Abstract
A total of 41 strains of lactic acid bacteria (LAB) isolated from durum wheat sourdoughs used to produce Cornetto di Matera bread, were identified by SDS-PAGE of whole cell proteins (WCP) and screened for acid production ability, antimicrobial activity and exopolysaccharide (EPS) production. The isolates were identified as Lactobacillus plantarum (49%), Leuconostoc mesenteroides (17%), Lactobacillus curvatus (15%), Lactobacillus paraplantarum (12%), Weissella cibaria (5%) and Lactobacillus pentosus (2%). Several strains of Lb. plantarum and Leuc. mesenteroides showed a high acid production ability. The antagonistic activity was tested using an agar-spot deferred antagonism assay against a set of five indicators. The species had different profiles of inhibition. Lb. plantarum had the largest spectrum of inhibition, while no isolates of W. cibaria and Leuc. mesenteroides showed antimicrobial activity. No strains had antimicrobial activity against Bacillus cereus. The inhibitory activity of five strains was confirmed to be sensitive to proteolytic enzymes and thus potentially due to bacteriocin production. All Leuc. mesenteroides and W. cibaria strains produced EPS from sucrose. Some Lb. plantarum and Lb. paraplantarum strains produced EPS from different sugars in solid media. EPS production in liquid media was different within the species, with the highest production in liquid media containing glucose and maltose. A defined strain starter culture (W. cibaria DBPZ1006, Lb. plantarum DBPZ1015 and S. cerevisiae MTG10) was selected on the basis of technological properties and tested in model sourdough fermentations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sourdough is an important cereal fermentation, involved in bread production, that greatly contributes to the flavor and functional properties of the final product. It is a mixture of wheat or rye flour and water, fermented by an association of lactic acid bacteria and yeasts, whose composition depends on the technology applied for its production (Vogel et al. 1999). Differences in the type of flour, ingredients and technology influence the microbial composition of sourdough and the characteristics of baked goods (Corsetti et al. 2001).
Lactic acid bacteria (LAB) play a major role in sourdough fermentations (De Vuyst and Vancanneyt 2007). Facultative heterofermentative LAB are important for the production of sourdough bread with a good grain and porous crumb and contribute to the sensory quality, while heterofermentative LAB, with their metabolic products, influence the flavor and promote the leavening (Arendt et al. 2007).
In Italy more than 200 types of traditional breads have been classified by the Istituto Nazionale di Sociologia Rurale (INSOR 2000). Sourdoughs are used in more than 30% of bakery products, some of which originate from a very old tradition and differ in the type of flour, ingredients, type of sourdough technology and shelf-life (De Vuyst and Neysens 2005).
Cornetto is a traditional bread produced in small bakeries in Matera (Basilicata, Southern Italy), that recently has obtained the Protected Geographical Indication (PGI) from the European Community (decree 9/6/2007, Gazzetta Ufficiale dell’Unione Europea n. 239, 11/10/2004). According to the standard of identity for PGI, the bread is to be manufactured by using durum wheat flours, NaCl (2.5–3% w/w), water and sourdough as leavening agent. Sourdoughs used for its production can be classified as type Ib (De Vuyst and Neysens 2005): the fermentation is performed at room temperature (20–30°C) with a final pH about 4.0 and is characterized by repeated daily refreshments to keep the mixed culture in an active state.
The objective of this study was the identification and the technological characterization of lactic acid bacteria isolated from sourdoughs used for the production of Cornetto, in order to select strains with interesting technological properties that are relevant for their rational exploitation as starters in bread production.
Materials and methods
Strains and culture conditions
Forty-one strains of lactic acid bacteria (LAB), isolated from sourdoughs used for the production of Cornetto di Matera and identified by phenotypic tests (Ricciardi et al. 2002), were used in this study.
The strains were routinely propagated by overnight cultivation in MRS broth (Oxoid, Basingstoke, Hampshire,UK) at 30°C.
Lactobacillus paracasei DSM4905, Lb. plantarum DSM20174, Micrococcus flavus DSM1790 (obtained from Deutsche Sammlüng von Mikroorganismen und Zellkülturen, Braunschweig, Germany), Listeria innocua BL86/26 (obtained from the culture collection of the Moorepark Food Research Centre, Fermoy, Co. Cork IRL) and Bacillus cereus ATCC9139 (obtained from American Type Culture Collection, commercialized by LGC Promochem s.r.l., Italy) were used as indicators for the deferred antagonism assay (see below). Lb. paracasei DSM4905 and Lb. plantarum DSM20174 were routinely cultivated in MRS broth at 30°C for 16 h, while M. flavus DSM1790, L. innocua BL86/26 and B. cereus ATCC9139 were routinely cultivated in Tryptone Soya Broth (Oxoid), with 0.6% (w/v) Yeast extract (Oxoid) (TSBYE) at 30°C for 16 h.
All strains were maintained as freeze-dried stocks in 11% (w/v) reconstituted skim milk containing ascorbic acid (0.1% w/v) in the culture collection of Dipartimento di Biologia, Difesa e Biotecnologie Agro-forestali, Università degli Studi della Basilicata, Potenza, Italy.
