Abstract
Plant growth-promoting rhizobacteria (PGPR) are beneficial microorganisms to develop microbial fertilizers. Biofertilizers can accelerate plant growth and enhance crop yields. The current research aimed to isolate and identify rhizobacterium with plant growth-promoting activity in the rhizospheric region of pistachio trees in arid and salty region of Iran. In the present study, 26 bacterial isolates were isolated from the rhizospheric region of the pistachio trees. Plant growth-promoting characteristics of isolated bacteria, including the ability to solubilize phosphate and zinc, produce hydrolyzing enzymes, and hydrogen cyanide (HCN), as well as synthesize indole-3-acetic acid (IAA) were evaluated through in vitro assays. Based on these activities, five multifunctional bacterial strains designated P1, P10, P11, P17, and P19 were then applied and their effect was studied on the growth and physiological properties of Pistacia vera L. seedlings by pot experiments under normal conditions. Finally, the most efficient strain has been identified by analysis of the 16S rRNA gene sequence. According to the results, all the isolated bacteria exhibited considerable plant growth-promoting properties. They could produce amylase (n = 26, 2 ± 0.00–13 ± 0.42 mm), lipase (n = 24, 2 ± 0.00–9 ± 0.23 mm), protease (n = 20, 1 ± 0.00–17 ± 0.0 mm), indole-3-acetic acid (n = 26, ranging from 5.05 ± 0.08 to 11.5 ± 0.11 μg/mL) and HCN (n = 24). Six isolates showed significant growth at 20% w/v NaCl. Inoculation of P1, P17, and P19 increased chlorophyll, carotenoid, and phenolic content in treated Pistacia vera L. seedlings. P1 and P11 inoculated plants showed an enhanced level of anthocyanin and proline. These most effective strains were catalase and Gram-positive bacterium and showed antibiotic sensitivity. They can consider as halotolerant PGPR, due to the growth in the presence of NaCl (20% w/v). Finally, P1 inoculated plants exhibited higher levels of sugar content. This strain showed the most similarity (99.92%–1322 bp) to Paenarthrobacter nitroguajacolicus based on 16S rRNA gene sequence. Based on the results, Paenarthrobacter nitroguajacolicus P1 with multiple PGPR can be applied as a promising candidate in the soil-Pistacia vera L. system to improve their productivity and health by increasing available nutrient content, improving photosynthetic parameters, and producing phytohormones and HCN.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pistachio (Pistacia vera L.) is known as one of the most significant exporting nut crops in arid and semi-arid countries especially Iran [1]. The Pistacia genus belongs to the Anacardiaceae family [2]. It has been reported that more than 500,000 hectares of bearing and non-bearing pistachio orchards exist in Iran. Iran is the third largest producer of pistachios worldwide after the United States of America and Turkey [3].
Pistacia vera L. is among the relatively tolerant plants to adverse effects of environmental stresses in the ecosystems like drought and salinity of the soil and water [4]. However, salinity stress causes unfavorable growth and reduced yield of pistachio trees in recent years in Iran [5].
It has been reported that abiotic stresses, such as salinity, low bioavailability P, K, Zn, Fe, Mn, and Cu and high content of CaCO3 in the soils as well as high temperature are responsible for the low yield of this nut crop in Iran despite the abundance of areas under Pistacia vera L. cultivation [6].
Decreased level of soluble micronutrients such as P and Zn causes reduced photosynthetic and transpiration rates [7], and increased osmotic stress as well as generates free radicals in various tissues of plants [1, 8]. Also, overuse of chemical fertilizers could results in declined fertility of the soil, suppression of the beneficial microorganisms, and pollution of the groundwater [9].
Therefore, there is an emergent need for promising alternative to minimize the usage of chemical fertilizers and improve plant growth. Applying beneficial plant-associated microorganisms in the form of biofertilizers is a promising and safe alternative approach to achieve sustainable agriculture with no adverse effect on the environment, human and animal health [9]. These bacteria can transform insoluble nutrients and minerals to biologically available forms through biosorption, complexation by metabolites, dissolution, reduction–oxidation processes as well as the production of organic acids and siderophores. These microorganisms are known as plant growth-promoting rhizobacteria (PGPR) [9]. PGPR appear to promote plant growth by solubilizing and enhancing the bioavailability of essential nutrients, producing and modulating phytohormones, synthesizing hydrolytic enzymes and various compounds with inhibitory effects on phytopathogens, as well as ameliorating abiotic stresses [10].
In this regard, there are limited studies indicating positive effects of PGPR on Pistacia vera L. growth. For example, Kalilpour et al., reported that Pistacia vera L. seedlings inoculated with stress-tolerant PGPR, including Arthrobacter endophyticus, Zobellella denitrificans, and Staphylococcus sciuri exhibited increased shoot and root dry weight, leaf area, leaf number, shoot and root K+ concentration and relative water content under high levels of salinity and drought stresses compared to the non-inoculated seedlings[11]. Also, It has been demonstrated that application of Pseudomonas fluorescens VUPF5 and Bacillus subtilis VRU1 considerably increased the root length, proliferation of Pistacia vera L. seedlings [12], enzymatic activities and levels of phenolic compounds [13].
The current study aimed to isolate rhizobacteria from the soil of pistachio orchards in Damghan, Iran, and screen their growth-promoting properties, including phosphate and zinc solubilization, hydrolyzing enzyme, indole acetic acid and HCN production and their tolerance to NaCl. Subsequently, the Pistacia vera L. growth-promoting activities of the most efficient strains were evaluated through a pot experiment.
