below the water surface. The mouth of the bottle was placed opposite the water current. If there was no current, it was created artificially by pushing the bottle forward. The bottle was filled leaving about 30 mm of empty space to allow mixing during laboratory analysis (Buckalew et al. 2006). Water samples were analysed immediately after collection and stored at 4°C for further use. The temperature of the water samples was determined in situ using a mercury thermometer.
Enumeration of the total coliform and faecal coliform populations
Membrane filtration technique was carried out to enumerate the total coliform and faecal coliform populations in the different water samples as described elsewhere (Britton and Greeson 1987). Fifty milliliter of the appropriate dilutions of the water samples were filtered through 0.45 μm pore size membrane filters which were transferred to 45 mm petri plates containing m-FC agar and m-Endo agar. The m-FC agar plates were incubated for 24 h at 44.5°C, while m-Endo agar plates were incubated for 24 h at 35°C for the detection and enumeration of faecal coliforms and total coliforms, respectively (Buckalew et al. 2006). Total and faecal coliform population was expressed as colony forming units per milliliter (CFU/ml).
Isolation and identification of E. coli isolates
Individual colonies from the m-FC plates were subcultured on to Eosin Methylene Blue (EMB) agar and incubated for 24 h at 37°C (Holt-Harris and Teague 1916). Colonies showing a green metallic sheen on EMB agar were confirmed using biochemical tests (Edward and Ewing 1972; Forbes et al. 1998). Confirmation of the E. coli isolates from other faecal coliform bacteria was done using IMViC (Indole, Methyl-red, Voges-Proskauer and Citrate) tests as described by Leclerc et al. (2001).
Antibiotic sensitivity tests
The antibiotic sensitivity of the isolates was determined using the disc diffusion method (NCCLS 2000). Standardized inoculum of the overnight grown LB broth cultures were spread on Mueller-Hinton agar plates using sterile swabs. The plates were dried at room temperature for 2 h before placing the antibiotic discs at equidistance. The plates were incubated for 24 h at 37°C and the diameter of zone of inhibition was measured. Organisms were classified as sensitive, intermediate or resistant, based on the NCCLS standards. E. coli ATCC 25922 was used as the MIC control in accordance with the NCCLS standards. A total of 15 antibiotics (belonging to 8 classes) were used in this study as shown in Table 1.
Results
Total coliform and faecal coliform populations in the water samples
Results of this study indicated that the river water sources were of poor microbiological quality. The faecal coliform (FC) and total coliform (TC) counts were found to be generally high in both rivers ranging from 50 to 255.5 CFU/ml and 0.8865 to 215.75 × 103 CFU/ml, respectively. The FC and TC counts for Palmiet River were highest at sampling point A1 and lowest at sampling point A3 (Fig. 1), while the highest FC and TC counts were obtained at sampling point B3 for Umgeni River (Fig. 1). Total coliform and faecal coliform counts varied from site to site, however, Palmiet River seemed to be more polluted compared to Umgeni River evidenced by the overall higher TC and FC population observed at the different sampling points of the Palmiet River.
Antibiotic resistance profiles of the isolates
The Antibiotic Resistance Profiles (ARPs) of the isolates from both rivers are presented in Tables 1 and 2. All isolates from both river waters were resistant to at least one antibiotic tested, with higher levels of antibiotic resistance observed in isolates from Umgeni River than those from the Palmiet River, however, no isolate was resistant to all the antibiotics. All the isolates were found to be susceptible to cefotaxime, and cefoxitin. All the isolates, with the exception of two Umgeni River isolates, were also sensitive to gentamicin, cefuroxime, ceftriaxone and tobramycin. Ciprofloxacin and nalidixic acid inhibited the growth of all Palmiet River isolates while 97% of the isolates from the same river were resistant to cephalothin. Other classes of antibiotics that generally showed high activity toward the isolates were the aminoglycosides, tetracyclines and β-lactams. The aminocoumarin class of antibiotics showed the least activity toward the isolates, as all isolates from both rivers were resistant to novobiocin. Proportional resistance of the total number of E. coli isolates from both rivers to different classes of antibiotics ranged from one antibiotic class to six classes. One (2.94%) of the E. coli isolates from Palmiet River was resistant to only one class of antibiotic while 97.1% were multi-resistant. Similarly, 28.85% of the Umgeni River isolates were resistant to one antibiotic class and 71.15% were multi-resistant. The ARPs of the Palmiet River E. coli isolates revealed that majority was resistant to four classes of antibiotics while majority of the Umgeni River E. coli isolates were resistant to just one class of antibiotic.
