Abstract
The marine microalga Pavlova viridis (Prymnesiophyceae) is widely used in marine aquaculture industries of China for feeding bivalves and has been proposed as an alternative source of eicosapentaenoic acid (EPA). To investigate variation of its lipid and fatty acid compositions during laboratory and outdoor cultivation, a 60-1 photobioreactor was established in Nanjing, China. Outdoor cultivation, paralleled with laboratory cultures in mid-October, was performed from autumn through midwinter. The results showed that the total lipid and EPA contents of outdoor cultures were both lower than those of indoor cultures. When the outdoor temperature and illumination decreased, total lipid experienced no significant change. Although the level of saturated fatty acids decreased, polyunsaturated fatty acids, especially EPA, increased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyunsaturated fatty acids (PUFAs), especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are essential in human development and physiology and might also prevent some diseases (Kogteva and Beruglov 1998; Borowitzka 1995; Wen and Chen 2000). At present, the main commercial source of EPA is fish oil, but its purification involves many problems (Cohen and Cohen 1991; Cohen 1994; Belarbi et al. 2000). Therefore, searching for alternative sources of EPA is necessary and significant.
Microalga have been recognized as one of the most promising EPA producers. The commercial mass culture of microalga has been developed in many countries (Cohen et al. 1995; Spolaore et al. 2006). The marine microalga Pavlova viridis (Prymnesiophyceae) is extensively used in marine aquaculture industries of China for feeding bivalves at all stages of growth, mainly for its richness in EPA. Many works about optimizing of its laboratory culture have been reported (Jiang et al. 1997; Zhao and Sun 2005). The effects of temperature, illumination intensity, illumination time, pH value and nitrogen on the growth of P. viridis were examined and optimal conditions for normal growth indoors were obtained (Zhao and Sun 2004). However, there is no work concerning outdoor culture of this species reported. The effect of outdoor natural climate conditions on its chemical compositions also remains unclear.
Microalgal production systems can be kept indoors under relative stable conditions, which are supposed to improve the reliability of their output. In tropical and subtropical countries, microalga may also be grown outdoors at a lower cost and for the benefit of commercial production of EPA. In this case, biomass yields and biochemical compositions may vary widely, depending on the season and meteorological conditions (López-Elías et al. 2003).
The lipid content and the fatty acid compositions of P. viridis are primary determinants for producing EPA as well as feeding animals properly. These parameters show some changes during outdoor mass cultivation compared with those of laboratory culture. The objective of this study was to document variation of the total lipid and EPA content under laboratory and outdoor culture conditions. The lipid content and fatty acid compositions in P. viridis under two different outdoor climate conditions were also examined.
Materials and methods
Organism and culture conditions
The marine microalga Pavlova viridis (3012) was provided by the Institute of Oceanology, Chinese Academy of Sciences, Qingdao. It was maintained in sterilized artificial seawater (g l−1): NaCl, 25; MgSO4·7H2O, 3.5; MgCl2·6H2O, 2.5; CaCl2, 1.2; KCl, 0.8; NaHCO3, 0.2; KNO3, 0.25 and KH2PO4, 0.015 (Yang et al. 2002) enriched with f/2 medium (Guillard and Ryther 1962), and the vitamins were doubled (B1 200 μg l−1, B2 1 μg l−1, B12 1 μg l−1). Cultures were grown and maintained at 23 ± 1°C under 12 h:12 h light:dark cycle provided by cool white fluorescent lights at 85–90 μmol photons m−2 s−1 irradiance.
Design of photobioreactor
This study was conducted in Jiangning district (Nanjing, China; latitude 31° 57′ N, longitude 118° 51′ E) in autumn and early winter, 2005.
The photobioreactor was consisted of 6 transparent PVC tubes (8 cm diameter, 0.2 mm wall thickness), which were laid horizontally and connected with two cement troughs (Fig. 1). The troughs were covered with plastic sheets at both ends. The sheets could be uncovered for sampling. One of the troughs was connected with an upper pool and a lower pool. During the working process, microalgal culture was lifted continuously from the lower to the upper pool through a pipeline powered with a pump, and flowed back to the lower pool after traveling in the tubes and troughs. Thus the culture cycled in the photobioreactor. The working volume of the bioreactor was maintained at 60-1 and the flow rate of the culture in tubes was maintained at 0.2 m3 min−1. Shading sheets 3 m above were adapted to avoid overheating and damage coming from sunshine during the strongest irradiation hours. The artificial seawater was prepared with the same nutrient recipe as that of indoors and filtered through 10 μm polypropylene filters.