Material and Methods
Soil Sampling and Isolation of Bacterial Strains
Rhizospheric soil samples were collected in 2022 from a pistachio orchard, in Raziabad, a village in Ghahab Sarsar, Amirabad district, Damghan city, Semnan province, Iran. This orchard is located at latitude 36.11616585209723 N and longitude 54.21172885290508 E. The pistachio trees in the orchard were 5–7 years old. They were in good condition. Autoclaved glass cans with a depth of 15 cm and a uniform diameter of 10 cm were used for soil collection. The collected soil sample was subsequently transported to the laboratory to isolate potential PGPR [14]. To isolate bacterial strains, the soil samples (1 g) were suspended in saline solution (10 mL) and vortexed (10 min) to obtain a uniform suspension. Subsequently, serial dilutions were prepared and surface plated on nutrient agar (Ibresco, Iran). The inoculated plates were incubated at 30 °C for 24 h. At the end of the incubation period, colonies with different morphologies were selected, and purified cultures were prepared. Isolated bacterial strains were stored in glycerol (20% v/v) at − 80 °C for further studies. All isolated strains were tested for plant growth-promoting attributes. [15].
Production of Hydrolyzing Enzymes
Overnight grown bacterial broth cultures were spot inoculated on 1% Tween-20 Luria–Bertani (LB) agar, starch agar, and skim milk agar plates to qualitatively identify lipase, amylase, and protease synthesis, respectively. The inoculated cultures were incubated at 30 °C for 3–4 days. The enzymatic activity was detected as halos around the colonies [16].
Assessment of Phosphate Solubilization Activity
All the bacterial isolates grown for 24 h were spot inoculated on the Pikovskaya medium containing 10 g/L of glucose, 5 g/L of Ca3(PO4)2 [as an insoluble source of phosphorus], 0.5 g/L of (NH4)2SO4, 0.5 g/L of yeast extract, 0.5 g/L of NaCl, 0.2 g/L of KCl, 0.1 g/L of MgSO4.7H2O, 0.002 g/L of MnSO4.H2O, 0.002 g/L of FeSO4.7H2O, and 15 g/L of agar, pH adjusted to 7.0 [17]. The ability to solubilize tricalcium phosphate was detected by the observation of a halo zone around colonies after incubation at 28 °C for 7 days. The diameter of the colony was subtracted from the total diameter to calculate the halo size [18].
Evaluation of Zinc Solubilization Ability
To screen zinc solubilization activity, bacterial isolates were inoculated as spots on a minimal agar medium that was composed of 10 g/L of glucose, 1 g/L of (NH4)2SO4, 0.2 g/L of KCl, 0.1 g/L of K2HPO4, 0.2 g/L of MgSO4, 1 g/L of zinc oxide, and 15 g/L of agar. The inoculated plates were incubated at 28 °C for 7 days in the dark condition. The diameter of the halo zone around the colony was measured after 7 days. The diameter of the colony was subtracted from the total diameter to calculate the halo size [19].
Measurement of Indole Acetic Acid Synthesis
IAA synthesis by bacterial isolates was estimated according to the method of Gordon and Weber [20]. Bacterial culture (50 μl, 24 h old-OD600 = 0.1) was inoculated in nutrient broth (5 mL) containing L-tryptophan [0.1% v/v (100 mg/L)]. The inoculated media were incubated at 28 ± 0.1 °C for 2 days in the dark condition. At the end of the incubation, the bacterial cultures were centrifuged at 10,000 × g for 10 min (Eppendorf, Germany). An equal volume of Salkowski reagent [150 mL of 95–98% H2SO4, 7.5 mL of 0.5 M FeCl3.6H2O, and 250 mL distilled water] and collected supernatant was mixed and incubated for 30 min. The development of pink color indicates IAA production. Subsequently, absorbance was read at 530 nm by using a UV/Visible spectrophotometer (BioTek, USA) to quantify IAA. The IAA concentration was estimated based on the IAA standard curve.
Evaluation of HCN Production
The isolated bacteria were cultured on an LB agar medium containing glycine (4.4 g/L). A Whatman paper No. 1 was saturated with alkaline picrate (solution of 2% Na2CO3 in 0.5% picric acid) and placed in the lid of each Petri plate. The dishes were sealed with parafilm and incubated at 28 °C for 4 days. The observation of a red–orange color confirms the synthesis of HCN [21].
Salinity Tolerance of the Isolated Bacteria
Each bacterial isolate was screened for halotolerant properties. For this purpose, they were spot inoculated on nutrient agar medium supplemented with various levels of NaCl (5, 10, 20% w/v). The inoculated plates were incubated at 28 °C for 7 days [22].
Seed Sterilization and Inoculation
One Pistacia vera L. cultivar (cv. Akbari) was used in the present study. Seeds were soaked into sodium hypochlorite solution (5% v/v) for 10 min for disinfection. Subsequently, they were washed with sterile dH2O to remove disinfectant (Cheng et al., 1997). Bacterial suspensions were prepared into the nutrient broth. The inoculated media were incubated at 28 °C on a shaker at 120 rpm overnight. Subsequently, the media were centrifuged to get biomass. The biomass was re-suspended in dH2O. Surface-disinfected seeds were soaked in PGPR suspension for 2 h. Autoclaved dH2O was used for seeds in the control group. One milliliter microbial suspension (CFU = 108 cells/mL) for each seed was applied. The inoculated seeds were put between two water-moistened filter papers (Whatman No. 1) in a Petri dish to germinate at 28 °C for 4 days in the dark condition. Germinated seeds were transferred to each pot containing sterilized soil. At sufficient intervals, irrigation was done with sterilized water (pH 7). Data was recorded after 60 days to determine the effect of PGPR on the growth and physiological characteristics of Pistacia vera L. seedlings [6].
