Abstract
Plant growth-promoting rhizobacteria (PGPR) are known to influence plant growth by various direct or indirect mechanisms. A total of 216 phosphate-solubilizing bacterial isolates were isolated from different rice rhizospheric soil in Northern Thailand. These isolate were screened in vitro for their plant growth-promoting activities such as solubilization of inorganic phosphate, ammonia (NH3), catalase and cell wall-degrading enzyme activity. It was found that 100% solubilized inorganic phosphate, 77.77% produced NH3 and most of the isolates were positive for catalase. In addition, some strains also produced cell wall-degrading enzymes such as protease (7%), chitinase (1%), cellulase (3%) and β-glucanase (3%), as evidenced by phenotypic biochemical test and quantitative assay using spectrophotometry. The isolates could exhibit more than two or three plant growth-promoting (PGP) traits, which may promote plant growth directly or indirectly or synergistically. Part of this study focused on the effect of NaCl, temperature, and pH on a specific the bacterial isolate Acinetobacter CR 1.8. Strain CR 1.8 was able to grow on up to 25% NaCl, between 25 and 55°C, and at pH 5–9. Maximum solubilization of tricalcium phosphate and aluminium phosphate was obtained at neutral pH, and 37°C. Strain CR 1.8 had protease activity but no cellulase, β-glucanase and cellulase activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa) is an important cereal crop of Thailand whose yields accounts for 85% of global rice production. Around 45% of the output is exported, earning about 69,000 million baht per year. During 2000–2005, sales of Thai rice captured 27% of the world market share, and is likely to continue holding its markets (The Institute of Science and Technology 2003).
Rice rhizosphere soil contains a high diversity of plant growth-promoting rhizobacteria (PGPR) such as N2-fixing Azospirilla, phosphate-solubilizing bacteria (PSB) and fluorescent bacteria (FB), and the inocula of these bacteria have been shown to enhance growth and yield of rice (Hira and Khera 2000). Rice is the staple food of the Thai people and other South East Asian countries and it s cultivation covers 85% of cultivable land, but yields could be greatly improved, since the demand for rice has been increasing, and so the amount of available phosphate fertilizer needs to be increased. Optimization of a biological phosphate solubilizer in the form of the rhizosphere flora seems a suitable tool to release some of the soil-bound phosphate and reduce the use of phosphate fertilizer for rice. Thus the potential for using PGPB inocula in the rice production system merits investigation as a means of increasing rice growth and yield (Mishra 1968).
Phosphorus (as phosphate) is an essential macronutrient for plant growth and development and is normally applied to soil in the form of phosphatic fertilizer. Phosphate in soil mostly exists in insoluble (bound) forms and the concentration of soluble phosphate in soil solution is very low (400–1,200 mg kg−1 of soil) (Rodriguez and Fraga 1999). Plants are able to utilize only a small proportion of phosphatic fertilizers that are applied, as much is rapidly converted into insoluble complexes in soil (Hilda and Fraga 1999; Cisse and Amar 2000). Thus in acid soil such as in the rice rhizosphere, the reaction products are aluminum and iron phosphates. As a result most of the phosphate applied (90%) is rendered unavailable for rice uptake but is retained in insoluble form. Thus soils have a large reserve of insoluble phosphate that could be used if phosphate-solubilizing microbes were able to free some of this essential element to improve long term crop production.
Some soil microorganism have previously been shown to be effective in releasing phosphate from bound inorganic soil phosphate through solubilization and mineralization. Microorganisms that convert insoluble phosphates into soluble forms are termed phosphate-solubilizing microorganism (PSMs). This is achieved through the acidification, chelation, ion exchange reactions and production of low molecular weight organic acids such as gluconic acids. These processes have the potential to decrease the use of phosphate fertilizer by ‘mobilizing’ the fertilizer constituents present in the soil and at the same time reducing costs and improving crop yields. Although phosphate-solubilizing microorganisms are abundant in soils and in rhizosphere of most plants application of phosphate-solubilizing microorganisms in such soils as a biofertilizer or bioconverter as a means of solubilizing fixed phosphorus has not as yet been successfully practised for rice in full scale agricultural applications. As the establishment and performance of phosphate-solubilizing microorganisms are highly likely to be affected by environmental factors, especially under stressful conditions (Nautiyal 1999), it is essential to isolate microorganisms from the relevant microbial niches to maximize their chances for use as a means of improved phosphate-solubilizing ability in the field.