Culture process and sampling regime
The growing process involved a batch scale-up routine. Using the 60-1 photobioreactor, the outdoor experiment was operated from September 10 through November 25, 2005. Three parallel samples were taken at 16:00 each day from the three separate sites (the ends of three tubes in the cement trough) in the bioreactor.
Laboratory experiments which paralleled with the early stage (from October 13 through October 22, Stage 1) of outdoor cultures were conducted in 3-l flasks (triplet) which were previously autoclaved at 121°C for 20 min with working volume 1-l. The cultivation condition was similar to the laboratory culture methods above. Daily work included shaking cultures 3 times and taking samples at 16:00 for the biochemical composition analysis.
We also chose the culture, which was from November 4 to November 17 (Stage 2) to compare with that of Stage 1. The main goal was to examine the changing of total lipid and fatty acid of P. viridis, which grew under lower temperature and illumination intensity than Stage 1 (with relative suitable growth conditions close to indoors).
Analytical procedures
Temperature reading was taken at 8:00, 14:00 and 16:00 each day during the whole culture process. Outdoor light levels were measured with a luminometer (TES-1332A, Taiwan) at the selected times. Cell counting was done using a 0.1-mm deep haemacytometer.
To determine the contents of total lipid and fatty acid profiles, biomass was collected directly from the photobioreactor or laboratory flasks, centrifuged at 800×g for 5 min. The biomass cake was washed with distilled water to remove non-biological material and then lyophilized. Total lipid was extracted according to Bligh and Dyer (1959) and quantified with the method described in Pande et al. (1963). Fatty acids were extracted and methylated according to Bousfield et al. (1983). The methyl esters were analysed by gas chromatography using a fused silica capillary column (30 m × 0.25 mm × 0.2 μm, Innowax, Hewlett-Packard, USA). The flow of carrier gas was helium and 1 μl methyl ester solution of sample was injected for each analysis. The temperature program was as follows: the initial temperature was 180°C, and rose to 200°C at a rate of 2°C min−1, then from 200°C to 250°C at a rate of 5°C min−1. The final oven temperature of 250°C was kept for 5 min. Fatty acids were identified from retention times of already known standards (Sigma) and mass spectra acquired by gas chromatography-mass spectrometry.
Results and discussion
The total light intensity received by indoor cultures in daytime remained constant. Outdoors, however, conditions varied according to the natural climate (Table 1). Water temperatures varied between 14.6°C (reading at 8:00) and 30.2°C (reading at 14:00) during Stage 1. Stage 2 was characterized by a much lower water temperature (from 9.6°C to 25.5°C) and illumination (from 23.3 to 286.4 μmol photons m−2 s−1). Temperature could be lower than 9.6°C at night. The pH of outdoor medium was at the range of 7.2–9.0.
Jiangning is located in the subtropical zone and characterized by a long spring and autumn, which benefits the outdoor cultivation of microalga. Outdoor cultivation can be performed from April to November according to the report of Zhao and Sun (2004). We chose Stage 1 to compare with indoors, for mean daytime temperature around this period is close to the optimal growth temperature of P. viridis.
The biochemical compositions of microalga can change with their growth rates and/or environmental conditions and with the phase of their life cycle (Richmond 1986). Unlike laboratory conditions, outdoor natural climates are complex and rigorous to microalga. Therefore, it might be possible that variations of outdoor natural conditions influence microalgal growth rate and biochemical constituents. At the same time, cultivation of microalga in outdoor photobioreactors is hampered by problems including overheating, fouling and accumulation of oxygen to toxic levels (Tredici and Materassi 1992). In our work, the bioreactor was shaded with black sheets 3 m above to prevent overheating during hours-long periods of strongest irradiation. The culture suffered a slight contamination. Some bacterial species in very low concentrations were observed in outdoor cultures of P. viridis.