Discussion
The lack of safe drinking water and adequate sanitation measures lead to a number of diseases that claim millions of lives every year in developing countries (Zamxaka et al. 2004). Major factors affecting the microbiological quality of surface waters are discharges from sewage treatment plants and runoff from informal settlements (Fatoki et al. 2001). In this study, total coliform (TC) and faecal coliform (FC) populations were assessed to determine whether both rivers were microbiologically safe for domestic use. According to DWAF (1998), the maximum limit for no risk of faecal coliforms is 0 CFU/100 ml and 10 CFU/100 ml for total coliforms. All FC and TC counts for both rivers were above the South African recommended limits indicating poor microbiological quality of the water and its unfitness for human consumption without prior treatment. The FC levels also did not fall within the guideline value of 0–130 counts/100 ml set for full contact recreation (DWAF 1996), indicative of the risk of contracting gastrointestinal-related illnesses as a result of full contact recreation or direct consumption of the water, without treatment (DWAF 1996).
Although the microbial pollution in both rivers is generally high and unacceptable, the TC and FC population obtained in this study are low compared to those reported for Levubu River and the rivers Mutale, Makonde and Mudaswali in rural Venda communities of South Africa (Obi et al. 2002). Since the sampling points were in the proximity of human activities, the quality of the river sources depend on local conditions. The high TC and FC load of both rivers is an indication of poor sanitation and hygiene conditions as well as lack of environmental awareness among the people of the river bank communities. The highest total and faecal coliform counts were recorded at sampling points A1 and B3, where informal settlements lacking proper sanitation facilities lined the banks of the rivers. The relatively low temperature observed at the Umgeni River sites (data not shown) could contribute towards the maintenance of the quality of water, hence the lower microbial population in this river. According to Zamxaka et al. (2004), relatively low temperatures can retard microbial growth especially coliforms, which may explain the low total coliform counts, observed at the Umgeni sites. The high coliform count observed at the Palmiet River sites may have resulted from runoff from settlements during summer rains. Other possible sources of contamination of the river waters in the study area could be the presence of pit latrines close to the water sources and poor catchment management.
Previous studies revealed that river sources serve as natural habitats of pathogenic E. coli strains that possess virulence factors that could play an important role in the disease process (Kuhnert et al. 2000; Müller et al. 2001; Obi et al. 2004a; Ahmed et al. 2007). The virulent strains of E. coli are categorically divided into enterotoxigenic E. coli [ETEC], enteropathogenic E. coli [EPEC], enteroaggregative E. coli [EAEC], enterohaemorrhagic E. coli [EHEC] and necrotoxigenic E. coli [NTEC] (Huang et al. 2006). The detection of specific virulence attributes of a given strain allows for the determination of potential reservoirs of virulence genes, which are expected to play a key role as the origin of emerging diseases caused by pathogenic E. coli (Orsi et al. 2007). Virulence gene detection is also a useful tool in the analysis and detection of new E. coli strains (Kuhnert et al. 2000). Preliminary screening of the E. coli strains isolated from the two river waters in this study (results not shown) revealed the presence of; stx1 (encoding for shiga toxin 1), cnf1 (encoding for cytotoxic necrotizing factor 1), and eaeA (encoding for enteropathogenic attachment and effacement) genes in some of the isolates. This further suggests the potential danger associated with the ingestion of these waters. However, research is on-going in our laboratory to comprehensively determine the virulence signatures of these isolates for their proper classification into pathogenic groups.