Determining Protein and Sugar Content
Pistacia vera L. leaflet tissue (0.25 g fresh leaves) was extracted in chilled acetone (80%). Subsequently, centrifugation was done at 8000 × g for 10 min at 4 °C. The supernatant was used to measure protein content. Each sample (100 µL) was mixed with Bradford reagent (1.0 mL). Bovine serum albumin was used as the standard. The test tubes were incubated at 37 °C for 10–15 min, and absorbance was read at 595 nm [23]. To determine reducing sugars, the dry plant sample (1 g) was dispersed in dH2O (10 mL). The mixture was boiled for 10 min and then cooled. The solution (1 mL), potassium ferrocyanide (0.1 mL, 15%), and zinc acetate (0.2 mL, 30%) were mixed. The obtained mixture was centrifuged at 112 × g for 5 min to recover the reducing sugars in the supernatant. The content of reducing sugars was measured using dinitrosalycilic acid (DNS). For this purpose, supernatant (0.1 mL) was diluted with dH2O (0.9 mL). Then diluted samples were treated with DNS (3 mL). The mixture was boiled for 10 min. The absorbance was measured at 550 nm. A standard range of maltose was used to determine sugar content [24].
Determination of Chlorophyll, Carotenoids, and Anthocyanin Contents
The amounts of chlorophyll a, b, and total chlorophyll were analyzed in the fresh leaves. Briefly, the leaves (100 mg) were ground in acetone (80%) and the mixture was incubated for 6 h in the dark condition. Subsequently, it was centrifuged at 10,000 × g for 10 min at 4 °C. Absorbance was recorded at 663 and 645 nm [25]. The concentration of chlorophylls was calculated as follows:
Carotenoid absorbance of the extract was recorded at 480 and 510 nm;
Anthocyanin level was calorimetrically determined. For this purpose, leaves have meshed with acetone (2 mL) to extract the green-colored pigment. Then, the filtered extract (0.05 mL) was added to 0.95 mL of buffered acetone (80%) to quantify anthocyanin via the following formulas [26].
The Determination of Total Phenolic Compounds
The total phenolic contents were determined using the Folin–Ciocalteau method [27]. In brief, the fresh leaves of Pistacia vera L. (0.5 g) were extracted by ethanol (5 mL, 95%), and the extract was kept in the dark condition for 48 h. Subsequently, an equal volume of the supernatant and ethanol was mixed and 1.5 mL of distillation water was added to the mixture. Then, folin regent (50%, 0.25 mL) and carbonate sodium solution (5% 0.5 mL) were added to the mixture. The samples were incubated for 1 h, and their absorbance was read at 725 nm.
Proline Measurement
The content of free proline was measured according to the method described by Bates et al. [28]. The fresh leaves (100 mg) were homogenized in 5 mL sulfosalicylic acid (3%) and an equal volume of filtrate and ninhydrin reagent (1.25 g ninhydrin in 30 mL glacial acetic acid) was mixed. The mixture was vortexed for a short time and heated at 100 °C via a water bath for 1 h. After cooling the samples in an ice bath, toluene (2 mL) was added to it and vortexed (15–20 s). The upper phase containing proline was taken and its absorbance was read at 520 nm. The standard curve of the proline with determined concentration was used to calculate proline concentration.
RNA Extraction, cDNA Synthesis, Primer Design, and RT-PCR Reaction
The effect of P1 and P11 strains on the enhancement of proline content was also confirmed using RT-PCR. According to the manufacturer’s instruction and using RNX plus solution (CinaClon, Tehran, Iran), total RNA was extracted from leaves of Pistacia vera L. seedlings inoculated with P1 and P11 strains. DNase (Thermo Fisher Scientific, USA) was used for removing DNA. In addition, 2% agarose gel electrophoresis was used to show the integrity of the isolated RNA. RNA samples with OD 260/280 and OD 260/230 > 2 were used for cDNA synthesis. The strand cDNA was synthesized with 200 ng of total RNA, using the cDNA synthesis kit (CinaClon, Iran) according to the manufacturer’s protocol. P5CS primers for amplification with a length of 241 base pairs and melting temperature of 60 °C were designed for RT-PCR with the following sequences: P5CSF: 5′-GAC CTC GAG CAA GTG GTG AA-3′ and P5CSR:5′- CTC TGC ACC AAG CCC AAA AC-3′. JZ896672 was selected as specific internal control for Pistacia vera L. According to Jazi et al. 2015, forward primer β-Tubulin, 5′-TGG GAC CCA CGT GAA GTC AG-3′ and reverse primer β-Tubulin 5′-GAG TGG TGT AAC TTG CTG CTT G-3′ produce a product with 138 base pairs length at 60 °C melting temperature. RT-PCR was performed using PCR Master Mix (Amplicon), as the initial denaturation at 94 °C for 3 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 57–59 °C for 30 s, and extension at 72 °C for 50 s with a final extension at 72 °C for 10 min [29].
Gram Staining, Catalase Activity, and Antibiotic Susceptibility Test
The most efficient strains (P1 and P11) were subjected to Gram staining and catalase test. The disk diffusion test was performed to evaluate the antibiotic susceptibility of the most efficient strains based on the Clinical and Laboratory Standards Institute (CLSI) guidelines [30]. The overnight cultures of these strains (1.5 × 108 CFU/mL) were cultured on the Muller Hinton agar plates. Antibiotic discs, including erythromycin (15 µg), tetracycline (30 µg), streptomycin (10 µg), chloramphenicol (30 µg), ampicillin (10 µg) were placed onto inoculated Muller Hinton agar medium. At the end of incubation (24 h, 37 °C), the diameter of the growth inhibition zone was measured around discs [31, 32].