In addition to the mineralization abilities discussed above, plant growth-promoting bacteria (PGPB) are soil and rhizosphere bacteria that can benefit plant growth by several different mechanisms, such as asymbiotic N2 fixation, ammonia production, solubilization of mineral phosphate and other essential nutrients, production of plant hormones and control of phytopathogenic microorganisms (Rangarajan et al. 2003). Specifically, biological control of plant pathogens and deleterious microbes, through the production of antibiotics, cell wall-degrading enzymes, hydrogen cyanide and siderophores or through competition for nutrients and space, can improve significantly plant health and promote growth as evidenced by increases in seedling emergence, vigor and yield (Antoun and Kloepper 2001). This mechanism is a recent indirect mechanism of action of PGPR. All these traits that can be present in PGPR can act collectively to promote of improved plant growth. Given the negative environmental impacts of chemical fertilizers and their increasing costs, the use of PGPB is advantageous in sustainable agricultural practices.
To date with rice, there have been only few studies on the influence of phosphate-solubilizing bacteria. Baldani et al. (2000) inoculated phosphate-solubilizing bacteria, Herbaspirillum seropedicae and Burkholderia spp. to rice soil, and the result was shown that these bacteria increased the weight of rice 1.5–21% over uninoculated controls. Bashan and Holguin (1997) showed the beneficial effect of Azospirillum on plants which increased both growth and yield, and also showed improved solubilization of phosphorus and other inorganic nutrients. Bacillus megaterium, B. subtilis and Pseudomonas have also been used to improve the grain yield of rice in pot and field trials (Trivedi et al. 2003). Bacillus sp. z 3-4 and Azospirillum sp. z 3-1 have similarly been used as a biofertilizer for rice. These bacteria have been shown to improve the biomass, root area and total N and P contents in rice (Yasmin et al. 2004).
The present study was therefore designed to screen phosphate-solubilizing bacteria from rice rhizosphere to demonstrate in vitro solubilization of inorganic mineral phosphates and rock phosphate and to examine their tolerance to high levels of pH, temperature, salt concentration, production of ammonia and lytic enzyme production. It is hoped that these experiment will explore the easibility of assessing plant growth-promoting activity through inoculation of the rice soil with phosphate-solubilizing bacteria, with a view to trialing any potentially successful inocula.
Materials and methods
Isolation of phosphate-solubilizing bacteria
One hundred and fifty rice fields at different locations in Northern Thailand were selected for the collection of rice rhizosphere samples. From each field site, two rhizospheric rice-growing soils were randomly selected and the plants uprooted. A composite sample of 10 g rhizosphere soil (root + adhering soil) was carried to the laboratory in an ice-box and isolation of rhizobacteria was completed within 48 h. Loosely adhering soil was removed from the roots by washing with sterile distilled water. Serial dilutions of soil samples were spread on modified Pikoskaya (PVK) agar (Nautiyal 1999) containing: 10 g glucose; 5 g Ca3(PO4)2; 0.2 g KCl; 0.5 g (NH4)2SO4; 0.2 g NaCl; 0.1 g MgSO4 · 7H2O; 0.002 g MnSO4 · H2O; 0.002 g FeSO4 · 7H2O and 15 g Bacto-agar per liter. The pH was adjusted to 7.0 before autoclaving. Bacterial colonies surrounded by a halo (indicating removal of phosphate) on Pikoskaya agar were assumed to be phosphate-solubilizers. Pure cultures were obtained by restreaking three to four times in the fresh medium. A total of 216 isolates originally obtained in this manner were maintained on nutrient agar slants at 30°C as stock cultures.
The bacterial isolates obtained above were re-tested by plate assay using PVK medium. These bacteria were stabbed in triplicate using sterile toothpicks. The halo zone around the colony was presumptive confirmation of phosphate solubilization and was measured after 7 days of incubation at 30°C. Halo size was calculated by subtracting colony diameter from the combined diameter of colony and halo. Tricalcium phosphate (TCP, Sigma, USA) was used as a source of phosphate in the medium.