The antibiotic resistance patterns of the isolates obtained in this study corroborate results from previous investigations (Wasfy et al. 2000; Obi et al. 2004b). Nalidixic acid is a synthetic chemotheraputic agent effective against Gram-negative bacteria by binding to DNA gyrase enzyme (topoisomerase), thus inhibits DNA duplication. It is mainly used in the treatment of urinary tract infections. Chloramphenicol inhibits translation during protein synthesis and causes aplastic anemia in a small percentage of patients, and its use is very minimal in non life-threatening situations. The observed rare bacterial resistance to chloramphenicol has been attributed to the restricted use of the drug (Goni-Urriza et al. 2000). Cefotaxime and cefoxitin inhibited the growth of all isolates from both rivers, while cefuroxime, ceftriaxone, gentamicin and tobramycin inhibited the growth of all the isolates except two Umgeni River isolates. Aminoglycosides are protein synthesis inhibitors binding to bacterial ribosomes to prevent the initiation of protein synthesis. Aminoglycosides usage has been limited because prolonged use was found to cause kidney damage and injury to the auditory nerves leading to deafness (Goni-Urriza et al. 2000). The reduced use of this class of antibiotics may explain the low resistance levels of the isolates to the antibiotics in this class, except streptomycin which is primarily used for treating tuberculosis patients. Beta-lactam antibiotics inhibit the last step in the bacterial cell wall synthesis, while tetracyclines block protein synthesis. The extremely low toxicity of the antibiotics in these classes has resulted in their overuse in the medical community, hence the observed increased resistance. The observed 100% resistance to novobiocin in this study corroborates the findings of Kobori et al. (2004) and Schroeder et al. (2002) also observed a 50% resistance to ampicillin, cephalothin, tetracycline, and streptomycin among 752 STEC strains. Also, Al-Jebouri (1985) and Al-Ghazali et al. (1988) found that 45 and 90%, respectively, of E. coli strains were resistant to ampicillin, which are higher rates than those observed in this study.
Industrial and human activities might contribute to the multi-drug resistance patterns and since rivers are the primary receptacle of industrial effluents, exposure to environmental pollutants and changes in nutrient composition can lead to selective pressure that favours antibiotic resistance in certain organisms (Furuya and Lowy 2006; Ram et al. 2008b). Indiscriminate and inappropriate use of antibiotics, and related compounds, for various purposes also causes significant antibiotic contamination of the natural environment (Mellon et al. 2001; McEwen and Fedorka-Cray 2002) and the development and proliferation of antimicrobial resistant pathogens (Martinez and Baquero 2002). Multiple antimicrobial resistances may partly result from the spread of genetic elements including plasmids, transposons, and integrons that may confer resistance to numerous antimicrobials (Obi et al. 2004b). It should be noted that susceptibility of bacteria to antibiotics is not static and resistance may be due to antibiotic abuse, antibiotic overuse or may be chromosomally or plasmid mediated (Obi et al. 1998). Antibiotic usage must therefore be carefully regulated and monitored.
Conclusion
The bacteriological quality of the river water investigated in this study, as suggested by the total and faecal coliform counts, did not meet the recommended limits and could pose a serious health risk to consumers if used without treatment. This emphasises the importance of safe water supply and provision of proper sanitation facilities for the inhabitants of the informal settlements along the river banks. Some of the E. coli isolates obtained from the river water samples harboured virulence markers; stx1, cnf1 and eaeA genes, showing the potential pathogenicity of the E. coli strains resident in these rivers. This could also lead to the emergence of strains with new combinations of virulence genes, since most virulence factors are encoded on mobile genetic elements. The E. coli isolates showed a high level of resistance to antimicrobial agents and multi-drug resistance was extremely common. This is of serious concern and calls for caution in the indiscriminate and inappropriate use of antibiotics, and related compounds on animals and humans. Although ciprofloxacin, nalidixic acid, gentamicin, ceftriazone, cefoxitin, and tobramycin demonstrated to be effective against the isolates, periodic monitoring of antibiograms is necessary to detect any change in patterns and in characterizing the isolates. A continued surveillance of E. coli isolates from surface waters used for recreational or domestic purposes and the development of adequate prevention strategies to diminish the spread of multi-resistant bacteria and/or the mobile resistance elements are needed for public health reasons.