Molecular Identification of the Most Efficient Plant Growth-Promoting Strain
The most efficient strain was inoculated in the nutrient broth medium, and incubated at 30 °C with shaking (160 rpm) for 24 h. The biomass was precipitated by centrifugation, and genomic DNA was extracted by using phenol–chloroform-isopropanol and precipitated by adding ethanol. The 16S rRNA gene was amplified using primers 27 F (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 1492R (5′-GGT TAC CTT GTT ACG ACT T-3′) (Galkiewicz and Kellogg, 2008). The PCR condition was 95 °C for 5 min, 35 cycles of 95 °C for 30 s, 55 °C for 60 s, and 72 °C for 60 s, followed by a final extension at 72 °C for 10 min. The PCR product was qualified by 1% agarose gel electrophoresis and then sequenced. The phylogenetic tree was constructed via the maximum likelihood method of the software package MEGA 6.0 and the topology of the tree was evaluated by a bootstrap test with 1000 replicates (Tamura et al., 2013). The 16S rRNA gene sequence of the most efficient strain (P1) was deposited in the GenBank database [33].
Statistical Analysis
All data are expressed as the mean ± the standard deviation. Each test was repeated three times. One Way ANOVA in IBM SPSS Statistics was used to determine the existence of the significant difference. Differences were considered significant at the level of P < 0.05.
Results
Physicochemical Properties of Soil Samples
The physicochemical properties of the soil samples, including cation exchange capacity (CEC) (mEq/100 g) [27.247 Cmolc/kg] and total organic carbon (TOC %) [0.853] were determined. The soil consisted of sand (50%), clay (19%), and silt (31%), and chemical analysis showed that the soil sample contained P (14.8 μg/g), K (450 μg/g), Fe (10.98 μg/g), Zn (5.9 μg/g), Mn (8.61 μg/g), and Cu (2.4 μg/g). The electrical conductivity of the soil was 6.8 dSm−1 and its pH was 8.3. In the current study, 26 strains were isolated from the rhizospheric soil of pistachio trees.
Enzymatic Activities of Isolated Rhizobacteria
The ability of isolated bacteria to synthesize hydrolytic enzymes such as protease, lipase, and amylase was investigated using the media containing enzyme substrates. All bacterial strains (except isolate 26) produced at least two different hydrolytic enzymes. The results showed that bacterial strains were able to produce amylase (2 ± 0.0–13 ± 0.42 mm). Most of the isolates showed lipolytic (84%, 2 ± 0.0–9 ± 0.23 mm) and proteolytic (76%, 1 ± 0.0–17 ± 1.00) activities. Isolates P1, P11, and P15 showed the best enzymatic activities among other isolates (Table 1).
Determination of Phosphate and Zinc Solubilization
Among the 26 strains isolated from the rhizospheric soil of Pistachio trees, clear zones were formed around the colonies of 18 bacterial strains on Pikovskaya medium supplemented with Ca3(PO4)2 which showed their ability to solubilize phosphate. Clear zones with diameters in the range of 1–14 mm were formed around the colonies after 5–7 days of incubation (Table 2). Maximum phosphate-solubilizing ability was observed with strain P1, followed by the strain P11 and P13. Ten out of 26 bacterial strains showed the ability to solubilize ZnO in agar plate assay. Clear zones with diameters of 13 ± 0.36 mm to 1 ± 0.0 mm were observed. Maximum Zn solubilization was related to isolate P1 which was followed by isolate P11 (11 ± 1.00). Isolates P10 and P19 showed minimum solubilization zone (Table 2).
Evaluation of the Indole Acetic Acid and the Hydrogen Cyanide Production
According to the results, all isolates were able to synthesize IAA. The concentration of IAA produced by the rhizobacteria showed a variation between 5.05 ± 0.08–11.50 ± 0.11 μg/mL (Table 3). The production of IAA was highest in the strain of the P17 (11.50 ± 0.11 μg/mL) followed by the isolates P15, (10.25 ± 0.39 μg/mL) and the isolate P4 (9.30 ± 0.20 μg/mL). At the same time, strain P12 produced the lowest amount of IAA (5.05 ± 0.08 μg/mL). Twenty-four rhizobacteria recovered from the rhizospheric region of the pistachio trees showed an ability to produce hydrogen cyanide (HCN). Among them, strong (+ + +), moderate (+ +), and weak ( +) HCN production were observed in five, nine and ten isolates, respectively (Table 3).
Halotolerance of PGPR
Most isolated bacteria (except isolate 14) showed considerable growth in the presence of 5% w/v NaCl (Table 4). The NaCl at a concentration of 10% w/v inhibited the growth of isolate P14. This level of salinity significantly reduced the bacterial growth of 31% of the strains, including isolates with codes of P8, P10, P15, P17, P18, P20, P23, and P26. The growth of P5, P6, P7, P9, P14, P18, P19, P21, and P22 isolates was suppressed in the presence of 20% w/v NaCl. The growth of isolates including, P3, P4, P8, P12, P13, P15, P17, P20, P23, P25, and P26 was reduced in the presence of 20% w/v NaCl. Remained isolates including, P1, P2, P10, P11, P16, and P24 isolates grew well at this salinity level and their growth was not affected by salinity (Table 4).