Quantitative estimation of phosphate solubilization in broth was carried out using Erlenmeyer flasks (250-ml) containing 50 ml of Pikoskaya medium. The test bacteria were stored on Nutrient agar (Difco, USA) slant at 4°C. Before inoculation, the bacteria were cultured on Nutrient Broth (Difco, USA) at 30°C for 24 h. The final concentration of cell suspensions were adjusted to a cell density of approximately 3 × 108 c.f.u. ml−1, assessed by spectrophotometer (A600). The Pikoskaya media were inoculated in triplicate with each presumptive isolate (100 μl inoculum with approximately 3 × 108 c.f.u. ml−1) and incubated at 30°C for 7 days on incubator shaker at 180 rev min−1. After incubation period, the cultures were harvested by centrifugation at 6,000 rev min−1 for 15 min. Sterile uninoculated medium served as control. Remaining phosphate in the culture supernatant was estimated using the Fiske and Subbarow method (1925), modified culture supernatant 500 μl was mixed with 500 μl of 10% (w/v) Trichloroacetic acid in a test tube to which was added 4 ml of color reagent (1:1:1:2 ratio of 3 M H2SO4/2.5% (w/v) Ammonium molybdate/10% (w/v) Ascorbic acid and distilled water) and incubated at room temperature (26 ± 2°C), 15 min. The absorbance of the developing blue color was measured at 820 using spectrophotometer. The amount of soluble phosphorus was detected from the standard curve of KH2PO4 (Sigma, USA). All phosphate determinations were made in triplicate.
Solubilization of phosphate sources by Acinetobacter CR 1.8 (one of the strain showing high levels of phosphate solubilization) was examined in more detail.
Quantitative estimation of tricalcium phosphate (TCP) solubilization was undertaken in PVK broth as described above. The solubilization of different inorganic phosphate sources was studied by replacing TCP from the modified PVK broth with 0.5% aluminum phosphate (AP, Sigma, USA) and rock phosphate (RP). The Erlenmeyer flask (250-ml) containing 50 ml Pikoskaya broth with their different phosphate salts were each inoculated with 100 μl bacterial suspension (3 × 108 c.f.u. ml−1) and incubated at 30°C on incubator shaker at 180 rev min−1. The remaining phosphorus in the culture filtrate was estimated on days 3, 6, 9 and 12 of incubation by the method of Fiske and Subbarow (1925) as describe above. Uninoculated medium with different phosphate substrates served as controls.
Based on the colony growth, selected rhizobacteria were screened for some additional functional characteristics such as NH3, catalase production, phosphatase and cell wall-degrading enzyme activities.
Stress tolerance of the selected bacteria
The general principle of stress adaptation can be brought about when a bacterium which is exposed to sub-lethal stress can become more resistant to subsequent applications of the same stress (or in some instances, to a different stress). Preliminary experiments presented in this paper study stress responses of the selected bacteria with in respect of growth temperature, growth pH and salt (NaCl) concentration by growing the bacteria for 5 days in Erlenmeyer flasks (250 ml) containing 50 ml of nutrient broth inoculated with the bacterial strain (100 μl inoculum with approximately 3 × 108 c.f.u. ml−1) to produce stressful conditions. The optimum temperature for the bacterial growth was 30°C and optimum pH was 7.0. The effect of temperature was studied by incubating the cultures over a range of 25–55°C at pH 7.0. The influence of pH on bacteria growth was studied by growing the bacteria at 30°C in the medium made in citrate buffer pH 5–6, phosphate buffer pH 7 and Tris–HCl buffer pH 8–10 for maintaining pHs of 5–6, 7 and 8–10, respectively. In addition the selected strains (including strain Acinetobacter CR 1.8) were grown over different concentrations of NaCl (2.5, 5, 7.5, 10, 12.5, 15, 20, 25 and 30%) at pH 7.0 and 30°C. After incubation, the bacterial cells were then pelleted by centrifugation at 10,000g for 15 min at 4°C and the pelleted cells were resuspended in 1 ml of distilled water to determine the turbidity of the bacterial suspension at 600 nm by spectrophotometer.
Phosphate solubilization under stress conditions
The solubilization of phosphate by the selected bacterium under stress conditions was studied by growing the bacteria for 5 days in Erlenmeyer flasks (250-ml) containing 50 ml of nutrient broth inoculated with the bacterial strain (100 μl inoculum with approximately 3 × 108 c.f.u. ml−1). The solubilization of TCP, AP and RP by selected bacteria was determined under different temperatures (25, 30, 37, 45 and 55°C) at pH 7.0 and different pH (5, 7 and 9). The pH of medium was adjusted with 0.1 M HCl.
Production of ammonia
Bacterial isolates were tested for the production of ammonia in peptone water. Overnight broth cultures (100 μl inoculum with approximately 3 × 108 c.f.u. ml−1) were inoculated in 10 ml peptone water and incubated at 30°C for 48–72 h. Nessler’s reagent (0.5 ml) was added to each tube. Development of brown to yellow color was recorded as a positive test for ammonia production (Cappucino and Sherman 1992).