Identification by SDS-PAGE of whole cell proteins
LAB strains were identified by SDS-PAGE of whole cell proteins as described in Ricciardi et al. (2005). Isolates and reference strains were cultivated in modified APT broth. Cells were harvested by centrifugation (12,000×g, 5 min), washed twice in 50 mmol/l Tris/HCl, pH 8.0, and resuspended in 150 μl of the same buffer containing 2 mg of egg white lysozime/ml (Sigma-Aldrich, Milan, Italy). Glass beads (0.15–0.212 mm diameter, Sigma) were added, followed by vortexing at high speed for 3 min. Cells suspensions were incubated at 37°C for 1 h and mixed by three cycles of sonication (10 min for each treatment at power 10%; Sonorex Super 10P, Bandelin, Berlin). After incubation, glass beads and cells debris were removed by centrifugation (12,000×g, 5 min) and the supernatants were used for the SDS-PAGE analysis. Protein concentration in the cell lysates was determined by using the Bradford Reagent for Protein Determination (Sigma), as described by the manufacture.
Electrophoretic runs were carried out in a MiniProtean III apparatus (Bio-Rad Laboratories) using the running gel (10% w/v total monomer concentration, T, 2.67% w/v crosslinking monomer concentration, C) and the stacking gel (4% w/v T, 2.67% w/v C) system described in Piraino et al. (2002). Eleven samples (standardized to load 5–10 μg proteins) and two molecular weight standards (Sigma Marker Wide range, Sigma) were applied to each gel. Gels were run at a constant intensity of 20 mA for 3 h using a Power Pack 3,000 unit (Bio-Rad). Gel images were digitized in Diversity Database™ software (Bio-Rad Laboratories Ltd., Watford, Herts, UK) and processed for detection of the protein bands. Gel reports containing sample name, molecular mass and band intensities in lane were exported from Diversity Database™ software and imported in Microsoft Excel™ software (Microsoft Corporation, 2003) for data processing.
Characterization of the isolates
Acid production ability
A sub-culture in Sour Dough Bacteria (SDB) broth (Kline and Sugihara 1971) was obtained from the active stock culture by 1% (v/v) inoculum and incubation overnight at 30°C. The sub-culture was standardized to a final OD650 = 1 and used to inoculate (5% v/v) sterile SDB broth. The pH was measured on aliquots of the modified SDB broth after 6 and 24 h. The decrease in pH (ΔpH) was calculated for each incubation time as the difference between the values immediately after inoculation (t = 0 h) and the values at the successive time points.
Antimicrobial activity
Inhibitory activity against Lb. paracasei DSM4905, Lb. plantarum DSM20174, M. flavus DSM1790, L. innocua BL86/26 and B. cereus ATCC9139 was tested using a deferred antagonism assay as described in Parente et al. (2001).
The overlay medium was modified MRS agar (MRS broth buffered at pH 6.5 containing 0.1 mol/l morpholin propanesulphonic acid, Sigma, and 0.6% w/v Bacteriological Agar, Oxoid) for Lb. paracasei DSM4905 and Lb. plantarum DSM20174, and TSBYE with 0.6% w/v agar for L. innocua BL86/26, M. flavus DSM1790 and B. cereus ATCC9139.
The diameter of the inhibition zones was measured using a calliper. Strains were considered positive when showing an inhibition zone >10 mm diameter.
To evaluate if the inhibition was due to bacteriocin-like inhibitory substances (BLIS), the assay was repeated by spotting 5 μl of trypsin (Sigma), chymotrypsin (Sigma) or pronase (Boehringer) solutions close to the bacterial spots before the overlay. Enzyme solutions was prepared by dissolving 0.2% (w/v) of each enzyme in a 50 mmol/l Tris water solution buffer (pH 7.5) containing 5 mmol/l CaCl2, filtered through a Millex-GV filter (Millipore SpA, Milan, Italy), and spotted next to the inoculum spot. A negative control (sterile buffer without enzymes) was included and negation of the inhibition zone near the enzyme spot was taken as evidence of the presence of BLIS.
EPS production
LAB strains were screened for exopolysaccharides (EPS) production by using a pick test on MRS agar plates containing 2% (w/v) of glucose (MRS) or maltose (mMRS) or 5% (w/v) of sucrose (sMRS) as carbon sources. The plates were incubated at 30°C for 48 h and the strains which produced slimy colonies were recorded as capable of producing EPS.
LAB strains that produced EPS on agar plates were selected and tested for EPS production in liquid MRS medium, supplemented with the same sugars, using the microhaematocrit capillaries method, as described by Ricciardi et al. (1997).
EPS concentration in liquid media (MRS, mMRS, sMRS) was measured by using the Dubois method, after removing simple carbohydrates with desalting gel according to Ricciardi et al. (1998). Eight strains identified as Lb. plantarum and Lb. paraplantarum showing an EPS concentration >50 mg/l (data not shown) were used for a further experiment in mMRS liquid measuring EPS concentration (Dubois method) and viscosity with a digital viscosimeter (Brookfield DV-I+; Brookfield Engineering Laboratories, Stoughton, MA, USA) with a small sample adapter and a S21C coaxial cylinder spindle at 100 rev/min at 10°C.
Leavening in simulated doughs
Two strains (Lb. plantarum DBPZ1015 and W. cibaria DBPZ1006) selected on the basis of technological properties were tested in sourdough fermentation, in pure culture and in association with Saccharomyces cerevisiae MTG10 isolated from Cornetto di Matera sourdoughs (Ricciardi et al. 2002).
Cells were incubated up to the exponential growth phase (ca. 12 h), harvested by centrifugation (4800×g, 20 min, 4°C), washed once with sterile 0.85% (w/v) saline solution and resuspended in saline solution to obtain a cell suspensions of 108 colony-forming units (cfu)/ml.