Influence of Microbial Inoculants on Pistacia vera L. Growth Parameters
According to the results, isolates P1, P10, P11, P17 and P19 due to having different levels of ability to improve plant growth including, various amylolytic, lipolytic,and proteolytic activities, phosphate, and zinc solubilization activities, as well as the different ability to produce HCN and IAA and finally diverse tolerance to a high level of salinity were selected for a pot experiment. Inoculation of seeds with these PGPR influenced the growth characteristics of Pistacia vera L. seedlings. The content of chlorophyll a and b was enhanced (P < 0.05) by inoculation of strains P1 and P11 and P17 compared to untreated plants (control). Compared with the control, 47.42%, 57.73%, and 32.98% increases were observed in the total chlorophyll content of seedlings treated with P1, P11, and P17 inoculants, respectively. It was found that the level of carotenoid was increased in P1, P11, and P17 treated plants by 53.12%, 32.81 and 25%, respectively (Fig. 1a). The chlorophyll and carotenoid contents of treated seeding with P10 and P19 have no significant different with untreated seedling (P > 0.05) (Fig. 1a). Also, the treated seedling with P1, P11, and P19 exhibited more anthocyanin level in comparison with untreated seedlings (Fig. 1b). The inoculation with PGPR strains was found to significantly influence (P < 0.05) on phenolic contents in the leaves of Pistacia vera L.. It was found that in comparison to the control phenolic contents of P1, P10, P11, P17, and P19 treated Pistacia vera L. were increased by 66.66, 50.66, 61.33, 33.33, and 36%, respectively (Fig. 2 a). Also, inoculation of P1 and P11 caused a significant effect on the enhancement of proline contents (Fig. 2b). Moreover, the results of RT-PCR confirmed that the gene expression level of 1-Pyrroline-5-Carboxylate Synthetase (P5CS) in Pistacia vera L. seedlings treated with P1 and P11 strains has increased compared to the control (Fig. 3). Based on obtained results microbial inoculation has no significant effect on the protein content of treated Pistacia vera L. in comparison with untreated ones (Fig. 4a). The P1 inoculated plants showed an enhanced level of sugar content (50%) compared to uninoculated seedlings (Fig. 4b). P1 and P11 as the most efficient strains were catalase and Gram-positive and showed considerable antibiotic susceptibility (Table 5).
Effects of PGPR on chlorophyll a (black column), chlorophyll b (white column), total chlorophyll (grey column) carotenoid (dotted column) (a) and anthocyanin (b) content of pistachio treated by most prominent PGPR. Measurements were made after two months (n = 3). Control: no-inoculation with microbial inoculants, Treatment: inoculation with microbial inoculants. * and NS showed the presence and absence of significant difference [at confidence interval of 95% (P < 0.05) between] control group and experimental group, respectively
Effects of microbial inoculants on the content of phenolic compounds (a) and proline content (b) (n = 3). Control: no-inoculation with PGPR microbial inoculants, Treatment: inoculation with PGPR microbial inoculants. * and NS showed the presence and absence of significant difference [at confidence interval of 95% (P < 0.05) between] control group and experimental group, respectively
Effects of PGPR on protein (a) and sugar (b) of Pistacia vera L seedling treated by most prominent PGPR. Measurements were made after two months (n = 3). Control: no-inoculation with PGPR microbial inoculants, Treatment: inoculation with PGPR microbial inoculants. * and NS showed the presence and absence of significant difference [at confidence interval of 95% (P < 0.05) between] control group and experimental group, respectively
Sequencing of 16S rRNA Gene of P1 Strain
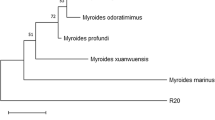
Strain P1 was selected as the most efficient PGPR for Pistacia vera L. seedlings. Therefore, the sequence of its 16S rRNA gene was submitted to NCBI GenBank using BLAST (basic local alignment tool), and a phylogenetic tree was constructed (Fig. 5). The strain P1 showed the most similarity to Paenarthrobacter nitroguajacolicus (99.92%–1322 bp). Sequences of strains were submitted in NCBI GenBank with the accession number OP810958.
Discussion
The soils of pistachio orchards are extremely saline and alkaline. These two stresses limit plant growth and development and decrease soil quality in the long term. Salinity adversely affects photosynthetic machinery. It leads to water deficit within the plant and ion toxicity, decreases nutrient uptake or their transportation to the shoot, and finally generates reactive oxygen species (ROS) and ethylene. Consequently, plant growth is adversely affected (Egamberdieva et al., 2019). So, cost-effective, eco-friendly, and sustainable strategies are critically needed to restore saline-alkaline degraded lands to improve Pistacia vera L. growth.
It has been known that the PGPR can adapt themselves to harsh conditions and, through various mechanisms can encourage plant growth under stressful conditions. Thus, in this study, we screened the rhizospheric region of pistachio trees and isolated 26 multifunctional PGPR.
Saline and alkaline soils in Iran have a deficiency of soluble and bioavailable zinc and phosphate that limit the growth and yield of the pistachio trees. So, applying biofertilizers containing phosphate (PSM) and zinc solubilizing microbes (ZSM) enhances the fertility of the soil and also decreases the consumption of hazardous phosphate and zinc fertilizers [34].
Some of the isolated rhizobacteria in the current study showed an ability to solubilize tricalcium phosphate (n = 18, 14 ± 0.5–1 ± 0.0) and zinc oxide (n = 10, 13 ± 0.19–1 ± 0.0) on plate assays and considered as PSM and ZSM, respectively. The P1 strain showed the best phosphate and zinc solubilization which was followed by the P11 strain.
Also, PGPR improve the breakdown of organic compounds in the soil through their hydrolytic enzymes which increase soil nutrient content and provide a favorable soil condition for the growth of plants [35]. For example, Nadeem et al. (2014) showed that applying fertilizers containing PGPR improve the physiochemical characteristics of the soil by enhancing the enzymatic activities of soil like invertase, phosphatase, peroxidase, and urease activities, which finally accelerate plant growth [36]. These results are also consistent with the finding of the present study. Some of the isolated bacteria were amylolytic (n = 26, 13 ± 0.10–2 ± 0.00), proteolytic (n = 20, 17 ± 1.00–1 ± 1.00), and lipolytic (n = 22, 9 ± 0.23–2 ± 0.00) strains. P11 isolate showed the most proteolytic and lipolytic activities. Also, P9 and 19 isolates showed the best amylolytic activities. These hydrolytic enzymes are involved the nutrient recycling through organic matter decomposition. Thereby, these strains with enzymatic activities increase the nutrient source around the host plant.