References
Ahmed W, Tucker J, Bettelheim KA, Neller R, Katouli M (2007) Detection of virulence genes in Escherichia coli of an existing metabolic fingerprint database to predict the sources of pathogenic E. coli in surface waters. Water Res 41:3785–3791
Al-Ghazali MR, Jazrawi SF, Al-Doori ZA (1988) Antibiotic resistance among pollution indicator bacteria isolated from Al-Khair River, Baghdad. Water Res 22:641–644
Al-Jebouri MM (1985) A note on antibiotic resistance in the bacterial flora of raw sewage and sewage-polluted river Tigris in Mosul, Iraq. J Appl Bacteriol 58:401–405
Ash RJ, Mauck B, Morgan M (2002) Antibiotic resistance of Gram negative bacteria in rivers. Emerging Infect Dis 8:7–12
Britton LJ, Greeson PE (eds) (1987) Methods for collection and analysis of aquatic biological and microbiological samples, p 363. U.S. Geological Survey Techniques of Water-Resources Investigation, chap A4
Buckalew DW, Hartman LJ, Grimsley GA, Martin AE, Register KM (2006) A long-term study comparing membrane filtration with Colilert defined substrates in detecting faecal coliforms and Escherichia coli in natural waters. J Environ Managt 80:191–197
Coker AO, Adefeso AO (1994) The changing patterns of Campylobacter jejuni/coli in Lagos, Nigeria after ten years. East Afr Med J 71:437–440
Department of Water Affairs and Forestry (DWAF) (1996) South African water quality guidelines 2: recreational water use, vol 2. Department of Water Affairs and Forestry, Pretoria
Department of Water Affairs and Forestry (DWAF) (1998) Quality of domestic water supplies—vol 1, assessment guide. Department of Water Affairs and Forestry, Pretoria
Edward PR, Ewing WH (1972) Identification of Enterobacteriaceae, 3rd ed. International Student Publication, Burgess, pp 26–28
El-Sheikh SM, El-Assouli SM (2001) Prevalence of viral, bacterial and parasitic enteropathogens among young children with acute diarrhoea in Jeddah, Saudi Arabia. J Health Pop Nutr 19:25–30
Engberg J, Aerestrup FM, Taylor DE, Gerner-Smidt Nachamkin (2001) Quinolone and macrolide resistance in Campylobacter jejuni and Campylobacter coli: resistance and trends in human isolates. Emerg Infect Dis 7:24–34
Falagas M, Gorbach S (1995) Practice guidelines: urinary tract infections. Infect Dis Clin Pract 4:241–257
Fatoki OS, Muyima NYO, Lujiza N (2001) Situation analysis of water quality in the Umtata River catchment. Water SA 27:467–473
Forbes BA, Sahm DF, Weissfeld AS (1998) Diagnostic microbiology, vol 10. Mosby, St. Louis, pp 384–388
Furuya YE, Lowy FD (2006) Antimicrobial-resistant bacteria in the community setting. Microbiol Nature Rev 4:36–45
Galane PM, Le Roux M (2001) Molecular epidemiology of Escherichia coli isolated from young South African children with diarrhoeal diseases. J Health Pop Nutr 19:31–37
Goni-Urriza M, Capdepuy M, Arpin C, Raymond N, Caumette P, Quentin C (2000) Impact of an urban effluent on antibiotic resistance of riverine Enterobacteriaceae and Aeromonas spp. Appl Environ Microbiol 66:125–132
Hoge CW, Gambel JM, Srijan A, Pitarangsic C, Echevervia P (1998) Trends in antibiotic resistance among diarrhoeal pathogens isolated in Thailand over 15 years. Clin Infect Dis 26:341–345
Holt-Harris JE, Teague O (1916) A new culture medium for the isolation of Bacillus typhosa from stools. J Infect Dis 18:596
Huang DB, Mohanty A, DuPont HL, Okhuysen PC, Chiang T (2006) A review of an emerging enteric pathogen: enteroaggregative Escherichia coli. J Med Microbiol 55:1303–1311
