Abstract
Urbanization and accelerated industrialization have led to significant waste generation following the accumulation of massive amounts of solid waste in open dump sites. Groundwater contamination is one of the critical ecological concerns associated with the percolation of leachate from dump sites. E.coli O157 is a particular virulent serotype which produces Intimin and Shiga toxins, that cause severe diseases including Hemorrhagic Colitis, Hemolytic Uremic Syndrome and Thrombotic Thrombocytopenic Purpura in humans. The focus of the present study is to study the virulence potential and antibiotic resistance profiles in E. coli isolates from selected Karadiyana, Methotamulla and Kerawalapitiya control open dump sites in Sri Lanka. The E.coli was isolated following the standard MPN method and 5 randomly selected ( n = 5) colonies from each location were subjected to the virulence potential tests and antibiotic resistivity study. The results showed the total coliform count was ranged from 0–120 MPN/mL around the Kardiyana dump site whereas 0–75 MPN/mL and 3–115 MPN/mL recorded in the Methotamulla and the Kerawalapitiya dump sites. Overall, resistance in isolated E.coli against AMX, AMP, SUF/ TRI, SDI, CLOX, TET and ERM was high (> 70%) compared with the other tested antibiotics namely CIP, GEN and AZY (< 40%). According to the results, the Enteropathogenic E. coli pathotype was identified in 17 samples, whereas the Enterohaemorrhagic E. coli pathotype was found in only 3 samples.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In most developed countries, the technology for the treatment of landfill leachate has been maintained well and monitored strictly. However, for developing countries, waste classification and sealed management systems have not yet been perfectly established due to the lack of waste recycling legislation, technique equipment, and public awareness [18, 23]. In landfills, a significant volume of human waste, animal waste, and industrial waste with high antibiotic levels have been discarded [2, 11, 14]. Antibiotic-resistant bacteria (ARB) may become the predominant communities in landfills as a result of the presence and ongoing input of such antibiotics [4, 15]. Additionally, the presence of mobile genetic elements would encourage the frequency of horizontal gene transfer between pathogenic bacteria and ARB, making the landfill a hotspot for pathogenic bacteria and ARGs [7]. This is especially true for landfills without proper seepage control facilities [16]. A serious threat to public health and environmental safety exists at these sites because mixed pollutants (ARB and ARGs) migrate with landfill leachate and contaminate surface or underground water [7, 16]. Therefore, it is critical to create regulating technologies to lessen the prevalence of dangerous bacteria and ARG spread.
One of the most prevalent facultative pathogens in human health, Escherichia coli (E. coli), is also a crucial component of the study of water quality, particularly concerning faecal contamination [3, 21]. According to studies, a significant amount of the E. coli population detected in surface water already has at least one acquired resistance, and multi-resistant isolates are no longer unusual.
However, some strains of E. coli have developed virulence factors and genes for antibiotic resistance, which can cause serious infections and represent a serious risk to the general public's health [5, 20]. Due to the risk of environmental contamination and subsequent spread of antibiotic resistance to other ecological niches, the prevalence and antimicrobial resistance patterns of E. coli in landfill leachates are of the utmost importance [5, 20].
The management of garbage, including the eradication of municipal solid waste, is a major concern in Sri Lanka, a developing country that is experiencing fast urbanization and industrialization. Landfills are frequently used to dispose of garbage, and the leachates they produce frequently find their way into the surface and groundwater in the area, endangering ecosystems and human health [19] Since they may serve as reservoirs for antimicrobial resistance genes that can spread to other bacteria in the environment, the existence of antimicrobial-resistant E. coli strains in landfill leachates can exacerbate this issue.
To evaluate the possible dangers connected with these waste disposal sites, it is essential to understand the prevalence and antibiotic resistance profiles of E. coli isolated from landfill leachates. We can learn a lot about the prevalence and antibiotic resistance trends of E. coli strains, which will help us understand how landfill leachates affect the propagation of antimicrobial resistance and the risk of human exposure to contaminated water sources.
This study aims to determine the prevalence and antimicrobial resistance profiles of E. coli strains isolated from landfill leachate sites in Sri Lanka. The objectives include: (1) identifying the prevalence and distribution of E. coli in landfill leachates, (2) characterizing the antimicrobial resistance patterns of the isolated E. coli strains, and (3) exploring potential correlations between the occurrence of antimicrobial resistance and the physicochemical parameters of the leachate.
The results of this study will be crucial for the creation of efficient waste management plans and for reducing any potential health risks brought on by landfill leachate contamination in Sri Lanka.