Moreover, these rhizobacteria can exert their beneficial effect by producing IAA that positively affects the root system which led to improved uptake of water and nutritional compounds. In the present study, all strains showed IAA production ability (5.05 ± 0.08–11.5 ± 0.11 µg/mL). Continuous production and slow release of phytohormones produced by rhizobacteria make them promising candidates for promoting plant growth [37]. Also, 24 bacterial isolates showed an ability to produce HCN.
These in vitro assays showed that isolated bacteria could be considered potential PGPR. Among the studied bacterial strains, the Pistacia vera L. growth-promoting ability of the P1, P10, P11, P17, and P19 strains was further screened in a pot experiment. It has been shown that inoculating PGPR can significantly improve the physiological properties of pistachio [38]. For example, Acar et al., showed that inoculation of Pistacia vera L. seedlings with PGPR including, Chlorobium limicola, C. vibrioforme, Nitrosomonas spp., Nitrobacter spp., Pseudomonas vulgaris under salinity stress imposes positive effect on the development of Pistacia vera L. seedlings and through establishing symbiosis between Pistacia vera L. roots and bacteria protected them against salinity[39]. Also, Atajan et al., reported that Pseudomonas sp. inoculation with Pistacia vera L. seedlings improves dry weight, Mn uptake, and chlorophyll content of the Pistacia vera L. seedlings in the presence of salinity stress [40]. Inoculation of P1, P11, and P17 isolates remarkably increased the level of the total chlorophyll of Pistacia vera L. in pot experiments. Therefore, P1, P11, and P17 had a meaningful promoting effect on the photosynthesis of seedlings. Chlorophyll concentration is indicative of a plant’s health in the presence of salinity [41]. Similar results were reported by Azarmi et al. that showed that the chlorophyll content of Pistacia vera L. seedlings was more than that of the control after the treatment by fluorescent pseudomonads strains (14–19%) [42]. In the current study, inoculated Pistacia vera L. seedlings with PGPR stains showed higher accumulation of phenolic content in relation to non-inoculated plants. Phenolic compounds and anthocyanin play important roles in different plant processes and play important role in the defense mechanisms of plants against various stresses [43].
Pistacia vera L. seedlings inoculated by the P1 strain showed an enhanced level of sugar and proline content compared to untreated seedlings. In this regard, the inoculated seedlings by P1 increased proline and sugar concentrations by 48.27% and 50%, respectively. The increase in sugars can presumably be due to increased photosynthesis and nutrient availability to the inoculated plants as a result of the application of PGPR [44]. Also, Azarmi et al. reported that fluorescent pseudomonads (pf1, pf2, and pf3) treated Pistacia vera L. seedlings showed enhanced concentrations of proline (11–21%) and sugar (20–31%) compared to control [6].
The P1, P10, and P11 strains tolerated high concentrations of NaCl (20% w/v). So, it can be stated that they are extremely halotolerant owning to growing in the absence as well as in the presence of high salt concentrations [45]. The growth of the P17 strain was reduced in the presence of 20% w/v NaCl and the P19 strain have no growth at this salinity level.
Halotolerant rhizobacteria with plant growth-promoting properties are considered one of an outstanding group of bacteria that can improve physicochemical properties of rhizospheric regions in the presence of salinity stress and enhance yield [46, 47].
It seems that the higher efficiency of P1 and P11 isolates compared to other investigated isolates, including P10, P17, and P19 in promoting the growth of Pistacia vera L. seedlings is attributed to higher enzymatic activities and their considerable ability to solubilize zinc and phosphate.
P1 and P11 isolates were catalase-positive and Gram-positive bacteria. It has been reported that catalase positive PGPR can protect plants against chemical, environmental, and mechanical stress [48]. The antibiotic sensitivity of the P1 strain to studied antibiotics may assure that this strain is safe to use in the field and there is a lower probability that its application results in the spread of antibiotic resistance. Although further studies are needed to investigate the presence or absence of transferable antibiotic resistance genes.
According to the molecular identification, the P1 strain showed the most similarity to Paenarthrobacter nitroguajacolicus. Paenarthrobacter nitroguajacolicus is a rare actinobacterium. Vergani et al. reported the isolation of P. nitroguajacolicus from the rhizosphere of Centaurea nigrescens in northern Italy which was mostly contaminated with polychlorinated biphenyls and metals. They reported that this strain could produce auxin and ACC deaminase under drought-stress conditions [49].
It has been reported that P. nitroguajacolicus 2–50 significantly enhanced fresh weight, shoot development, shoot, and root length of treated tomato plants compared to the non-inoculated plant. This strain exhibited a considerable ability to improve the efficiency of water use and reduce plant water stress under severe irrigation deficit [50].
The current study documented the supportive role of Paenarthrobacter nitroguajacolicus P1 in the growth improvement of Pistacia vera L. that was associated with the elevated levels of chlorophyll, anthocyanin content, proline accumulation and increased level of phenolic compounds. Paenarthrobacter nitroguajacolicus P1 showed resistance to salt stress, and enhanced sugar content in Pistacia vera L seedlings. It can be suggested that the phosphorus, and zinc solubilization, as well as IAA, HCN, and hydrolytic enzyme production capacities of this strain, might be possible mechanisms to promote the growth of pistachio.
