Abstract
In this study, Pseudomonas species were isolated from the rhizospheres of two plant hosts: rice (Oryza sativa cultivar Pathum Thani 1) and maize (Zea mays cultivar DK888). The genotypic diversity of isolates was determined on basis of amplified rDNA restriction analysis (ARDRA). This analysis showed that both plant varieties selected for two distinct populations of Pseudomonas. The actual biocontrol and plant promotion abilities of these strains was confirmed by bioassays on fungal (Verticillum sp., Rhizoctonia solani and Fusarium sp.) and bacterial (Ralstonia solanacearum and Bacillus subtilis) plant pathogens, as well as indole-3-acetic acid (IAA) production and carbon source utilization. There was a significant difference between isolates from rice and maize rhizosphere in terms of biological control against R. solanacearum and B. subtilis. Interestingly, none of the pseudomonads isolated from maize rhizosphere showed antagonistic activity against R. solanacearum. This study indicated that the percentage of pseudomonad isolates obtained from rice rhizosphere which showed the ability to produce fluorescent pigments was almost threefold higher than pseudomonad isolates obtained from maize rhizosphere. Furthermore, the biocontrol assay results indicated that pseudomonad isolated from rice showed a higher ability to control bacterial and fungal root pathogens than pseudomonad isolates obtained from maize. This work clearly identified a number of isolates with potential for use as plant growth-promoting and biocontrol agents on rice and maize.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modern agriculture is heavily dependent on the application of chemical inputs, including fertilizers and pesticides. Because of concerns regarding both human health and environmental protection, viable alternatives to these chemicals are being sought (Morrissey et al. 2004; Franks et al. 2006). It has been long recognized that many naturally occurring rhizospheric bacteria and fungi are antagonistic towards crop pathogens and, as a result, may offer a viable substitute for the use of these chemicals.
Of particular interest are the soil-borne pseudomonads, which can be utilized in low-input sustainable agriculture applications such as biocontrol. Numerous Pseudomonas strains display plant growth-promoting activities and have been investigated due to their widespread distribution in soil, ability to colonize the rhizospheres of host plants, and ability to produce a range of compounds antagonistic to a number of serious plant pathogens (Rodriguez and Pfender 1997; Ross et al. 2000). The study of genotypic and phenotypic diversity of Pseudomonas spp. and their plant growth-promoting potential is important not only for understanding their ecological role in the rhizosphere and the interaction with plants, but also for any biotechnological application (Berg et al. 2002).
Plant roots influence soil-borne microbial communities via several mechanisms, including excretion of specific organic compounds, competition for nutrients, and providing a solid surface for attachment. It has been previously been demonstrated that an effective biological control strain isolated from one region may not perform as effectively in other soils or plants (Kiely et al. 2006). For this reason it has become important to screen different environments to understand the role that the rhizosphere plays in selection for bacterial populations.
The purpose of this study was to investigate bacterial populations of the wetland soil of rice (Oryza sativa L.) and desiccated soil of maize (Zea mays L.) on the abundance and diversity of plant growth-promoting Pseudomonas spp. Pseudomonas isolates were selected from both plant host and profiled using amplified 16S rDNA restriction analysis (ARDRA). These isolates were further screened for phenotypes associated with plant growth promotion, such as, indole-3-acetic acid (IAA) production and the antagonistic activity of soil-borne disease bacteria and fungi.
Materials and methods
Soil sample collection and isolation of bacterial strains
Rhizosphere samples were collected from two different crops, rice (Oryza sativa cultivar Pathum Thani 1) and maize (Zea mays cultivar DK888) in Nakhon Ratchasima province, Thailand. The intact root systems were collected. Loosely adhering soils were shaken and detached from the roots and discarded. These root portions with just a layer of closely adhering rhizosphere soil was then transferred to 10 ml sterilized water and vigorously shaken for 10–15 min. The suspensions from all samples were serially diluted up to 10−6 with three replications for each sample. From 10−1 to 10−6 dilutions, 100 μl of each dilution was spread on Pseudomonas isolation agar and Pseudomonas selective medium based on King’s medium B (KMB) (McSpadden Gardener et al. 2001). Three replicate plates were maintained for each dilution. All Pseudomonas strains isolated in this study were then conserved at −80°C after an addition of glycerol to a final concentration of 40% (v/v). All strains were checked for fluorescent pigment under u.v. light (Sharifi-Tehrani et al. 1998) and routinely maintained on KMB agar.