Methodology
Study Area
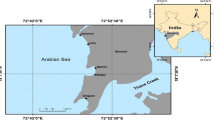
The Karadiyana, Meethotamulla and Kerawalapitiya dumping sites were selected as the study area for the present study (Fig. 1). Those sites are identified as major solid waste disposal sites in the Colombo area. The number of groundwater sampling locations depended on the availability of groundwater sources in each area.
Water Samples Collection
For the study, 17 dug-well water samples were collected from the Karadiyana (10), Meethotamulla (4) and Kerawalapitiya (3) open dump sites respectively on 15th and 16th August, 2023 (Table 1). Pre-cleaned polypropylene bottles and amber-coloured sterile glass bottles were used to collect water for chemical and microbial analysis respectively. Water samples were transported to the laboratory in a refrigerated condition within 24 h and stored in a cold room. Microbiological and chemical analyses were performed within 24 h after the collection of samples. The GPS coordinates were recorded via a hand-held GPS receiver (Model -etrex® 22x) at the site.
Physico Chemical Analysis in Water Samples
Water quality parameters; water temperature, pH, Dissolved Oxygen (DO), and Electrical Conductivity (EC) were measured using a thermometer (Immersion, Philip Harris, and England), pH meter (330 I/ Set, WTW Co., Weilheim, Germany), DO meter (HQD portable multimeter -HACH—HQ 40D) and a conductivity meter (340A-Set 1) respectively at the site itself. Chemical parameters such as N- Nitrate (as NO3−), N-nitrite (as NO2−), N-Ammonia (as NH3), total inorganic nitrogen and total phosphorous were measured in the laboratory using Standard Methods for the Examination of Water and Wastewater published by American Public Health Association (APHA 2012). The Chemical Oxygen Demand (COD) of the water was measured following the closed reflux method [17, 22].
Microbiological Analysis
Total and Faecal Coliform Bacteria (Most Probable Number (MPN) Method)
The Most Probable Number method was performed to determine the Total coliform (TC) and E. coli count per 0.1 dm3 of the water samples. Presumptive test, confirmed test and completed test were carried out to isolate and identify E.coli and Total coliform in the samples [Manjula et al., 2011 and WHO,2012, SLSI 2013, [22]].
Colonies developed on EMB agar, were further identified as coliforms or faecal coliforms (Escherichia coli) using colony characteristics, morphology and biochemical tests. For faecal coliforms, cell smears prepared from green metallic sheen were Gram stained and the IMVIC test was carried out to identify the colony as E.coli colonies. The MPN per 100 mL water was determined using the completed test.
Isolation and Confirmation of Pure Cultures of E.coli
Five colonies were randomly selected from each plate. For plates with ≤ 5 colonies, all the isolates were selected for further purification. Following incubation, a single colony was selected and streaked further on EMB agar to obtain pure isolates, which were then used for virulence and antibiotic susceptibility profiling.
Screening of Antibiotic Resistance in Isolated E.coli
Following incubation in nutrient broth, the turbidity of the broth culture was adjusted to a 0.5 McFarland standard before inoculating onto a pre-prepared nutrient agar medium. Filter sterilized (0.2 μm) antibiotics; Tetracycline (TET), Amphicillin (AMP), Amoxicillin (AMX), Cloxacillin (CLOX) and Ciprofloxacin (CIP) at a final concentration of 60 µg/mL were spiked to each molting nutrient agar media (40 0C) before inoculating bacteria [9, 12]. Then equalized bacterial samples were inoculated on the prepared nutrient agar medium according to CLSI guidelines and screened for antibiotic resistance in each bacterium [11].
Determination of Virulence Potentials of Isolates
Extraction of DNA
Following [8], the genomic DNA of isolated bacteria was extracted. Resuspended purified DNA was kept at -20 0C in 50 µL of TE buffer.
Detection of the Virulence Genes by PCR
The extracted DNA was used as the template DNA in different real-time PCR assays for the identification of genes associated with virulence in two (02) E. coli pathotypes. The various genes tested and the associated pathotypes are shown in Table 2.
The primers and PCR conditions used for the various genes were previously described by [1]. All controls were obtained from the Medical Research Laboratory in Sri Lanka. Reaction mixtures without DNA, which were used as negative controls, were also included in each PCR assay. All PCR assays were performed on a BIORAD PCR machine (Qiagen, Hilden, Germany). From Integrated DNA Technologies (IDT), primer sets were acquired. For each, PCR master mixture was prepared as follows (Table 3).