Conclusions
In the current study, multipotent PGPR with the ability to produce IAA and HCN, amylase, lipase and protease as well as solubilize phosphate and zinc were isolated from the Pistacia vera L. rhizosphere. Paenarthrobacter nitroguajacolicus P1 as the most efficient halotolerant plant growth-promoting bacterium showed an improving effect on the growth of Pistacia vera L. seedling in a pot experiment. Therefore, it can be applied as a halotolerant bioinoculant in the cultivation of pistachio in stressed soil. Biofertilizer application is a sustainable and environmentally friendly approach to enhance the productivity and health of pistachio orchards over the time.
References
Jamshidi Goharrizi K, Amirmahani F, Salehi F (2020) Assessment of changes in physiological and biochemical traits in four pistachio rootstocks under drought, salinity and drought+ salinity stresses. Physiol Plant 168(4):973–989
Mozaffari Nejad A (2011) Global pistachio production and marketing challenges in Iran. Int Symp Mycotoxins Nuts Dried Fruits 963:133–141
FAOSTAT F (2020) Food and agriculture organization of the United Nations (FAO). https://www.fao.org/faostat/en/#data/QCL
Bagheri V, Shamshiri M, Shirani H, Roosta H (2011) Effect of mycorrhizal inoculation on ecophysiological responses of pistachio plants grown under different water regimes. Photosynthetica 49(4):531–538
Habibi G, Norouzi F, Hajiboland R (2014) Silicon alleviates salt stress in pistachio plants. Progress Biol Sci 4(2):189–202
Azarmi F, Mozafari V, Abbaszadeh Dahaji P, Hamidpour M (2016) Biochemical, physiological and antioxidant enzymatic activity responses of Pistacia vera L seedlings treated with plant growth promoting rhizobacteria and Zn to salinity stress. Acta Physiol Plant 38(1):1–16
Tavallali V, Rahemi M, Maftoun M, Panahi B, Karimi S, Ramezanian A, Vaezpour M (2009) Zinc influence and salt stress on photosynthesis, water relations, and carbonic anhydrase activity in pistachio. Sci Hortic 123(2):272–279
Fattahi M, Mohammadkhani A, Shiran B, Baninasab B, Ravash R, Gogorcena Y (2021) Beneficial effect of mycorrhiza on nutritional uptake and oxidative balance in pistachio (Pistacia spp) rootstocks submitted to drought and salinity stress. Sci Horticult 281:109937
Salimi F, Hamedi J (2021) Biofertilizers: Microbes for agricultural productivity: soil microbiomes for sustainable agriculture. Springer, pp 407–469
Salimi F, Hamedi J (2021) Biopesticides: microbes for agricultural sustainability: soil microbiomes for sustainable agriculture. Springer, pp 471–501
Khalilpour M, Mozafari V, Abbaszadeh-Dahaji P (2021) Tolerance to salinity and drought stresses in pistachio (Pistacia vera L) seedlings inoculated with indigenous stress-tolerant PGPR isolates. Sci Horticult 289:110440
Moradipour M, Saberi-Riseh R, Mohammadinejad R, Hosseini A (2019) Nano-encapsulation of plant growth-promoting rhizobacteria and their metabolites using alginate-silica nanoparticles and carbon nanotube improves UCB1 pistachio micropropagation. J Microbiol Biotechnol 29:1096–1103
Saberi Riseh R, Fathi F, Moradi M (2019) The effects of biocontrol bacillus and pseudomonas strains on plant growth and biochemical defense echanisms in Pistacia vera L seedlings inoculated with phytophthora drechsleri. Pistachio Health J 1(3):15–26
Eziuzor C, Okpokwasili G (2009) Bioremediation of hydrocarbon contaminated mangrove soil in a bioreactor. Nigerian J Microbiol 23(1):1777–1791
Etminani F, Harighi B (2018) Isolation and identification of endophytic bacteria with plant growth promoting activity and biocontrol potential from wild pistachio trees. Plant Pathol J 34(3):208
Zahra T, Hamedi J, Mahdigholi K (2020) Endophytic actinobacteria of a halophytic desert plant Pteropyrum olivieri: promising growth enhancers of sunflower. 3 Biotech 10(12):1–13
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170(1):265–270
Mehta S, Nautiyal CS (2001) An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol 43(1):51–56
Bhatt K, Maheshwari DK (2019) Decoding multifarious role of cow dung bacteria in mobilization of zinc fractions along with growth promotion of C. annuum L. Sci Rep 9(1):1–10
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26(1):192
Lorck H (1948) Production of hydrocyanic acid by bacteria. Physiol Plant 1(2):142–146
Ramadoss D, Lakkineni VK, Bose P, Ali S, Annapurna K (2013) Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. Springerplus 2(1):1–7
Bradford N (1976) A rapid and sensitive method for the quantitation microgram quantities of a protein isolated from red cell membranes. Anal Biochem 72(248):e254
Miller G (1959) Modified DNS method for reducing sugars. Anal Chem 31(3):426–428
Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1
Zameer M, Zahid H, Tabassum B, Ali Q, Nasir IA, Saleem M, Butt SJ (2016) PGPR potentially improve growth of tomato plants in salt-stressed environment. Turk J Agric—Food Sci Technol 4(6):455–463
Agbor GA, Vinson JA, Donnelly PE (2014) Folin-Ciocalteau reagent for polyphenolic assay. Int J Food Sci Nutr Diet 3(8):147–156
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Moazzam Jazi M, Rajaei S, Seyedi SM (2015) Isolation of high quality RNA from pistachio (Pistacia vera L.) and other woody plants high in secondary metabolites. Physiol Mol Biol Plants 21:597–603. https://doi.org/10.1007/s12298-015-0319-x