Igbinosa EO, Okoh AI (2009) Impact of discharge wastewater effluents on the physico–chemical qualities of a receiving watershed in a typical rural community. Int J Environ Sci Tech 6:175–182
Kobori D, Rigobelo EC, Macedo C, Marin JM, Avila FA (2004) Virulence properties of shiga toxin-producing Escherichia coli isolated from cases of bovine mastitis in Brazil. Revue Élev Méd vét Pays trop 57:15–20
Kroening SE (1999) Faecal Coliform and Escherichia coli Bacteria in the St. Croix National Scenic Riverway, Summer. Water-Resources Investigations Report, 00-4214 U.S. Geological Survey, p 1–8
Kuhnert P, Boerlin P, Frey J (2000) Target genes of virulence assessment of Escherichia coli isolates from water, food and the environment. Fed Europ Microbiol Soc Rev 24:107–117
Leclerc H, Mossel DAA, Edberg SC, Struijk CB (2001) Advances in the bacteriology of the coliform group: their suitability as markers of microbial water safety. Ann Rev Microbiol 55:201–234
Lehloesa LJ, Muyima NYO (2000) Evaluation of impact of household treatment procedures on the quality of groundwater supplies in the rural community of the Victoria District, Eastern Cape. Water SA 26:285–290
Lo Presti F, Riffard S, Jarraud S, Legallou F, Richet H, Vandenesch F, Etienne J (2000) Isolation of Legionella oakridgensis from two patients with pleural effusion living in the same geographical area. J Clin Microbiol 38:3128–3130
Martinez JL, Baquero F (2002) Interactions among strategies associated with bacterial infection: pathogenicity, epidemicity, and antibiotic resistance. Clin Microbiol Rev 15:647–679
McEwen SA, Fedorka-Cray PJ (2002) Antimicrobial use and resistance in animals. Clin Infect Dis 34:93–106
Mellon M, Benbrook C, Benbrook K (2001) Hogging it: estimates of antimicrobial abuse in livestock. UCS Publications, Cambridge
Müller EE, Ehlers MM, Grabow WOK (2001) The occurrence of E. coli O157:H7 in South African water sources intended for direct and indirect human consumption. Water Res 35:3085–3088
Nataro JP, Kaper JB (1998) Diarrhoeagenic Escherichia coli. Clin Microbiol Rev 11:142–201
National Committee for Clinical Laboratory Standards (NCCLS) (2000) Performance standards for antimicrobial disk susceptibility tests. NCCLS document M2-A7. National Committee for Clinical Laboratory Standards, Wayne
Nevondo TS, Cloete TE (1999) Bacterial and chemical qualities of water supply in Dertig Village Settement. Water SA 25:215–220
Obi CL, Coker AO, Epoke J, Ndip RN (1998) Distributional pattern of bacterial diarrhoeagenic agents and antibiograms of isolates from diarrhoeic and non-diarrhoeic patients in urban and rural areas of Nigeria. Cent Afr J Med 44:223–229
Obi CL, Potgieter N, Bessong PO, Matsaung G (2002) Assessment of the microbial quality of river water sources in rural communities in South Africa. Water SA 28:287–292
Obi CL, Green E, Bessong PO, de Villiers B, Hoosen AA, Igumbor EO, Potgieter N (2004a) Gene encoding virulence markers among Escherichia coli isolates from diarrhoeic stool samples and river sources in rural Venda communities of South Africa. Water SA 30:37–41
Obi CL, Bessong PO, Momba MNB, Potgieter N, Samie A, Igumbor EO (2004b) Profiles of antibiotic susceptibilities of bacterial isolates and physico-chemical quality of water supply in rural Venda communities, South Africa. Water SA 30:515–519