PCR amplification was performed to screen for eaeA, stx1 and stx2 (Table 2) genes. Initial denaturation at 94 °C for 3 min was followed by 30 cycles at 94 °C for 10 s, 50 °C for 20 s, and 72 °C for 60 s in the PCR for eaeA. Initial denaturation at 94 °C for 10 min was followed by 30 cycles at 94 °C for 30 s, 45 °C for 30 s, and 72 °C for 60 s for stx1 and stx2.
Results
Physico-Chemical Water Quality Parameters in Dug Well Water Samples
Tables 4 and 5 describe the recorded physicochemical parameters of the well water in the dug wells of the Kardiyana, Meethotamulla and Kerawalapitiya dump sites. According to the recorded values, the pH was recorded from 4.25 ± 0.52 to 7.8 ± 0.42 in all the dumping sites which indicates the water pH was within the standards for inland water (SLS- 6.5—8.5, WHO- 6.5–8.5). The DO ranged from 0.5 mg/L to 7.64 mg/L in all selected wells around dump sites. The concentrations of NO3−, NO2− and NH4+ were ranged from 1.05 – 15.25 mg/L, 1.02 – 5.04 mg/L, and 0.05 >—0.58 mg/L respectively. The total phosphate concentrations ranged from 0.28 – 1.65 mg/L around the Karadiyana dump site and it ranged from 0.25 – 3.52.mg/L (Table 4) in the Meethotamiulla dump site respectively (Table 5). All the recorded total phosphate concentrations were within the SLS standard level. Importantly the the COD values ranged from 224 -596 mg/L, 224 – 488 mg/L and 150 – 428 mg/L in dug wells around Karadiyana, Meethotamulla and Kerawalapitiya dump sites respectively. Further, the recorded COD of all the dug well water exceeded the maximum permissible tolerance level given by SLS Sri Lanka.
A correlation analysis was performed to elucidate a possible relationship between the water quality parameters, distance of the sampling site from the dumping site and covered and uncovered wells. There were no significant variations detected between water quality parameters and covered and uncovered wells even from distance (Fig. 1). The correlation analysis revealed that K1U, K2U, K3C, M1U, and K4C showed the highest total nitrogen, total coliform, E.coli and total phosphate.
Total and Fecal Coliform Bacteria
Figures 2 and 3 represent the distribution pattern of faecal and total coliform levels in groundwater around the dump sites. According to the figures, the total coliform counts were greater in the nearby wells around the dump site and comparatively lower faecal coliform value was recorded in the distance wells. The total coliform count ranged from 0—120 MPN/mL around the Kardiyana dump site. The contamination pattern of fecal coliform also was similar to the total coliform distribution pattern around the Kardiyana dump site which ranged from 0 – 75 MPN/mL. The well water around the Meethotamulla dump site recorded the faecal and total coliform counts ranging from 0–94 MPN/mL and 3–115 MPN/mL respectively. Moreover, compared to the other two dump sites the well water around the Kerawalpitiya dump site recorded a lower value of total and fecal coliform count which ranged from 3–11 MPN/mL and 7–60 MPN/mL respectively (Table 6).
Antibiotic Resistance
Overall bacteria isolates showed that the highest resistance against AMX (100%) and AMP (100%), following descending order SUF (Karadiyana: 95%, Meethotamulla; 100%; Kerawalapitiya; 80%), SDI (Karadiyana: 95%, Meethotamulla; 90%; Kerawalapitiya; 80), ERM (Karadiyana: 85%, Meethotamulla; 60%; Kerawalapitiya; 90%), TET (Karadiyana: 80%, Meethotamulla; 85%; Kerawalapitiya; 70), CLOX (Karadiyana: 45%, Meethotamulla; 40%; Kerawalapitiya; 0), GEN (Karadiyana: 10%), and AZY (0) respectively. Resistance against AMX, AMP, SUF/ TRI, SDI, CLOX, TET and ERM was high (>70%) compared with the other tested antibiotics namely CIP, GEN and AZY (<40%) (Table 7).
Detection of the Virulence Genes by PCR
E. coli isolated from three different dumpsites were screened for virulence genes eae A, stx 1, stx 2 by direct PCR. PCR running conditions for these virulence markers were optimized for any deviation from the earlier reported conditions to suit the reagents and thermal cycler used in the present experiment (Table 3) [1].