CLSI (2017) Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute, Wayne, PA
Vlková E, Rada V, Popelářová P, Trojanová I, Killer J (2006) Antimicrobial susceptibility of bifidobacteria isolated from gastrointestinal tract of calves. Livest Sci 105(1–3):253–259
Saif FAA, Sakr EA (2020) Characterization and bioactivities of exopolysaccharide produced from probiotic Lactobacillus plantarum 47FE and Lactobacillus pentosus 68FE. Bioactive Carbohydrates Diet Fibre 24:100231
Katsu K, Nijo T, Yoshida T, Okano Y, Nishikawa M, Miyazaki A, Maejima K, Namba S, Yamaji Y (2021) Complete genome sequence of pleioblastus mosaic virus, a distinct member of the genus Potyvirus. Arch Virol 166(2):645–649
Zaidi A, Khan MS, Rizvi A, Saif S, Ahmad B, Shahid M (2017) Role of phosphate-solubilizing bacteria in legume improvement. Microbes for legume improvement. Springer, pp 175–197
Saia S, Rappa V, Ruisi P, Abenavoli MR, Sunseri F, Giambalvo D, Frenda AS, Martinelli F (2015) Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00815
Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M (2014) The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 32(2):429–448
Mohite B (2013) Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J Soil Sci Plant Nutr 13(3):638–649
Damodharan K, Palaniyandi SA, Le B, Suh J-W, Yang SH (2018) Streptomyces sp. strain SK68, isolated from peanut rhizosphere, promotes growth and alleviates salt stress in tomato (Solanum lycopersicum cv. Micro-Tom). J Microbiol 56(10):753–759
Acar I, Sarpkaya K. Ak BE (2022) Morphological responses of some Pistacia species to salinity under the effect of Pgpr application. Available at SSRN 4090391
Atajan FA, Mozafari V, Abbaszadeh-Dahaji P, Hamidpour M (2019) Fractionation and speciation of manganese in rhizosphere soils of Pseudomonas sp rhizobacteria inoculated Pistachio (Pistacia vera L.) seedlings under salinity stress. Commun Soil Sci Plant Anal 50(7):894–908
Akhter MS, Noreen S, Mahmood S, Ashraf M, Alsahli AA, Ahmad P (2021) Influence of salinity stress on PSII in barley (Hordeum vulgare L) genotypes, probed by chlorophyll-a fluorescence. J King Saud Univ-Sci 33(1):101239
Azarmi F, Mozafari V, Abbaszadeh Dahaji P, Hamidpour M (2016) Biochemical, physiological and antioxidant enzymatic activity responses of Pistacia vera L seedlings treated with plant growth promoting rhizobacteria and Zn to salinity stress. Acta Physiol Plant 38(1):1–16
del Rosario CL, Chiappero J, Santoro MV, Giordano W, Banchio E (2017) Inducing phenolic production and volatile organic compounds emission by inoculating Mentha piperita with plant growth-promoting rhizobacteria. Sci Hortic 220:193–198
Riachi LG, De Maria CA (2015) Peppermint antioxidants revisited. Food Chem 176:72–81
Remonsellez F, Castro-Severyn J, Pardo-Esté C, Aguilar P, Fortt J, Salinas C, Barahona S, León J, Fuentes B, Areche C (2018) Characterization and salt response in recurrent halotolerant Exiguobacterium sp SH31 isolated from sediments of Salar de Huasco. Chilean Altiplano. Front Microbiol 9:2228
Stringlis IA, Proietti S, Hickman R, Van Verk MC, Zamioudis C, Pieterse CM (2018) Root transcriptional dynamics induced by beneficial rhizobacteria and microbial immune elicitors reveal signatures of adaptation to mutualists. Plant J 93(1):166–180
Wang Q, Bai L, Luo S, Wang T, Yang F, Xia J, Wang H, Ma K, Liu M, Wu S (2020) TMEM16A Ca2+-activated Cl− channel inhibition ameliorates acute pancreatitis via the IP3R/Ca2+/NFκB/IL-6 signaling pathway. J Adv Res 23:25–35
Kumar A, Kumar A, Devi S, Patil S, Payal C, Negi S (2012) Isolation, screening and characterization of bacteria from Rhizospheric soils for different plant growth promotion (PGP) activities: an in vitro study. Recent Res Sci Technol 4(1):1–5
Vergani L, Mapelli F, Marasco R, Crotti E, Fusi M, Di Guardo A, Armiraglio S, Daffonchio D, Borin S (2017) Bacteria associated to plants naturally selected in a historical PCB polluted soil show potential to sustain natural attenuation. Front Microbiol 8:1385
Riva V, Mapelli F, Dragonetti G, Elfahl M, Vergani L, Crepaldi P, La Maddalena N, Borin S (2021) Bacterial inoculants mitigating water scarcity in tomato: the importance of long-term in vivo experiments. Front Microbiol 12:1328
Acknowledgements
The authors of the study are thankful to Damghan University for the financial support of the current research.
Funding
No funding was received for the current study.
Author information
Authors and Affiliations
Contributions
All authors have contributed substantially to the manuscript and approved the final submission. FS performed supervision, validation, writing—review & editing. MKh, FA, AA did the investigation and methodology.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salimi, F., Khorshidi, M., Amirahmadi, F. et al. Effectiveness of Phosphate and Zinc Solubilizing Paenarthrobacter nitroguajacolicus P1 as Halotolerant Rhizobacterium with Growth-Promoting Activity on Pistacia vera L. Curr Microbiol 80, 336 (2023). https://doi.org/10.1007/s00284-023-03448-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03448-0