Production of catalase
The test bacteria were grown on nutrient agar at 30°C for 24–48 h. A loopful of each culture was mixed with 50 μl of 3% (v/v) H2O2 on a glass slide and incubated at room temperature (26 ± 2°C) for 1 min to observe the evolution of oxygen which was recorded as a positive catalase reaction.
Cell wall-degrading enzyme activities
Proteolytic activity was tested by inoculation of isolated bacteria into skim milk agar medium (containing 5 g pancreatic digest of casein, 2.5 g yeast extract, 1 g glucose, 15 g agar and 100 ml of 7% skim milk solution per liter). After 2 days incubation at 30°C, a clear zone around the cells indicated positive proteolytic activity (Smibert and Krieg 1994). Chitinase activity was tested on a chitin agar plate containing 1.62 g nutrient broth (Difco, USA), 0.5 g NaCl, 6 g M 9 salts (Difco, USA), 8 g colloidal chitin and 15 g agar per liter; formation of clear halos surrounding growth after 7 day incubation at 30°C indicated positive chitinase activity (Sahoo et al. 1999). Cellulase and β-glucanase activities were detected following standard methods (Cattelan et al. 1999).
Enzyme assays
A total of 216 phosphate-solubilizing bacterial isolates were grown at 30°C for 5 days on a rotary shaker (150 rev min−1) in 250-ml Erlenmeyer flasks containing 50 ml nutrient broth (8.0 g l−1) with colloidal chitin (10 g l−1) for chitinase assay, carboxymethylcellulose (1 g l−1) for cellulase and β-glucanase assays and skim milk (10 g l−1) for protease assay. After centrifugation of each broth culture at 10,000 rev min−1 for 15 min at 4°C, the culture supernatants were examined for chitinase, cellulase and β-glucanase activities by measuring the reducing sugars released from colloidal chitin, carboxymethylcellulose and larminarin, respectively, using the dinitrosalicylic acid method as described by Sahoo et al. (1999).
Assay of β-1,3-glucanase
The activity of β-1,3-glucanase was determined by measuring the release of reducing sugars by using laminarin as substrate and glucose as standard. The reaction mixture consisted of 500 μl of culture supernatant, 500 μl of 1 M citrate buffer (pH 5.0) and 500 μl of 4% laminarin. The reaction was carried out at 37°C for 30 min. The reaction was stopped by adding 2 ml of dinitrosalicylic acid and heating for 5 min on a boiling water bath, vortexes and its absorbance measured at 500 nm.
Assay of cellulase
The activity of cellulase was determined by measuring the release of reducing sugars using carboxymethylcellulose as substrate and glucose as standard. The reaction mixture consisted of 500 μl of culture supernatant, 500 μl of 1 M citrate buffer (pH 5.0) and 500 μl of 1% carboxymethyl cellulose. The reaction was carried out at 37°C for 30 min. The reaction was stopped by adding 2 ml of dinitrosalicylic acid and heating the reaction mixtures for 5 min on a boiling water bath, vortexing and measuring absorbance at 500 nm.
Assay of chitinase
Colloidal chitin was prepared from shrimp shell chitin (Sigma, USA). The reaction mixture consisted of 500 μl of 1 M phosphate buffer (pH 7.0), 500 μl of culture supernatant and 500 μl of colloidal chitin (10 mg). After 30 min incubation at 37°C, the reaction was stopped by centrifugation at 8,000g for 3 min. An aliquot of the supernatant (1 ml) was pipetted into a test tube and the reaction was stopped by adding 2 ml of dinitrosalicylic acid and heating for 5 min on a boiling water bath, vortexing and measuring absorbance at 575 nm. One unit (U) of chitinase, cellulase and β-glucanase activities was defined as the amount of enzyme that produced reducing sugars corresponding to 1 μmol of N-acetyl-d-glucosamine and glucose equivalents from colloidal chitin, carboxymethylcellulose and larminarin per minute, respectively, under the assay conditions.
Assay of protease
The activity of protease was determined by measuring the release of reducing sugars by using azocasein as substrate and tyrosine as standard. The reaction mixture consisted of 500 μl of culture supernatant, 500 μl of 0.2 M phosphate buffer (pH 7.0) and 500 μl of 1% azocasein. The reaction was carried out at 37°C for 30 min. The reaction was stopped by adding 2 ml of 10% (w/v) trichloroacetic acid (TCA) and incubated at room temperature (26 ± 2°C) for 5 min. The reaction mixture was mixed with 1,000 μl of 1 M NaOH and its absorbance measured at 440 nm. One unit of enzyme activity was defined as the amount of the enzyme resulting in the release of 1 μmol of tyrosine per min under the assay condition. In all cases, the average value from three replicates and standard deviation were calculated.