Among the 70 isolates of E. coli samples analyzed, 17 samples tested positive for the eaeA gene, while 10 and 4 samples were positive for the stx1 and stx2 genes, respectively. According to the results, the Enteropathogenic E. coli pathotype was identified in 17 samples, whereas the Enterohaemorrhagic E. coli pathotype was found in only 3 samples.
The percentage of E. coli isolates positive for the eaeA gene, ranging from 20% to 40%, as compared to the positive percentages for stx1 (12.5% to 20%) and stx2 (2.5% to 10%) in isolated E. coli strains (Fig. 4). Among the E. coli isolates the highest number of positive samples was recorded for the eaeA gene in Kerawalapitiya (40%), followed by Meethotamulla (25%) and Karadiyana (20%) in descending order (Fig. 4). For stx1, the highest number of positive isolates was observed in Kerawalapitiya (20%), while the highest detection of stx2 was found in isolates from Meethotamulla and Kerawalapitiya (10% each).
Discussion
Escherichia coli (E. coli) stands as a dependable biological indicator of faecal contamination in water sources, notably accounting for causing various waterborne infections in humans, particularly gastrointestinal diseases [5]. Furthermore, the presence of antimicrobial-resistant pathogenic strains of E. coli in water sources can potentially facilitate the transfer of antimicrobial resistance and virulence genes to other environmental bacteria [19]. In addition, the release of leachate from landfills has significant implications for the physical, chemical, biological, and groundwater attributes associated with agriculture and human well-being [6, 23].
Excessive levels of nitrates and nitrites in groundwater can lead to serious health risks, including methemoglobinemia, blue baby syndrome, cancer, and central nervous system disorders in humans [2, 23]. According to the ambient water quality guidelines set by the Sri Lanka Standard Institute (SLSI), certain wells around the Karadiyana dump site have surpassed the maximum acceptable nitrate concentration, raising concerns about potential health risks for the general public [22]. Moreover, the Chemical Oxygen Demand (COD) levels in all the selected wells exceeded the SLSI standard concentration significantly [2]. COD is a crucial parameter for assessing groundwater contamination by a wide range of organic and inorganic pollutants [1, 22]. The results of the present studies showed, that the COD levels around the Karadiyana dump site were greater than those at the other two sites, likely due to the continuous disposal of a substantial amount of municipal solid waste from the Western Province in Karadiyana. There are three main types of soil: sand, silt and clay. Particle size and distribution will affect a soil's capacity for holding water and therefore the movement of pollutants [1].
The contamination of well water around open dump sites is a global concern due to the potential spread of pathogenic microorganisms within the groundwater system [1]. Most of the wells studied in the present study were contaminated with faecal and total coliform, indicating severe groundwater pollution in the vicinity of the open solid waste dump area. Landfills often containing expired medications, used diapers, and sanitary products from households and healthcare facilities were observed during the sampling. When these waste items are mixed with general refuse, they can be exposed to various medications, including antibiotics. E. coli can acquire antibiotic resistance traits during prolonged incubation within landfills [1, 5]. Antibiotic resistance genes (ARGs) contribute to bacterial antibiotic resistance development and can be transferred via conjugation, transformation, and transduction to pathogenic or environmental bacteria in the environment through horizontal gene transfer [5, 11, 20]. The presence of E. coli is a significant risk factor for human infectious diseases caused by these microorganisms, as coliform pathogens can acquire resistance genes from bacterial populations in aquatic environments through horizontal gene transfer, neutralizing various classes of antibiotics [10, 14]. The findings of this study suggest that landfills could contribute to the proliferation of antibiotic-resistant bacteria in the environment, which has implications for human health.
The battle against antibiotic resistance remains unresolved, with bacteria increasingly developing resistance even to newly developed antibiotics [13]. In the present study, 70 E. coli isolates were tested for resistance to 10 antibiotics from seven different classes. All of these isolates (100%) exhibited resistance to at least one of the tested antibiotics. Resistance was particularly high (> 70%) against AMX, AMP, SUF/TRI, SDI, CLOX, TET, and ERM, in contrast to CIP, GEN, and AZY, which showed lower resistance levels (< 40%).
To the best of our knowledge, this is the first documented report on antibiotic resistance in E. coli isolated from open controlled solid waste dump sites. While the precise reasons for this phenomenon are not easily explained, it is suggested that, in addition to exposure to antibiotics in the environment, other stressors such as exposure to heavy metals may contribute to increased antibiotic resistance in environmental strains [16].