Orsi RH, Stoppe NC, Inês ZSM, Gomes TAT, Prado PI, Manfio GP, Ottoboni LMM (2007) Genetic variability and pathogenicity potential of Escherichia coli isolated from recreational water reservoirs. Res Microbiol 158:420–427
Pandey S (2006) Water pollution and health—review article. Kathmandu Univ Med J 4:128–134
Ram S, Vajpayee P, Shanker R (2008a) Contamination of potable water distribution system by multi-antimicrobial resistant enterohaemorrhagic Escherichia coli. Environ Health Perspect 116:448–452
Ram S, Vajpayee P, Tripathi U, Singh RL, Seth PK, Shanker R (2008b) Determination of antimicrobial resistance and virulence gene signatures in surface water isolates of Escherichia coli. J Appl Microbiol 105:1899–1908
Ram S, Vajpayee P, Singh RL, Shanker R (2009) Surface contamination of a perennial river exhibits multi-antimicrobial resistant shiga toxin and enterotoxin producing Escherichia coli. Ecotoxcol Environ Safety 72:490–495
Schroeder CM, Zhao C, DebRoy C, Torcolini J, Zhao S, White DG, Wagner DD, McDermott PF, Walker RD, Meng J (2002) Antimicrobial resistance of Escherichia coli O157 isolated from humans, cattle and swine food. Appl Environ Microbiol 68:576–581
Schubert S, Rakin A, Karch H, Carniel E, Heesemann J (1998) Prevalence of the ‘high-pathogenicity island’ of Yersinia species among Escherichia coli strains that are pathogenic to humans. J Infect Immun 66:480–485
Snyder JD, Merson MH (1982) The magnitude of the global problem of acute diarrhoea disease: a review of active surveillance data. Bull World Health Org 60:603–613
Venter SN (2001) Microbial water quality in the 21st century. SA Water Bull 27:16–17
Wasfy MO, Oyofo BA, David JC, Ismail TF, El-Gendy AM, Mohran ZS, Sultan Y, Peruski LF (2000) Isolation and antibiotic susceptibility of Salmonella, Shigella and Campylobacter from acute enteric infections in Egypt. J Health Pop Nutr 18:33–38
White DG, Hudson C, Maurer JJ, Ayers S, Zhao S, Lee MD, Bolton L, Foley T, Sherwood J (2000) Characterization of chloramphenicol and florfenicol resistance in Escherichia coli associated with bovine diarrhoea. J Clin Microbiol 38:4593–4598
Wilkes G, Edge T, Gannon V, Jokinen C, Lyautey E, Medeiros D, Neumann N, Ruecker N, Topp E, Lapen DR (2009) Seasonal relationships among indicator bacteria, pathogenic bacteria, Cryptosporidium oocysts, Giardia cysts, and hydrological indices for surface waters within an agricultural landscape. Water Res 43:2209–2223
Zamxaka M, Pironcheva G, Muyima NYO (2004) Microbiological and physico-chemical assessment of the quality of domestic water sources in selected rural communities of the Eastern Cape Province, South Africa. Water SA 30:333–340
Acknowledgments
The Authors would like to thank Prof. U. Reischl (Institute of Medical Microbiology and Hygiene, University of Regensburg, Germany) for the kind supply of DNA samples of positive control E. coli strains used in this study. This study was supported by the National Research Foundation and Water Research Commission of South Africa and the University of KwaZulu-Natal, Durban, South Africa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olaniran, A.O., Naicker, K. & Pillay, B. Antibiotic resistance profiles of Escherichia coli isolates from river sources in Durban, South Africa. World J Microbiol Biotechnol 25, 1743–1749 (2009). https://doi.org/10.1007/s11274-009-0071-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-009-0071-x