Identification of selected bacterial isolates
Selected bacterium isolates was identify based on morphological and biochemical characteristics as described in Bergey’s Manual of Determinative Bacteriology (Holt et al. 1994).
Statistical analysis
One-way analyses of variance (ANOVA) followed by LSD tests (P < 0.01) (Sokal and Rohlf 1999) were used to detect all treatment in this study. Theses analyses were performed with the computer program SYSTATTM v 5.05, for WindowsTM.
Results and discussion
Population of phosphate solubilizer
The viable bacterial population present in the rice rhizosphere soil (c.f.u. g−1) detected using isolation methods described, ranged from 1.5 to 4.5 × 108. A total of 216 isolates of phosphate-solubilizing bacteria from paddy field in this study were screened for in vitro PGP activities. It was found that 100% of strains solubilized inorganic phosphate on PVK plates at 30°C and 77.77% produced NH3. In addition, some strains also produced cell wall-degrading enzymes such as protease (7%), chitinase (1%), cellulase (3%) and β-glucanase (3%). Catalase production was exhibited by most of the rhizobacterial isolates and this may be potentially very advantageous to the survival of these bacteria in the environment.
Since the direct measurement of phosphate solubilization in broth assay is likely to give more reliable results (Johri et al. 1999) than a regular plate assay, the 216 phosphate-solubilizing strains were further tested for their ability to solubilize tricalcium phosphate in PVK broth. In the broth assay Acinetobacter strains CR 1.8 gave maximum solubilization (334.4 ± 0.4 μg ml−1) and produced acid phosphatase (259 ± 2.19 Unit l−1) and hence was selected as a key strain. The final pH of the medium decreased from 7 to 4.5 after growth of this strain. The decrease in pH clearly indicates the production of organic acid and phosphatase, which is considered to be responsible for phosphate solubilization. It has been suggested that microorganisms that decrease the medium pH during growth are efficient phosphate solubilizers (Khan et al. 2006). Acinetobacter CR 1.8 was selected for further phosphate-solubilizing condition analysis.
Phosphatase (acid phosphatase, alkaline phosphatase etc.) produced by soil microorganisms play a major role in mineralization of organic forms of bound soil phosphate to release unbound soluble forms of phosphate (e.g. P2O5) (Raghothama 1999). Indeed, high levels of phosphatase activity may be one of the causes of highest phosphate solubilization in the case of Acinetobacter CR 1.8 and similar strains. Also, the activity of this enzyme might play a role in lowering the pH of the medium. Acid phosphatase participates in the total substrate dephosphorylation action of this enzyme and the production of acids and resultant lowered pH of this growth medium and environment. Low pH values have been shown to favor the production of acid phosphatase and phytase activity by soil microorganisms (Gargova et al. 1997).
Biochemical characterization of the selected isolates
As described above, isolate CR 1.8 was the best phosphate-solubilizer (Table 1) isolated in this study. Identification based on morphological and biochemical characteristics according to Bergey’s Manual of Determinative Bacteriology (Holt et al. 1994), indicated that CR 1.8 was Acinetobacter sp. (Table 2).
Solubilization of phosphate sources by the selected bacteria
The zone of TCP solubilization by strain CR 1.8 appeared on day 5 in modified PVK agar. The quantification of the phosphate liberated showed that TCP solubilization was significantly highest, followed by the solubilization of AP but no RP was solubilized by this strain (Table 2). The incubation period also exhibited significant influence on the quantity of phosphate solubilization. The level of soluble phosphorus in the culture filtrate, in case of strain CR 1.8 increased significantly from the third day and remained high for nine days. However, subsequently a significant drop in soluble phosphorus levels was observed on later days (Table 2). The decrease could be due to the availability of soluble form of phosphate, which has an inhibitory effect on further phosphate solubilization (Varsha-Narsian et al. 1994) and the formation of an organo-phosphate compound induced by organic metabolites released, which in turn, reduce the amount of available phosphate (Illmer and Schinner 1995). The decline in pH of the culture medium was highest in the solubilization of AP, followed by the solubilization of TCP and RP. Quantitative estimation of phosphate solubilization by the bacteria in PVK broth containing TCP showed high phosphate solubilization. There was a significant variation in the quantity of phosphorus liberated by strain CR 1.8 from the different inorganic phosphates tested. The quantity of phosphate solubilized was highest for TCP among the inorganic phosphates. The solubilization of aluminum phosphate was also high, whereas this bacterium solubilized less rock phosphate (Table 2). Solubilization of inorganic phosphates by these bacteria agreed with other workers who found that rock phosphate and aluminum phosphate are less amenable to microbial solubilization than TCP (Shin et al. 2006). The maximum solubilization of different phosphate sources was obtained on day 9. The decrease in phosphate solubilization during the incubation period could be attributed to the depletion of nutrients in the culture medium (Kang et al. 2002). Phosphate solubilization was accompanied by the decrease in pH of the medium, with the pH 7.0 coinciding with the day of highest solubilization of the phosphate source (Table 2). Decreasing in pH of the culture filtrates containing various inorganic phosphates suggested secretion of organic acids by the bacteria (Vyas et al. 2007).