Some pathogenic strains of E. coli, such as Enteropathogenic E. coli and Enterohaemorrhagic E. coli, have been recognized as emerging bacterial pathogens [15]. Various pathogenic E. coli strains have been isolated from diverse aquatic environments worldwide, including rivers [4], lakes, seas, and groundwater resources [15]. In the present study, 8% of the pure isolates carried at least one of the tested virulence genes. It is worth noting that the majority of the isolates (approximately 90%) were negative for the genes examined, which could be attributed to the specific selection of genes for testing.
Conclusion
The assessment of virulence factors in the E. coli isolates indicated that a proportion of these bacteria carried genes associated with pathogenicity. This finding raises concerns about the potential for these isolates to cause diseases in humans and other organism. The study also demonstrated a concerning level of antibiotic resistance among the E. coli isolates. This implies that these bacteria have developed resistance mechanisms against commonly used antibiotics, which can complicate the treatment of infections caused by these strains. The study opens avenues for further research into the specific sources and routes of contamination in open dump sites, as well as the potential impact on nearby water sources and communities.
Data Availability
The datasets generated during and analysed during the current study are available from the corresponding author upon reasonable request.
References
Abia, W. A., Ezekiel, C.N., Sarkanj, B., Turner, P.C., Marko, D., Krska, R. and Sulyok, M.: Uncommon toxic microbial metabolite patterns in traditionally home-processed maize dish (fufu) consumed in rural Cameroon. Food Chem. Toxicol. 107, 10–19 (2017)
Ashokkumar, V., Flora, G., Venkatkarthick, R., SenthilKannan, K., Kuppam, C., Stephy, G.M., Kamyab, H., Chen, W.H., Thomas, J. and Ngamcharussrivichai, C.: Advanced technologies on the sustainable approaches for conversion of organic waste to valuable bioproducts: Emerging circular bioeconomy perspective. Fuel. 324, 124313 (2022)
Devane, M.L., Moriarty, E., Weaver, L., Cookson, A., Gilpin, B.: Fecal indicator bacteria from environmental sources; strategies for identification to improve water quality monitoring. Water Res. 185, 116204 (2020)
Felis, E., Kalka, J., Sochacki, A., Kowalska, K., Bajkacz, S., Harnisz, M., Korzeniewska, E.: Antimicrobial pharmaceuticals in the aquatic environment-occurrence and environmental implications. Eur. J. Pharmacol. 866, 172813 (2020)
Haley, B.J., Kim, S.W., Salaheen, S., Hovingh, E., Van Kessel, J.A.S.: Virulome and genome analyses identify associations between antimicrobial resistance genes and virulence factors in highly drug-resistant Escherichia coli isolated from veal calves. PLoS ONE 17(3), e0265445 (2022)
Idroos, F.S., Wijerathna, P.A.K.C., Abeysiri, H.A.S.N., Manage, P.M.: Nitrite, Nitrate, Ammonia and Phosphate Pollution and Their Remediation. In: Karn, S.K., Bhambri, A. (eds.) Microbial Technologies and Their Applications. Nova science Publishers (2023)
Junaid, M., Liu, S., Liao, H., Liu, X., Wu, Y., Wang, J.: Wastewater plastisphere enhances antibiotic resistant elements, bacterial pathogens, and toxicological impacts in the environment. Sci. Total Environ. 841, 156805 (2022)
Kim, S.J., Ogo, M., Oh, M.J. and Suzuki, S.: Occurrence of tetracycline resistant bacteria and tet (M) gene in seawater from Korean coast. Interdisciplinary studies on environmental chemistry—environmental pollution and ecotoxicology. TERRAPUB, Tokyo. 367–375 (2012)
Liyanage, G.Y., Manage, P.M.: Quantification of Oxytetracycline and Amphicillin in two waste water discharging points in Colombo, Sri Lanka. Environ. Nat. Resour. J. 1, 195–198 (2014)
Liyanage, G.Y., Manage, P.M.: Presence of tetracycline and oxytetracycline resistant bacteria and resistant genes in effluent water of zoological garden, Sri Lanka. Proceeding of 12th International academic conference on development in science and technology (IACDST-2015), pp. 15–19 (2015)
Liyanage, G.Y., Illango, A., Manage, P.M.: Prevalence and quantitative analysis of antibiotic resistance genes (ARGs) in surface and groundwater in meandering part of the Kelani River Basin in Sri Lanka. Water Air Soil Pollut. 232, 1–11 (2021)