Total genomic DNA isolation
Pseudomonas isolates were grown in a nutrient broth at 28°C overnight. Bacterial cells were harvested by centrifugation at 5,000×g for 5 min and washed twice in 500 μl of TEN buffer (50 mM Tris, 20 mM disodium EDTA, and 50 mM NaCl, pH 8.0). Cell lysates were prepared by mixing the cell pellet with 200 μl of 20% (w/v) sucrose in TEN buffer to this 20 μl of 2 mg/ml of lysozyme and 20 μl of 10 mg/ml of RNase was added. Cell mixtures were incubated at 37°C for 60 min. Then 75 μl of 5 M NaCl and 100 μl of 10% SDS were added before gentle mixing. The solution was purified twice by using phenol:chloroform:isoamyl-alcohol (25:24:1, by volume). The upper phase was collected and precipitated by using isopropanol and 3 M sodium acetate. The DNA pellet was resuspended in sterilized deionized-water and total genomic DNA was kept at -20°C before use (Sambrook and Russell 2001).
Identification of the genus Pseudomonas
16S rDNA region was amplified using the Pseudomonas genus-specific 16S rRNA gene PCR primers. The forward primer Ps-for (20-mer [5′-GGTCTGAGAGGATGATCAGT-3′]) and reverse primer Ps-rev (18-mer [5′-TTAGCTCCACCTCGCGGC-3′]) (Cirvilleri et al. 2005; Rangarajan et al. 2002) were used to amplify the total genomic DNA. The total volume of the reaction mixture was 25 μl containing 10–50 ng of template DNA, 10× reaction buffer, 2.5 mM dNTPs, 20 pmol of each primer, and 1 U taq DNA polymerase (invitrogen). PCR amplification was performed as detailed in previously study (Rangarajan et al. 2002). To estimate the product size, the products were run on 1% agarose gels along with 1 kb ladder as marker and stained with ethidium bromide. The Pseudomonas type strain was P. fluorescens 96.578 obtained from soil microbiology research group, Department of Agriculture, Thailand (DOA).
Phenotypic characterization and carbon source utilization
Carbon sources solution were filter sterilized and added at 0.1% (w/v) final concentration to LG medium (Lipman 1904) plus 1 ml of 1 M KNO3 pH 7.2. Pseudomonas strains were streaked onto the medium and incubated at 28°C for 3 days. Growth was compared to the carbon source-free medium. The carbon substrates tested included myo-inositol, arabinose, glucose, sorbitol, mannitol, sucrose, lactose, xylose, glycerol, fructose, and the oxidase test, and the ability to grow in nitrogen-free medium (Desnoues et al. 2003).
Indole-3-acetic acid (IAA) production
Indole-3-acetic acid production was determined by adding 2 ml of 0.01 M FeCl3 in 35% HClO4 into 1 ml of Tris-TMRT (d-mannitol 10 g, yeast extract 0.2 g, CaCl2 × 2H2O 0.2 g, MgSO4 × 7H2O 0.25 g, Tris-base 1.21 g, l-tryptophan 0.061 g/l, pH 6.8) culture broth after being incubated at 28°C for 10 days. The mixture was incubated in the dark at 30°C for 30 min. Results were compared with positive control of 1 g of IAA in distilled water and ethanol (1:1, by volume for 1.0 ml) (Nuntagij et al. 1997).