Bacterial growth under stress conditions
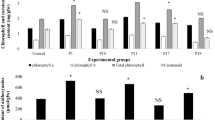
The bacterial growth under stress condition by selected isolates was found to increase linearly until days 5 at 25–55°C, pH 5–9 and salt concentration up to 25% but in 30% salt concentration the bacterial growth was decreased (Fig. 1). The results broadly revealed that the strains isolated from acid soils have the potential to solubilize phosphate under stressful conditions (high salt and pH). Stress tolerance potential of phosphate-solubilizing strains isolated from acid soils have been reported earlier (Thakuria et al. 2004).
Phosphate solubilization by Acinetobacter CR 1.8 under stress conditions
Acinetobacter CR 1.8 was tested for the ability to solubilize inorganic phosphate at different pH values and temperatures as described above. There were significant differences in solubilization of various forms of inorganic phosphate. The culture medium at pH 7 had a more positive effect on solubilized inorganic phosphate than those with acidic and alkaline pH values (Table 3). At pH 7 with TCP as substrate a significant increase in the solubilization of phosphate substrate was also recorded in the culture at various temperature 25–55°C. Maximum phosphate solubilization of this strain occurred at 25°C. The maximum phosphate solubilization was recorded at pH 7 (3,820.6 μg ml−1) followed by pH 5 (1,042.2 μg ml−1) (Table 4). The increase in incubation temperature also influenced the decrease in pH of the culture medium, with the minimum decrease in the pH at 25°C. The solubilization of TCP, AP and RP in the liquid medium by CR 1.8 are shown in Tables 2 and 3. Statistically a strong positive correlation (P < 0.01) between pH and soluble-P source was observed. The low phosphate solubilization by the bacteria under acidic and alkali pH could be attributed to the availability of initial content of soluble phosphate in the medium, which was estimated at 2,983.3, 831.3, 6.0 μg ml−1 for TCP, AP and RP at pH 7.0 in comparison with 1,350.6, 0, 0 μg ml−1 for TCP, AP and RP at pH 9, respectively.
Significant difference in the fastest rate of phosphate-solubilization with strain CR 1.8 was observed at 25°C, but the pH drop of the medium had no effect at other temperatures (Table 4). The relationship between pH drop and soluble phosphate content in PVK broth was not statistically significant for phosphate-solubilizing bacteria strains (Tables 3 and 4).
The results indicate that the production of organic acid by the microorganisms is one of the major factors but not the sole factor responsible for phosphate solubilization by bacteria (Chen et al. 2005). Therefore, the protons associated with extracellular polysaccharides secreted by the microbes are also responsible for dissolution of phosphate in PVK broth (Illmer et al. 1995). The results of this study are in agreement with other studies on the effect of pH on the solubilization of rhizobacteria (Chen et al. 2005), Chryseobacterium sp. (CCBC 05) produced highest amounts of soluble-P (289.8 mg l−1) when the pH was 6.0 as was in the case of Azospirillum, Bacillus and Pseudomonas in this study by Thakuria et al. (2004). They found the solubilization of TCP and AP was appreciable while solubilization of RP was very low.
The solubilization of TCP and AP was appreciable, while solubilization of RP was very low. The solubilization of rock phosphates depends on their structural complexity and particle size as well as the nature and quantity of organic acids secreted by the microorganisms (Pradhan and Sukla 2005). TCP and AP were found to be more vulnerable to solubilization as compared with RP (Illmer and Schinner 1995). A similar trend of decreasing phosphate solubilization with an increase in the incubation period has been reported for some microorganisms, which could be attributed to the depletion of nutrients in the culture medium (Kang et al. 2002).