The doughs were prepared mixing tap water, commercial wheat flour (type “00”; Divella S.p.A, Bari, Italy) and NaCl (1% w/w) manually for 5 min to obtain a dough yield (DY) of 150. The final concentration of cells was 107 cfu/g of dough. After mixing, the doughs were put in sterile cylinders and incubated at 30°C for 24 h. The decrease in pH (ΔpH) and the dough leavening were calculated at intervals of 1 h during incubation time as the difference between the values immediately after inoculation (t = 0 h) and the values at the successive time points. The pH values were measured using a spear tip pH electrode (Orion Research Inc. Runnings Center, Beverly, USA), while the dough leavening was determined measuring the volume increase in sterile cylinders.
Statistical analysis
Statistical analysis of SDS-PAGE patterns of whole cell proteins was carried out as described by Piraino et al. (2002), using a logarithmic transformation of molecular weight (log kDa). Classes (23; class width = 0.050 log kDa) were obtained in the range from 10 kDa (starting class) to 126 kDa (last class); flat range (FR) around the class centre and the membership in the flat range (MFR) were 30% and 99%, respectively, in all cases. Hierarchical cluster analysis (Unweighted Pair Group Method Using Average Linkage, UPGMA) was carried out on the Goodman-Krustal’s γ similarity matrix of the profiles.
All statistical analyses were performed using Systat 11.0 for Windows (SPSS, Chicago, IL, USA).
Results and discussion
Identification of lactic acid bacteria from sourdoughs
Forty-one strains of lactic acid bacteria (LAB) isolated from sourdoughs used for the production of the Cornetto, were randomly selected among a set of isolates obtained by numerical analysis of phenotypic profiles. In a previous study a total of 407 LAB strains were randomly isolated and characterized using a set of 25 phenotypic tests. 39 clusters were identified at the 80% similarity level using hierarchical cluster analysis. Of the isolates 85% were identified as omofermentative LAB (Lactobacillus plantarum, Lb. paracasei), 15% as heterofermentative LAB (Lb. fermentum, Weissella ssp., W. confusa) (Ricciardi et al. 2002). In order to obtain a representative subsample of the original composition of the microflora, for each phenotypic cluster, 10% of the isolates were randomly selected and identified using SDS-PAGE of whole cell proteins (WCP) (Ricciardi et al. 2002).
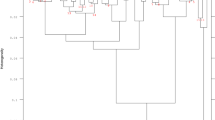
The similarity relationships among the WCP patterns of LAB and related reference or type strains are shown in Fig. 1. Eight major clusters were found at an arbitrary distance value of 0.24 (1-Kruskal γ). Most strains were identified as facultative heterofermentative lactobacilli belonging to Lactobacillus paraplantarum (cluster 2), Lb. plantarum (cluster 3), Lb. pentosus (cluster 4) and Lb. curvatus (cluster 6) species. Obligate heterofermentative strains belonging to Leuconostoc mesenteroides and Weissella cibaria were also identified (clusters 1 and 5, respectively). The strains of Leuc. mesenteroides were not identified at subspecies level because the SDS-PAGE pattern of Leuc. mesenteroides spp. mesenteroides and Leuc. mesenteroides spp. cremoris were very similar. SDS-PAGE of WCP, when carried out under standardized conditions is considered a highly reproducible technique whose results are comparable with DNA–DNA hybridization (Vandamme et al. 1996). We have found that it provides an excellent classification of lactic acid bacteria (Piraino et al. 2006).
Abridged dendrogram showing the distance between the 16 reference strains of lactic acid bacteria and the 41 bacterial isolates obtained from Cornetto di Matera sourdoughs. Hierarchical cluster analysis (Unweighted Pair Group Method Using Average Linkage, UPGMA) was carried out on the Goodman-Krustal’s γ similarity matrix of the profiles. The electrophoretic fingerprints are shown as processed profiles, by using a grey scale to report the intensities of the 23 classes of molecular mass that were defined in the gel. The number of isolates belonging each cluster is also shown
The percentage of obligate heterofermentative species found in our study is lower than that found in other Italian wheat sourdoughs (Corsetti et al. 2001; Gobbetti et al. 1994), in which Lb. sanfranciscensis was the dominant bacterial species in association with Lb. plantarum or with Lb. alimentarius. However, a similar composition in terms both of percentage of facultative and obligate heterofermentative strains and species association, was found in the sourdoughs used for the production of Altamura bread, in which the 88% of the isolates were identified as Lb. plantarum, Lb. casei and Lb. paracasei, 12% as Lb. brevis and Leuc. mesenteroides (Ricciardi et al. 2005). A study on the bacterial population in Sicilian traditional sourdoughs has also confirmed that Lb. plantarum strains may have a high prevalence: among the 45 sourdough LAB, 17 isolates were identified as Lb. sanfranciscensis, 14 as Lb. plantarum, 4 as Lb. kimchii/Lb. alimentarius and 3 as Lb. casei (Randazzo et al. 2005). High percentages of facultative heterofermentative LAB (Lb. plantarum and Lb. pentosus from 68% of facultative heterofermentative species in sourdoughs for Carasau bread to 98% in Moddizzosu bread) were found in the sourdoughs used to produce some typical breads of Sardinia region (Catzeddu et al. 2006). In this study the structure and the diversity of LAB communities demonstrated that obligate heterofermentative species did not dominate the microflora of traditional sourdoughs, with the exception of some samples of Zichi sourdoughs.