Biological control assay
An inhibition of phytopathogens by the Pseudomonas strains on Potato dextrose agar (PDA) plates was performed as detailed in previously study (Keel et al. 1996). Briefly fungi and bacteria were grown overnight in LG broth, and 10 ml of each culture was spotted 2 cm from the edge of the plate (four spots per plate) and 0.1–0.3-cm square plug from a culture of Verticillum sp., Rhizoctonia solani, Fusarium sp. and Ralstonia solanacearum were placed at the center of the plate. The results were assessed after 3 days by measuring the distance between the edges of the bacterial colony and the fungal mycelium. An inhibition of Bacillus subtilis by the Pseudomonas strains on SA (Fenton et al. 1992) plates containing 100 μM FeCl3 was performed. Bacillus subtilis was grown overnight in LB at 37°C by shaking and was sprayed onto the plates contain test strains. Results were assessed after overnight incubation. A zone of inhibition of Bacillus around the test strain is indicative of a positive result.
Amplified rDNA restriction analysis (ARDRA)
The 16S rDNA universal primers fD1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and rD1 (5′-AAGGAGGTGATCCAGCC-3′) (Weisburg et al. 1991) were used to amplify a 1.5-kb internal region of the 16S rRNA gene. This primer pair was capable of amplifying nearly full-length 16S ribosomal DNA from a wide variety of bacterial taxa. Amplification was performed as previously described (Picard et al. 2004). Restriction analysis was performed with 5 μl of amplified product and 10 μl of restriction buffer containing 2 U of either the restriction enzymes AluI, HinfI, MspI or RsaI. After a 3 h digestion at the appropriate temperature, the enzyme was inactivated by heating the preparations at 70°C for 15 min. For each isolate, PCR amplification and restriction analysis were performed at least three times. Calculation of the pair-wise coefficients of similarity was based on the presence or absence of bands. A cluster analysis with the UPGMA algorithm was performed with the NTSYS-pc numerical taxonomy and multivariate analysis system (Raaijmakers and Weller 2001).
Results
Isolation and preliminary investigation of the genus Pseudomonas
The number of cultivable bacterial isolates were obtained by growth on Pseudomonas isolation agar from rhizosphere samples was significantly different between rice and maize rhizospheres. Specifically, 103 out of 138 total bacterial isolates were obtained from rice rhizosphere and 35 isolates were obtained from maize rhizosphere. Amplification of a 16S rRNA gene approximately 950 bp–1 kb, from all isolates confirmed that each isolate was closely related to pseudomonads. Isolates were designated with a strain number and an alphabetical prefix denoting the site from which it was obtained (R for rice and M for maize) (Fig. 1).
Cluster dendogram, based on ARDRA analysis with four restriction enzymes (AluI, HinfI, MspI, RsaI) and phenotypic characteristics of Pseudomonas isolates that were isolated from maize and rice rhizosphere. Key: +, positive result (Production of IAA, oxidase test positive, fluorescence detected, grow in N-free medium, grow on a variety of carbon sources, zone of inhibition detected) −, negative result (No production of IAA, oxidase test negative, no fluorescence detected, not growing on a variety of carbon sources, no zone of inhibition detected). Data includes a control typed strain: P. fluorescens 96.578
Genotypic characterization
The cluster dendogram of ARDRA analysis of pseudomonad isolates obtained from rice and maize rhizospheres is illustrated in Fig. 1. One hundred and thirty eight isolates selected at random but representing each field site were analysed by ARDRA. Digestion of amplified 16S rDNA with four restriction enzymes revealed four main clusters of ARDRA dendogram after hierarchical cluster analysis by Nei’s genetic similarity statistic. The rhizospheres of rice and maize clearly selected from different soil bacterial ribotypes. ARDRA group 1 contained isolates found in maize rhizosphere except one isolate, R24, was found in rice rhizosphere.