Liyanage, G. Y., Weerasekera, M. M., Manage, P. M.: Screening and quantitative analysis of antibiotic resistance genes in hospital and aquaculture effluent in Sri Lanka as an emerging environmental contaminant. J. Natl. Sci. Found. 50(2), 361–370 (2022)
Liyanage, G. Y., Manage, P. M.: Evaluation of amoxicillin and sulfonamide removal by Bacillus cereus, Enterobacter ludwigii and Enterobacter sp., Environ. Nat. Resour. J. 14(1), 39–43 (2016)
Manage, P.M., Liyanage, G.Y.: Antibiotics induced antibacterial resistance. In: Pharmaceuticals and personal care products: waste management and treatment technology, pp. 429–448. Butterworth-Heinemann (2019)
Morgado-Gamero, W.B., Parody, A., Medina, J., Rodriguez-Villamizar, L.A., Agudelo-Castañeda, D.: Multi-antibiotic resistant bacteria in landfill bioaerosols: Environmental conditions and biological risk assessment. Environ. Pollut. 290, 118037 (2021)
Ondon, B.S., Li, S., Zhou, Q., Li, F.: Sources of antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARGs) in the soil: A review of the spreading mechanism and human health risks. Rev. Environ. Contam. Toxicol. 256, 121–153 (2021)
Pathmalal, M.M., Hemantha, R.S.K.W.D., Dilena, P.K., Liyanage, G.Y., Chalani, H.T.R., Bandara, K.R.V., Wijerathna, P.A.K.C., Abeysiri, H.A.S.N.: Impact of the MV X-Press Pearl ship disaster on the coastal environment from Negambo to Benthota in Sri Lanka. Reg. Stud. Mar. Sci. 58, 102788 (2023). https://doi.org/10.1016/j.rsma.2022.102788
Roy, H., Alam, S.R., Bin-Masud, R., Prantika, T.R., Pervez, M.N., Islam, M.S., Naddeo, V.: A Review on Characteristics, Techniques, and Waste-to-Energy Aspects of Municipal Solid Waste Management: Bangladesh Perspective. Sustainability 14(16), 10265 (2022)
Saja, A.M.A., Zimar, A.M.Z., Junaideen, S.M.: Municipal solid waste management practices and challenges in the southeastern coastal cities of Sri Lanka. Sustainability 13(8), 4556 (2021)
Shen, W., Zhang, H., Li, X., Qi, D., Liu, R., Kang, G., Liu, J., Li, N., Zhang, S., Hu, S.: Pathogens and antibiotic resistance genes during the landfill leachate treatment process: Occurrence, fate, and impact on groundwater. Sci Total Environ. 903, 165925 (2023)
Wijerathna, P.A.K.C., Ekanayake, M.S., Idroos, F.S., Manage, P.M.: Biological Wastewater Treatment Technology. In: Karn, S.K., Bhambri, A. (eds.) Microbial Technologies and Their Applications. Nova science Publishers (2023). https://doi.org/10.52305/JUTX4763
Wijerathna, P.A.K.C., Idroos, S.F., Manage, P.M.: A review of the green approach to the treatment of solid waste leachate. Int. J. Environ. Waste Manag. 1(1), (2023). https://doi.org/10.1504/IJEWM.10050942
Wijerathna P.A.K.C., Idroos F.S. Manage P.M.: A review of the green approach to the treatment of solid waste leachate. Int. J. Environ. Waste Manag. 35, 2 (2022). https://doi.org/10.1504/IJEWM.2023.10050942
Funding
The authors would like to acknowledge the University of Sri Jayewardenepura, Sri Lanka, and the Centre for Water Quality and Algae Research for providing the laboratory facilities for this study.
Author information
Authors and Affiliations
Contributions
P. A. K. C. Wijerathna- samples collection, microbiological analysis, water quality analysis manuscript writing and editing.
G. Y. Liyanage – Molecular analysis, manuscript writing editing and proofreading.
S.M.T.V Bandara- sample collection, microbiological analysis.
P. M. Manage conceptualization, sample collection, manuscript writing, editing and proofreading.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Statement of Novelty
This is the first study conducted to investigate open dump sites in Sri Lanka as potential environmental reservoirs of antibiotic-resistant pathogenic E. coli and their virulence potential. These organisms could represent a potential health threat through the contamination of groundwater.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liyanage, G.Y., Wijerathna, P.A.K.C., Bandara, S.M.T.V. et al. Assessment of Virulence Potential and Antibiotic Resistance Profiles in E. coli Isolates from Selected Ground Water Samples Around the Control Open Dump Sites in Sri Lanka. Waste Biomass Valor 15, 5475–5485 (2024). https://doi.org/10.1007/s12649-024-02544-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-024-02544-x