The identification of W. cibaria strains in our study confirms the results obtained by De Vuyst et al. (2002). W. cibaria, a species which is closely related to W. confusa (Bjorkroth et al. 2002), was first isolated from Greek traditional wheat sourdough manufactured without the addition of baker’s yeasts. The obligate heterofermentative species was associated with strains belonging to Lb. sanfranciscensis, Lb. brevis and Lb. paralimentarius to constitute an exclusive microbial consortium in Greek products. Strains of W. confusa, instead, were isolated from organic flours and sourdoughs produced in the Centre and Southern Italy together to strains of Lb. sanfranciscensis, Lb. brevis, Lb. alimentarius, Lb. plantarum, Lb. farciminis and Lb. fructivorans (Corsetti et al. 2003).
Association of Lb. plantarum, Lb. brevis and Lb. fermentum, and association of Lb. acidophilus and Lb. plantarum species dominate in Russian and Finnish rye sourdoughs, respectively (De Vuyst and Neyssens 2005). LAB belonging to Lb. brevis and Lb. curvatus species are the most frequently isolated in Portuguese sourdoughs manufactured with maize flours (Rocha and Malcata 1999).
Technological characterization of lactic acid bacteria
In order to select a multiple strain starter culture for the production of Cornetto, a preliminary technological characterization of the 41 LAB strains was performed. The strains were characterized on the basis of acid production ability, antimicrobial activity, exopolysaccharides (EPS) production, which are relevant technological properties for bread production.
Figure 2 shows the distribution of acid production ability of the strains after 6 and 24 h of incubation at 30°C in a synthetic medium (panels a and b, respectively). The decrease in pH (ΔpH) ranged from 0.1 to 1.1 at 6 h and from 0.7 to 1.8 at 24 h. A high variability among the species Lb. curvatus and Leuc. mesenteroides was found at both 6 and 24 h. On the contrary, the isolates belonging to W. cibaria and Lb. plantarum group (including Lb. plantarum, Lb. paraplantarum and Lb. pentosus species) showed a similar levels of acid production. W. cibaria and some isolates of Lb. plantarum and Lb. paraplantarum had the highest acid production ability after 6 h of incubation, while Lb. curvatus isolates had the lowest values of decrease in pH. At the end of incubation time, the majority of Lb. plantarum group isolates and few Leuc. mesenteroides isolates had the highest levels of acid production capability.
Box and whiskers plots showing the distribution of decrease in pH after 6 h (a) and 24 h (b) within strains of LAB isolated from sourdoughs for the production of Cornetto di Matera. Lb. curvatus (Lbcu); Lb. paraplantarum, Lb. pentosus, Lb. plantarum (Lbplgroup); Leuc. mesenteroides (Leme); W. cibaria (Wecb)
Dough acidification, due to the production of lactic acid from facultative heterofermentative LAB and lactic and acetic acids from heterofermentative strains, is an important metabolic activity in bread making. Production of organic acids during fermentation to achieve a pH value lower than 5.0 is effective in preventing rope spoilage of wheat bread which is usually caused by strains of Bacillus spp., especially Bacillus subtilis and B. licheniformis (Pepe et al. 2003; Pepe et al. 2004). Lactic and acetic acids produced by LAB during sourdough fermentation also have little direct effects on bread flavor. However, when combined with ethanol and other products of dough fermentation they enhance the perception of aroma (Röcken 1996). In fact, when the molar ratio lactate/acetate (the fermentation quotient, FQ) is in the range of 2.0–2.7, a pleasant odour of bread is perceived. pH decrease is also essential in obtaining the correct rheological and sensory properties of breads (Arendt et al. 2007). Production of organic acids decreases the digestion and absorption of the starch affecting positively the diet of diabetic subjects, cholesterol reduction and glucose tolerance (Pepe et al. 2004). Additionally, Leenhardt et al. (2005) found that acidification of dough due to LAB metabolism improves the nutritional properties of bread promoting the activation of endogenous flour phytases which leads the reduction of phytic acid, an anti-nutritional compound that chelates proteins, amino acids and divalent cations such Mg2+, Ca2+, Fe2+, Zn2+, hindering their adsorption by human intestinal mucosa.
Our data showed that facultatively and obbligately heterofermentative strains generate a significant drop in pH in 6 h of incubation; since the fermentative process for the production of Italian wheat sourdough breads occur in few hours (5–10 h; Gobbetti et al. 1996) some isolates of W. cibaria and Lb. plantarum group could be selected as producer of antifungal activity and to improve the nutritional properties of bread.
The stability of bread is also due to the production of antimicrobial compounds by sourdough lactic microflora. To evaluate the antimicrobial activity of the LAB isolates, a deferred antagonism assay was carried out, using as indicators strains that are starters or contaminants in sourdough baked products. Since antimicrobial compounds produced by the sourdough microflora play an important role in the regulation of the complex interactions within the starter microflora and between the starter and contaminant microflora (Messens and De Vuyst 2002), the indicators were chosen to be representative of facultatively heterofermentative bacteria isolated from sourdoughs (Lb. plantarum and Lb. paracasei) (De Vuyst and Neysens 2005; Ricciardi et al. 2005), of spore formers found in flours (B. cereus) or because of high sensitivity to class I or class II bacteriocins (M. flavus and L. innocua) (Klaenhammer 1993).