Phenotypic characterization of isolate for plant protection and biocontrol traits
The pseudomonad isolates were further analysed for phenotypic traits associated with biocontrol and plant promotion. Firstly, their ability to produce indole-3-acetic acid (IAA) in presence of l-tryptophan as precursor was tested. One hundred and two isolates showed the ability to produce IAA, 17.14% of isolates obtained from maize rhizosphere and 93.2% of isolates obtained from rice rhizosphere. The pseudomonad isolates from rice rhizosphere, R4, R8, R31 and R35, were the most productive of IAA while 36 isolates produced no IAA. Interestingly, all pseudomonad isolates in cluster 1 did not show the ability to produce IAA except strain M22 (Fig. 1). The percentage of pseudomonad isolates obtained from rice rhizosphere which showed the ability to produce IAA were almost fourfold higher than pseudomonad isolates obtained from maize rhizosphere which showed the ability to produce IAA (Fig. 2).
Sixty-four isolates showed the ability to produced fluorescence under u.v. light, 17.14% of isolates obtained from maize rhizosphere and 56.31% of isolates were obtained from rice rhizosphere. Most isolates in cluster 1 had no ability in fluorescence production, but most of isolates in cluster 2, 3 and 4 were capable of fluorescing. The percentage of pseudomonad isolates obtained from rice rhizosphere which showed the ability to produce fluorescence were almost threefold higher than pseudomonad isolates with the same property obtained from maize rhizosphere (Fig. 2).
Eighty-nine isolates showed the ability to grow in nitrogen-free medium, 14.3% of isolates obtained from maize rhizosphere and 81.6% of the isolates obtained from rice rhizosphere. Most isolates in cluster 1 could not grow in nitrogen-free medium, but most isolates in cluster 2, 3 and 4 could do so. Pseudomonad isolates obtained from rice rhizosphere showed higher ability to grow in nitrogen-free medium than pseudomonad isolates obtained from maize rhizosphere (Fig. 2).
One hundred and eight isolates gave a positive oxidase test, 77.14% of isolates obtained from maize rhizosphere and 52.43% of isolates obtained from rice rhizosphere. Most isolates in cluster 1 showed a positive oxidase test, but most isolates in cluster 2, 3 and 4 were oxidase negative (Figs 1, 2).
Differences in utilization of carbon sources between pseudomonad isolates obtained from maize and rice rhizosphere are summarized in Figs. 1 and 2. All pseudomonad isolates in ARDRA group 4 showed ability to grow on all ten sources of substrate (Fig. 1). However, not all of the isolates used the same set of substrates. Ten isolates, M1, M3, M4, M17, M35, R24, R55, R56, R65, and R84, grew significantly on fewer substrates than the other isolates. Pseudomonad isolates obtained from maize rhizosphere showed lower reduced ability to utilize a variety carbon sources than pseudomonad isolates obtained from rice rhizosphere, except one that is glucose (Fig. 2).
Plant protection assays
In this study Verticillum sp., R. solani, Fusarium sp., R. solanacearum and B. subtilis were used for biocontrol assay because they are considered to be widely distributed and destructive plant pathogens in agriculture. Verticillum sp. and R. solanacearum cause wilt of a wide range of broad hosts (NSW Department of Environment and Climate Change 2007). Rhizoctonia solani and Fusarium sp. cause sheath blight diseases (Inagaki 1998) and sheath rot disease in rice (Abbas et al. 1998). Bacillus subtilis cause seed rot-seedling blight disease in maize (Shurtleff et al. 1993).
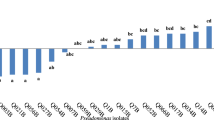
In vitro antagonism experiments with Verticillum sp., R. solani, Fusarium sp., R. solanacearum and B. subtilis revealed that 53.6% of the 138 isolates tested detectable antifungal activity, 31.4% of isolates obtained from maize rhizosphere and 61.2% of isolates obtained from rice rhizosphere. Seventy-four isolates had the ability to control plant pathogens and 64 isolates gave no control of plant pathogen. Four isolates from rice rhizosphere in ARDRA group 3 and 4, R2, R36, R85, and R102, were the most antagonistic against all pathogens, while the other isolates displayed various degrees of antagonistic activity. Figure 3(a) shows that there was a significant difference between isolates from rice and maize rhizosphere in terms of biological control against R. solanacearum and B. subtilis. Interestingly, none of the pseudomonads isolated from maize rhizosphere showed antagonistic activity against R. solanacearum and 50% of antagonist isolates against B. subtilis were found in ARDRA group 3 (Figs. 1, 3).