Growth of P. viridis
Both indoor and outdoor Stage 1 cultures were grown for 10 days until the early stationary phase was achieved (Fig. 2). Outdoor culture of Stage 1 experienced a relative lower growth rate than indoors during the first 4 days. The cell densities at the 10th day were 1.03 × 107 cells ml−1 indoors and 9.95 × 106 cells ml−1 outdoors, respectively. At Stage 2, the microalga showed a rather slower growth rate and reached the early stationary phase with the cell density 6.5 × 106 cells ml−1 after 14 days.
The outdoor 60–1 culture was inoculated by a batch scale-up routine and a higher initial inoculation cell density was adopted. Meanwhile, enough illumination was obtained in Stage 1. Therefore, the culture showed a promising growth rate. As to Stage 2, growth of P. viridis was much slower than that of Stage 1 due to the rather low temperature and light irradiance. Indoor culture exhibited a typical microalgal growth pattern, which may be attributed to the optimal culture conditions.
Variation of total lipid and fatty acids
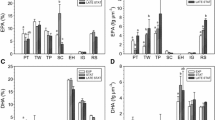
Compared to indoor culture, total lipid and EPA of outdoor Stage 1 both decreased by 14.7% and 12%, respectively. In Stage 2, total lipid kept decreasing and dropped to 10.4% in dry weight, while EPA increased to 25.2% in total lipid (Fig. 3). Figures 4 and 5 show the variation of total lipid content in Stage 1 and Stage 2, respectively. Both stages presented the same tendency that total lipid increased in the first 2 days, and gradually decreased in the early several days of the exponential phase, and then followed by another increase. Total lipid decreased to about 10% in dry weight during the stationary phase.
The major fatty acids in P. viridis were 14:0, 16:0, 16:1 and EPA. DHA was present in lower quantities. Compared with those of Stage 1, saturated fatty acids (SFAs) of Stage 2 decreased, whereas PUFAs, with the majority of EPA and DHA, experienced an increase. Monounsaturated fatty acids (MUFAs) remained rather stable (Table 2). EPA occupied about 2.1% in dry weight in Stage 1 and 2.5% in Stage 2.
Our study showed that percentage of total lipid in dry weight decreased during outdoor culture, whereas no significant changes were observed during cultivation of Stage 1 and Stage 2. Change of total lipid in Stage 1 and Stage 2 exhibited the same tendency. It appeared that content of total lipid decreased in the exponential phase and increased in early stationary phase. About 10% of the dry weight was total lipid at the end of both stages, despite differences of culture conditions and growth rates.
The fatty acid content of many microalgal species is affected by environmental factors. Light availability plays a major role in the lipid content of cells grown outdoors, mainly due to its effect on the energy supply to many of the biosynthetic pathways, as well as its effect on the ultrastructure of the cell organelles where lipids are an important component of their membrane composition. According to Renaud’s (1995) report, maximum lipid content coincided with an optimal range in growth temperature in many species. The content was lower at temperatures below and above this range.
Dong et al. (2004) found that percentages of EPA and DHA in total lipid of P. viridis were 23.3% and 11.5%, respectively. Our laboratory results were 24.1% and 12.8%, basically agreed with their reports. Hua et al. (1999) investigated the influence of temperature, light intensity on growth, total lipid and fatty acid compositions, and found that while P. viridis could grow at 15–30°C, it could grow faster and produce more lipid and EPA at 20°C, 96.15 μmol m−2 s−1, light:dark = 18 h:6 h.
Temperature has a major effect on the types of fatty acids produced by microalga. An inverse relationship has been recognized between temperature and fatty acid unsaturation in microalga (Mortensen et al. 1988; James et al. 1989; Thompson 1992b). However, the response to growth temperature varies from species to species, with no overall consistent relationship between temperature and fatty acid unsaturation.