Acid tolerance is important in the growth and survival of microorganisms in rice soil. The ability to adapt to temperature stress may be important in the survival of the microorganisms during drought. The bacteria tested in this study demonstrated a linear increase in growth until day 5 at 25–55°C and tolerated 25% salt concentration, but at 30% salt concentration the growth was decreased (Fig. 1) Acinetobacter CR 1.8 had the ability to solubilize inorganic phosphate under stress conditions. The results showed the potential of strain CR 1.8 as a phosphate-solubilizing inoculant in rice soil.
Cell wall-degrading enzyme activities
Production of fungal cell wall-degrading enzymes was determined in the 216 strains isolated in this study because this is an important mechanism of fungal inhibition in terms of biocontrol potential of selected strains. From these studies, as reported, only 6 (3%) isolates showed cellulose activity and four isolates (3%) showed β-1,3-glucanase activity. Chitinase was detected from three isolates (1%) and fifteen isolates (7%) could produce protease (Table 5).
Significant differences in the highest activity of cellulase, β-glucanase and chitinase were observed only in Ochrobactrum anthropi D 5.2 while the highest activity of protease was detected from isolate TS 4. Degrading activities, which were detected by the estimation for each selected strain at primary screening, were confirmed by the estimation of chitinase, cellulase, β-glucanase and protease activities in corresponding culture supernatants. A good correlation between the halo diameter of colonies grown on agar plates and the estimated level of enzyme production in liquid medium was observed (Table 5).
The ability of antagonistic bacteria to produce proteolytic enzymes, chitinase and cellulase was poor and varied among isolates. However, the production of cell wall-degrading enzymes was not expected, as expected in Acinetobacter CR 1.8 which was negative for cellulose, chitin and protein hydrolysis. Some of the above-tested isolates could exhibit more than two or three PGP traits, which may promote plant growth directly or indirectly or synergistically. Similar multiple PGP activities among PGPR have been reported by some other workers (Farah et al. 2006).
In conclusion, the characterization and screening of rhizobacteria from rice rhizosphere helped in the selection of various phosphate-solubilizing organisms. Ammonia, catalase and cell wall-degrading enzyme producers are superior strains for use as possible bio-inoculants and have the potential to increase the available phosphate in the soil, which in turn will help to minimize the P-fertilizer application, reduce environmental pollution, promote sustainable agriculture and increase yields of rice in Thailand and other regions of the world. Field trials with selected strains from this study are now planned.
Abbreviations
- c.f.u.:
-
Colony forming units
- g :
-
Centrifugal field
- A :
-
Absorbance (−log T)
- PGP:
-
Plant growth-promoting
References
Antoun H, Kloepper JW (2001) Plant growth-promoting rhizobacteria (PGPR). In: Brenner S, Miller JF (eds) Encyclopedia of genetics. Academic Press, New York, pp 1477–1480
Baldani JL, Reis VM, Baldani VLD, Dobereiner J (2000) A brief story of nitrogen fixation in sugarcane—reasons for success in Brazil. Funct Plant Biol 29:417–423. doi:10.1071/PP01083
Bashan Y, Holguin G (1997) Azospirillum–plant relationships: environmental and physiological advances (1990–1996). Can J Microbiol 43:103–121
Cappucino JC, Sherman N (1992) Microbiology: a laboratory manual. Benjamin/Cummings Publishing Company, New York, pp 125–179
Cattelan AJ, Hartel PG, Fuhrmann JJ (1999) Screening of plant growth-promoting rhizobacteria to promote early soybean growth. Soil Sci Soc Am J 63:1670–1680
Chen YP, Rekha PD, Arun AB, Shen FT (2005) Phosphate-solubilizing bacteria from subtropical soil and their tricalcium phosphate-solubilizing abilities. Appl Soil Ecol 34:33–41. doi:10.1016/j.apsoil.2005.12.002
Cisse L, Amar B (2000) The importance of phosphate fertilizer for increased crop production in developing countries. AFA 6th international annual conference, 31 January–2 February 2000, Cairo, Egypt
Farah A, Iqbal A, Khan MS (2006) Screening of free-living rhizospheric bacteria for their multiple plant growth-promoting activity. Microbiol Res 63:11–19