(a) Percentage of antagonistic activity at each sampling site which was obtained by dividing the number of isolates from each site represented in a particular group by the total number of isolates tested from that site. Key: a, There was significantly difference between isolates from rice rhizosphere and maize rhizosphere (P < 0.05, T-test). (b) Antagonistic activity of Rhizoctonia solani by pseudomonad isolates R2 which was carried out on 9 cm diameter agar plate. (c) Antagonistic activity of Fusarium sp. by pseudomonad isolates M22 and R2 which was carried out on 9 cm diameter agar plate
Discussion
Pseudomonas spp. are common soil bacteria easily cultured from most agricultural soils and rhizospheres (Morrissey et al. 2004). They have been studied intensively because of their ability to promote plant growth, either by directly stimulating the plant or by suppressing root pathogens. Moënne-Loccoz et al. (2001) studied the culturable fluorescent pseudomonad community associated with the roots of field-grown sugarbeet seedlings using ARDRA, which suggested that the root-associated Pseudomonas community was flexible to the change that may be caused by adding a similar inoculant. Bakker et al. (2002) further confirmed this using ARDRA by showing effects of biocontrol agents on the entire bacterial community in the rhizosphere of wheat.
To date studies in biological control have focused mainly on fluorescent pseudomonads, producing fluorescent pigments and siderophores like pyoverdin (Haas and Défago 2005), because of their metabolic versatility, their excellent root colonization ability and antimicrobial metabolites (O’Sullivan and O’Gara 1992). The results using ARDRA in this study indicated that the rhizospheres of rice and maize clearly select for different soil bacterial ribotypes (Fig. 1).
This study indicated that the percentage of pseudomonad isolates obtained from rice rhizosphere which showed the ability to produce fluorescent pigments was almost threefold higher than pseudomonad isolates obtained from maize rhizosphere (Fig. 2). Furthermore, the biocontrol assay results indicated that pseudomonads isolated from rice showed a higher ability to control bacterial and fungal root pathogens than pseudomonad isolates obtained from maize (Fig. 3). Compared to all fluorescent pseudomonad isolates obtained from rice and maize rhizosphere which had the ability to control phytopathogens, the fluorescent pseudomonad isolates obtained from rice rhizosphere showed antagonistic activity of 91.7, 85.7, 100, 100 and 77.8% against Verticillum sp., R. solani, Fusarium sp., R. solanacearum and B. subtilis, respectively. However the fluorescent pseudomonad isolates obtained from maize rhizosphere showed a much lower antagonistic activity against the pathogens with 16.7, 16.7, 0, 0 and 33.3% against Verticillum sp., R. solani, Fusarium sp., R. solanacearum and B. subtilis, respectively. It is of some note that this result seen from maize isolates is in conflict with data already published (Nielsen et al. 1998), as fluorescent Pseudomonads have correlated with the level of pathogen antagonistic activity seen, but in our study with the low number of isolates taken direct correlation is difficult. In this present work, we observe in isolates from cluster 3, a correlation between the members of this cluster and antagonistic activity against particularly R. solanacearum and B. subtilis (Fig. 1).
Previously, Berg et al. (2002) explored the effect of different plant species on the abundance and diversity of bacteria antagonistic to plant pathogens, isolated originating from the rhizospheres of three host plants of Verticillium dahliae-strawberry, potato, and oilseed rape and from soil were analysed for their antagonistic properties. Plants can have strong effects on soil microbial communities viewed from the functional perspective. The proportion of isolates with antagonistic activities was the highest for the strawberry rhizosphere (9.5%), followed by oilseed rape (6.3%), potato (3.7%), and bulk soil (3.3%). Hence, plants affect their associated communities also in a functional way.