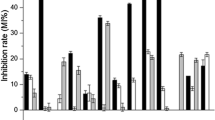
W. cibaria and Leuc. mesenteroides strains did not show any antimicrobial activity, while Lb. curvatus isolates did not show inhibitory activity against M. flavus and B. cereus. Lb. plantarum had the largest spectrum of inhibition with differences among the isolates. Several isolates, in fact, produced inhibition zones against L. innocua and Lb. plantarum, others against M. flavus and Lb. paracasei. All the strains exhibited low antagonistic activity against B. cereus.
Table 1 shows the results of the six LAB that presented an inhibitory activity of proteinaceus nature (bacteriocin-like inhibitory substance, BLIS). The resistance to proteolytic enzymes was tested using as indicators the strains that showed the widest inhibition diameter zones. Except for Lb. plantarum DBPZ1019, all other inhibitory activities were sensitive to the action of the three proteolytic enzymes. All the strains were inhibitory against Lb. plantarum, while only two strains (Lb. curvatus DBPZ1024 and Lb. plantarum DBPZ1019) showed inhibition against B. cereus.
Studies on the production of antimicrobial compounds by LAB sourdoughs are important to reduce microbial contamination during fermentation (Pepe et al. 2003; Corsetti et al. 1996) and to explain competition among sourdough microflora, in order to select competitive starters (Torodov et al. 1999). As reported by Messens and De Vuyst (2002) the screening of LAB strains for bacteriocin production is most promising to isolate strains that are adapted to this food ecosystem and that may produce bacteriocins in situ. In our study, all the isolates produced inhibition zones against other Lactobacillus strains, even if the profiles were strain specific (data not shown), in agreement with Corsetti et al. (1996). In this work, the authors found and isolated a bacteriocin-like inhibitory substance (BLIS) from Lb. sanfranciscensis C57. In a later work, Corsetti et al. (2004) confirmed the presence of BLIS in two strains of each Lb. plantarum and Lb. pentosus isolated from sourdoughs. The BLIS compounds were characterized by a limited inhibitory spectrum and showed no inhibition against Bacillus spp., L. innocua and yeasts. Settanni et al. (2005) demonstrated that the in situ activity of a BLIS produced by Lc. lactic ssp. lactis M30 during sourdough fermentation influences the sourdough microflora and may support the desired implantation of selected BLIS-insensitive bacteria (Lb. sanfranciscensis) useful to confer good characteristics to the dough, inhibiting other LAB frequently emerging (Lb. plantarum) as dominant bacteria during sourdough propagation (Corsetti et al. 2004). The production of bacteriocins by Lb. plantarum species isolated from sourdoughs was demonstrated by Todorov et al. (1999) which found and characterized the bacteriocin plantaricin ST341 from Lb. plantarum ST31. Lb. amylovorus DCE 471, an important starter culture of type II rye sourdoughs because of its strong and fast acidifying capability, has been also recognized as producer of bacteriocin (amylovorin) during sourdough fermentation (Messens and De Vuyst 2002).
The production of bacteriocins from sourdough lactobacilli is an important technological trait, and some strains of Lb. plantarum tested in our study, showing a wide spectrum of inhibition, could be selected for further experiments in order to select a starter culture to use protective culture, contributing to the safer products and reducing the addition of chemical preservative used by food industry.
The 41 strains of LAB were also tested for the production of EPS on solid and liquid media (Table 2 ). Lb. curvatus strains were not able to produce EPS in solid media, whereas all strains of Leuc. mesenteroides and W. cibaria produced dextran from sucrose. Some strains of Lb. plantarum, Lb. paraplantarum and Lb. pentosus were able to produce EPS from different sugars in solid media. The isolates of Lb. plantarum and Lb. paraplantarum showed the highest EPS production when grown in liquid medium containing maltose as carbon source. However, relative high efflux times were observed in several strains of Lb. plantarum and two strains of Lb. paraplantarum when grown on MRS broth containing glucose or sucrose, respectively, as a carbon source. On the contrary, all strains of Leuc. mesenteroides and W. cibaria had the highest EPS production from liquid MRS medium containing sucrose.
Eight strains belonging to Lb. plantarum and Lb. paraplantarum species showing an EPS concentration >50 mg/l (Table 3) were used for a further experiment in mMRS liquid in which EPS concentration and viscosity were measured.
The amount of EPS produced by Lb. plantarum and Lb. paraplantarum strains ranged from 140 to 297 mg/l. The highest value of viscosity among Lb. plantarum group was observed for the strain Lb. plantarum DBPZ1014. Viscosity and EPS concentration were not correlated. When Lb. plantarum DBPZ0998 or Lb. plantarum DBPZ1015 were grown on mMRS, the liquid medium had high viscosity, but the production of EPS was very low; on the contrary, the medium fermented with Lb. paraplantarum DBPZ0997 had the lowest value of viscosity but high EPS concentration.
EPS production during sourdough fermentation can potentially affect rheological properties of the dough, as volume, texture and keepability of the bread (Di Cagno et al. 2006; Korakli et al. 2001). EPS produced in situ have been found to have positive effect on the technological properties of dough and bread improving water absorption of the dough, dough rheology and machinability, dough stability during frozen storage, loaf volume and bread staling (Arendt et al. 2007, Tieking and Ganzle 2005).