As P. viridis has a high EPA content, study on how to improve EPA content by optimizing culture conditions is of commercial value. The effect of temperature on EPA biosynthesis appears to be complex and has not yet been clarified. Many works have been conducted with Nannochloropsis sp. (Zittelli et al. 1999) to enhance its EPA content. Seto et al. (1992) found that cells grown at 20°C contained 60% more EPA than those grown at the optimal growth temperature of 25°C. Sukenik et al. (1993b) showed that cultures grown at a low temperature (20°C) were characterized by a high rate of galactolipid synthesis and a high level of EPA, as compared with cells grown at a high temperature (30°C). However, Teshima et al. (1983) found maximal EPA synthesis at the optimal growth temperature of 25°C. In contrast to these findings, Zittelli et al. (1999) suggested that even when the fatty acid profile was deeply modified in response to an environmental stimulus, the total content of EPA in the biomass was not significantly affected. According to Sukenik’s (1993a) report, the highest cellular EPA content (3.8%) of Nannochloropsis sp was observed during winter, with water temperatures between 8°C and 16°C and under low irradiances, i.e. with conditions of very low biomass productivity. In our work with P. viridis, the EPA percentage in dry weight of Stage 2 (which was about 2.5%) was lower than this result, but it was higher than those of Stage 1 and indoors. Because temperature and irradiance vary during the day, their individual effect is not easy to establish in outdoor cultures. But Zittelli et al. (1999) supposed that lower night temperature plays a key role in influencing the fatty acid level and compositions.
Although EPA content increased in Stage 2, outdoor culture in conditions as Stage 2, which was characterized rather low temperature and low irradiance, may not be adoptable, because these climates could lead to a low productivity. In our study, only 6.5 × 106 cells ml−1 was achieved in Stage 2. Therefore, a balance between lipid content and microalgal productivity must be a major consideration.
Conclusion
In summary, using the 60-1 photobioreactor established outdoors, we tested lipid and EPA of P. viridis and compared them with laboratory cultures. The total lipid of outdoor cultures was lower than that of indoor cultures. When outdoor temperature and light declined, biomass productivity and lipid decreased; however, EPA experienced an increase. The present study may benefit further commercial mass culture of the marine microalga P. viridis for production of EPA. Because outdoor climate factors exert complex influences on the growth of this species, further work is required to optimize culture conditions. The mechanism of lipid synthesis at low temperature needs to be elucidated.
References
Belarbi EH, Molina E, Chisti Y (2000) A process for high yield and scaleable recovery of high purity eicosapentaenoic acid esters from microalgae and fish oil. Enzyme Microb Tech 26:516–529
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borowitzka MA (1995) Microalgae as sources of pharmaceuticals and other biologically active compounds. J Appl Phycol 7:3–15
Bousfield IJ, Smith GL, Dando TR, Hobbs G (1983) Numerical analysis of total fatty acid profiles in the identification of Coryneform, Nocardioform and some other bacteria. J Gen Microbiol 129:375–394
Cohen Z, Cohen S (1991) Preparation of eicosapentaenoic acid (EPA) concentrate from Porphyridium cruentum. Am Oil Chem Soc 68:16–19
Cohen Z (1994) Production potential of eicosapentaenoic acid by Monodus subterraneus. J Am Oil Chem Soc 71:941–945
Cohen Z, Norman HA, Heimer YM (1995) Microalgae as a source of ω-3 fatty acids. In: Simopoulos AP (ed) Plants in human nutrition, vol 77. Karger Basel, pp 1–32
Dong LH, You JT, Lin QQ, Hu R. Han BP (2004) Comparison of fatty acids composition in marine and freshwater microalgae. J Trop Subtrop Bot 12:226–232
Guillard RRL, Ryther JH (1962) Studies on marine planktonic diatoms I. Cyclotella nana Hustedt and Detonula comfervacea (Cleve). Gran Can J Microbiol 8:229–239