This study established the presence of individual populations of antagonistic Pseudomonas strains and IAA productive strains in maize and rice rhizosphere environment and is an important step towards fully understanding the functional roles of the organisms in these natural environments, in different plant host and soil environments. We conclude that selection of P. fluorescens strains showing multi-antagonism and secondary metabolite production, as represented by isolate R2 and other isolates belonging to cluster 3, is promising for future applications of these bacteria in biocontrol. However, it is becoming important to map these pseudomonad populations in order to select “consortia” of bacteria for biological control for each plant species.
References
Abbas HK, Cartwright RD, Shier WT, Abouzied MM, Bird CB, Rice LG, Ross PF, Sciumbato GL, Meredith FI (1998) Natural occurrence of fumonisins in rice with Fusarium sheath rot disease. Plant Dis 82:22–25
Bakker PA, Glandorf DC, Viebahn M, Ouwens TW, Smit E, Leeflang P, Wernars K, Thomashow LS, Thomas-Oates JE, van Loon LC (2002) Effects of Pseudomonas putida modified to produce phenazine-1-carboxylic acid and 2,4-diacetylphloroglucinol on the microflora of field grown wheat. Antonie van Leeuwenhoek 81:617–624
Berg G, Roskot N, Steidle A, Eberl L, Zock A, Smalla K (2002) Plant-dependent genotypic and phenotypic diversity of antagonistic rhizobacteria isolated from different Verticillium host plants. Appl Environ Microbiol 68:3328–3338
Cirvilleri G, Spina S, Scuderi G, Gentile A, Catara A (2005) Characterization of antagonistic root-associated fluorescent pseudomonads of transgenic and non-transgenic citrange troyer plants. J Plant Pathol 87:179–186
Desnoues N, Lin M, Guo X, Ma L, Carreño-Lopez R, Elmerich C (2003) Nitrogen fixation genetics and regulation in a Pseudomonas stutzeri strain associated with rice. Microbiology 149:2251–2262
Fenton AM, Stephens PM, Crowley J, O’Callaghan M, O’Gara F (1992) Exploitation of gene(s) involved in 2,4-diacetylphloroglucinol biosynthesis to confer a new biocontrol capability to a Pseudomonas strain. Appl Environ Microbiol 58:3873–3878
Franks A, Ryan RP, Abbas A, Mark GL, O’Gara F (2006) Molecular tools for studying plant growth-promoting rhizobacteria (PGPR). In Molecular techniques for soil and rhizosphere microorganisms. CABI Publishing, Wallingford, Oxfordshire, UK
Haas D, Défago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319
Inagaki K (1998) Dispersal of rice sheath blight fungus, Rhizoctonia solani AG-1(IA), and subsequent disease development in paddy fields, from survey of vegetative compatibility groups. Mycoscience 39:391–397
Keel C, Weller DM, Natsch A, Défago G, Cook RJ, Thomashow LS (1996) Conservation of the 2,4-diacetylphloroglucinol biosynthesis locus among fluorescent Pseudomonas strains from diverse geographic locations. Appl Environ Microbiol 62:552–563
Kiely DP, Haynes JM, Higgins CH, Franks A, Mark GL, Morrissey JP, O’Gara F (2006) Exploiting new systems-based strategies to elucidate plant-bacterial interactions in the rhizosphere. Microb Ecol 51:257–266
Lipman JG (1904) Soil bacteriological studies. Further contributions to the physiology and morphology of the members of the Azotobacter group. Report of the New Jersey State Agricultural Experiment Station 25:237–289
McSpadden Gardener BB, Mavrodi DV, Thomashow LS, Weller DM (2001) A rapid polymerase chain reaction-based assay characterizing rhizosphere populations of 2,4-diacetylphloroglucinol-producing bacteria. Phytopathology 91:44–54