Korakli et al. (2003) found that Lb. sanfranciscensis LTH2590 was able to produce a levan-type fructan from sucrose, which positively affected dough rheology and bread texture, while Lb. reuteri LB121 was shown to produce glucan and levan by the same sugar source (van Geel-Schutten et al. 1998). EPS may influence the intestinal flora, because oligofructose and fructans of the levan and inulin types are known to selectively stimulate the growth of bifidobacteria (Tieking et al. 2003) improving the nutritional properties of sourdough fermented products and selectively stimulated the growth of bifidobacteria during cultivation of human faecal microflora in vitro (Di Cagno et al. 2006; Arendt et al. 2007).
Lacaze et al. (2007) found that the dextran produced by Leuc. mesenteroides LMGP-16878 isolated from sourdough have multiple advantages in bakery applications; in particular, dextran being a hydrocolloid can bind high amounts of water improving freshness of the end product. Dextran enhances dough stability and gas retention increasing bread volume and crumb softness.
Our results showed that W. cibaria and Lb. plantarum strains were able to produce EPS in MRS agar containing sucrose according to Di Cagno et al. (2006). In this work the authors found that the strains belonging to W. cibaria, Lb. plantarum and P. pentosaceus species, selected after the screening in solid media, produced amounts of EPS ranging from 153 and 388 mg/l after 1 day of growth on MRS broth with sucrose as carbon source. These levels of EPS production were similar to those obtained in our study by some strains of Lb. plantarum (140–297 mg/l) cultivated in MRS broth containing maltose. Considering that in situ production of EPS by W. cibaria WC4 e Lb. plantarum PL9, the best-producing EPS strains selected by Di Cagno et al. (2006), after 7 h of fermentation in dough reached the final concentration of 2.5 g/kg of dough, our strains could be selected for further study on the production and purification of EPS.
Selection of a defined strains starter culture
In order to select a defined strain starter culture for the production of the Cornetto di Matera, W. cibaria DBPZ1006 and Lb. plantarum DBPZ1015 were tested in sourdough fermentations in pure culture and in association with a sourdough yeast (S. cerevisiae MTG10).
In a previous work (Zotta et al. 2006) we found that the fermentation of doughs started with Lb. plantarum DBPZ1015 resulted in a considerable hydrolysis of salt-soluble protein (albumins and globulins) after 6 h fermentations, increasing the low MM proteins fractions. After 24 h of fermentations, the hydrolysis of gliadin and glutenin fractions resulted in new protein fragments of 20 and 57 kDa, respectively. In a later work, Zotta et al. (2007) confirmed the high proteolytic activity of Lb. plantarum DBPZ1015 and demonstrated the presence of high β-glucosidase and phosphatase activity for W. cibaria DBPZ1006. On the basis of these technological properties (high proteolytic activity of Lb. plantarum and the presence of high β-glucosidase and phosphatase activity for W. cibaria) and those found in this work (high acidifying capability, EPS production and antimicrobial activity), the two strains were selected as possible defined starter culture to improve the flavour, aroma, texture and nutritional value of bread. The percentage of the volume increase (% Vol) for all doughs is shown in Fig. 3a. All yeasts/LAB associations and the yeasts alone caused an increase in volume around 140% after 2 h of incubation. The doughs started with S. cerevisiae in association (D4, D5 and D6) showed the same values of volume increase. The LAB in pure culture had different time courses: as expected, W. cibaria was capable to produce a dough with sufficient leavening (143% increased volume) at 24 h of fermentation when used alone (D3) or in association with the yeast (D5), with no additive effect. The yeast in pure colture (D1) showed a similar volume increase (around 154%) at the end of fermentation, while in association with Lb. plantarum and W. cibaria (D6) the volume increase was only 149%, probably due to a negative effect of the interaction yeast/LAB. In mixed culture, yeast growth might be negatively affected by the rapid pH drop caused by the LAB and the metabolites produced, i.e. lactic and acetic acid (Paramithiotis et al. 2007). It is known that sourdough yeasts and LAB have different kinetics for carbohydrate uptake. Most yeasts found in sourdoughs take up hexoses and maltose by high affinity transport systems, while the disaccharide uptake of LAB is strictly dependent on the external sugar concentration and is less effective (Gobbetti 1998). These results were confirmed by the plate count of the strains in pure and in mixed culture: the yeasts and LAB in mixed cultures had a reduced growth (data not shown).
Acid production and leavening ability in model sourdough fermentation: (a) percentage of the volume increase (%Vol) and (b) decrease in pH of the doughs during incubation at 30°C. S. cerevisiae MTG10 (●, D1); Lb. plantarum DBPZ1015 (▴, D2); W. cibaria DBPZ1006 (■, D3); S. cerevisiae MTG10 + Lb. plantarum DBPZ1015 (○, D4); S. cerevisiae MTG10 + W. cibaria DBPZ1006 (△, D5); S. cerevisiae MTG10 + Lb. plantarum DBPZ1015 + W. cibaria DBPZ1006 (□, D6)
As to the acidification (Fig. 3b), the pH decreased after 24 h of incubation to reach ΔpH values of 2.1 and 1.7, respectively for Lb. plantarum in pure culture and in association with S. cerevisiae. The interaction yeasts/LAB showed a reduction of the acidification ability: the sourdoughs started with associations of LAB and yeasts showed a different acid production and acidifying ability, with pH values between 4.49 and 4.71.
Bacterial growth and production of lactic and acetic acids decreased when S. cerevisiae was associated with LAB strains probably because of the faster consumption of maltose and glucose by yeast. The imbalance between consumption by yeast and starch hydrolysis by flour enzymes may led to the rapid depletion of soluble carbohydrates during sourdough fermentation which, in turn, decreases LAB acidification. In particular, in a previous study (Zotta et al. 2006) cell counts of facultative heterofermentative LAB were affected by the presence or absence of yeasts to a lesser extent than cell counts of heterofermentative LAB.