Hua XM, Zhou HQ, Ding ZP (1999) Effect of temperature and illumination on the microalgae’s growth, total lipid and fatty acid composition. J Shanghai Fish Univ 8:309–315
James CM, Al-Hinty S, Salman AE (1989) Growth and n-3 fatty acid and amino acid composition of microalgae under different temperature regimes. Aquaculture 77:337–351
Jiang XM, Wang GC, Zhong MJ (1997) A testing Study on ecological conditions of cultivation of Pavlova viridis. J Zhejiang College Fis 16:175–182
Kogteva GS, Beruglov VV (1998) Unsaturated fatty acids as endogenous bioregulators. Biochemistry-Moscow 63:4–12
López-Elías JA, Voltolina D, Chavira-Ortega CO, Rodríguez-Rodríguez BB, Sánz-Gaxiola LM, Cordero-Esquivel B, Nieves M (2003) Mass production of microalgae in six commercial shrimp hatcheries of the Mexican northwest. Aquacult Eng 29:155–164
Mortensen SH, Borsheim KY, Rainuzzo JK, Knutsen G (1988) Fatty acid and elemental composition of the marine diatom Chaetoceros gracilis Schutt. Effects of silicate deprivation, temperature and light intensity. J Exp Mar Biol Ecol 122:173–185
Pande SV, Khan RP, Venkitasubramanian TA (1963) Microdetermination of lipids and serum total fatty acid. Anal Biochem 6:415–423
Renaud SM, Zhou HC, Parry DL, Loung-Van T, Woo KC (1995) Effect of temperature on the growth, total lipid content and fatty acid composition of recently isolated tropical microalgae Isochrysis sp., Nitzschia closterium, Nitzschia paleacea, and commercial species Isochrysis sp., (clone T.ISO). J Appl Phycol 7:595–602
Richmond A (1986) Cell response to environmental factors. In: Richmond A (ed) CRC handbook of microalgae mass culture. CRC Press, Boca Raton, pp 69–106
Seto A, Kumasaka K, Hosaka M, Kojima E, Kashiwakura M, Kato T (1992) Production of eicosapentaenoic acid by marine microalgae and its commercial utilization for aquaculture. In: Kyle DJ, Ratledge C (eds) Industrial application of single cell oils. American Oil Chemists’ Society, Champaign, IL, pp 219–234
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Sukenik A, Yamaguchi Y, Livne A (1993b) Alterations in lipid molecular species of the marine eustigmatophyte Nannochloropsis sp. J Phycol 29:620–626
Sukenik A, Zmora O, Carmeli Y (1993a) Biochemical quality of marine unicellular algae with special emphasis on lipid composition. II. Nannochloropsis sp. Aquaculture 117:313–326
Teshima S, Yamasaki S, Kanazawa A, Hirata H (1983) Effects of water temperature and salinity on eicosapenatenoic acid level of marine Chlorella. Bull Jpn Soc Sci Fish 49:805
Thompson PA (1992b) Effect of variation in temperature: II. On the fatty acid composition of eight species of marine-phytoplankton. J Phycol 28:488–497
Tredici MR, Materassi R (1992) From open ponds to vertical alveolar panels: the Italian experience in the development of reactors for the mass cultivation of phototrophic microorganisms. Appl Phycol 4:221–231
Wen Z, Chen F (2000) Production potential of eicosapentaenoic acid by the diatom Nitzchia laveis. Biotechnol Lett 22:27–33
Yang QY, Shi QQ, Chen BL, Wu SG (2002) Study on advancing the growth rate of Pavlova viridis Tseng with plant hormone. J Fujian Teach Univ (Nat Sci) 16: 80–83
Zhao SF, Sun HQ (2004) Studies on the ecological factors to the growth of Pavlova viridis. Fish Sci 23:9–11
Zhao SF, Sun HQ (2005) Effects of nitrogen, phosphorus and three heavy metals on the growth of Pavlova viridis. J Zhanjiang Ocean Univ 25:60–63
Zittelli GC, Lavista F, Bastianini A, Rodolfi L, Vincenzini M, Tredici MR (1999) Production of eicosapentaenoic acid by Nannochloropsis sp. cultures in outdoor tubular photobioreactors. J Biotecnol 70:299–312
Acknowledgements
This work was supported by grants from the China Postdoctoral Foundation (No. 2005037121) and the Agricultural Three Item Projects of Jiangsu Province (SX (2005) 098). We would like to thank Prof. Y.X. Ou for his kind advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, C., Li, M., Li, J. et al. Variation of lipid and fatty acid compositions of the marine microalga Pavlova viridis (Prymnesiophyceae) under laboratory and outdoor culture conditions. World J Microbiol Biotechnol 24, 1209–1214 (2008). https://doi.org/10.1007/s11274-007-9595-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-007-9595-0