Moënne-Loccoz Y, Tichy H-V, O’Donnell A, Simon R, O’Gara F (2001) Impact of 2,4-diacetylphloroglucinol producing biocontrol strain Pseudomonas fluorescens F113 on intraspecific diversity of resident culturable fluorescent pseudomonads associated with the roots of field-grown sugar beets seedlings. Appl Environ Microbiol 67:3418–3425
Morrissey JP, Dow JM, Mark GL, O’Gara F (2004) Are microbes at the root of a solution to world food production? EMBO Rep 5:922–926
Nielsen MN, Sorensen J, Fels J, Pedersen HC (1998) Secondary metabolite- and endochitinase-dependent antagonism toward plant-pathogenic microfungi of Pseudomonas fluorescens isolates from sugar beet rhizosphere. Appl Environ Microbiol 64:3563–3569
NSW Department of Environment and Climate Change, Sydney Australia (2007) Botanic gardens trust. http://www.rbgsyd.nsw.gov.au/. Cited 28 May 2007
Nuntagij A, Abe M, Uchimi T, Seki Y, Boonkerd N, Higashi S (1997) Characterization of bradyrhizobium strains isolated from soybean cultivation in Thailand. J Gen Appl Microbiol 43:183–187
O’Sullivan DJ, O’Gara F (1992) Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol Rev 56:662–676
Picard C, Frascaroli E, Bosco M (2004) Frequency and biodiversity of 2,4 diacetylphloroglucinol-producing rhizobacteria are differentially affected by the genotype of two maize inbred lines and their hybrid. FEMS Microbiol Ecol 49:207–215
Raaijmakers JM, Weller DM (2001) Exploiting genotypic diversity of 2,4-diacetylphloroglucinol-producing Pseudomonas spp.: characterization of superior root-colonizing P. fluorescens strain Q8r1-96. Appl Environ Microbiol 67:2545–2554
Rangarajan S, Saleena LM, Nair S (2002) Diversity of Pseudomonas spp. isolated from rice rhizosphere populations grown along a salinity gradient. Microb Ecol 43:280–289
Rodriguez F, Pfender WF (1997) Antibiosis and antagonism of Sclerotinia homoeocarpa and Drechslera poae by Pseudomonas fluorescens PF-5 in vitro and in planta. Phytopathology 87:614–621
Ross IL, Alami Y, Harvey PR, Achouak W, Ryder MH (2000) Genetic diversity and biological control activity of novel species of closely related pseudomonads isolated from wheat field soils in south Australia. Appl Environ Microbiol 66:1609–1616
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, New York
Sharifi-Tehrani A, Zala M, Natsch A, Moënne-Loccoz Y, Défago G (1998) Biocontrol of soil-borne fungal plant diseases by 2,4-diacetylphloroglucinol-producing fluorescent pseudomonads with different restriction profiles of amplified 16S rDNA. Eur J Plant Pathol 104:631–643
Shurtleff MC, Edwards DI, Noel GR, Pederson WL, White DG (1993) Diseases of corn or maize (Zea mays L.). In: Common names of plant diseases. The American Phytopathological Society. Available via DIALOG. http://www.apsnet.org/online/common/names/corn.asp. Cited 27 May 2007
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Acknowledgements
This work was supported by a grant from the Royal Golden Jubilee Ph.D. program (Thailand Research Fund) and Suranaree University of Technology. F.O. was supported in part by grants awarded by Science Foundation Ireland (SFI 02/IN.1/B1261 and 04/BR/B0597 to F.O.) and the Health Research Board (RP/2004/145 and RP/2006/271 to F.O.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lawongsa, P., Boonkerd, N., Wongkaew, S. et al. Molecular and phenotypic characterization of potential plant growth-promoting Pseudomonas from rice and maize rhizospheres. World J Microbiol Biotechnol 24, 1877–1884 (2008). https://doi.org/10.1007/s11274-008-9685-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9685-7