Conclusions
In this study lactic acid bacteria isolated from sourdoughs used for the production of Cornetto were identified and characterized on the basis of technological properties, in order to select strains to be used as starters in bread production.
Recent studies (Ferchichi et al. 2007; Catzeddu et al. 2006; Randazzo et al. 2005; Ricciardi et al. 2005) showed that facultative heterofermentative species like Lb. casei, Lb. paracasei, Lb. pentosus, Lb. plantarum and species of the genera Leuconostoc and Weissella are important members of sourdough microflora.
On the basis of their technological characteristics (high acidifying capability, antimicrobial activity and EPS production) the mixed culture selected in this work could be used, to formulate a mixed culture for bread production.
References
Arendt EK, Ryan LAM, Dal Bello F (2007) Impact of sourdough on the texture of bread. Food Microbiol 24:165–174
Bjorkroth KJ, Schillinger U, Geisen R, Weiss N, Hoste B, Holzapfel WH, Korkeala HJ, Vandamme P (2002) Taxonomic study of Weissella confusa and description of Weissella cibaria sp. nov., detected in food clinical samples. Int J System Evol Microbiol 52:141–148
Catzeddu P, Mura E, Parente E, Sanna M, Farris GA (2006) Molecular characterization of lactic acid bacteria from sourdough breads produced in Sardinia (Italy) and multivariate statistical analyses of results. Syst Appl Microbiol 29:138–144
Corsetti A, Gobbetti M, Smacchi E (1996) Antibacterial activity of sourdough lactic acid bacteria: isolation of a bacteriocin-like inhibitory substance from Lactobacillus sanfrancisco C57. Food Microbiol 13:447–456
Corsetti A, Lavermicocca P, Morea M, Baruzzi F, Tosti N, Gobbetti M (2001) Phenothypic and molecular identification and clustering of lactic acid bacteria and yeasts from wheat (species Triticum durum and Triticum aestivum) sourdoughs of Southern Italy. Int J Food Microbiol 64:95–104
Corsetti A, De Angelis M, Dellaglio F, Paparella A, Fox PF, Settani L, Gobbetti M (2003) Characterization of sourdough lactic acid bacteria based on genotypic and cell-wall protein analyses. J Appl Microbiol 94:641–654
Corsetti A, Settanni L, Van Sinderen D (2004) Characterization of bacteriocin-like inhibitory substances (BLIS) from sourdough lactic acid bacteria and evaluation of their in vitro and in situ activity. J Appl Microbiol 96:521–534
De Vuyst L, Neysens P (2005) The sourdough microflora: biodiversity and metabolic interactions. Trends Food Sci Technol 16:1–14
De Vuyst L, Vancanneyt M (2007) Biodiversity and identification of sourdough lactic acid bacteria. Food Microbiol 24:120–127
De Vuyst L, Schrijvers V, Paramithiotis S, Hoste B, Vancanneyt M, Swings J, Kalantzopoulos G, Tsakalidou E, Messens W (2002) The biodiversity of lactic acid bacteria in Greek traditional wheat sourdoughs is reflected in both composition and metabolic formation. Appl Environ Microbiol 68:6059–6069
Di Cagno R, De Angelis M, Limitone A, Minervini F, Carnevali P, Corsetti A, Gänzle M, Ciati R, Gobbetti M (2006) Glucan and Fructan production by sourdough Weissella cibaria and Lactobacillus plantarum. J Agric Food Chem 54:9873–9881
Ferchichi M, Valcheva R, Prévost H, Onno B, Dousset X (2007) Molecular identification of the microbiota of French sourdough using temporal temperature gradient gel electrophoresis. Food Microbiol 24:678–686
Gobbetti M (1998) The sourdough microflora: interactions of lactic acid bacteria and yeasts. Trends Food Sci Technol 9:267–274
Gobbetti M, Corsetti A, Rossi J, La Rosa F, De Vincenzi S (1994) Identification and clustering of lactic acid bacteria and yeasts from wheat sourdoughs of Central Italy. Ital J Food Sci 1:85–94
Gobbetti M, Smacchi E, Fox P, Stepaniak L, Corsetti A (1996) The sourdough microflora. Cellular localization and characterization of proteolytic enzymes in lactic acid bacteria. Lebensm Wiss Technol 29:561–569
INSOR (2000) Atlante dei Prodotti tipici: il pane (Eds. Agra-Rai Eri) pag. 13. Roma, Italy
Klaenhammer TR (1993) Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev 12:39–85
Kline L, Sugihara TF (1971) Microorganisms of the San Francisco sour dough bread process. II. Isolation and characterization of undescribed bacterial species responsible for the souring activity. Appl Microbiol 21:459–465
Korakli M, Rossmann A, Gänzle MG, Vogel RF (2001) Sucrose metabolism and exopolysaccharide production in wheat and rye sourdoughs by Lactobacillus sanfranciscensis. J Agric Food Chem 49:5194–5200
Korakli M, Pavlovic M, Gänzle MG, Vogel RF (2003) Exopolysaccharides and kestose production by Lactobacillus sanfranciscensis LTH2590. Appl Environ Microbiol 69:2073–2079
Lacaze G, Wick M, Cappelle S (2007) Emerging fermentation technologies: development of novel sourdoughs. Food Microbiol 24:155–160