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Gargova S, Roshkova Z, Vancheva G (1997) Screening of fungi for phytase production. Biotechnol Tech 11:221–224. doi:10.1023/A:1018426119073
Hilda R, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–359
Hira GS, Khera KL (2000) Water resource management in Punjab under rice-wheat production system. Punjab Agricultural University, Ludhiana, India Research Bull. No 2/2000
Holt JG, Krieng NR, Sneath PHA, Staley JT, Williams ST (1994) In: Bergey manual of determinative bacteriology, 9th edn. Williams and Wilkins Pub., MD, USA
Illmer P, Schinner F (1995) Solubilization of inorganic calcium phosphates-solubilization mechanisms. Soil Biol Biochem 27:257–263. doi:10.1016/0038-0717(94)00190-C
Illmer P, Barbato A, Schinner F (1995) Solubilization of hardly soluble AlPO4 with P-solubilizing microorganisms. Soil Biol Biochem 27:265–270. doi:10.1016/0038-0717(94)00205-F
Johri JK, Surange S, Nautiyal CS (1999) Occurrence of salt, pH and temperature-tolerant phosphate-solubilizing bacteria in alkali soils. Curr Microbiol 39:89–93. doi:10.1007/s002849900424
Kang SC, Ha CG, Lee TG, Maheshwari DK (2002) Solubilization of insoluble inorganic phosphates by a soil-inhabiting fungus Fomitopsis sp. PS 102. Curr Sci 82:439–441
Khan MMK, Bhuiyan M, Kabir SM, Oki Y, Adachi T (2006) Effect of selected treatments on the production of rice (Oryza sativa) in acid sulfate soils in a stimulation study. Jpn J Trop Agric 50:109–115
Mishra R (1968) Ecology workbook. Oxford & IBA, Calcutta
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate-solubilizing microorganism. FEMS Microbiol Lett 29:221–229
Pradhan N, Sukla LB (2005) Solubilization of inorganic phosphates by fungi isolated from agriculture soil. Afr J Biotechnol 5:850–854
Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Biol 50:665–693. doi:10.1146/annurev.arplant.50.1.665
Rangarajan S, Saleena LM, Vasudevan P, Nair S (2003) Biological suppression of rice disease by Pseudomonas spp. under saline soil condition. Plant Soil 251:73–82. doi:10.1023/A:1022950811520
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 285:260–263
Sahoo A, Agarwal N, Kamra DN, Chudhary LC, Pathak NN (1999) Influence of the level of molasses in de-oiled rice bran-based concentrate mixture on rumen fermentation pattern incrossbred cattle calves. Anim Feed Sci Technol 80:83–90. doi:10.1016/S0377-8401(99)00055-3
Shin W, Ryu J, Kim Y, Yang J, Madhaiyan M, Sa T (2006) Phosphate solubilization and growth promotion of maize (Zea mays L.) by the rhizosphere soil fungus Penicillium oxalicum. 18th world conference of soil science, 9–15 July, Philadelphia
Smibert RM, Krieg NR (1994) Phenotypic characterization. In: Method for general and molecular bacteriology. Am Soc Microbiol 34:607–654
Sokal RP, Rohlf FJ (1999) Biometry: the principle and practice of statistics in biological research, 3rd edn. Freeman and Co., New York, pp 1–887
Thakuria D, Talukdar NC, Goswami C, Hazarika S, Boro RC, Khan MR (2004) Characterization and screening of bacteria from rhizosphere of rice grown in acidic soils of Assam. Curr Sci 86:978–985
The Institute of Science, Technology Development in Thailand (2003) High quality organic fertilizer (in Thai). Ministry of Science and Technology, Bangkok
Trivedi P, Kumar B, Pandey A, Palni LMS (2003) Growth promotion of rice by phosphate-solubilizing bioinoculants in a Himalayan location Plant. Soil Sci 102:291–299
Varsha-Narsian J, Thakkar J, Patel HH (1994) Inorganic phosphate solubilization by some yeast. Indian J Microbiol 35:113–118
Vyas P, Rahi P, Chauhan A, Gulati A (2007) Phosphate solubilization potential and stress tolerance of Eupenicillium parvum from tea soil. Mycol Res 111:931–938. doi:10.1016/j.mycres.2007.06.003
Yasmin S, Rahman Bakar MA, Malik KA, Hafeez FY (2004) Isolation, characterization and beneficial effects of rice associated plant growth-promoting bacteria from Zanzibar soil. J Basic Microbiol 44:241–252. doi:10.1002/jobm.200310344
Acknowledgement
A grant from The Commission of Higher Education is greatly appreciated. We thank Dr. Peter Green, NCIMB, UK for helping edit the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaiharn, M., Lumyong, S. Phosphate solubilization potential and stress tolerance of rhizobacteria from rice soil in Northern Thailand. World J Microbiol Biotechnol 25, 305–314 (2009). https://doi.org/10.1007/s11274-008-9892-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9892-2