Leenhardt F, Levrat-Verny M, Chanliaud E, Rémésy C (2005) Moderate decrease of pH by sourdough fermentation is sufficient to reduce phytate content of whole wheat flour through endogenous phytase activity. J Agric Food Chem 53:98–102
Messens W, De Vuyst L (2002) Inhibitory substances produced by Lactobacilli isolated from sourdoughs—a review. Int J Food Microbiol 72:31–43
Paramithiotis S, Gioulatos S, Tsakalidou E, Kalantzopoulos G (2007) Interactions between Saccharomyces cerevisiae and lactic acid bacteria in sourdough. Proc Biochem 41:2429–2433
Parente E, Grieco S, Crudele MA (2001) Phenotypic diversity of lactic acid bacteria isolated from fermented sausages produced in Basilicata (Southern Italy). J Appl Microbiol 90:943–952
Pepe O, Blaiotta G, Moschetti G, Greco T, Villani F (2003) Rope-producing strains of Bacillus spp. from wheat bread and strategy for their control by lactic acid bacteria. Appl Environ Microbiol 69:2321–2339
Pepe O, Blaiotta G, Anastasio M, Moschetti G, Ercolini D, Villani F (2004) Technological and molecular diversity of Lactobacillus plantarum strains isolated from naturally fermented sourdoughs. Syst Appl Microbiol 27:443–453
Piraino P, Ricciardi A, Lanorte MT, Malkhazova I, Parente E (2002) A procedure for data reduction in electrophoretic fingerprints. Biotechnol Lett 24:1477–1482
Piraino P, Ricciardi A, Salzano G, Zotta T, Parente E (2006) Use of unsupervised and supervised artificial neural networks for the identification of lactic acid bacteria on the basis of SDS-PAGE patterns of whole cell proteins. J Microbiol Methods 66:336–346
Randazzo CL, Heilig H, Restuccia C, Giudici P, Caggia C (2005) Bacterial population in traditional sourdough evaluated by molecular methods. J Appl Microbiol 99:251–258
Ricciardi A, Parente E, Clementi F (1997) A simple method for the screening of lactic acid bacteria for the production of exopolysaccharides. Biotechnol Tech 11:271–275
Ricciardi A, Parente E, Aquino M, Clementi F (1998) Use of desalting gel for the rapid separation of simple sugars from exopolysaccharides produced by lactic acid bacteria. Biotechnol Tech 12:649–652
Ricciardi A, Paraggio M, Salzano G, Andreotti G, De Fina M, Romano P (2002) La microflora di impasti utilizzati per la produzione del Cornetto di Matera e del Pane di Altamura. Tecnica Molitoria 8:780–787
Ricciardi A, Parente E, Piraino P, Paraggio M, Romano P (2005) Phenotypic characterization of lactic acid bacteria from sourdoughs for Altamura bread produced in Apulia (Southern Italy). Int J Food Microbiol 98:63–72
Rocha MJ, Malcata XF (1999) On the microbiological profile of traditional Portuguese sourdough. J Food Proteomics 62:1416–1429
Röcken W (1996) Applied aspects of sourdough fermentation. Adv Food Sci 18:212–216
Settanni L, Massitti O, Van Sinderen D, Corsetti A (2005) In situ activity of a bacteriocin-producing Lactococcus lactis strain. Influence on the interactions between lactic acid bacteria during sourdough fermentation. J Appl Microbiol 99:670–681
Tieking M, Gänzle M (2005) Exopolysaccharides from cereal-associated lactobacilli. Trends Food Sci Technol 16:79–84
Tieking M, Korakli M, Ehrmann MA, Gänzle MG, Vogel RF (2003) In situ production of exopolysaccharides during sourdough fermentation by cereal and intestinal isolates of lactic acid bacteria. Appl Environ Microbiol 69:945–952
Torodov S, Onno B, Sorokine O, Chobert JM, Ivanova I, Dousset X (1999) Detection and characterization of a novel antibacterial substance produced by Lactobacillus plantarum ST31 isolated from sourdough. Int J Food Microbiol 48:167–177
Vandamme P, Pot B, Gillis M, Kersters K, Swings J (1996) Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol Rev 60:407–438
van Geel-Schutten GH., Flesch F, ten Brink B, Smith MR, Dijkhuizen L (1998) Screening and characterization of Lactobacillus strains producing large amounts of exopolysaccharides. Appl Microbiol Biotech 50:697–703
Vogel RF, Knorr R, Muller MRA, Steudel U, Gänzle MG, Ehrmann MA (1999) Non-dairy lactic fermentations: the cereal world. Antonie van Leeuwenhoek 76:403–411
Zotta T, Piraino P, Ricciardi A, McSweeney PLH, Parente E (2006) Proteolysis in Model Sourdough Fermentations. J Agric Food Chem 54: 2567–2574
Zotta T, Ricciardi A, Parente E (2007) Enzymatic activities of lactic acid bacteria isolated from Cornetto di Matera sourdough. Int J Food Microbiol 115:165–172
Acknowledgements
This research was supported by funds from Agenzia Lucana di Sviluppo e di Innovazione in Agricoltura (ALSIA), Italy
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zotta, T., Piraino, P., Parente, E. et al. Characterization of lactic acid bacteria isolated from sourdoughs for Cornetto, a traditional bread produced in Basilicata (Southern Italy). World J Microbiol Biotechnol 24, 1785–1795 (2008). https://doi.org/10.1007/s11274-008-9671-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9671-0